Transcriptome Analysis of Adventitious Bulblet Initiation in Lilium lancifolium Thunb
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Sucrose, Glucose, and Fructose Content Determination
2.3. Histomorphological Observation During Scale Initiation
2.4. RNA Extraction and cDNA Synthesis
2.5. Gene Expression Analysis by qRT-PCR
2.6. Transcriptome Sequencing, RNA-Seq Data Processing, and Transcripts De Novo Assembly
2.7. Gene Annotation, KEGG Enrichment, and Differential Expression Analysis
2.8. Statistical Analysis
3. Results
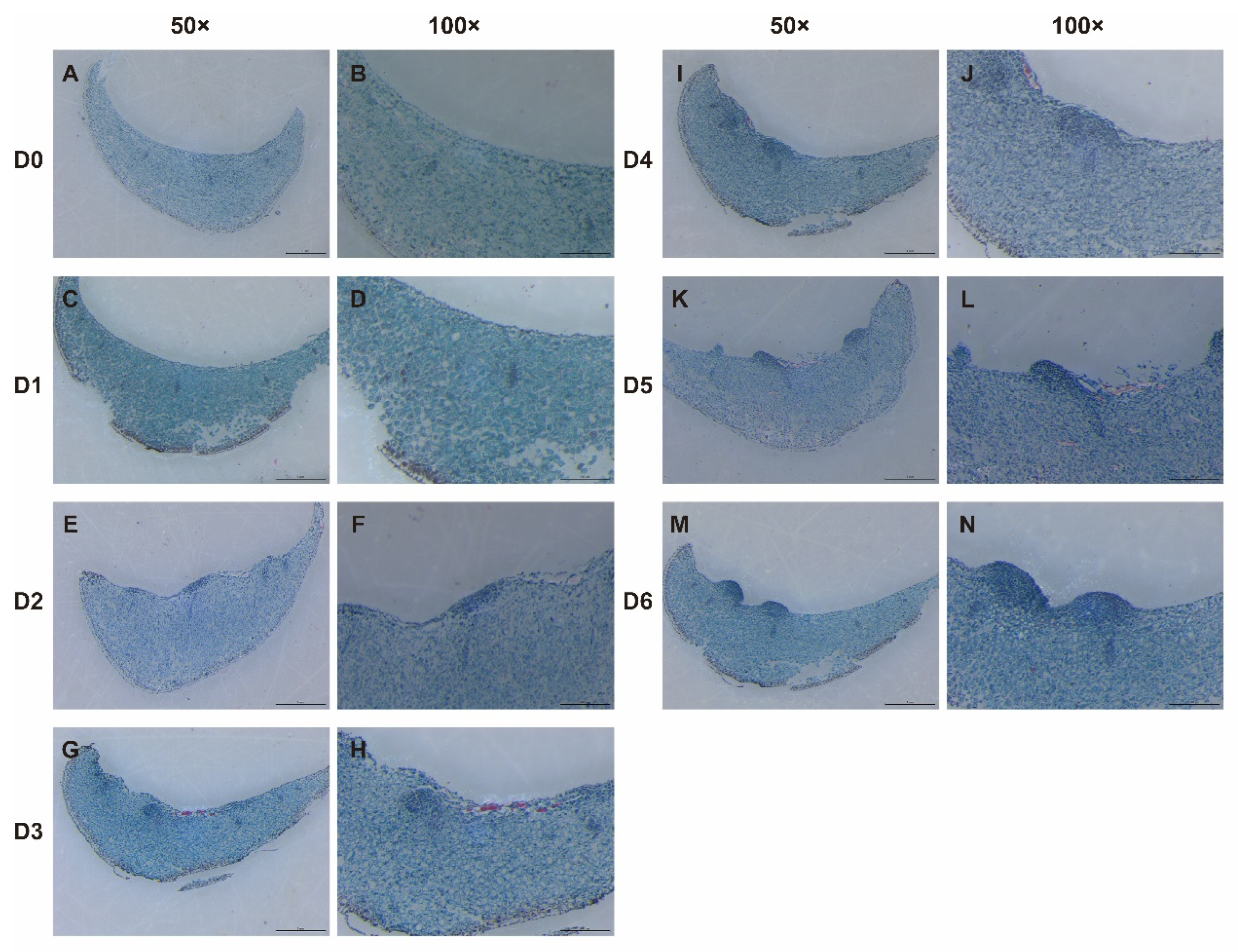
3.1. Histological Observation During Adventitious Bulblet Initiation
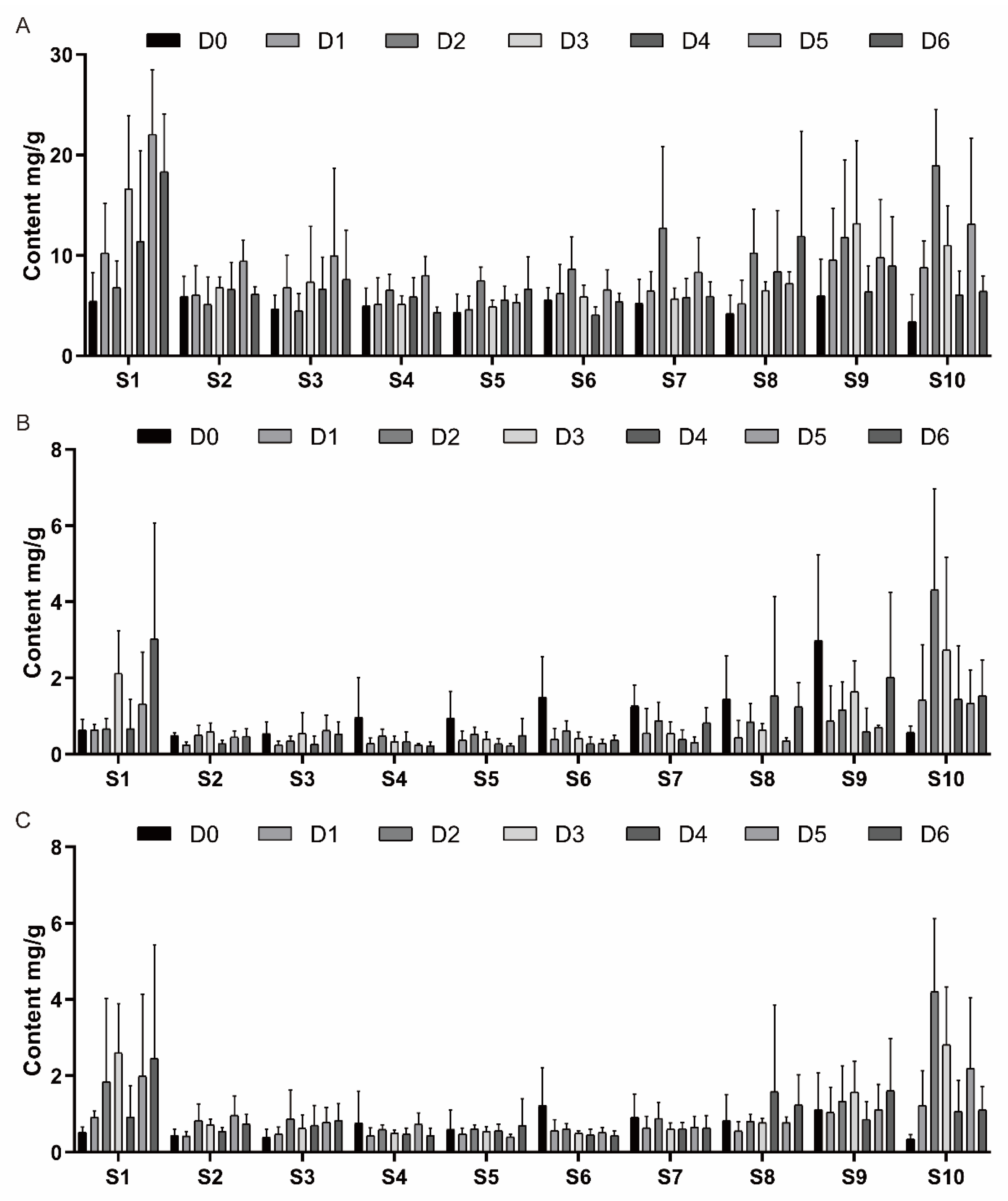
3.2. Changes in Sugar Contents During Scale Culture
3.3. RNA-Sequencing Statistics and Assembly Overview for L. lancifolium
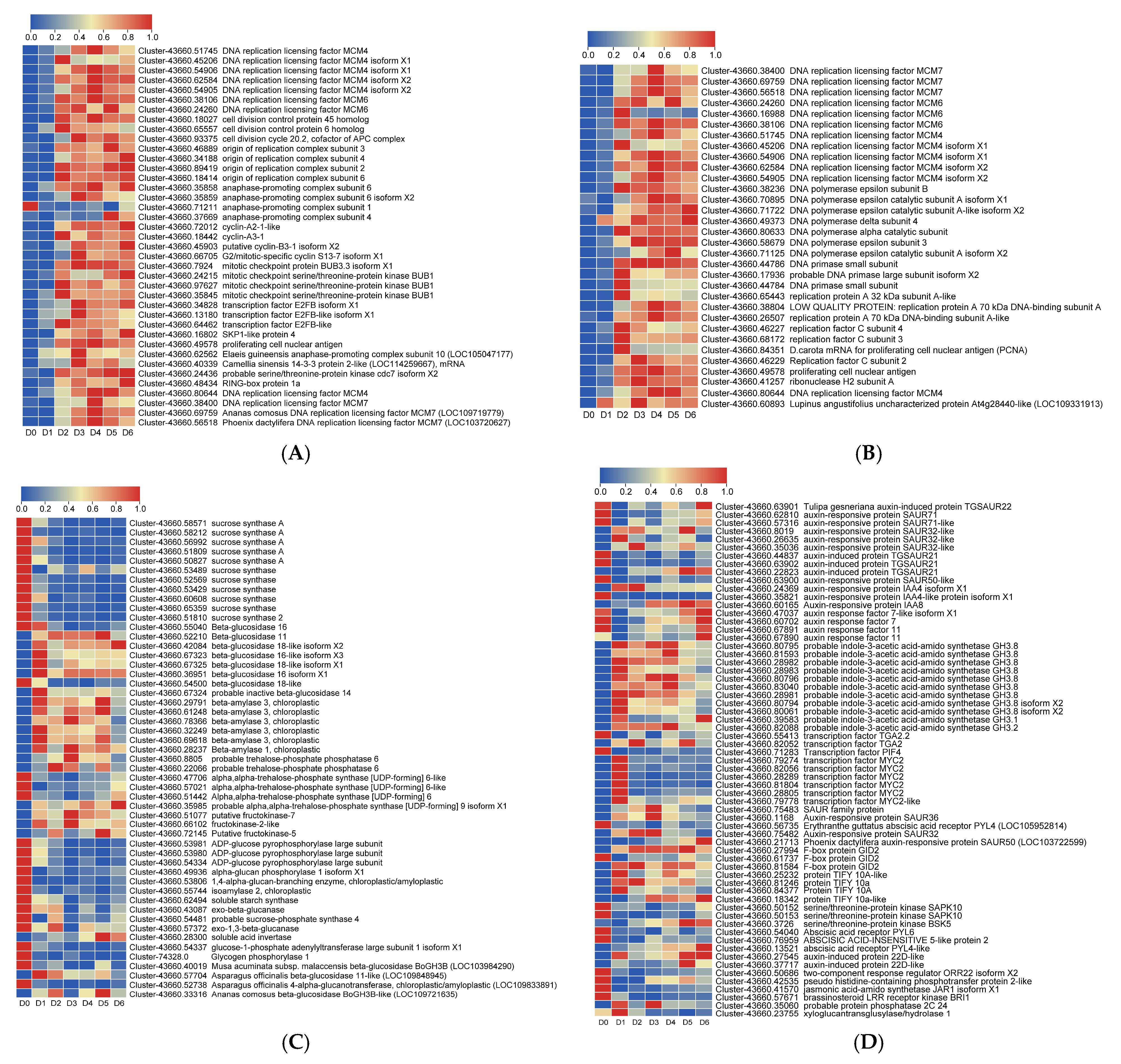
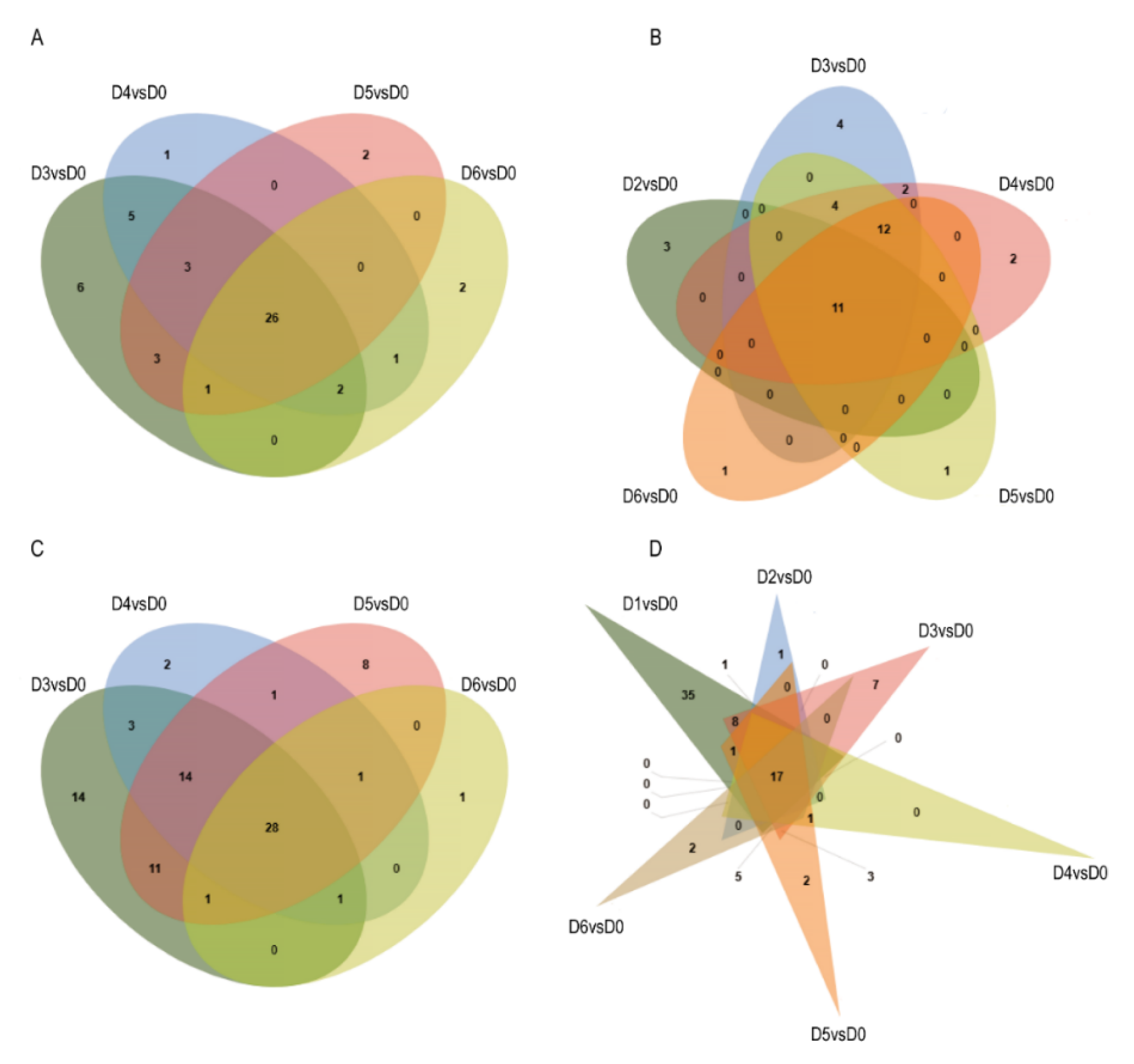
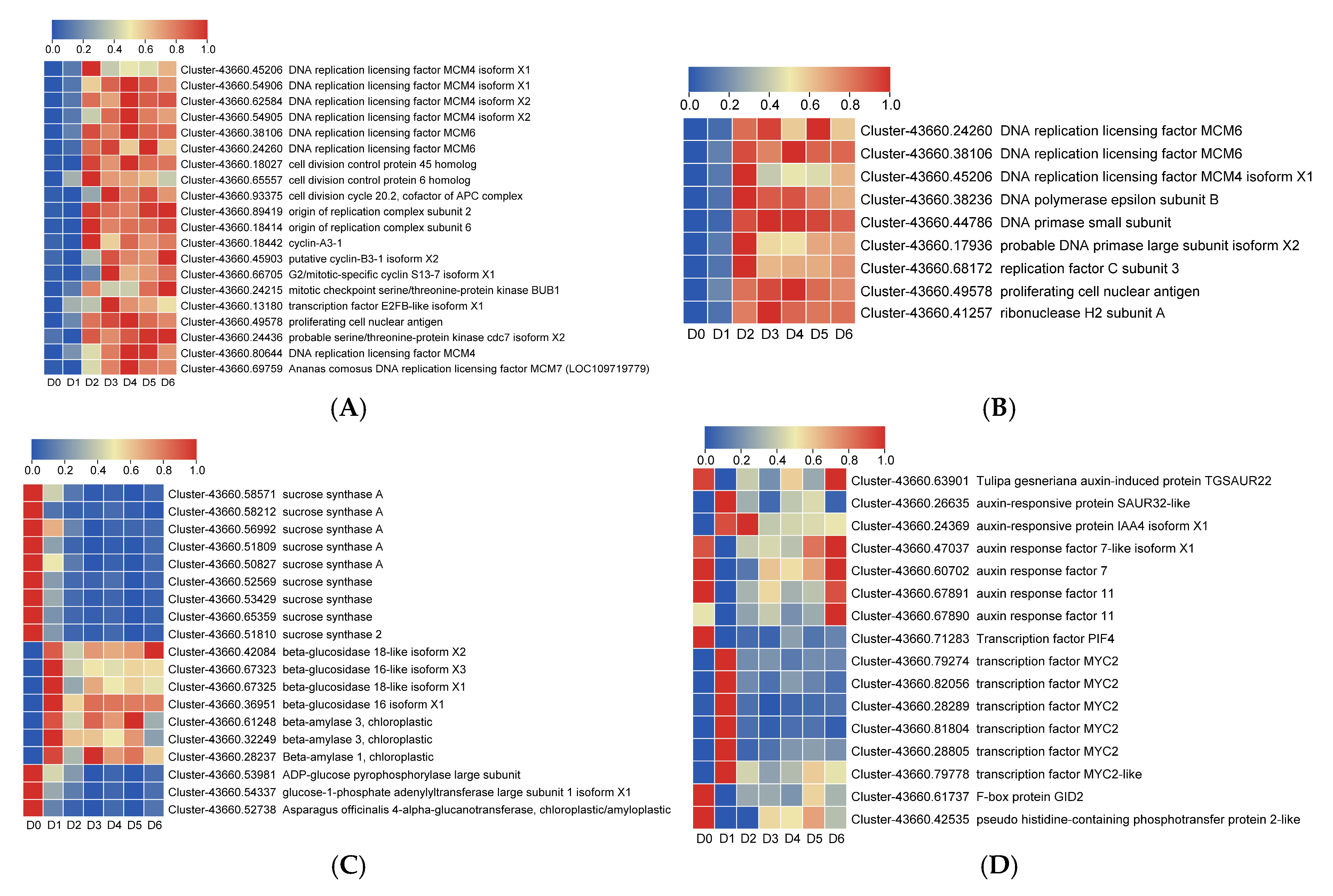
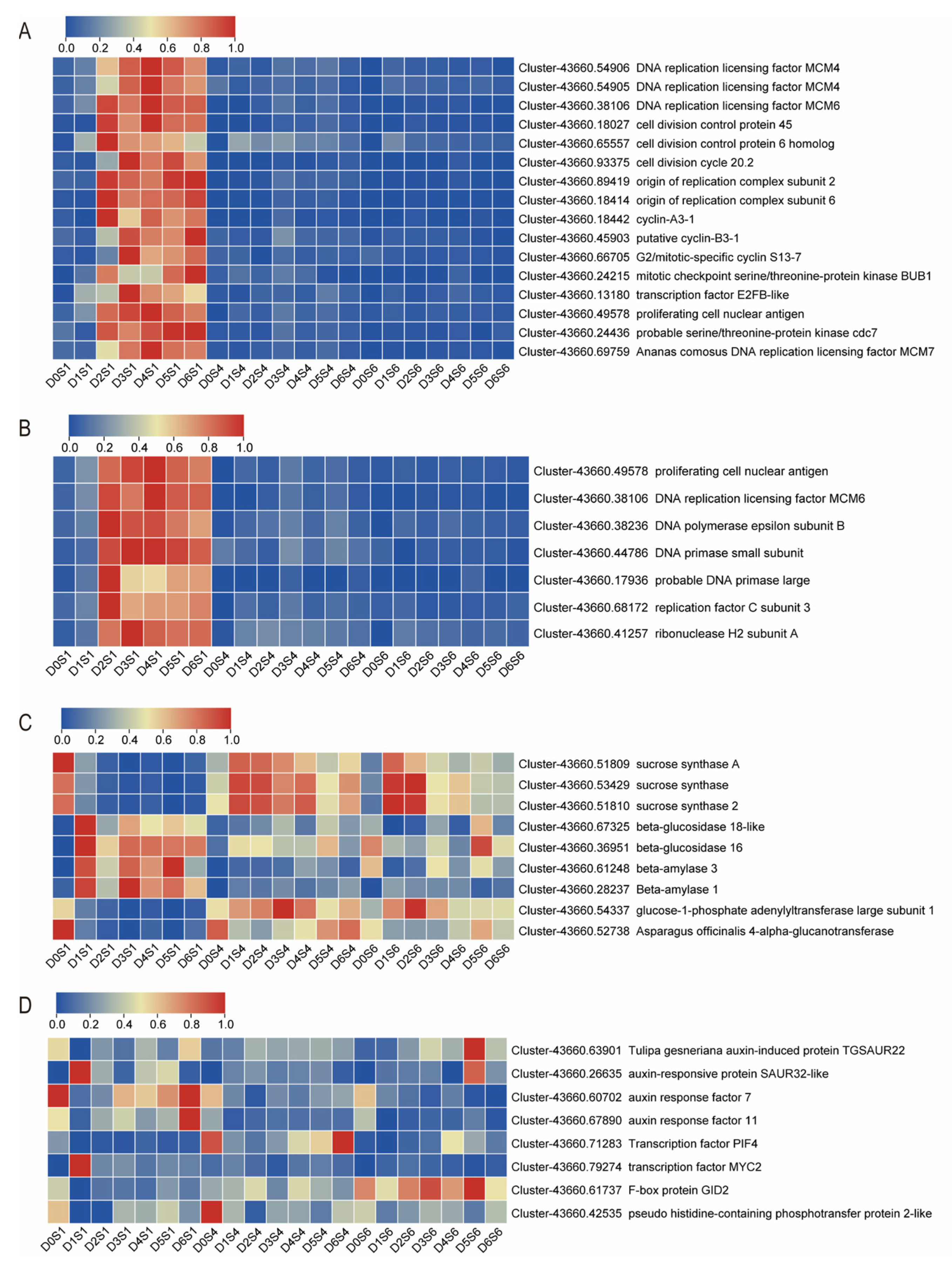
3.4. Screening of Differentially Expressed Genes (DEGs) Related to Bulblet Initiation
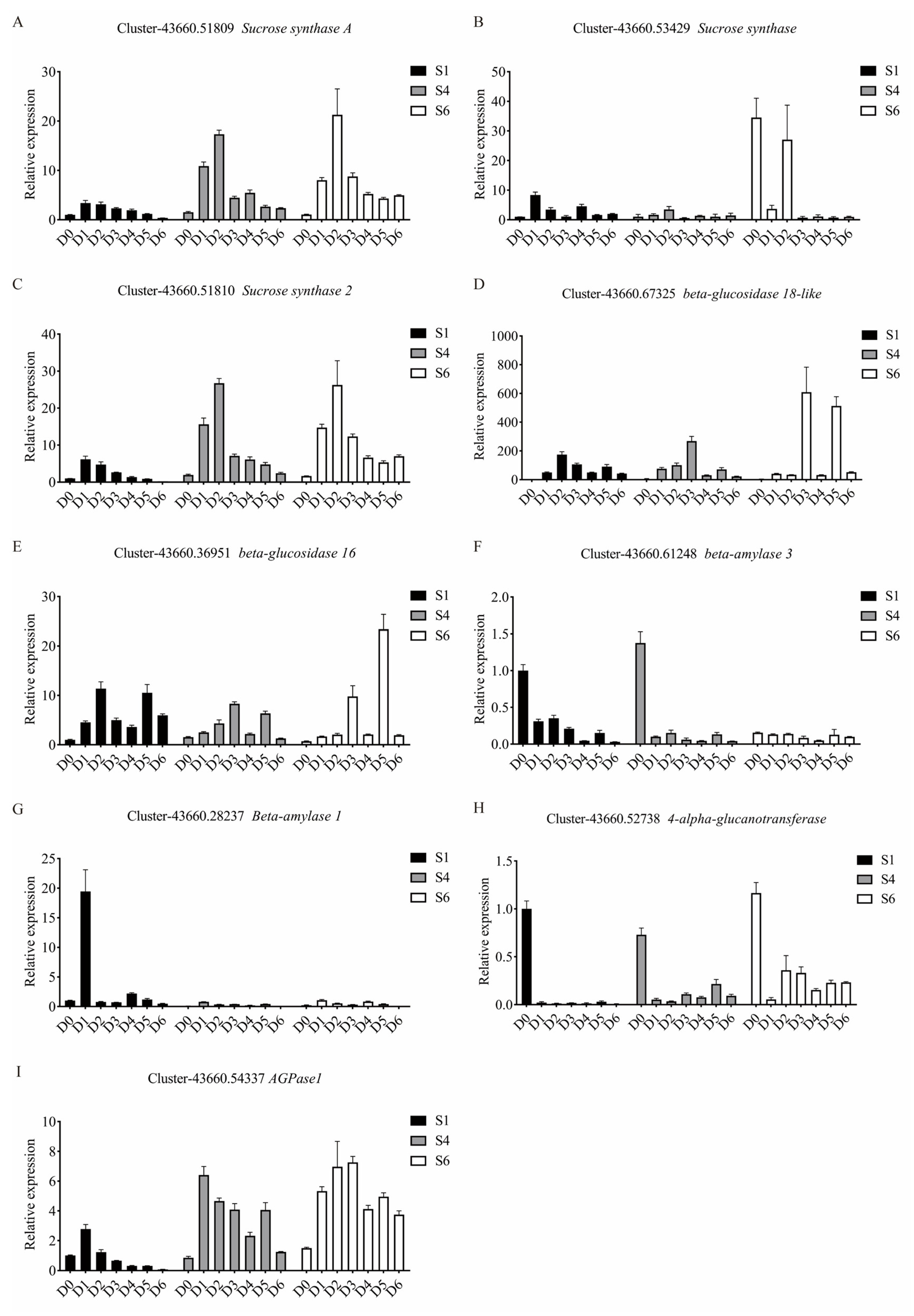
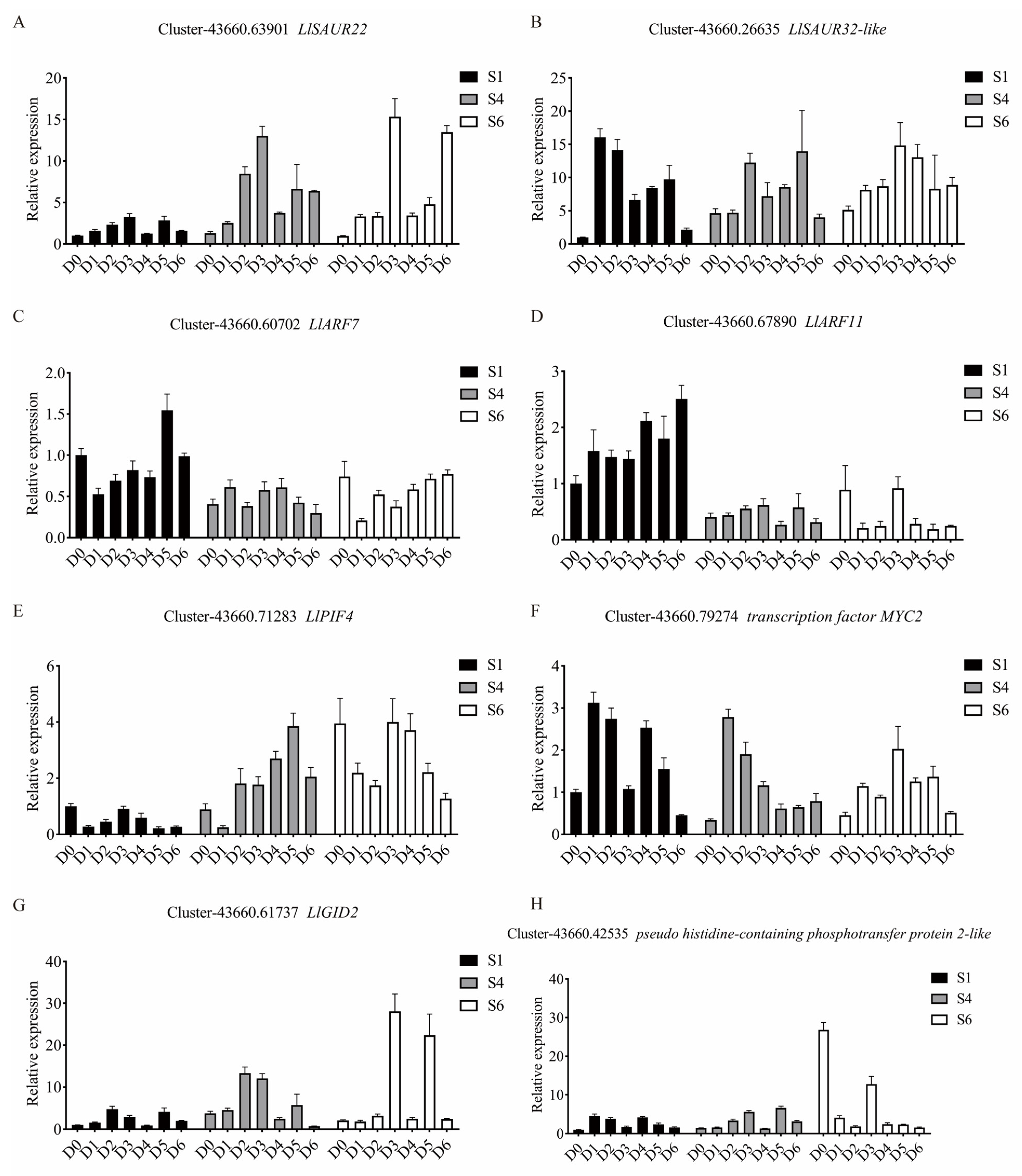
3.5. Validation of DEGs by qRT-PCR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, C.; Yang, P.; Qu, Y.; Hao, Z.; Yin, X.; Tang, Y.; Bi, M.; Xu, L.; Hu, F.; Ming, J. Sucrose function on the bulbil formation of Lilium lancifolium. Sci. Hortic. 2024, 323, 112538. [Google Scholar] [CrossRef]
- He, G.; Yang, P.; Cao, Y.; Tang, Y.; Wang, L.; Song, M.; Wang, J.; Xu, L.; Ming, J. Cytokinin Type-B Response Regulators Promote Bulbil Initiation in Lilium lancifolium. Int. J. Mol. Sci. 2021, 22, 3320. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Pan, W.; Sang, Q.; Liang, J.; Zhang, X.; Kang, S.; Zhang, M.; Zhang, X.; Yu, X.; et al. Auxin regulates bulbil development by affecting carbohydrate metabolism in Lilium lancifolium. Sci. Hortic. 2025, 341, 113972. [Google Scholar] [CrossRef]
- Askari, N.; Visser, R.G.F. The role of scale explants in the growth of regenerating lily bulblets in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 149, 589–598. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Xu, Y.; Liu, Y.; Xiao, X.; Cui, L.; Xia, Y.; Wu, Y.; Ren, Z. Full-length transcriptome analysis revealed that 2,4-dichlorophenoxyacetic acid promoted in vitro bulblet initiation in lily by affecting carbohydrate metabolism and auxin signaling. Front. Plant Sci. 2023, 14, 1236315. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in Sucrose Cleavage Pattern and Rapid Starch Accumulation Govern Lily Shoot-to-Bulblet Transition in vitro. Front. Plant Sci. 2020, 11, 564713. [Google Scholar] [CrossRef]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Plant stem cells and de novo organogenesis. New Phytol. 2018, 218, 1334–1339. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H. Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 2014, 108, 1–33. [Google Scholar]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Bavnhøj, L.; Driller, J.H.; Zuzic, L.; Stange, A.D.; Schiøtt, B.; Pedersen, B.P. Structure and sucrose binding mechanism of the plant SUC1 sucrose transporter. Nat. Plants 2023, 9, 938–950. [Google Scholar] [CrossRef]
- Li, W.; Huang, D.; Wang, B.; Hou, X.; Zhang, R.; Yan, M.; Liao, W. Changes of starch and sucrose content and related gene expression during the growth and development of Lanzhou lily bulb. PLoS ONE 2022, 17, e0262506. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Yang, L.; Li, X.; Wang, Z.; Zhang, Y. Changes in carbohydrate metabolism and endogenous hormone regulation during bulblet initiation and development in Lycoris radiata. BMC Plant Biol. 2020, 20, 180. [Google Scholar] [CrossRef]
- Sun, H.; He, L.; Wang, W.; Jia, Z.; Li, T. Mechanism of Starch-Sucrose Metabolism Regulated by IBA asWell as GA3 During Scale Cutting Propagation in Lilium. Sci. Agric. Sin. 2011, 44, 798–806. [Google Scholar]
- Ren, Z.M.; Zhang, D.; Jiao, C.; Li, D.Q.; Wu, Y.; Wang, X.Y.; Gao, C.; Lin, Y.F.; Ruan, Y.L.; Xia, Y.P. Comparative transcriptome and metabolome analyses identified the mode of sucrose degradation as a metabolic marker for early vegetative propagation in bulbs of Lycoris. Plant J. Cell Mol. Biol. 2022, 112, 115–134. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.H.; Gao, Z.; Shen, S.; Liang, X.G.; Zhao, X.; Lin, S.; Zhou, S.L. Regulation of maize kernel weight and carbohydrate metabolism by abscisic acid applied at the early and middle post-pollination stages in vitro. J. Plant Physiol. 2017, 216, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, M.; Sharma, D.R. In vitro regeneration and bulblet growth from lily bulbscale explants as affected by retardants, sucrose and irradiance. Biol. Plant. 2005, 49, 629–632. [Google Scholar] [CrossRef]
- Xin, Y.; Chen, X.; Liang, J.; Wang, S.; Pan, W.; Wu, J.; Zhang, M.; Zaccai, M.; Yu, X.; Zhang, X.; et al. Auxin regulates bulbil initiation by mediating sucrose metabolism in Lilium lancifolium. Hortic. Res. 2024, 11, uhae054. [Google Scholar] [CrossRef]
- He, G.; Yang, P.; Tang, Y.; Cao, Y.; Qi, X.; Xu, L.; Ming, J. Mechanism of exogenous cytokinins inducing bulbil formation in Lilium lancifolium in vitro. Plant Cell Rep. 2020, 39, 861–872. [Google Scholar] [CrossRef]
- Georgelis, N.; Fencil, K.; Richael, C.M. Validation of a rapid and sensitive HPLC/MS method for measuring sucrose, fructose and glucose in plant tissues. Food Chem. 2018, 262, 191–198. [Google Scholar] [CrossRef]
- Mo, J.; Qu, Y.; He, G.; Yang, P.; Wang, L.; Zhang, L.; Wu, X.; Zhang, D.; Li, L.; Ming, J. Effect of exogenous 6-BA induced Lilium lancifolium bulblets formation in aerial cultivation. Sci. Hortic. 2023, 309, 111644. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J.; Bai, M.; Chen, Y.; Wang, W.; Fan, C.; Liu, J.; Ning, G.; Wang, C. Phytochrome-interacting factors interact with transcription factor CONSTANS to suppress flowering in rose. Plant Physiol. 2021, 186, 1186–1201. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, M.Z.; Xue, B.Y.; Jia, N.N.; Sun, H.M. The use of miRNAs as reference genes for miRNA expression normalization during Lilium somatic embryogenesis by real-time reverse transcription PCR analysis. Plant Cell Tissue Organ Cult. 2016, 129, 105–118. [Google Scholar] [CrossRef]
- Nachamkin, I.; Panaro, N.J.; Li, M.; Ung, H.; Yuen, P.K.; Kricka, L.J.; Wilding, P. Agilent 2100 bioanalyzer for restriction fragment length polymorphism analysis of the Campylobacter jejuni flagellin gene. J. Clin. Microbiol. 2001, 39, 754–757. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Liang, J.; Wu, Z.; Zheng, J.; Koskela, E.A.; Fan, L.; Fan, G.; Gao, D.; Dong, Z.; Hou, S.; Feng, Z.; et al. The GATA factor HANABA TARANU promotes runner formation by regulating axillary bud initiation and outgrowth in cultivated strawberry. Plant J. Cell Mol. Biol. 2022, 110, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Cheng, J.; Zhang, J.; da Silva, J.A.; Liu, X.; Duan, X.; Li, T.; Sun, H. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol. 2014, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Yang, P.; Xu, L.; Xu, H.; Tang, Y.; He, G.; Cao, Y.; Feng, Y.; Yuan, S.; Ming, J. Histological and Transcriptomic Analysis during Bulbil Formation in Lilium lancifolium. Front. Plant Sci. 2017, 8, 1508. [Google Scholar] [CrossRef]
- Yang, P.P.; Xu, L.F.; Xu, H.; He, G.R.; Feng, Y.Y.; Cao, Y.W.; Tang, Y.C.; Yuan, S.X.; Ming, J. Morphological and anatomical observation during the formation of bulbils in Lilium lancifolium. Caryologia G. Citol. Citosistematica Citogenet. 2018, 71, 146–149. [Google Scholar] [CrossRef]
- Wu, S.S.; Chen, L.N.; Zhang, Q.X.; Lv, Y.M. Source and sink changes of lily bulb and the transportation role of the basal plate during the development of oriental hybrid lily ‘Sorbonne’. J. Food Agric. Environ. 2012, 10, 1213–1219. [Google Scholar]
- Liu, Z.; Li, J.; Wang, L.; Li, Q.; Lu, Q.; Yu, Y.; Li, S.; Bai, M.Y.; Hu, Y.; Xiang, F. Repression of callus initiation by the miRNA-directed interaction of auxin-cytokinin in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2016, 87, 391–402. [Google Scholar] [CrossRef]
- Zeng, M.; Hu, B.; Li, J.; Zhang, G.; Ruan, Y.; Huang, H.; Wang, H.; Xu, L. Stem cell lineage in body layer specialization and vascular patterning of rice root and leaf. Sci. Bull. 2016, 61, 847–858. [Google Scholar] [CrossRef]
- Marinangeli, P.A.; Hernández, L.F.; Pellegrini, C.P.; Curvetto, N.R. Bulblet Differentiation after Scale Propagation of Lilium longiflorum. J. Am. Soc. Hortic. Sci. 2003, 128, 324–329. [Google Scholar] [CrossRef]
- Xin, Y.; Pan, W.; Chen, X.; Liu, Y.; Zhang, M.; Chen, X.; Yang, F.; Li, J.; Wu, J.; Du, Y.; et al. Transcriptome profiling reveals key genes in regulation of the tepal trichome development in Lilium pumilum D.C. Plant Cell Rep. 2021, 40, 1889–1906. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Ren, H.; Guo, Z.; Li, N.; Zhao, C.; Xie, Z. Transcriptomic and physiological analyses identifying Lanzhou lily (Lilium davidii var. unicolor) drought adaptation strategies. Hortic. Plant J. 2023, 9, 13. [Google Scholar] [CrossRef]
- Long, Y.P.; Xie, D.J.; Zhao, Y.Y.; Shi, D.Q.; Yang, W.C. BICELLULAR POLLEN 1 is a modulator of DNA replication and pollen development in Arabidopsis. New Phytol. 2019, 222, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.; Kearsey, S.E. DNAReplication: A database of information and resources for the eukaryotic DNA replication community. Nucleic Acids Res. 2009, 37, D837–D839. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Hu, Y.; Deng, Y.; Liu, Y.; Li, G.; Zou, W.; Zhao, J. Arabidopsis replication factor C4 is critical for DNA replication during the mitotic cell cycle. Plant J. Cell Mol. Biol. 2018, 94, 288–303. [Google Scholar] [CrossRef]
- Pedroza-García, J.A.; Mazubert, C.; Del Olmo, I.; Bourge, M.; Domenichini, S.; Bounon, R.; Tariq, Z.; Delannoy, E.; Piñeiro, M.; Jarillo, J.A.; et al. Function of the Plant DNA Polymerase Epsilon in Replicative Stress Sensing, a Genetic Analysis. Plant Physiol. 2017, 173, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Jenik, P.D.; Jurkuta, R.E.; Barton, M.K. Interactions between the cell cycle and embryonic patterning in Arabidopsis uncovered by a mutation in DNA polymerase epsilon. Plant Cell 2005, 17, 3362–3377. [Google Scholar] [CrossRef] [PubMed]
- Springer, P.S.; Holding, D.R.; Groover, A.; Yordan, C.; Martienssen, R.A. The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G(1) phase and is required maternally for early Arabidopsis development. Development 2000, 127, 1815–1822. [Google Scholar] [CrossRef]
- Wang, J.W.; Wang, L.J.; Mao, Y.B.; Cai, W.J.; Xue, H.W.; Chen, X.Y. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell 2005, 17, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef]
- Schuetz, M.; Fidanza, M.; Mattsson, J. Identification of Auxin Response Factor-Encoding Genes Expressed in Distinct Phases of Leaf Vein Development and with Overlapping Functions in Leaf Formation. Plants 2019, 8, 242. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, R.; Gu, W.; Dong, X. The Solanum lycopersicum auxin response factor SlARF2 participates in regulating lateral root formation and flower organ senescence. Plant Sci. Int. J. Exp. Plant Biol. 2017, 256, 103–111. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.L.; Zhou, Y.K.; Guo, D.; Zhu, J.H.; Peng, S.Q. Transcriptomes analysis reveals novel insight into the molecular mechanisms of somatic embryogenesis in Hevea brasiliensis. BMC Genom. 2021, 22, 183. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 Regulate Lateral Root Formation via Direct Activation of LBD/ASL Genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Zhang, J.; Ni, Y.; Jiao, Z.; Li, H.; Wang, T.; Zhang, P.; Guo, W.; Li, L.; et al. Key auxin response factor (ARF) genes constraining wheat tillering of mutant dmc. PeerJ 2021, 9, e12221. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Chakrabarty, D.; Paek, K.Y. Sprouting rate, change of carbohydrate contents and related enzymes during cold treatment of lily bulblets regenerated in vitro. Sci. Hortic. 2002, 96, 195–204. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Luan, S.; Wi, S.G.; Bae, H.; Lee, D.S.; Bae, H.J. Pronounced Phenotypic Changes in Transgenic Tobacco Plants Overexpressing Sucrose Synthase May Reveal a Novel Sugar Signaling Pathway. Front. Plant Sci. 2015, 6, 1216. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Zhou, L.; Fu, X.; Lei, T.; Chen, Q.; Yu, X.; Zhou, Y.; Li, W.; Hu, J.; et al. Sucrose signaling function on the formation and swelling of bulblets of Lilium sargentiae E.H. Wilson. Plant Cell Tissue Organ Cult. Int. J. Vitr. Cult. High. Plants 2018, 135, 11. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, Y.; Lvy, X.; Zhang, D.; Gao, C.; Lin, Y.; Liu, Y.; Wu, Y.; Xia, Y. Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration In Vitro. Int. J. Mol. Sci. 2021, 22, 11890. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Lemoine, R. Sucrose transporters in plants: Update on function and structure. Biochim. Et Biophys. Acta 2000, 1465, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Bieniawska, Z.; Paul Barratt, D.H.; Garlick, A.P.; Thole, V.; Kruger, N.J.; Martin, C.; Zrenner, R.; Smith, A.M. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. Cell Mol. Biol. 2007, 49, 810–828. [Google Scholar] [CrossRef]
- Goren, S.; Lugassi, N.; Stein, O.; Yeselson, Y.; Schaffer, A.A.; David-Schwartz, R.; Granot, D. Suppression of sucrose synthase affects auxin signaling and leaf morphology in tomato. PLoS ONE 2017, 12, e0182334. [Google Scholar] [CrossRef]
- Barratt, D.H.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef]
- Seng, S.; Wu, J.; Liang, J.; Zhang, F.; Yi, M. Silencing GhAGPL1 Reduces the Quality and Quantity of Corms and Cormels in Gladiolus. J. Am. Soc. Hortic. Sci. 2017, 142, 119–125. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, C.; Guo, P.; Xue, J.; Zhang, X.; Wang, X.; Liu, H.; Qian, J.; Shan, G.; Chen, X. Transcriptome Analysis of Adventitious Bulblet Initiation in Lilium lancifolium Thunb. Horticulturae 2025, 11, 1084. https://doi.org/10.3390/horticulturae11091084
Xing C, Guo P, Xue J, Zhang X, Wang X, Liu H, Qian J, Shan G, Chen X. Transcriptome Analysis of Adventitious Bulblet Initiation in Lilium lancifolium Thunb. Horticulturae. 2025; 11(9):1084. https://doi.org/10.3390/horticulturae11091084
Chicago/Turabian StyleXing, Chuanji, Pengyu Guo, Jing Xue, Xiuhai Zhang, Xian Wang, Hua Liu, Ji Qian, Guilin Shan, and Xuqing Chen. 2025. "Transcriptome Analysis of Adventitious Bulblet Initiation in Lilium lancifolium Thunb" Horticulturae 11, no. 9: 1084. https://doi.org/10.3390/horticulturae11091084
APA StyleXing, C., Guo, P., Xue, J., Zhang, X., Wang, X., Liu, H., Qian, J., Shan, G., & Chen, X. (2025). Transcriptome Analysis of Adventitious Bulblet Initiation in Lilium lancifolium Thunb. Horticulturae, 11(9), 1084. https://doi.org/10.3390/horticulturae11091084





