Impact of Irrigation and Artificial Pollination on Macadamia: Fruit Set and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Sample Collection
2.4. Determination of Fruit Economic Indicators
2.5. Pollen Viability Determination
2.6. Physiological Index Determination
2.7. Statistical Methods
3. Results
3.1. Effects of Different Treatments on Morphology of Macadamia Flowers, Leaves, and Summer Shoots
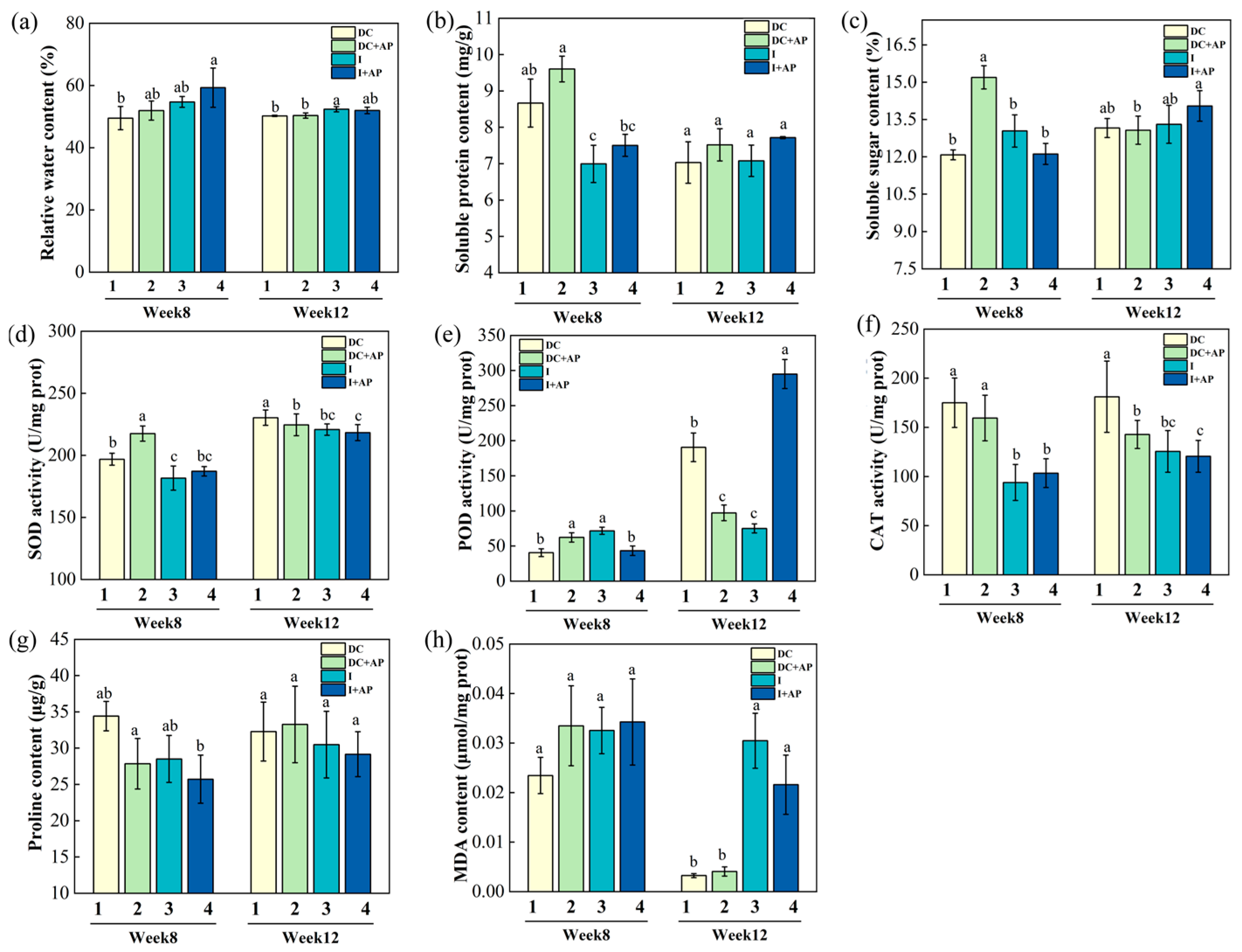
3.2. Effects of Different Treatments on Physiology of Macadamia Racemes
3.3. Effects of Different Treatments on Physiology of Macadamia Leaves
3.4. Effects of Different Treatments on Macadamia Fruit Setting Rate
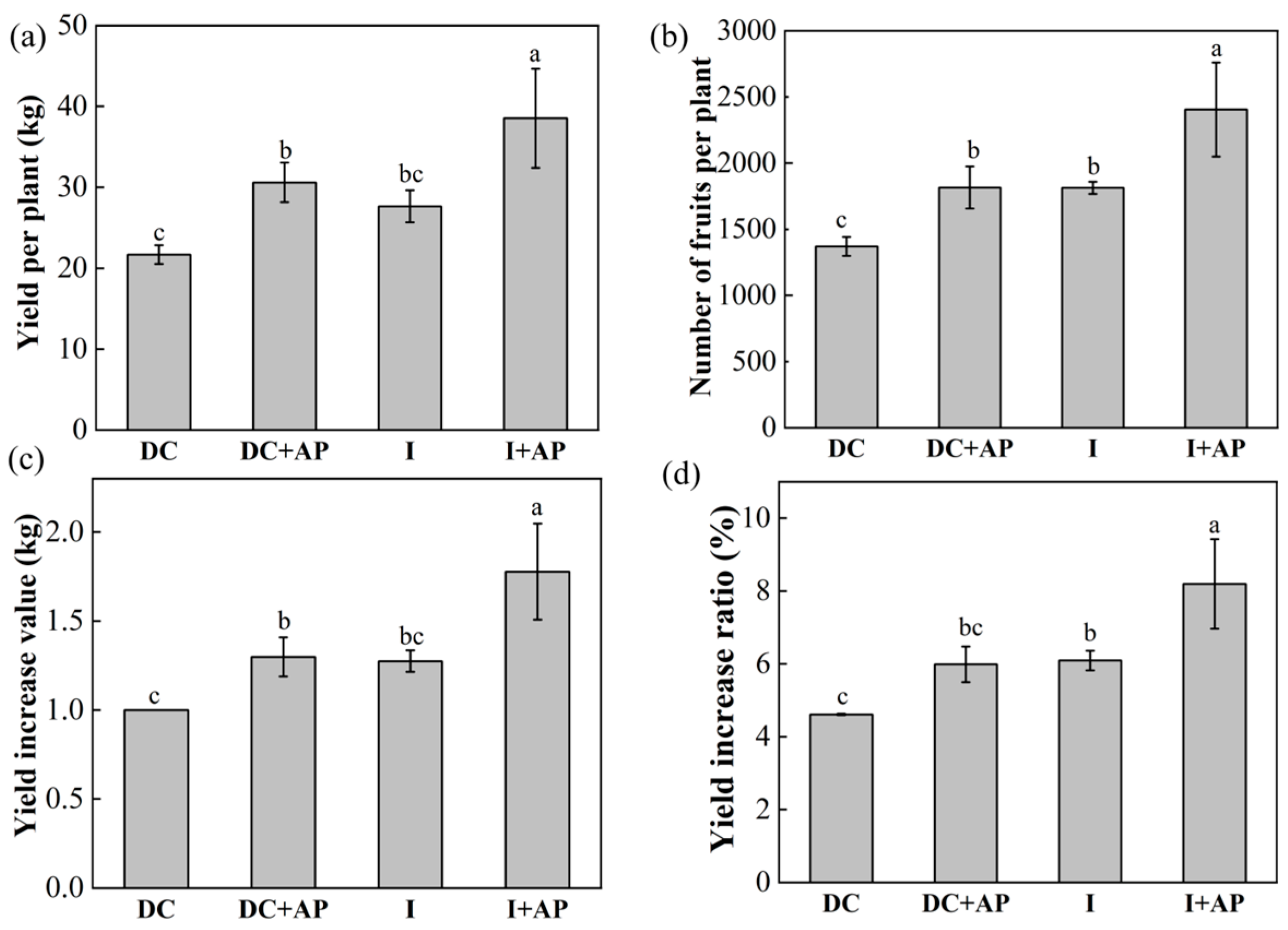
3.5. Effects of Different Treatments on Macadamia Yield
3.6. Effects of Different Treatments on the Economic Traits of Macadamia Nuts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOD | Superoxide Dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
References
- Rahman, A.; Wang, S.; Yan, J.S.; Xu, H.R. Intact macadamia nut quality assessment using near-infrared spectroscopy and multivariate analysis. J. Food Compos. Anal. 2021, 102, 104033. [Google Scholar] [CrossRef]
- Shabalala, M.; Toucher, M.; Clulow, A. The Macadamia bloom—What are the hydrological implications? Sci. Hortic. 2022, 292, 110628. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, S.; Tan, Q.; Wang, W.; Zhao, L.; Zhang, C.; Bao, Y.; Liu, Q.; Xiao, J.; Deng, K.; et al. Chromosomal-level genome of macadamia (Macadamia integrifolia). Trop. Plants 2022, 1, 3. [Google Scholar] [CrossRef]
- Nock, C.J.; Baten, A.; Mauleon, R.; Langdon, K.S.; Topp, B.; Hardner, C.; Furtado, A.; Henry, R.J.; King, G.J. Chromosome-scale assembly and annotation of the Macadamia genome (Macadamia integrifolia HAES 741). G3-Genes Genomes Genet. 2020, 10, 3497–3504. [Google Scholar] [CrossRef]
- Bringhenti, T.; Joubert, E.; Abdulai, I.; Hoffmann, M.; Moriondo, M.; Taylor, P.; Roetter, R. Effects of environmental drivers and irrigation on yields of macadamia orchards along an altitudinal gradient in South Africa. Sci. Hortic. 2023, 321, 112326. [Google Scholar] [CrossRef]
- Hardner, C.M.; Wall, M.; Cho, A. Global macadamia science: Overview of the special section. HortScience 2019, 54, 592–595. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Q.; Liu, Y.; Li, D. Managing Macadamia Decline: A Review and Proposed Biological Control Strategies. Agronomy 2024, 14, 308. [Google Scholar] [CrossRef]
- Ellis, K.L.; Anderson, J.M.; Yonow, T.; Kriticos, D.J.; Andrew, N.R. Biology and ecology of insect pests in macadamia: A review of the current status of IPM strategies in Australia. J. Integr. Pest Manag. 2023, 14, 26. [Google Scholar] [CrossRef]
- Herbert, S.W.; Walton, D.A.; Wallace, H.M. The influence of pollen-parent and carbohydrate availability on macadamia yield and nut size. Sci. Hortic. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Zhang, X.; Ma, X.; Zhang, S.; Chen, S.; Wang, Y.; Jia, H.; Liao, Z.; Lin, J.; et al. Signatures of selection in recently domesticated macadamia. Nat. Commun. 2022, 13, 242. [Google Scholar] [CrossRef]
- Hardner, C. Macadamia domestication in Hawai‘i. Genet. Resour. Crop Evol. 2016, 63, 1411–1430. [Google Scholar] [CrossRef]
- Langdon, K.S.; King, G.J.; Nock, C.J. DNA paternity testing indicates unexpectedly high levels of self-fertilisation in macadamia. Tree Genet. Genomes 2019, 15, 29. [Google Scholar] [CrossRef]
- Ni, S.-B.; Liu, J.-F.; Li, D.-G.; Jiang, J.-G.; Deng, Y.-Y.; He, X.-Y.; Tao, L.; Chen, G.-Y.; Xiao, G.-Z.; Chen, L.-L. Effects of water stress on Macadamia plants at their flowering stage. J. Southwest Agric. Univ. 2002, 24, 34–37. (In Chianese) [Google Scholar]
- Gong, L.; Ni, S.; He, X.; Tao, L.; Fang, Q.; Liu, J.; Li, Y.; Ma, J. Study on water consumption law and irrigation system of Macadamia. Chin. Agric. Sci. Bull. 2015, 31, 99–102. (In Chinese) [Google Scholar]
- Perdoná, M.J.; Soratto, R.P. Higher yield and economic benefits are achieved in the macadamia crop by irrigation and intercropping with coffee. Sci. Hortic. 2015, 185, 59–67. [Google Scholar] [CrossRef]
- Trochoulias, T.; Johns, G. Poor response of macadamia (Macadamia integrifolia Maiden and Betche) to irrigation in a high rainfall area of subtropical Australia. Aust. J. Exp. Agric. 1992, 32, 507–512. [Google Scholar] [CrossRef]
- Howlett, B.G.; Nelson, W.R.; Pattemore, D.E.; Gee, M. Pollination of macadamia: Review and opportunities for improving yields. Sci. Hortic. 2015, 197, 411–419. [Google Scholar] [CrossRef]
- Zhou, Z.-J.; Zhao, Z.-X.; Zhou, J.-J.; Yang, F.; Zhang, J.-Z. Boron supplementation and phytohormone application: Effects on development, fruit set, and yield in Macadamia cultivar ‘A4’ (Macadamia integrifolia, M. tetraphylla). Plants 2025, 14, 2461. [Google Scholar] [CrossRef]
- Howlett, B.G.; Read, S.F.J.; Alavi, M.; Cutting, B.T.; Nelson, W.R.; Goodwin, R.M.; Cross, S.; Thorp, T.G.; Pattemore, D.E. Cross-pollination enhances Macadamia yields, even with branch-level resource limitation. Hortscience 2019, 54, 609–615. [Google Scholar] [CrossRef]
- Sedgley, M.; Bell, F.D.H.; Bell, D.; Winks, C.W.; Pattison, S.J.; Hancock, T.W. Self- and cross-compatibility of macadamia cultivars. J. Hortic. Sci. 1990, 65, 205–213. [Google Scholar] [CrossRef]
- Tao, L.; Chen, L.L.; Yang, F.; Tao, L.; Ni, S.B.; Zhang, H.W.; He, X.Y. Study on pollination combination selection and self-fruitfulness of 7 Macadamia cultivars. South China Fruits 2018, 47, 55–58. (In Chinese) [Google Scholar] [CrossRef]
- He, X.-Y.; Tao, L.; Ni, S.-B.; Chen, L.-L.; Zhang, H.-W. Study on pollination variety selection and fruit characteristics of Macadamia Kau. South China Fruits 2016, 45, 38–42. (In Chinese) [Google Scholar] [CrossRef]
- Kaur, P.; Cowan, M.; De Faveri, J.; Alam, M.; Topp, B. Evaluating self-pollination methods: Their impact on nut set and nutlet abscission in Macadamia. Plants 2024, 13, 3456. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Bai, H.; Fan, S.; Wan, X.; Yang, J.; Li, Z.; Su, J.; Zhang, Y.; Wu, J.; Zhao, Y. Research advances in Macadamia breeding system. World For. Res. 2022, 35, 37–41. (In Chinese) [Google Scholar] [CrossRef]
- Kong, G.-H.; Tao, L.; He, X.-Y.; Ni, S.-B.; Chen, L.-L.; Chen, X.-M. Effects of different pollination combinations on fruit setting and fruit size of macadamia variety ‘HAES863’. China Fruits 2024, 3, 93–97. (In Chinese) [Google Scholar] [CrossRef]
- Tao, L.; He, X.-Y.; Chen, L.-L.; Xiao, X.-M.; Ni, S.-B. Pollination of Macadamia cultivar HAES900 and observation of the growth of pollen tubes under fluorescent microscope. Chin. J. Trop. Crops 2010, 31, 49–53. [Google Scholar]
- de Vargas, R.J.; Facchin, S.L.; Traini, C.; Cinosi, N.; Villa, F.; Portarena, S.; Sánchez-Piñero, M.; Brunetti, M.; Baiocco, A.; Stabile, M.; et al. The Efficiency of Artificial Pollination on the Hazelnut ‘Tonda Francescana®’ Cultivar and the Xenia Effects of Different Pollinizers. Horticulturae 2025, 11, 724. [Google Scholar] [CrossRef]
- Ferrari, T. Improving odds of success for supplemental pollination of almonds. Hortscience 2003, 38, 740. [Google Scholar]
- Gharaghani, A.; Javarzari, A.M.; Rezaei, A.; Nejati, R. Kaolin Spray Improves Growth, Physiological Functions, Yield, and Nut Quality of ‘Tardy Nonpareil’ Almond Under Deficit Irrigation Regimens. Erwerbs-Obstbau 2023, 65, 989–1001. [Google Scholar] [CrossRef]
- Kulahcilar, A.; Tonkaz, T.; Bostan, S.Z. Effect of irrigation regimes by mini sprinkler on yield and pomological traits in “Tombul” hazelnut. Acta Hortic. 2018, 1226, 301–308. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Gonzalez-Dugo, V.; García-Tejero, I.F.; López-Urrea, R.; Intrigliolo, D.S.; Egea, G. Quantitative analysis of almond yield response to irrigation regimes in Mediterranean Spain. Agric. Water Manag. 2023, 279, 108208. [Google Scholar] [CrossRef]
- Kadri, K.; Elsafy, M.; Makhlouf, S.; Awad, M.A. Effect of pollination time, the hour of daytime, pollen storage temperature and duration on pollen viability, germinability, and fruit set of date palm (Phoenix dactylifera L.) cv “Deglet Nour”. Saudi J. Biol. Sci. 2022, 29, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.-W.; Wang, F.-L.; Zhao, P.; Fu, G.-Q.; Tang, W.-D. Effects of drought stress on water content, photosynthetic characteristics and antioxidant enzymes of Artemisia desertorum leaves. J. Northwest For. Univ. 2025, 40, 42–50. (In Chinese) [Google Scholar]
- Chu, L.-L.; Zheng, W.-X.; Liu, H.-Q.; Sheng, X.-X.; Wang, Q.-Y.; Wang, Y.; Hu, C.-G.; Zhang, J.-Z. ACC SYNTHASE4 inhibits gibberellin biosynthesis and FLOWERING LOCUS T expression during citrus flowering. Plant Physiol. 2024, 195, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Deans, C.A.; Sword, G.A.; Lenhart, P.A.; Burkness, E.; Hutchison, W.D.; Behmer, S.T. Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. JoVE 2018, 138, e58164. [Google Scholar] [CrossRef]
- Sena, F.; Monza, J.; Signorelli, S. Determination of Free Proline in Plants. In ROS Signaling in Plants: Methods and Protocols; Corpas, F.J., Palma, J.M., Eds.; Springer: New York, NY, USA, 2024; pp. 183–194. [Google Scholar]
- Meyers, N.; Huett, D.; Morris, S.; McFadyen, L.; McConchie, C. Investigation of sampling procedures to determine macadamia fruit quality in orchards. Aust. J. Exp. Agric. 1999, 39, 1007–1012. [Google Scholar] [CrossRef]
- Stephenson, R.A.; Ko, H.L.; Gallagher, E.C. Plant-water relations of stressed, non-bearing macadamia trees. Sci. Hortic. 1989, 39, 41–53. [Google Scholar] [CrossRef]
- Smit, T.G.; Taylor, N.J.; Midgley, S.J.E. The seasonal regulation of gas exchange and water relations of field grown macadamia. Sci. Hortic. 2020, 267, 109346. [Google Scholar] [CrossRef]
- Wallace, H.M.; Vithanage, V.; Exley, E.M. The effect of supplementary pollination on nut set of Macadamia (Proteaceae). Ann. Bot. 1996, 78, 765–773. [Google Scholar] [CrossRef]
- Armand Hendrik, S. The impact of water stress at different phenological stages on the yield and quality of Macadamia (F. Muell). Masters Abstr. Int. 2021, 85, 30943040. [Google Scholar]
- Jian-Ju, L.; Shu-Bang, N.; Xi-Yong, H.; Qing-Lin, T.; Dao-Gao, L.; Chao-Ai, L. The relationship between water stress and pollen development of Australian. South China Fruits 2003, 5, 40–41. [Google Scholar]
- Trueman, S.J.; Penter, M.G.; Malagodi-Braga, K.S.; Nichols, J.; De Silva, A.L.; Ramos, A.T.M.; Moriya, L.M.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; et al. High outcrossing levels among global Macadamia cultivars: Implications for nut quality, orchard designs and pollinator management. Horticulturae 2024, 10, 203. [Google Scholar] [CrossRef]
- McFadyen, L.M.; Robertson, D.; Sedgley, M.; Kristiansen, P.; Olesen, T. Post-pruning shoot growth increases fruit abscission and reduces stem carbohydrates and yield in macadamia. Ann. Bot. 2011, 107, 993–1001. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Althiab-Almasaud, R.; Teyssier, E.; Chervin, C.; Johnson, M.A.; Mollet, J.-C. Pollen viability, longevity, and function in angiosperms: Key drivers and prospects for improvement. Plant Reprod. 2024, 37, 273–293. [Google Scholar] [CrossRef]
- Albert, B.; Ressayre, A.; Dillmann, C.; Carlson, A.L.; Swanson, R.J.; Gouyon, P.-H.; Dobritsa, A.A. Effect of aperture number on pollen germination, survival and reproductive success in Arabidopsis thaliana. Ann. Bot. 2018, 121, 733–740. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, H.; Tian, B.; Sheng, D.; Xu, C.; Zhou, H.; Huang, S.; Wang, P. Flowering dynamics, pollen, and pistil contribution to grain yield in response to high temperature during maize flowering. Environ. Exp. Bot. 2019, 158, 80–88. [Google Scholar] [CrossRef]
- Jian-Fu, L.; Chang-Ji, C.; Song-Bai, L.; Shu-Bang, N.; Xi-Yong, H.; Gao-Zhong, X. The effect of water stress on the fertility of Australian. South China Fruits 2002, 3, 34–35. [Google Scholar]
- Yu, J.; Jiang, M.; Guo, C. Crop pollen development under drought: From the phenotype to the mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef]
- Gong, L.-D.; Ma, J.; Tao, L.; He, X.-Y. The effect of sustained drought on the osmotic regulation ability of Australian nut seedlings. Trop. Agric. Sci. Technol. 2018, 41, 23–26. [Google Scholar] [CrossRef]
- Scoffoni, C.; McKown, A.D.; Rawls, M.; Sack, L. Dynamics of leaf hydraulic conductance with water status: Quantification and analysis of species differences under steady state. J. Exp. Bot. 2012, 63, 643–658. [Google Scholar] [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Tian, Y.; Ni, S.; Yue, H. Physiological Responses of Macadamia Seedlings to Water Stress. Fujian For. Sci. Technol. 2008, 3, 27–32+47. (In Chinese) [Google Scholar] [CrossRef]
- Kang, Z.; Wang, R.; Guo, G.; Wang, D.; He, F.; Song, X.; Xu, X.; Zeng, H.; Wang, W.; Tao, L.; et al. Physiological Responses of Five Macadamia Nut Germplasm Materials to Cold Stress in Leaf Tissues. Econ. For. Res. 2025, 43, 1–9. Available online: https://link.cnki.net/urlid/43.1117.S.20250627.1054.004 (accessed on 15 August 2025).
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Olayen, W.; Brini, F. Chapter 17—Roles of enzymatic antioxidants in stress response and signaling in plants. In Defense-Related Proteins in Plants; Upadhyay, S.K., Ed.; Academic Press: Oxford, UK, 2024; pp. 413–468. [Google Scholar]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.; Zhang, X.; Wei, C. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, W.e.; Guo, G.; Pan, X.; Huang, D.; Wang, R.; Shen, X. Morphological and physiological responses of 14 macadamia rootstocks to drought stress and a comprehensive evaluation of drought resistance. Environ. Exp. Bot. 2024, 219, 105630. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar] [CrossRef]
- Aroca, R. Plant Responses to Drought Stress. From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–5. [Google Scholar]
- Kang, Z.; Cai, H.; Guo, G.; Zeng, H.; Wang, W.; Tu, X. Physiological response of macadamia (Macadamia integrifolia) seedlings to drought stress. Horticulturae 2025, 11, 347. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Kumar, S.; Sachdeva, S.; Bhat, K.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–25. [Google Scholar]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]



| Fruit Diameter (cm) | Week 1 | Week 2 | Week 4 | Week 6 | Week 20 |
|---|---|---|---|---|---|
| Drought | 0.30 ± 0.06 c | 0.44 ± 0.05 c | 1.09 ± 0.11 d | 2.79 ± 0.08 c | 2.99 ± 0.17 c |
| Drought + artificial pollination | 0.32 ± 0.04 b | 0.46 ± 0.04 b | 1.17 ± 0.06 c | 2.86 ± 0.07 b | 3.04 ± 0.07 ab |
| Irrigation | 0.32 ± 0.03 b | 0.47 ± 0.04 b | 1.19 ± 0.04 b | 2.86 ± 0.19 b | 3.03 ± 0.07 b |
| Irrigation + artificial pollination | 0.36 ± 0.05 a | 0.49 ± 0.04 a | 1.24 ± 0.05 a | 2.96 ± 0.08 a | 3.06 ± 0.07 a |
| Treatment | Drought | Drought + Artificial Pollination | Irrigation | Irrigation + Artificial Pollination |
|---|---|---|---|---|
| Transverse diameter of green-skinned fruits (cm) | 2.99 ± 0.16 a | 3.00 ± 0.14 a | 2.98 ± 0.14 a | 3.03 ± 0.13 a |
| Longitudinal diameter of green-skinned fruits (cm) | 3.43 ± 0.17 a | 3.47 ± 0.17 a | 3.42 ± 0.18 a | 3.44 ± 0.18 a |
| Aspect ratio of green-skinned fruits (cm) | 1.15 ± 0.04 a | 1.14 ± 0.03 a | 1.15 ± 0.04 a | 1.15 ± 0.03 a |
| green-skinned fruit weight (g) | 16.63 ± 2.44 a | 17.30 ± 2.32 a | 16.65 ± 2.07 a | 17.28 ± 2.34 a |
| Transverse diameter of shells (cm) | 2.33 ± 0.13 a | 2.38 ± 0.18 a | 2.33 ± 0.13 a | 2.35 ± 0.15 a |
| Longitudinal diameter of shells (cm) | 2.59 ± 0.16 a | 2.61 ± 0.14 a | 2.57 ± 0.13 a | 2.65 ± 0.14 a |
| Aspect ratio of shells (cm) | 1.11 ± 0.04 a | 1.1 ± 0.04 a | 1.1 ± 0.04 a | 1.13 ± 0.04 a |
| Shell weight (g) | 7.94 ± 1.56 a | 8.31 ± 1.43 a | 7.78 ± 1.08 a | 8.10 ± 1.41 a |
| Kernel yield (%) | 47.52 ± 2.51 a | 47.84 ± 2.39 a | 46.69 ± 2.70 a | 46.71 ± 2.90 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.-X.; Zhou, Z.-J.; Zhou, J.-J.; Li, J.-X.; Yang, F.; Yang, H.-X.; Zhang, J.-Z. Impact of Irrigation and Artificial Pollination on Macadamia: Fruit Set and Yield. Horticulturae 2025, 11, 1111. https://doi.org/10.3390/horticulturae11091111
Zhao Z-X, Zhou Z-J, Zhou J-J, Li J-X, Yang F, Yang H-X, Zhang J-Z. Impact of Irrigation and Artificial Pollination on Macadamia: Fruit Set and Yield. Horticulturae. 2025; 11(9):1111. https://doi.org/10.3390/horticulturae11091111
Chicago/Turabian StyleZhao, Zi-Xuan, Zhang-Jie Zhou, Jing-Jing Zhou, Jin-Xue Li, Fan Yang, Hong-Xia Yang, and Jin-Zhi Zhang. 2025. "Impact of Irrigation and Artificial Pollination on Macadamia: Fruit Set and Yield" Horticulturae 11, no. 9: 1111. https://doi.org/10.3390/horticulturae11091111
APA StyleZhao, Z.-X., Zhou, Z.-J., Zhou, J.-J., Li, J.-X., Yang, F., Yang, H.-X., & Zhang, J.-Z. (2025). Impact of Irrigation and Artificial Pollination on Macadamia: Fruit Set and Yield. Horticulturae, 11(9), 1111. https://doi.org/10.3390/horticulturae11091111








