Organ-Specific Physiological and Metabolic Differentiation in Celery (Apium graveolens L.) to Supplemental Blue Light in Controlled Environment Agriculture
Abstract
1. Introduction
2. Materials and Methods
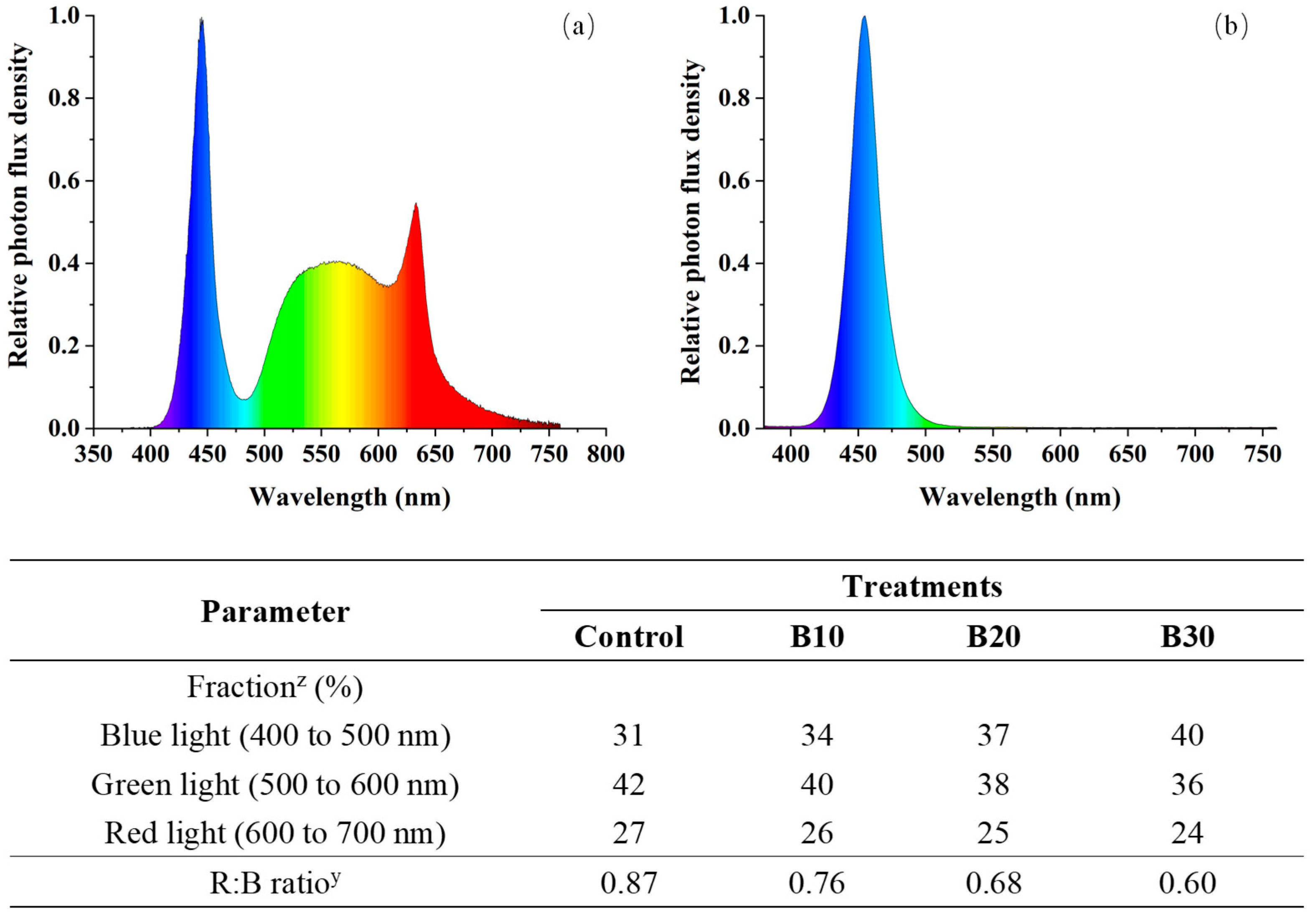
2.1. Plant Materials and Experimental Design
2.2. Measurements and Methods
2.2.1. Plant Morphology and Biomass Measurements
2.2.2. Chlorophyll Content, Photosynthetic Characteristics, and Chlorophyll Fluorescence
2.2.3. Biochemical Quality Parameters
2.2.4. Antioxidant Enzyme Activities
2.2.5. Volatile Compound Profiling via Electronic Nose
2.3. Statistical Analysis
3. Results
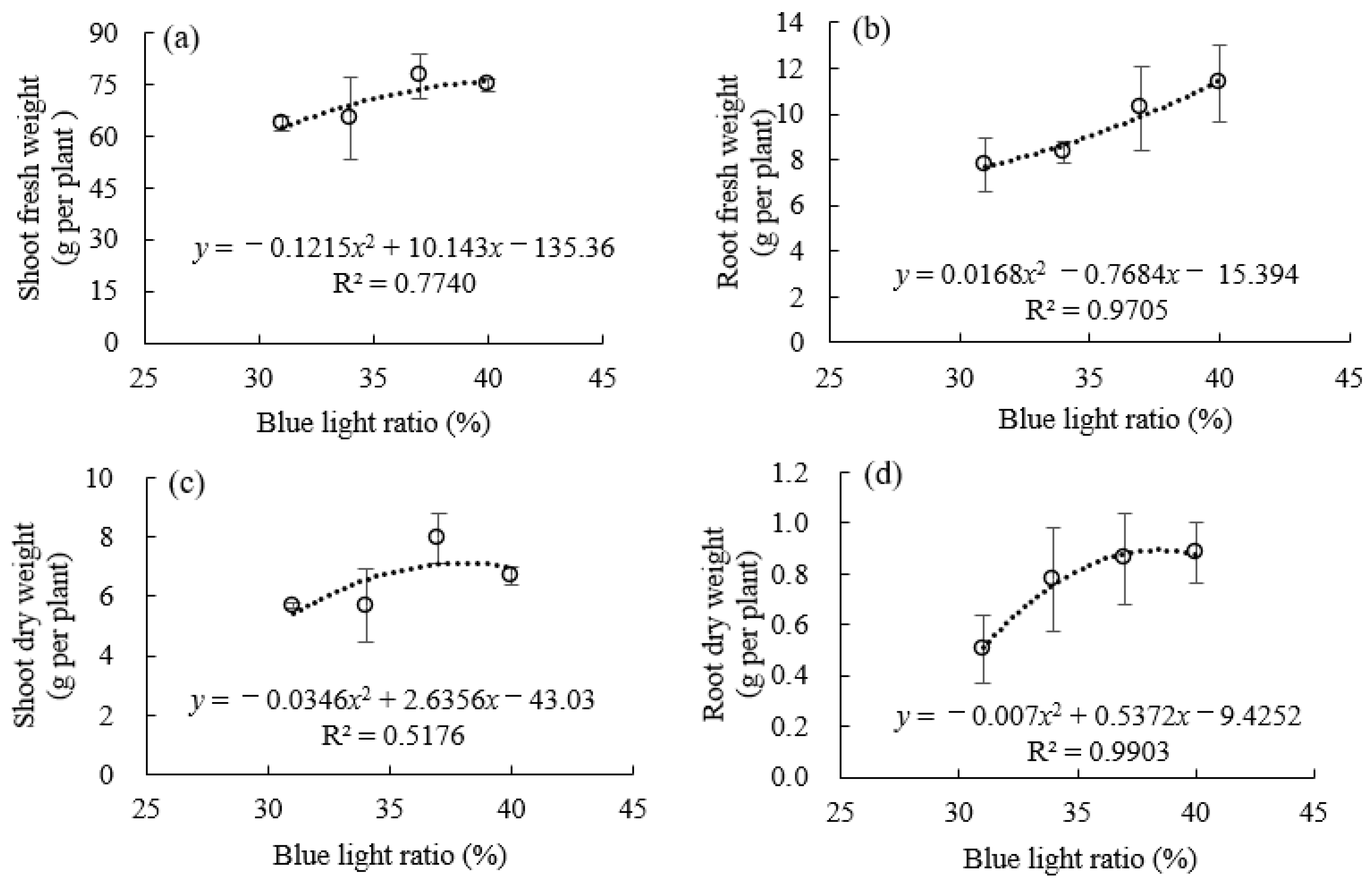
3.1. Morphological Traits and Biomass Accumulation Under Supplemental Blue Light
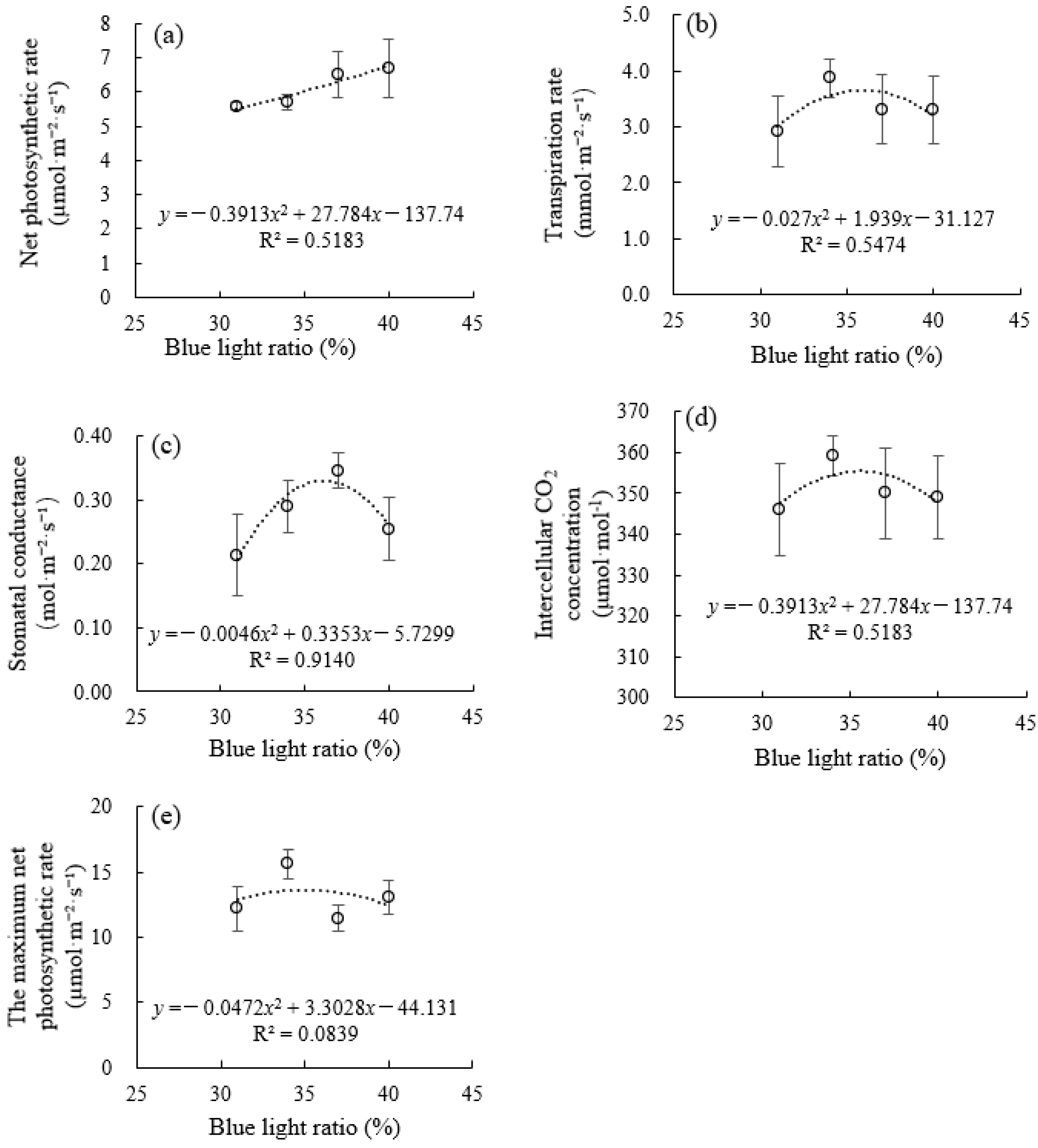
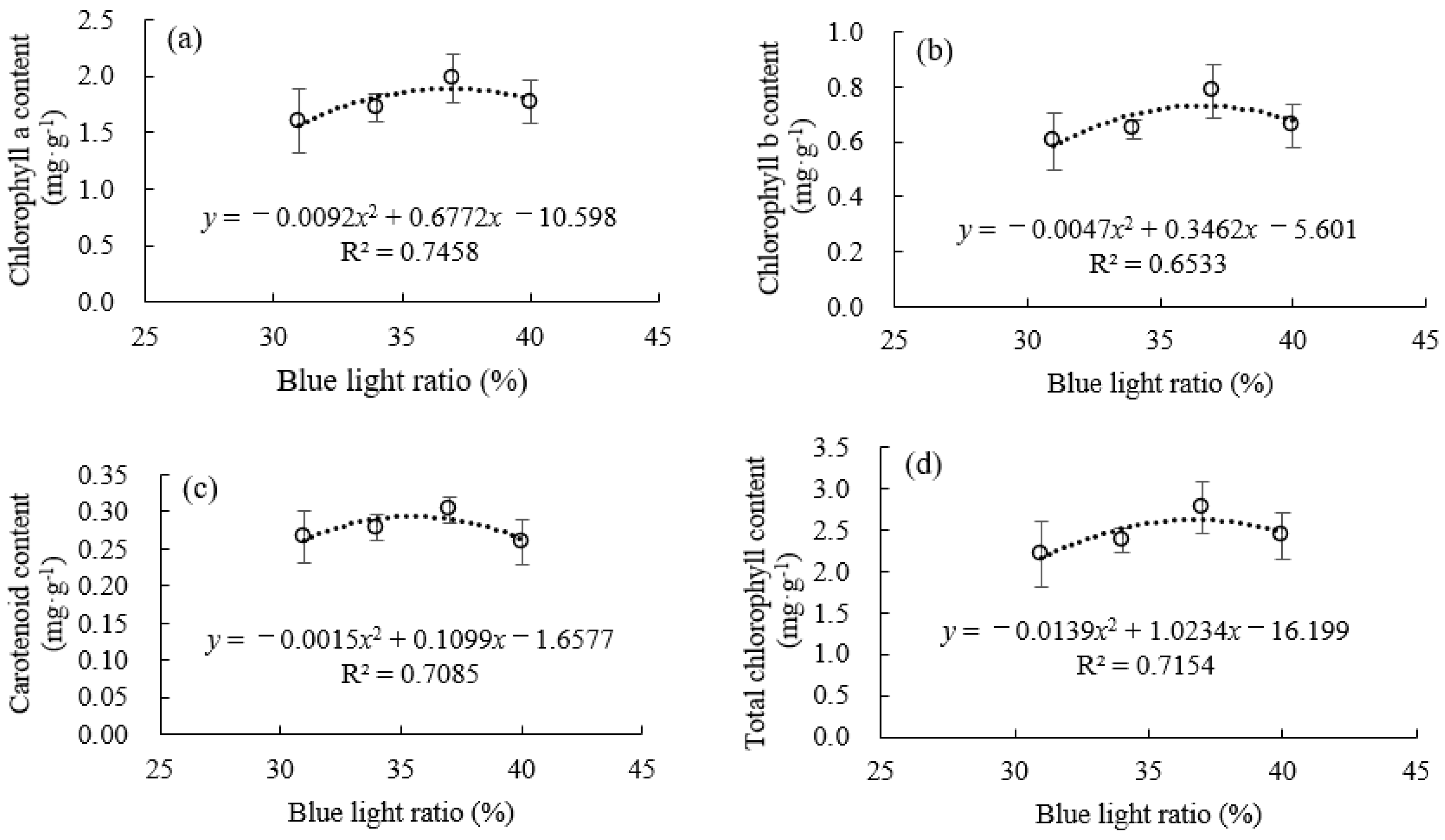
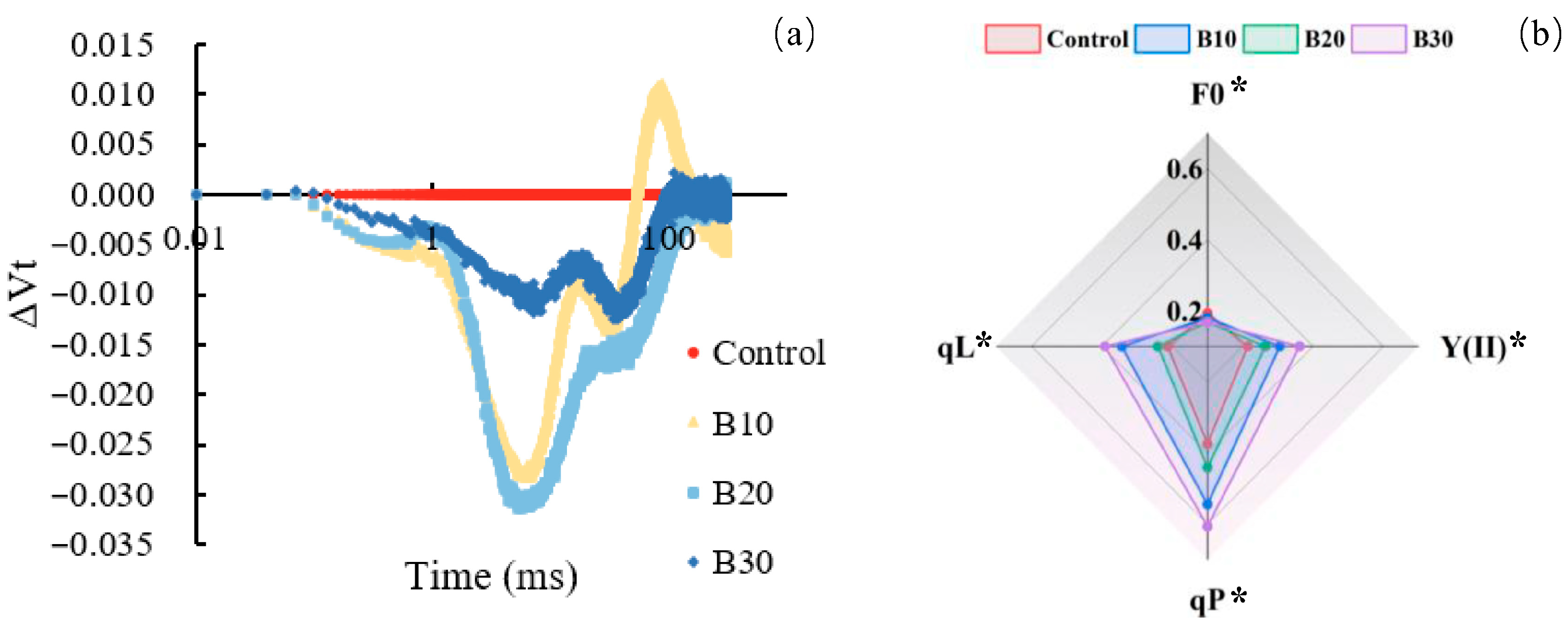
3.2. Photosynthetic Characteristics, Chlorophyll Content, and Chlorophyll Fluorescence Under Supplemental Blue Light
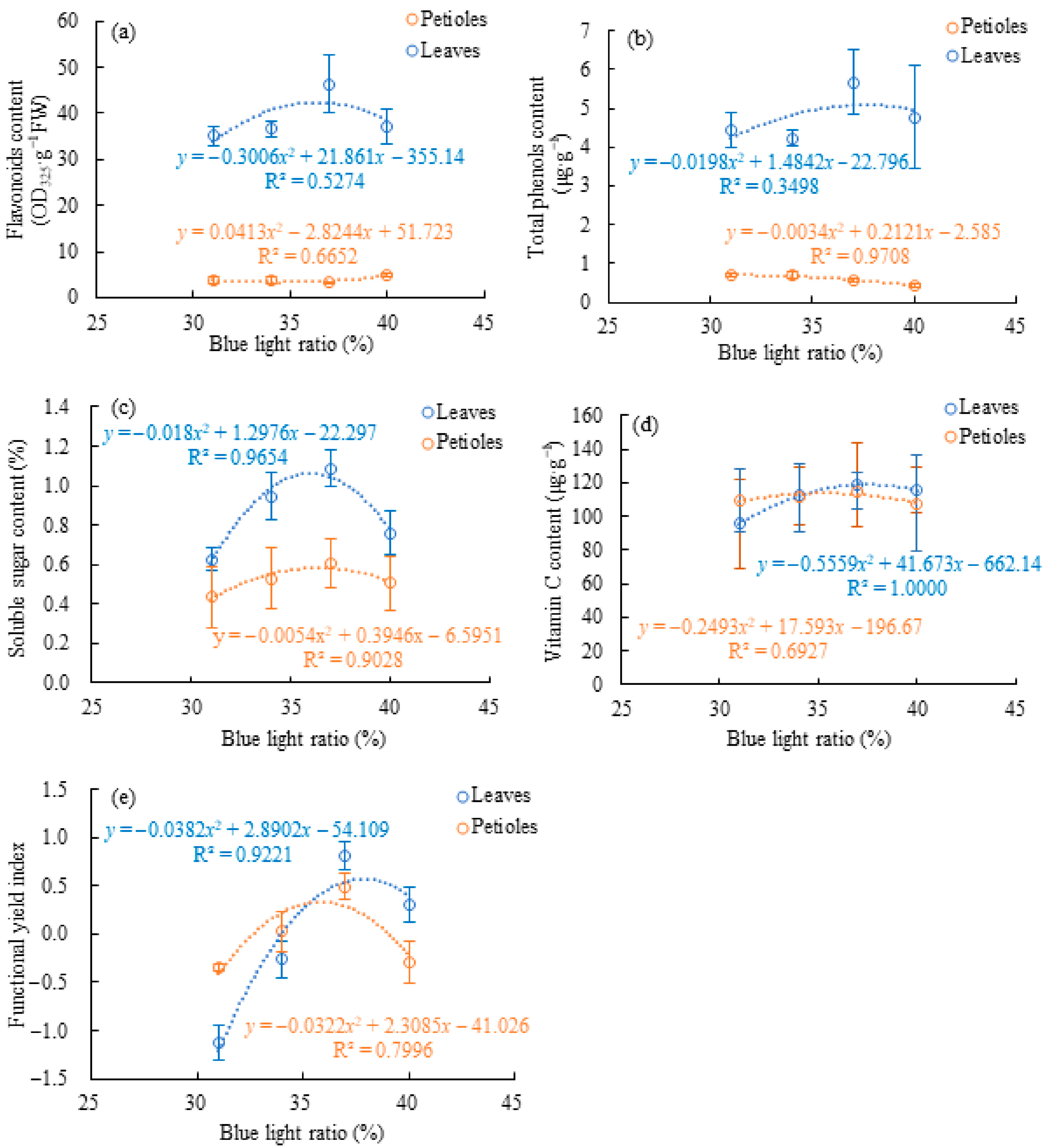
3.3. Organ-Specific Nutritional Traits Under Supplemental Blue Light
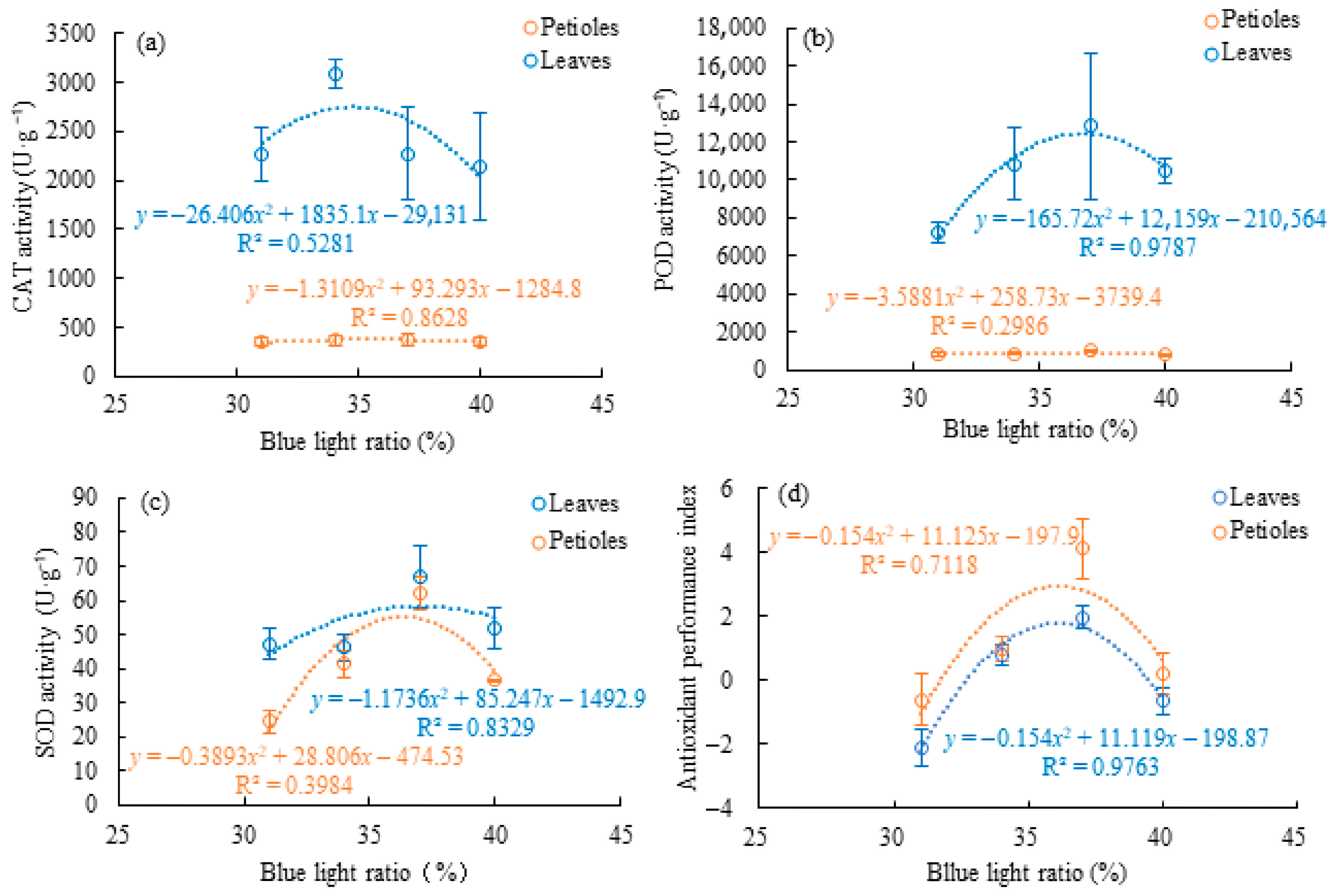
3.4. Organ-Specific Antioxidant Enzyme Activity Under Supplemental Blue Light
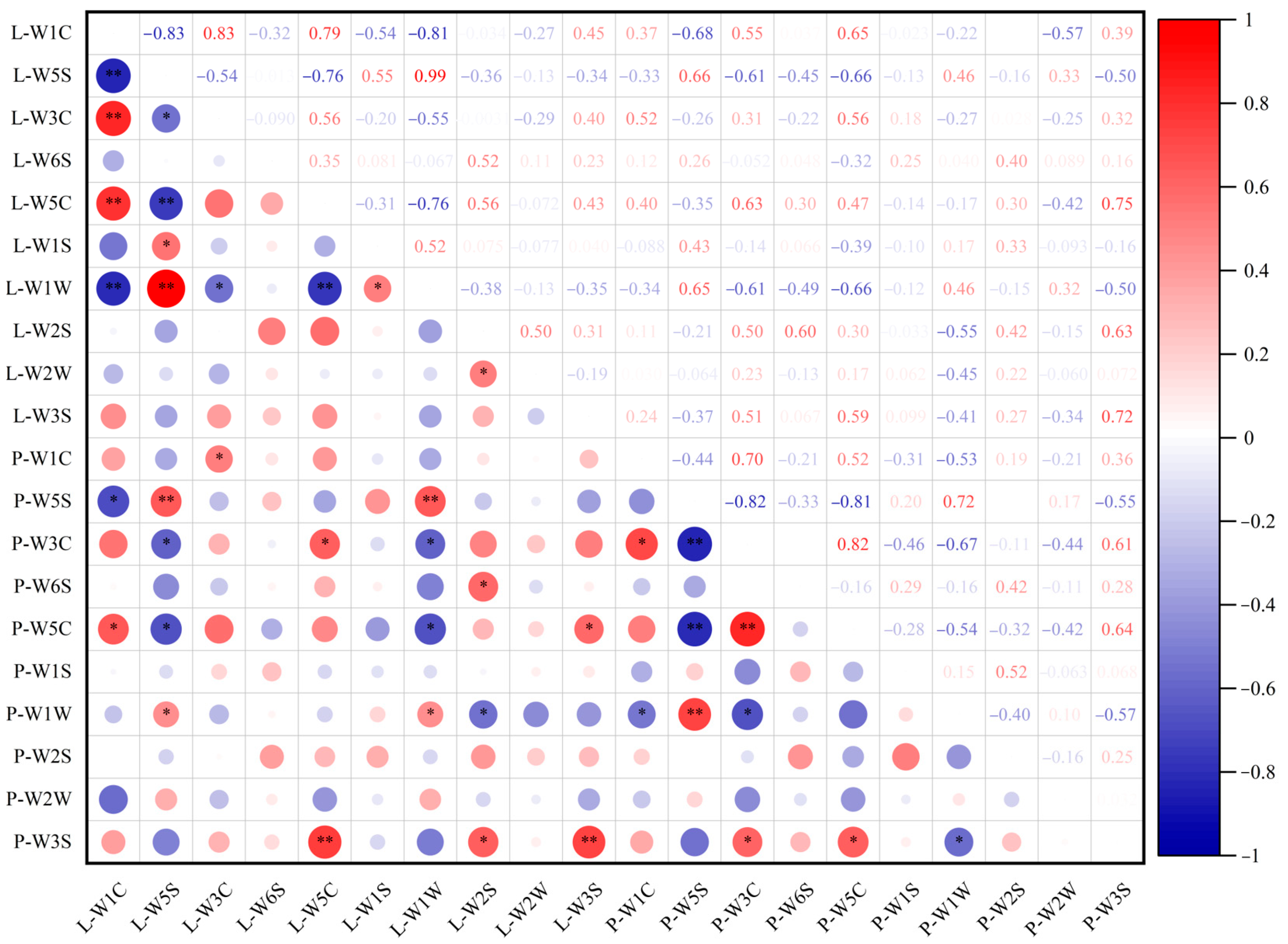
3.5. Volatile Compound Differentiation Under Supplemental Blue Light
3.6. Principal Component Analysis
4. Discussion
4.1. Moderate Red/Blue Light Ratio Balancing Photochemical Efficiency and Biomass Accumulation in Celery
4.2. Organ-Specific Metabolic Responses to Supplemental Blue Light in Celery Leaves and Petioles
4.3. Organ-Specific Modulation of VOCs Under Supplemental Blue Light
4.4. Practical Implications for Controlled Environment Agriculture and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both multi-segment light intensity and extended photoperiod lighting strategies, with the same daily light integral, promoted Lactuca sativa L. growth and photosynthesis. Agron. J. 2019, 9, 857. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Si, C.; Lin, Y.; Luo, S.; Yu, Y.; Liu, R.; Naz, M.; Dai, Z. Effects of LED light quality combinations on growth and leaf color of tissue culture-generated plantlets in Sedum rubrotinctum. Hortic. Sci. Technol. 2024, 42, 53–67. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Song, J.; Zhang, R.; Cai, W. Does the daily light integral influence the sowing density of tomato plug seedlings in a controlled environment. Horticulturae 2024, 10, 730. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of light intensity and temperature on the photosynthesis characteristics and yield of lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Bhattarai, T.; Ebong, A.; Raja, M.Y.A. A review of light-emitting diodes and ultraviolet light-emitting diodes and their applications. Photonics 2024, 11, 491. [Google Scholar] [CrossRef]
- Liu, K.; Tang, L.; Chu, Q.; Lu, Y.; Su, L.; Cheng, S.; He, Z.; Zhou, X. Effects of different LED light quality combinations on nutritional quality and physiological characteristics of celery. Horticulturae 2025, 11, 524. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue light improves photosynthetic performance and biomass partitioning toward harvestable organs in saffron (Crocus sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef]
- Chan, A.M.H.; Pay, M.L.; Christensen, J.; He, F.; Roden, L.C.; Ahmed, H.; Foo, M. Red, blue or mix: Choice of optimal light qualities for enhanced plant growth and development through in silico analysis. Silico Plants 2024, 6, diae008. [Google Scholar] [CrossRef]
- Sun, J.; Tan, X.; Liu, B.; Battino, M.; Meng, X.; Zhang, F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biol. Technol. 2024, 215, 113006. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Metallo, R.M.; Waterland, N.L.; Kopsell, D.E. Biomass, carbohydrates, pigments, and mineral elements in kale (Brassica oleracea var acephala) microgreens respond to LED blue-light wavelength. Sci. Hortic. 2024, 328, 112929. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Frutos-Tortosa, A.; Diaz-Mula, H.; Mestre, T.C.; Martínez, V. Effect of the intensity and spectral quality of LED light on growth and quality of spinach indoors. Horticulturae 2024, 10, 411. [Google Scholar] [CrossRef]
- Zhu, Y.; Singh, J.; Patil, B.S.; Zhen, S. End-of-production supplemental blue light intensity and duration co-regulate growth, anthocyanin, and ascorbic acid production in red leaf lettuce. Sci. Hortic. 2024, 335, 113333. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.B.; Li, X.; Choi, S.R.; Park, S.; Park, J.S.; Lim, Y.P.; Park, S.U. Accumulation of phenylpropanoids by white, blue, and red light irradiation and their organ-specific distribution in Chinese cabbage (Brassica rapa ssp. pekinensis). J. Agric. Food Chem. 2015, 63, 6772–6778. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Solikhah, T.I.; Ansori, A.N.M.; Hidayatullah, A.R.; Hartadi, E.B.; Ramandinianto, S.C.; Fadholly, A. Review on the pharmacological and health aspects of Apium Graveolens or celery: An update. Sys. Rev. Pharm. 2021, 12, 606–612. [Google Scholar]
- Liu, P.Z.; Wang, Y.H.; Wang, L.X.; Li, M.Y.; Liu, H.; Shu, S.; Tan, G.F.; Xiong, A.S. Current situation and future outlook of production, processing and marketing in the celery industry. Technol. Horticul. 2024, 4, e013. [Google Scholar] [CrossRef]
- Zhang, B.; Li, S.; Zhang, W.; Cheng, Y.; Liu, Z.; Zhang, N.; Xu, J.; Wu, X.; Dong, F.; Zheng, Y.; et al. Sensitive and portable intelligent detection platform construction and dietary risk assessment of procymidone in Chinese leek, cowpea and celery. Food Chem. 2025, 465, 142081. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Li, C.; Chu, Q.; Cheng, S.; Su, L.; Shao, D.; Guo, X.; He, Z.; Zhou, X. Effect of light intensity on celery growth and flavonoid synthesis. Front. Plant Sci. 2024, 14, 1326218. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Huang, X.; Wang, L.; Ren, Y.; Zhang, X.; Wang, Y. Characterization of selected Chinese soybean paste based on flavor profiles using HS-SPME-GC/MS, E-nose and E-tongue combined with chemometrics. Food Chem. 2022, 375, 131840. [Google Scholar] [CrossRef]
- Dai, C.; Huang, X.; Lv, R.; Zhang, Z.; Sun, J.; Aheto, J.H. Analysis of volatile compounds of Tremella aurantialba fermentation via electronic nose and HS-SPME-GC-MS. J. Food Saf. 2018, 38, e12555. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X.; Wang, S.; Gao, S.; Yang, K.; Zhang, R.; Zhang, M.; Wang, M.; Ren, L.; Yu, J. Electronic nose application for detecting different odorants in source water: Possibility and scenario. Environ. Res. 2023, 227, 115677. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Ye, Z.; Suggett, D.; Robakowski, P.; Kang, H. A mechanistic model for the photosynthesis light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytol. 2013, 199, 110–120. [Google Scholar] [CrossRef]
- Ullah, I.; Hanping, M.; Javed, Q.; Rasool, G.; Ali, M.; Ullah, A.A.M.S. Nitrogen fertilization effects on growth, leaf gas exchange and chlorophyll fluorescence of Brassica juncea. Int. J. Agric. Biol. 2020, 24, 1070–1076. [Google Scholar] [CrossRef]
- Zhang, C.; Akhlaq, M.; Yan, H.; Ni, Y.; Liang, S.; Zhou, J.; Xue, R.; Li, M.; Adnan, R.M.; Li, J. Chlorophyll fluorescence parameter as a predictor of tomato growth and yield under CO2 enrichment in protective cultivation. Agric. Water Manag. 2023, 284, 108333. [Google Scholar] [CrossRef]
- Popescu, S.; Chiș, T.Ș.; Chiricuţță, M.G.; Vârlan, A.C.; Pîrvulescu, L.; Velciov, A. Vitamin C determination in foods. Titrimetric methods—A review. J. Agroaliment. Process. Technol. 2024, 30, 471–476. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric analysis of phenolic compounds in grapes and wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean citrus peel flavonoids and their antioxidant capacity using an innovative UV-Vis spectrophotometric approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Chi, X.; Li, X.; Hou, X.; Guo, S.; Hu, X. Facile bioself-assembled crystals in plants promote photosynthesis and salt stress resistance. Amer. Chem. Soc. Nano 2021, 15, 5165–5177. [Google Scholar] [CrossRef]
- Yang, J.; Liu, K.; Chen, Y.; Ye, H.; Hao, G.; Du, F.; Wang, P. A supramolecular bactericidal material for preventing and treating plant-associated biofilms. Nat. Commun. 2025, 16, 2627. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, Y.C. Evaluation of antioxidative performance of tomato extracts obtained by different methods. J. Sci. Food Agric. 2008, 88, 612–618. [Google Scholar] [CrossRef]
- Wang, G.; Xu, H.; Zhao, H.; Wu, Y.; Gao, X.; Chai, Z.; Liang, Y.; Zhang, X.; Zheng, R.; Yang, Q.; et al. Screening optimal oat varieties for cultivation in arid areas in China: A comprehensive evaluation of agronomic traits. Agronomy 2023, 13, 2266. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Misra, A.N.; Misra, M.; Singh, R. Chlorophyll fluorescence in plant biology. Biophys. J. 2012, 7, 171–192. [Google Scholar]
- Yuan, J.; Ma, C.; Feng, Y.; Zhang, J.; Yang, F.; Li, Y. Response of chlorophyll fluorescence transient in leaves of wheats with different drought resistances to drought stresses and rehydration. J. Plant Physiol. 2018, 54, 1119–1129. [Google Scholar]
- Chen, X.; Li, Y.; Wang, L.; Yang, Q.; Guo, W. Responses of butter leaf lettuce to mixed red and blue light with extended light/dark cycle period. Sci. Rep. 2022, 12, 6924. [Google Scholar] [CrossRef]
- Shamsabad, M.R.M.; Esmaeilizadeh, M.; Roosta, H.R.; Dehghani, M.R.; Dąbrowski, P.; Kalaji, H.M. The effect of supplementary light on the photosynthetic apparatus of strawberry plants under salinity and alkalinity stress. Sci. Rep. 2022, 12, 13257. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Response of carbon assimilation and chlorophyll fluorescence to soybean leaf phosphorus across CO2: Alternative electron sink, nutrient efficiency and critical concentration. J. Photochem. Photobiol. B Biol. 2015, 151, 276–284. [Google Scholar] [CrossRef]
- Padmasree, K.; Padmavathi, L.; Raghavendra, A.S. Essentiality of mitochondrial oxidative metabolism for photosynthesis: Optimization of carbon assimilation and protection against photoinhibition. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 71–119. [Google Scholar] [CrossRef]
- Dierck, R.; Dhooghe, E.; Van Huylenbroeck, J.; Van Der Straeten, D.; De Keyser, E. Light quality regulates plant architecture indifferent genotypes of Chrysanthemum morifolium Ramat. Sci. Hortic. 2017, 218, 177–186. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Ali, S.S.; Gao, L.; Ni, X.; Li, X.; Wu, Y.; Jiang, J. Identification and expression analysis of Sorghum bicolor gibberellin oxidase genes with varied gibberellin levels involved in regulation of stem biomass. Ind. Crops Prod. 2020, 145, 111951. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mariod, A.A.; Mahunu, G.K.; Abdualrahman, M.A.; Tchabo, W. Assessment of antioxidant properties, instrumental and sensory aroma profile of red and white Karkade/Roselle (Hibiscus sabdariffa L.). J. Food Meas. Charact. 2017, 11, 1559–1568. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.W.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.H.; Yang, J.X.; Wang, X.Z.; Yang, Q.Q.; Zhu, X.J.; Cao, X.Y. Transcriptome and targeted metabolome analysis revealed the effects of combined red and blue light on the growth and secondary metabolism of Scutellaria baicalensis Georgi. Ind. Crops Prod. 2022, 188, 115598. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Venkitasamy, C.; Wu, B.; Pan, Z.; Ma, H. Effect of pulsed light on activity and structural changes of horseradish peroxidase. Food Chem. 2017, 234, 20–25. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Zhan, X.; Yang, Q.; Wang, S.; Wang, Y.; Fan, X.; Bian, Z. The responses of sucrose metabolism and carbon translocation in tomato seedlings under different light spectra. Int. J. Mol. Sci. 2023, 24, 15054. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Varshney, V.; Singh, J.; Salvi, P. Sugar signaling and their interplay in mitigating abiotic stresses in plant: A molecular perspective. In Smart Plant Breeding for Field Crops in Post-Genomics Era; Springer Nature: Singapore, 2023; pp. 369–393. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Xu, S.; Zhu, W. Light quality-controlled phytochemicals biosynthesis in vegetables and fruits. Agric. Sci. Technol. 2015, 16, 2029. [Google Scholar]
- Khalighi, S.; Mehrjerdi, M.Z.; Aliniaeifard, S. Light quality affects phytochemicals content and antioxidant capacity of Satureja hortensis. South West. J. Hortic. Biol. Environ. 2021, 12, 99–117. [Google Scholar]
- Zhang, X.; Zhang, M.; Xu, B.; Mujumdar, A.S.; Guo, Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Compr. Rev. Food Sci. Food Saf. 2021, 21, 106–126. [Google Scholar] [CrossRef]

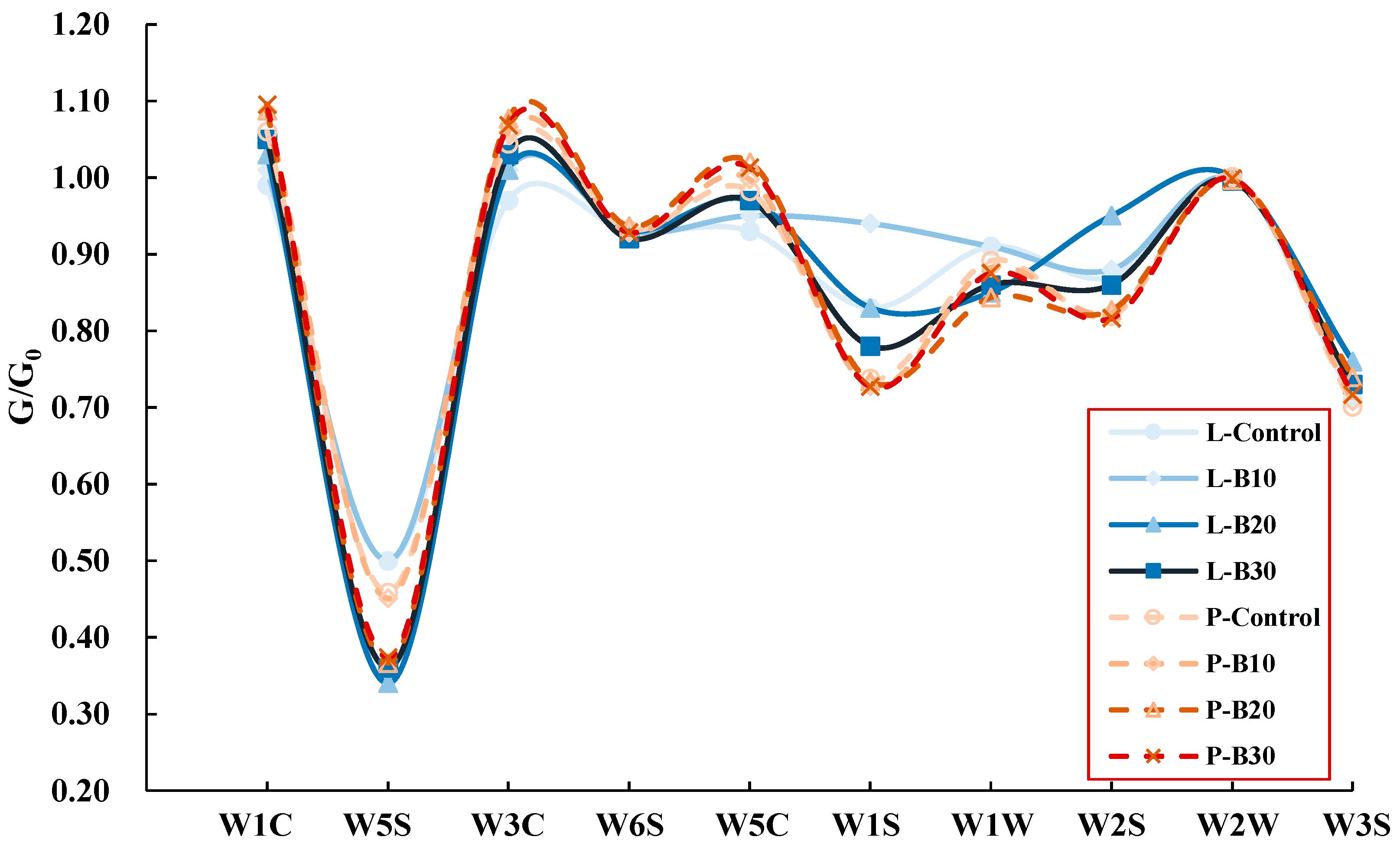
| Number | Name of Sensor | Description of Performance |
|---|---|---|
| 1 | W1C | Aromatic components |
| 2 | W5S | Highly sensitive to nitrogen oxides |
| 3 | W3C | Ammonia, sensitive to aromatic components |
| 4 | W6S | Selective mainly for hydrogen |
| 5 | W5C | Alkane aromatic components |
| 6 | W1S | Sensitive to methyl groups |
| 7 | W1W | Sensitive to sulfides |
| 8 | W2S | Sensitive to alcohols, aldehydes and ketones |
| 9 | W2W | Aromatic components, sensitive to organic sulfides |
| 10 | W3S | Sensitive to alkanes |
| Principal Component | Eigenvalue | Contribution Rate (%) | Cumulative Contribution Rate (%) | Control | B10 | B20 | B30 |
|---|---|---|---|---|---|---|---|
| PC1 | 34.50 | 59.48 | 59.48 | −6.40 | −1.68 | 7.72 | 0.35 |
| PC 2 | 14.20 | 24.49 | 83.97 | 3.03 | −0.53 | 2.63 | −5.12 |
| PC 3 | 9.30 | 16.03 | 100.00 | −1.97 | 4.47 | −0.57 | −1.92 |
| Composite Score | - | - | - | −3.38 | −0.41 | 5.14 | −1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, H.; Li, Z.; Liu, Q.; Jiang, P.; Song, J.; Ji, F.; Lu, N.; Xu, L.; Yan, Z. Organ-Specific Physiological and Metabolic Differentiation in Celery (Apium graveolens L.) to Supplemental Blue Light in Controlled Environment Agriculture. Horticulturae 2025, 11, 1074. https://doi.org/10.3390/horticulturae11091074
Dou H, Li Z, Liu Q, Jiang P, Song J, Ji F, Lu N, Xu L, Yan Z. Organ-Specific Physiological and Metabolic Differentiation in Celery (Apium graveolens L.) to Supplemental Blue Light in Controlled Environment Agriculture. Horticulturae. 2025; 11(9):1074. https://doi.org/10.3390/horticulturae11091074
Chicago/Turabian StyleDou, Haijie, Zhixin Li, Qi Liu, Pengyue Jiang, Jinxiu Song, Fang Ji, Na Lu, Ligang Xu, and Zhengnan Yan. 2025. "Organ-Specific Physiological and Metabolic Differentiation in Celery (Apium graveolens L.) to Supplemental Blue Light in Controlled Environment Agriculture" Horticulturae 11, no. 9: 1074. https://doi.org/10.3390/horticulturae11091074
APA StyleDou, H., Li, Z., Liu, Q., Jiang, P., Song, J., Ji, F., Lu, N., Xu, L., & Yan, Z. (2025). Organ-Specific Physiological and Metabolic Differentiation in Celery (Apium graveolens L.) to Supplemental Blue Light in Controlled Environment Agriculture. Horticulturae, 11(9), 1074. https://doi.org/10.3390/horticulturae11091074





