Strain- and System-Specific Enhancement of Artemisinin in Artemisia annua Composite Plants Grown in Hydroponic and Aeroponic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth and Experimental Setup for Artemisia annua
2.2. Agrobacterium rhizogenes Strains and Transformation
2.3. Exudate Collection and Root Exudate Extraction
2.4. Final Sampling of A. annua
2.5. Sample Preparation for UHPLC, Extraction and Quantification of Artemisinin
2.6. Total Phenolic Content (TPC) Extraction and Assay
2.7. Statistical Analysis
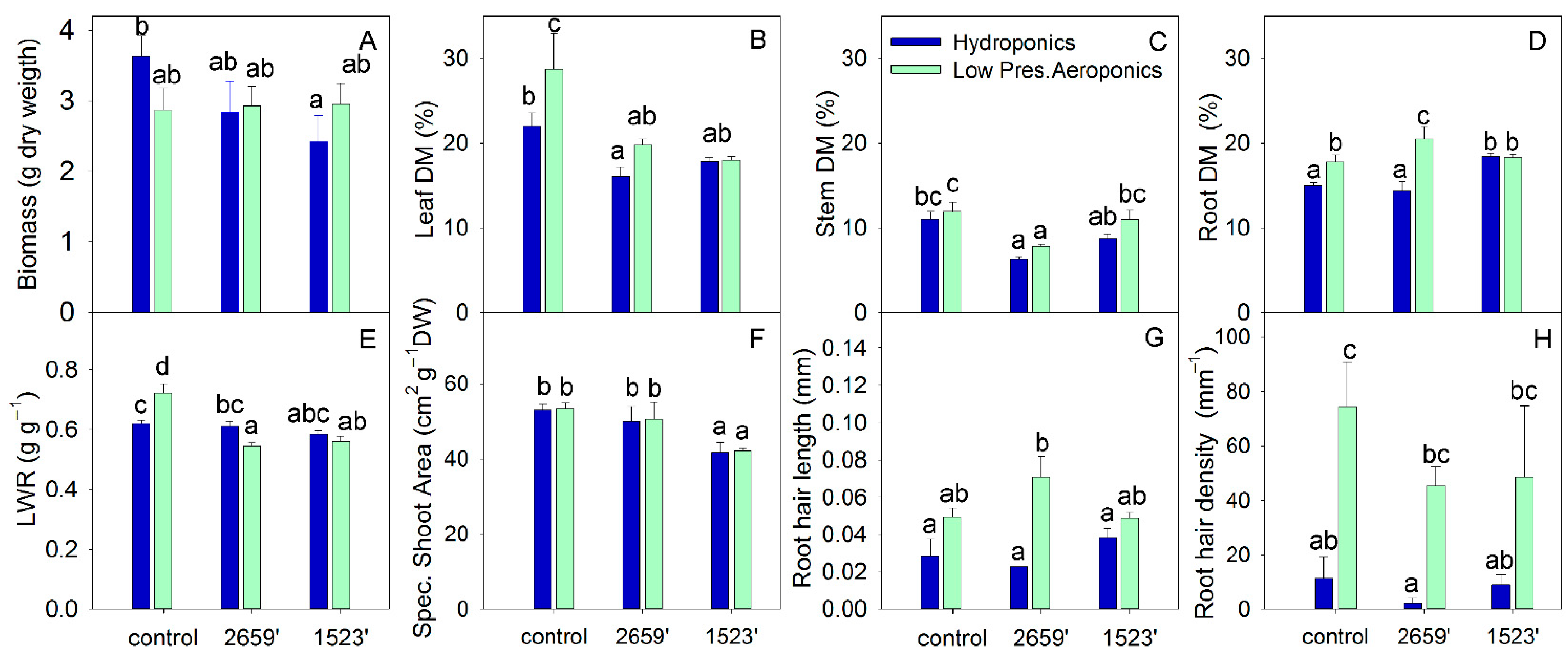
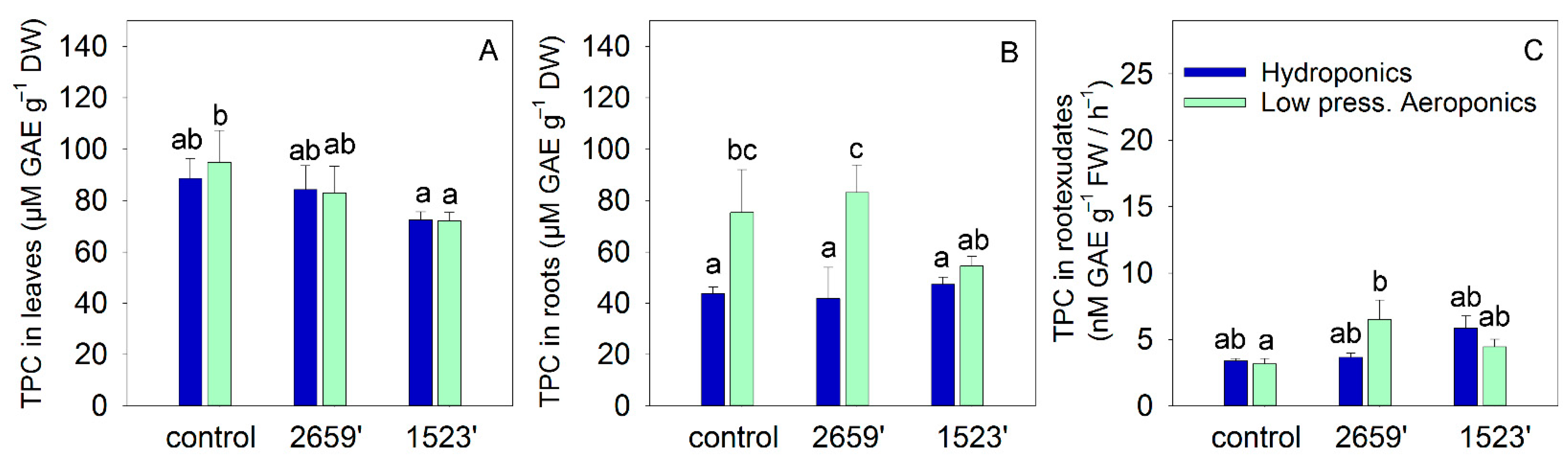
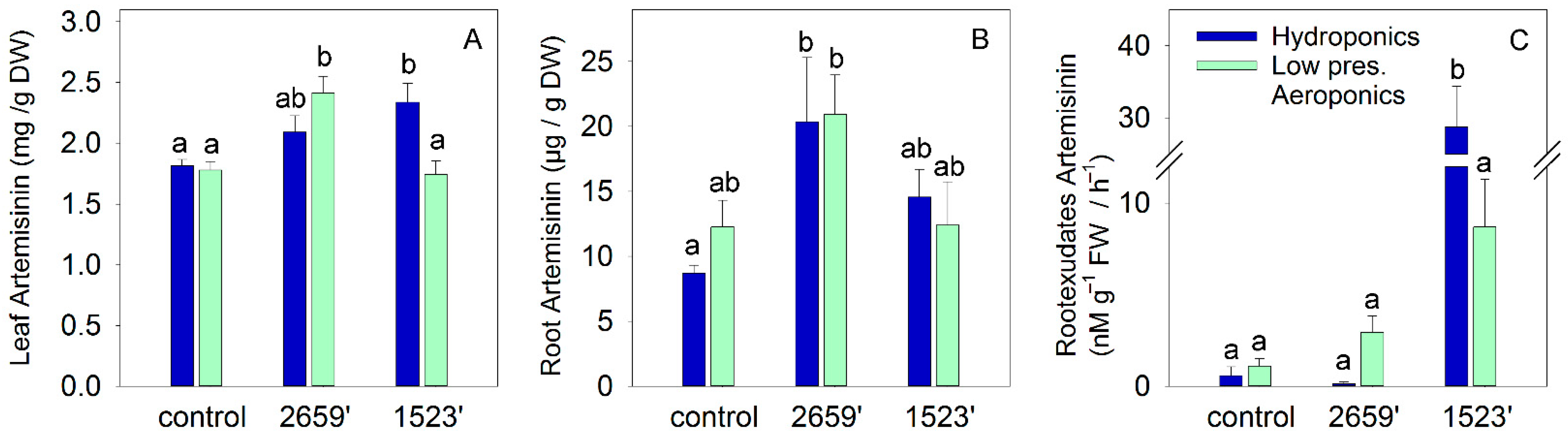
3. Results
4. Discussion
4.1. Enhancing Artemisinin Biosynthesis in Leaves via Composite Plants
4.2. Root Artemisinin Accumulation and Exudation: A Neglected but Significant Target
4.3. Tissue- and Compound-Specific Regulation of Phenolics
4.4. Physiological Trade-Offs and Biomass Allocation
4.5. A Scalable and Ecologically Relevant Production Platform
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atherton, H.R.; Li, P. Hydroponic cultivation of medicinal plants—plant organs and hydroponic systems: Techniques and trends. Horticulturae 2023, 9, 349. [Google Scholar] [CrossRef]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Walters, D.; Voelckel, C. Costs of resistance in plants: From theory to evidence. In Annual Plant Reviews Online; Wiley: Hoboken, NJ, USA, 2014; pp. 263–307. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants—To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Paponov, M.; Antonyan, M.; Slimestad, R.; Paponov, I.A. Decoupling of plant growth and accumulation of biologically active compounds in leaves, roots, and root exudates of Hypericum perforatum L. by the combination of jasmonate and far-red lighting. Biomolecules 2021, 11, 1283. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, P.; Kumari, R.; Kumar, S. Hairy root cultures for secondary metabolite production. In Genetic Manipulation of Secondary Metabolites in Medicinal Plant; Singh, R., Kumar, N., Eds.; Springer Nature: Singapore, 2023; pp. 205–223. [Google Scholar] [CrossRef]
- Vu, T.D.; Jousse, C.; Pawlicki-Jullian, N.; Schiltz, S.; Nguyen, T.K.O.; Tran, T.L.M.; Bouquet, L.A.; Hehn, A.; Boitel-Conti, M.; Moussaron, J.; et al. Datura innoxia plants hydroponically-inoculated with Agrobacterium rhizogenes display an enhanced growth and alkaloid metabolism. Plant Sci. 2018, 277, 166–176. [Google Scholar] [CrossRef]
- Mohammadparast, B.; Shirazi, Z. Enhancement of the production of terpenoid and flavonoid secondary metabolites in the ground and aerial parts of licorice composite plant in a hydroponic system. J. Biotechnol. 2025, 399, 164–171. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, R. Engineering secondary metabolite production in hairy roots. Phytochem. Rev. 2011, 10, 371–395. [Google Scholar] [CrossRef]
- Patel, P.K.; Rathod, K.D. Aeroponics: Transforming vegetable farming for the future. J. Adv. Biol. Biotechnol. 2025, 28, 155–165. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.H.; Abass, W.; Morsy, O.M.; Elghobashy, H.; Shaban, Y.; Egela, M. Production of basil (Ocimum basilicum L.) under different soilless cultures. Sci. Rep. 2021, 11, 12754. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.M.K.; Gao, J.; Tunio, M.H. Development and experiment of the intelligent control system for rhizosphere temperature of aeroponic lettuce via the internet of things. Int. J. Agric. Biol. Eng. 2022, 15, 225–233. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tan, H.; Zhang, L. Artemisia annua glandular secretory trichomes: The biofactory of antimalarial agent artemisinin. Sci. Bull. 2016, 61, 26–36. [Google Scholar] [CrossRef]
- Koehorst, R.; Laubscher, C.P.; Ndakidemi, P.A. Growth response of Artemisia afra Jacq. to different pH levels in a closed hydroponics system. J. Med. Plants Res. 2010, 4, 1617–1623. [Google Scholar]
- Hu, Z.-B.; Du, M. Hairy root and Its application in plant genetic engineering. J. Integr. Plant Biol. 2006, 48, 121–127. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, H.I. The Water-Culture Method for Growing Plants Without Soil; California Experimental Agriculture Station Circular: Berkeley, CA, USA, 1950; p. 32. [Google Scholar]
- Bahramnejad, B.; Naji, M.; Bose, R.; Jha, S. A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol. Adv. 2019, 37, 107405. [Google Scholar] [CrossRef]
- Paponov, M.; Flate, J.; Ziegler, J.; Lillo, C.; Paponov, I.A. Heterogeneous nutrient supply modulates root exudation and accumulation of medicinally valuable compounds in Artemisia annua and Hypericum perforatum. Front. Plant Sci. 2023, 14, 1174151. [Google Scholar] [CrossRef]
- Paponov, M.; Ziegler, J.; Paponov, I.A. Light exposure of roots in aeroponics enhances the accumulation of phytochemicals in aboveground parts of the medicinal plants Artemisia annua and Hypericum perforatum. Front. Plant Sci. 2023, 14, 1079656. [Google Scholar] [CrossRef]
- Gavarić, N.; Aćimović, M.; Kladar, N.; Hitl, M.; Drljača Lero, J.; Milić, N.; Radovanović, K. Unlocking the bioactivity of sweet wormwood (Artemisia annua L., Asteraceae) ethanolic extract: Phenolics, antioxidants, and cytotoxic effects. Pharmaceutics 2025, 17, 890. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Khodakovskaya, M.V.; Glazunov, V.P.; Radchenko, S.V.; Zvereva, E.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Solomatina, T.O.; Khopta, A.A.; Brodovskaya, E.V.; Gorpenchenko, T.Y.; Grigorchuk, V.P.; Bulgakov, D.V.; Bulgakov, V.P. Agropine-type rolA modulates ROS homeostasis in an auxin-dependent manner in rolA-expressing cell cultures of Rubia cordifolia L. Planta 2024, 261, 20. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sudheer, W.N.; Lakshmaiah, V.V.; Mukherjee, E.; Nizam, A.; Thiruvengadam, M.; Nagella, P.; Alessa, F.M.; Al-Mssallem, M.Q.; Rezk, A.A.; et al. Biotechnological approaches for production of artemisinin, an anti-malarial drug from Artemisia annua L. Molecules 2022, 27, 3040. Molecules 2022, 27, 3040. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Yu, M.; Lai, E.M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, O.C.; Nicolia, A.; Diretto, G. Terpenoid transport in plants: How far from the final picture? Plants 2023, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Veena, V.; Taylor, C. Agrobacterium rhizogenes: Recent developments and promising applications. Vitr. Cell. Dev. Biol.-Plant 2007, 43, 383–403. [Google Scholar] [CrossRef]
- Weathers, P.J.; Wyslouzil, B.E.; Wobbe, K.K.; Kim, Y.J.; Yigit, E. The biological response of hairy roots to O2 levels in bioreactors. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 286–289. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Secomandi, E.; De Gregorio, M.A.; Garcia-Perez, P.; Vaccari, F.; Puglisi, E.; Lucini, L. Waterlogging alone and combined with other abiotic stresses provides unique metabolic signatures at the plant-rhizosphere interface: A multi-omics perspective on root metabolome, root exudation and rhizomicrobiome. Plant Physiol. Biochem. 2025, 221, 109646. [Google Scholar] [CrossRef]
- Sharma, P.; Padh, H.; Shrivastava, N. Hairy root cultures: A suitable biological system for studying secondary metabolic pathways in plants. Eng. Life Sci. 2013, 13, 62–75. [Google Scholar] [CrossRef]
- Collier, R.; Fuchs, B.; Walter, N.; Kevin Lutke, W.; Taylor, C.G. Ex vitro composite plants: An inexpensive, rapid method for root biology. Plant J. 2005, 43, 449–457. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paponov, M.; Lama, P.S.; Ziegler, J.; Lillo, C.; Paponov, I.A. Strain- and System-Specific Enhancement of Artemisinin in Artemisia annua Composite Plants Grown in Hydroponic and Aeroponic Systems. Horticulturae 2025, 11, 1070. https://doi.org/10.3390/horticulturae11091070
Paponov M, Lama PS, Ziegler J, Lillo C, Paponov IA. Strain- and System-Specific Enhancement of Artemisinin in Artemisia annua Composite Plants Grown in Hydroponic and Aeroponic Systems. Horticulturae. 2025; 11(9):1070. https://doi.org/10.3390/horticulturae11091070
Chicago/Turabian StylePaponov, Martina, Pembi S. Lama, Jörg Ziegler, Cathrine Lillo, and Ivan A. Paponov. 2025. "Strain- and System-Specific Enhancement of Artemisinin in Artemisia annua Composite Plants Grown in Hydroponic and Aeroponic Systems" Horticulturae 11, no. 9: 1070. https://doi.org/10.3390/horticulturae11091070
APA StylePaponov, M., Lama, P. S., Ziegler, J., Lillo, C., & Paponov, I. A. (2025). Strain- and System-Specific Enhancement of Artemisinin in Artemisia annua Composite Plants Grown in Hydroponic and Aeroponic Systems. Horticulturae, 11(9), 1070. https://doi.org/10.3390/horticulturae11091070





