Effects of Different Light Qualities and Intensities of Blue Light on Flowering and Volatiles in Coriander (Coriandrum sativum)
Abstract
1. Introduction
2. Materials and Methods
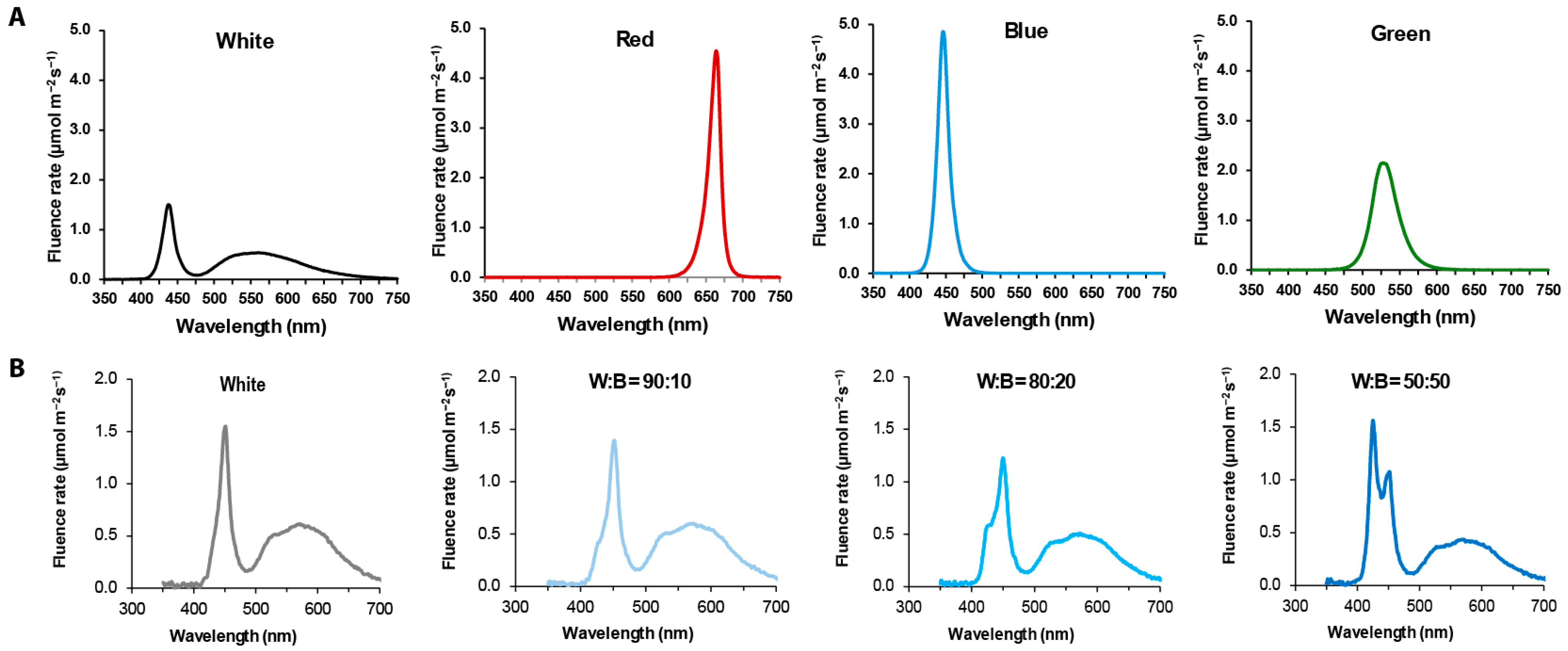
2.1. Plant Materials and Growth Conditions
2.2. Analyses of Growth and Volatiles
2.3. Statistical Data Analysis
3. Results
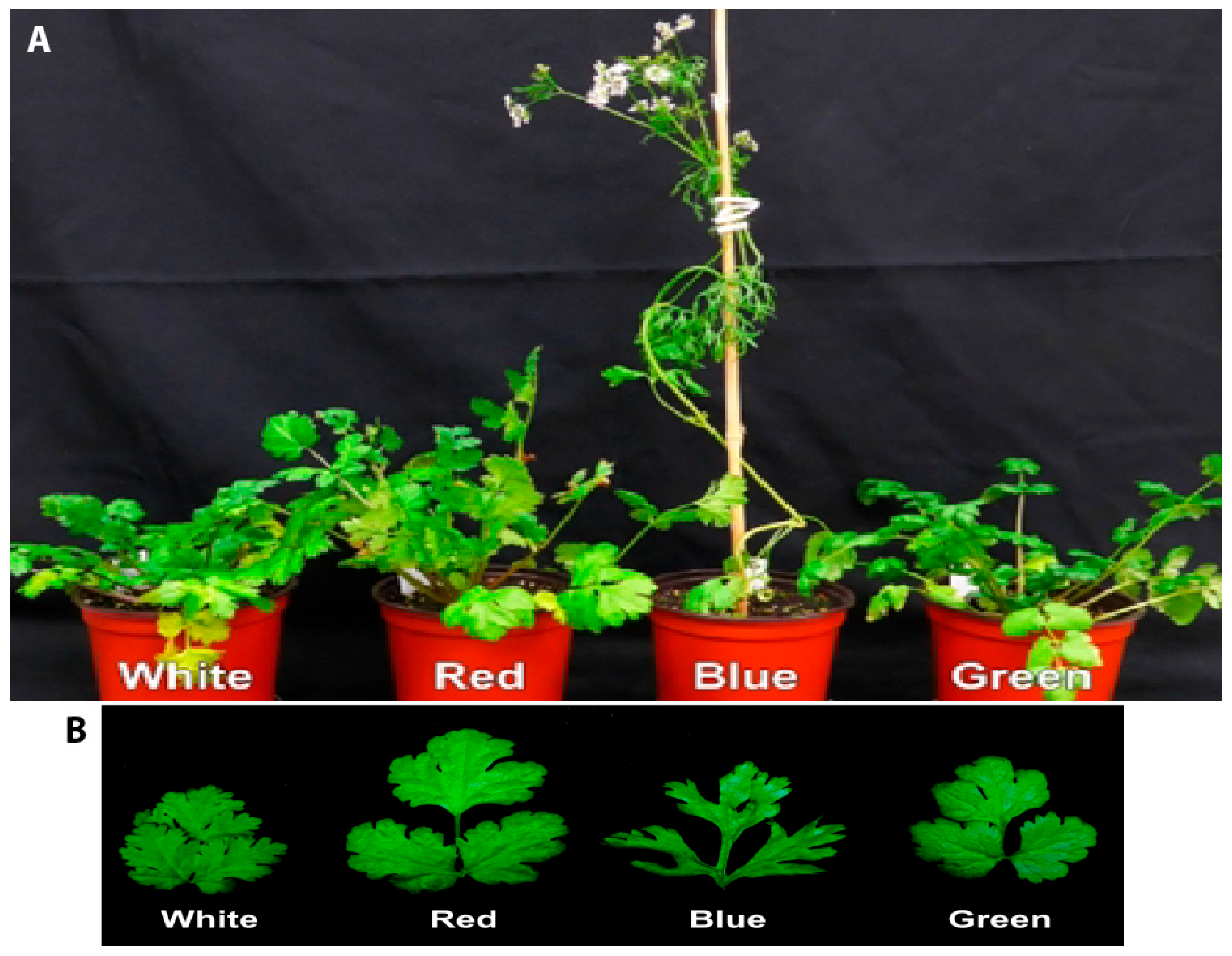
3.1. Analysis of the Growth of Coriander Plants Grown Under W, R, B, or G Light
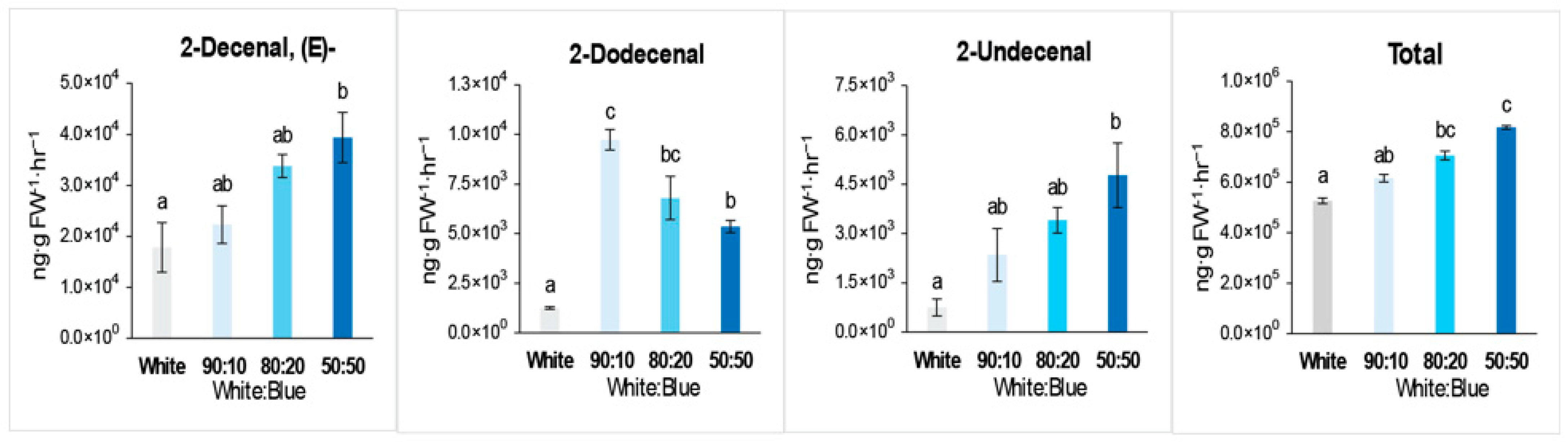
3.2. Analysis of the Growth in Coriander Plant Under Various Intensities of B Light
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Reg. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED red and Blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Diverse Flowering Response to Blue light Manipulation: Application of Electric Lighting in Controlled-Environment Plant Production. Horticulturae 2024, 10, 578. [Google Scholar] [CrossRef]
- Livadariu, O.; Maximilian, C.; Rahmanifar, B.; Cornea, C.P. LED Technology applied to plant development for promoting the accumulation of bioactive compounds: A review. Plants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Cammarisano, L.; Taylor, G.; Naznin, M.T.; Verdonk, J.C.; Ahamed, M.S. Impact of light spectral combinations on morphology, yield, and quality of indoor-grown cilantro. Front. Sustain. Food Syst. 2024, 8, 1499954. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–230. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kitayama, M.; Lu, N.; Takagaki, M. Improving secondary metabolite accumulation, mineral content, and growth of coriander (Coriandrum sativum L.) by regulating light quality in a plant factory. J. Hortic. Sci. Biotechnol. 2020, 95, 356–363. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Mockler, T.C.; Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998, 279, 1360–1363. [Google Scholar] [CrossRef]
- Fukuda, N.; Ishii, Y.; Ezura, H.; Olsen, J.E. Effects of light quality under red and B light emitting diodes on growth and expression of FBP28 in petunia. Acta Hortic. 2011, 907, 361–366. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue-light-promoted elongation and flowering are not artifacts from 24-h lighting: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 659–666. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kadowaki, M.; Che, J.; Takahashi, S.; Horiuchi, N.; Ogiwara, I. Influence of light quality on flowering characteristics, potential for year-round fruit production and fruit quality of blueberry in a plant factory. Fruits 2019, 74, 3–10. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Blue light supplemented at intervals in long-day conditions intervenes in photoperiodic flowering, photosynthesis, and antioxidant properties in chrysanthemums. Antioxidants 2022, 11, 2310. [Google Scholar] [CrossRef]
- Diederichsen, A. Coriander: Coriandrum sativum L.; Bioversity International: Rome, Italy, 1996; Volume 3, pp. 22–26. [Google Scholar]
- Diederichsen, A.; Hammer, K. The infraspecific taxa of coriander (Coriandrum sativum L.). Genet. Resour. Crop Evol. 2003, 50, 33–63. [Google Scholar] [CrossRef]
- Johnson, T.S.; Schwieterman, M.L.; Kim, J.Y.; Cho, K.H.; Clark, D.G.; Colquhoun, T.A. Lilium floral fragrance: A biochemical and genetic resource for aroma and flavor. Phytochemistry 2016, 122, 103–112. [Google Scholar] [CrossRef]
- Hiscox, J.T.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Buchenauer, H. Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f. sp. tritici. Physiol. Mol. Plant Pathol. 2000, 57, 25–34. [Google Scholar] [CrossRef]
- Folta, K.M.; Pontin, M.A.; Karlin-Neumann, G.; Bottini, R.; Spalding, E.P. Genomic and physiological studies of early cryptochrome 1 action demonstrate roles for auxin and gibberellin in the control of hypocotyl growth by blue light. Plant J. 2003, 36, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of light quality on vegetative cutting and in vitro propagation of coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of Band red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Fukuda, N.; Yoshida, T.; Olsen, J.E.; Senaha, C.; Jikumaru, Y.; Kamiya, Y. Short main shoot length and inhibition of floral bud development under red light can be recovered by application of gibberellin and cytokinin. Acta Hortic. 2012, 956, 215–222. [Google Scholar] [CrossRef]
- Gautam, P.; Terfa, M.T.; Olsen, J.E.; Torre, S. Red and blue light effects on morphology and flowering of Petunia × hybrida. Sci. Hortic. 2015, 184, 171–178. [Google Scholar] [CrossRef]
- Potter, T.L.; Fagerson, I.S. Composition of coriander leaf volatiles. J. Agric. Food Chem. 1990, 38, 2054–2056. [Google Scholar] [CrossRef]
- Carrubba, A.; Ascolillo, V.; Pagan Domenech, A.T.; Saiano, F.; Aiello, P. Modifications over time of volatile compounds in coriander (Coriandrum sativum L.). Acta Hortic. 2007, 826, 43–50. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tong, Y.-X.; Yang, Q.-C. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 2016, 7, 12699. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]


| White | Red | Blue | Green | |
|---|---|---|---|---|
| Height (cm) | 17.27 ± 0.72 a * | 20.70 ± 1.01 a | 42.57 ± 4.42 b | 21.40 ± 0.26 a |
| Leaf length (cm) | 2.97 ± 0.18 a | 4.27 ± 0.15 a | 3.50 ± 0.64 a | 3.13 ± 0.09 a |
| Leaf width (cm) | 3.83 ± 0.29 ab | 5.10 ± 0.23 b | 3.73 ± 0.38 a | 3.03 ± 0.26 a |
| Leaf number | 73.00 ± 5.51 b | 60.33 ± 9.91 b | 13.67 ± 6.77 a | 63.67 ± 6.23 b |
| Branch number | 10.67 ± 0.33 ab | 16.00 ± 2.31 b | 8.00 ± 1.53 a | 14.33 ± 0.67 ab |
| Flower number | 0.00 a | 0.00 a | 13.33 ± 3.76 b | 0.00 a |
| Days to 1st flower | 62 | 62 | 36 | 69 |
| White | Red | Blue | Green | |
|---|---|---|---|---|
| Chl a | 0.813 ± 0.087 ab * | 0.864 ± 0.042 b | 0.780 ± 0.005 a | 0.912 ± 0.071 ab |
| Chl b | 0.647 ± 0.085 a | 0.709 ± 0.076 a | 0.600 ± 0.005 a | 0.688 ± 0.050 a |
| Chl Total | 1.460 ± 0.171 a | 1.573 ± 0.118 a | 1.379 ± 0.009 a | 1.599 ± 0.120 a |
| Volatile Compounds | CAS | RT | White | Red | Blue | Green | Confirmed * |
|---|---|---|---|---|---|---|---|
| 2-Hexenal, (E)- | 6728-26-3 | 11.01 | 226.5 | 197.3 | 212.0 | 217.8 | Y |
| 3-Hexen-1-ol, (Z)- | 928-96-1 | 11.12 | 2080.8 | 637.0 | 1329.8 | 497.6 | Y |
| 2-Heptanone | 110-43-0 | 12.35 | 306.9 | 269.4 | 298.2 | 300.6 | Y |
| Nonane | 111-84-2 | 12.80 | 1528.3 | 2519.0 | 478.0 | 2412.0 | Y |
| Benzaldehyde | 100-52-7 | 15.12 | 1459.5 | 1765.0 | 2906.5 | 1979.0 | Y |
| 3-Hexen-1-ol, acetate, (Z)- | 3681-71-8 | 16.54 | 19,353.0 | 3348.6 | 15,976.0 | 2448.8 | Y |
| Dibutyl sulfide | 544-40-1 | 16.74 | 116.1 | 19.3 | 55.4 | 4.3 | |
| Benzyl alcohol | 100-51-6 | 17.65 | 114.0 | 132.0 | 159.8 | 100.4 | Y |
| D-Limonene | 138-86-3 | 17.71 | 124.1 | 104.0 | 117.8 | 112.6 | Y |
| Isobutyl isovalerate | 589-59-3 | 17.97 | 184.6 | 161.6 | 180.9 | 179.9 | |
| Phenylacetaldehyde | 122-78-1 | 18.09 | 1144.0 | 1591.0 | 3118.0 | 1794.8 | Y |
| Hexanoic acid, propyl ester | 626-77-7 | 19.63 | 194.4 | 167.6 | 184.0 | 199.7 | Y |
| Methyl benzoate | 93-58-3 | 19.94 | 1636.1 | 1583.1 | 1848.7 | 1939.0 | Y |
| Nonanal | 124-19-6 | 20.05 | 165.4 | 150.9 | 173.1 | 193.7 | Y |
| Octanoic acid, methyl ester | 111-11-5 | 20.65 | 286.6 | 236.0 | 258.4 | 294.3 | Y |
| Propane, 2-methoxy-2-methyl- | 1634-04-4 | 21.47 | 201.9 | 156.1 | 160.2 | 172.3 | |
| Hexyl butyrate | 2639-63-6 | 22.88 | 131.0 | 103.5 | 118.3 | 130.9 | Y |
| cis-4-Decenal | 21662-09-9 | 23.13 | 32.2 | 2.3 | 37.5 | 75.2 | |
| Naphthalene | 91-20-3 | 23.33 | 221,214.8 | 175,953.3 | 106,703.0 | 118,155.0 | |
| Decanal | 112-31-2 | 23.43 | 3617.8 | 2176.3 | 546.7 | 6277.0 | Y |
| 4-Undecene, 3-methyl-, (Z)- | 74645-87-7 | 24.80 | 65.3 | 13.7 | 55.2 | 172.7 | |
| 2-Decenal, (E)- | 3913-81-3 | 25.24 | 7766.7 | 1459.7 | 8007.3 | 19,012.1 | Y |
| 2-Decen-1-ol, (Z)- (citral) | 5392-40-5 | 25.37 | 541.3 | 131.7 | 1326.7 | 1939.2 | Y |
| 1-Decanol | 112-30-1 | 25.44 | 287.6 | 156.3 | 263.9 | 631.8 | Y |
| 2-Undecenal | 2463-77-6 | 28.33 | 556.7 | 138.3 | 795.3 | 1499.5 | |
| 3-Decen-1-ol, acetate, (Z)- | 81634-99-3 | 29.42 | 0.0 | 0.0 | 0.0 | 85.1 | |
| 2-Dodecenal | 4826-62-4 | 31.25 | 3200.2 | 909.4 | 776.8 | 3223.4 | |
| 8-Hexadecenal, 14-methyl-, (Z)- | 60609-53-2 | 33.99 | 64.3 | 18.8 | 30.4 | 151.8 | |
| 2-Tridecenal, (E)- | 7206-21-5 | 36.58 | 2021.2 | 241.3 | 403.6 | 1854.5 | |
| 13-Tetradecenal | 85896-31-7 | 39.02 | 70.1 | 9.3 | 11.8 | 119.4 | |
| Total | 303,560.0 | 223,623.2 | 175,786.2 | 197,814.7 |
| White | W:B = 90:10 | W:B = 80:20 | W:B = 50:50 | |
|---|---|---|---|---|
| Height (cm) | 33.77 ± 3.58 ab * | 35.70 ± 1.30 a | 34.60 ± 3.05 ab | 44.70 ± 2.66 b |
| Leaf length (cm) | 2.00 ± 0.20 a | 2.20 ± 0.53 a | 1.73 ± 0.09 a | 1.93 ± 0.15 a |
| Leaf width (cm) | 2.55 ± 0.15 a | 3.53 ± 0.47 a | 2.33 ± 0.15 a | 2.50 ± 0.21 a |
| Leaf number | 50.50 ± 9.50 a | 36.67 ± 11.14 a | 32.67 ± 5.84 a | 31.33 ± 8.41 a |
| Branch number | 16.67 ± 2.03 a | 15.33 ± 1.45 a | 13.00 ± 3.51 a | 12.00 ± 1.53 a |
| Stem diameter (cm) | 0.73 ± 0.09 a | 0.77 ± 0.24 a | 0.87 ± 0.19 a | 1.07 ± 0.17 a |
| Days to 1st flower | 52 | 52 | 52 | 38 |
| Flower cluster | 13.00 ± 4.36 ab | 5.67 ± 0.88 a | 11.67 ± 1.20 ab | 20.33 ± 2.33 b |
| Flower number | 30.67 ± 13.86 ab | 4.30 ± 1.86 a | 38.67 ± 1.45 ab | 79.67 ± 20.58 b |
| Flower node length (cm) | 2.70 ± 0.47 ab | 1.31 ± 0.14 a | 3.97 ± 0.53 bc | 5.32 ± 0.35 c |
| White | W:B = 90:10 | W:B = 80:20 | W:B = 50:50 | |
|---|---|---|---|---|
| Chlorophyll a | 0.575 ± 0.016 ab * | 0.640 ± 0.010 a | 0.489 ± 0.015 b | - |
| Chlorophyll b | 0.607 ± 0.042 a | 0.700 ± 0.023 a | 0.504 ± 0.015 a | - |
| Total chlorophyll | 1.182 ± 0.056 a | 1.339 ± 0.028 a | 0.993 ± 0.051 a | - |
| Free phenolics | 76.728 ± 0.788 b | 58.128 ± 1.615 a | 84.589 ± 1.580 c | 75.989 ± 0.975 b |
| Wall-bound phenolics | 155.869 ± 1.866 c | 102.970 ± 0.694 b | 150.779 ± 0.777 c | 76.758 ± 0.750 a |
| Total phenolics | 232.597 ± 2.673 b | 161.098 ± 1.140 a | 235.368 ± 2.103 b | 52.747 ± 1.722 a |
| Volatile Compounds | CAS | RT | White | W:B = 90:10 | W:B = 80:20 | W:B = 50:50 | Confirmed * |
|---|---|---|---|---|---|---|---|
| Nonane | 111-84-2 | 12.40 | 249.3 | 2905.5 | 135.2 | 2021.2 | Y |
| Pyrazine, 2,6-dimethyl- | 108-50-9 | 12.74 | 2348.8 | 3295.2 | 4082.1 | 2462.1 | Y |
| Benzaldehyde | 100-52-7 | 14.69 | 424.4 | 580.3 | 577.2 | 622.9 | Y |
| 1-Heptanol, 6-methyl- | 1653-40-3 | 15.61 | 226.0 | 224.0 | 304.7 | 304.8 | |
| Octanal | 124-13-0 | 16.07 | 796.1 | 606.4 | 509.4 | 643.6 | Y |
| 1-Pentanol, 2-methyl-, acetate | 7789-99-3 | 16.34 | 341.9 | 294.8 | 321.3 | 282.8 | |
| 3-Penten-2-one | 6976-27-8 | 16.99 | 433.8 | 455.0 | 666.8 | 658.8 | |
| 1-Heptanol, 3-methyl- | 1070-32-2 | 17.12 | 748.6 | 763.6 | 1009.3 | 1045.7 | |
| D-Limonene | 5989-27-5 | 17.27 | 229.9 | 219.3 | 256.8 | 321.9 | Y |
| (S)-(+)-5-Methyl-1-heptanol | 57803-73-3 | 17.43 | 187.6 | 199.0 | 280.3 | 284.9 | |
| 1-Octanol | 111-87-5 | 18.36 | 2740.6 | 2353.3 | 2583.6 | 2222.8 | Y |
| Octane, 1,1’-oxybis- | 629-82-3 | 18.87 | 158.5 | 132.5 | 216.1 | 181.7 | |
| Linalool | 78-70-6 | 19.50 | 5090.2 | 2063.7 | 3216.1 | 3055.1 | Y |
| Nonanal | 124-19-6 | 19.61 | 670.5 | 659.4 | 862.6 | 739.2 | Y |
| Undecane, 5,7-dimethyl- | 17312-83-3 | 19.80 | 188.1 | 173.1 | 282.3 | 229.6 | |
| Octanoic acid, methyl ester | 111-11-5 | 20.22 | 255.7 | 252.5 | 417.7 | 332.8 | Y |
| Benzyl nitrile (Benzyl cyanide) | 140-29-4 | 20.88 | 1554.0 | 1499.6 | 1895.1 | 1028.8 | Y |
| Lilac aldehyde B | 53447-45-3 | 21.37 | 0.0 | 162.3 | 0.0 | 65.1 | |
| cis-4-Decenal | 21662-09-9 | 22.69 | 247.2 | 315.4 | 313.3 | 371.9 | |
| Naphthalene | 91-20-3 | 22.85 | 15,474.4 | 17,116.1 | 21,508.1 | 22,775.7 | |
| Ocyl acetate | 112-14-1 | 23.02 | 6908.9 | 5436.5 | 4714.9 | 6480.2 | Y |
| 2-Decenal, (E)- | 3913-81-3 | 24.79 | 17,847.8 | 22,300.7 | 33,795.6 | 39,401.8 | Y |
| 2-Decen-1-ol, (E)- | 40642-37-3 | 24.92 | 5157.4 | 5583.1 | 4369.8 | 3225.3 | |
| Undecane, 3,8-dimethyl- | 17301-30-3 | 25.38 | 240.1 | 254.9 | 336.6 | 323.4 | |
| 10-Methylnonadecane | 56862-62-5 | 26.74 | 433.9 | 445.6 | 615.9 | 588.8 | |
| 2-Undecenal | 2463-77-6 | 27.87 | 757.7 | 2347.2 | 3407.0 | 4770.6 | |
| Ethyl decanoate | 110-38-3 | 28.62 | 93.3 | 105.3 | 85.1 | 398.5 | Y |
| 3-Heptadecenal | 1000143-48-7 | 28.82 | 124.1 | 191.0 | 169.0 | 198.5 | |
| Dodecanal | 2765-11-9 | 29.12 | 52.1 | 196.9 | 74.1 | 146.6 | |
| 2-Dodecenal | 4826-62-4 | 30.77 | 1247.3 | 9730.3 | 6791.9 | 5361.1 | |
| 2-Tridecenal, (E)- | 7206-21-5 | 33.49 | 28.9 | 91.4 | 74.6 | 72.3 | |
| 13-Tetradecenal | 85896-31-7 | 36.09 | 2312.3 | 4070.6 | 1192.3 | 1024.6 | |
| Total | 526,663.7 | 616,242.0 | 706,269.1 | 817,545.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Gennaro, M.D.; Cho, K.H.; Patt, J.M.; Colquhoun, T.A. Effects of Different Light Qualities and Intensities of Blue Light on Flowering and Volatiles in Coriander (Coriandrum sativum). Horticulturae 2025, 11, 1093. https://doi.org/10.3390/horticulturae11091093
Kim JY, Gennaro MD, Cho KH, Patt JM, Colquhoun TA. Effects of Different Light Qualities and Intensities of Blue Light on Flowering and Volatiles in Coriander (Coriandrum sativum). Horticulturae. 2025; 11(9):1093. https://doi.org/10.3390/horticulturae11091093
Chicago/Turabian StyleKim, Joo Young, Madelyn D. Gennaro, Keun Ho Cho, Joseph M. Patt, and Thomas A. Colquhoun. 2025. "Effects of Different Light Qualities and Intensities of Blue Light on Flowering and Volatiles in Coriander (Coriandrum sativum)" Horticulturae 11, no. 9: 1093. https://doi.org/10.3390/horticulturae11091093
APA StyleKim, J. Y., Gennaro, M. D., Cho, K. H., Patt, J. M., & Colquhoun, T. A. (2025). Effects of Different Light Qualities and Intensities of Blue Light on Flowering and Volatiles in Coriander (Coriandrum sativum). Horticulturae, 11(9), 1093. https://doi.org/10.3390/horticulturae11091093





