Abstract
Cauliflower, a widely cultivated vegetable crop valued for its edible curds, faces a persistent threat from insect pests, which are typically managed using synthetic insecticides. This study evaluated the benefits of intercropping practices as part of an ecological pest management strategy in cauliflower cultivation during the winter seasons of 2017–18 and 2021–22. Nine insect pests belonging to six families of three orders were recorded. The calendula intercropping system (IS) consistently showed the lowest infestation by Plutella xylostella and Pieris brassicae/plant. Calendula IS had attracted the highest numbers of syrphids, Cotesia glomerata, Diaeretiella rapae, Cotesia vestalis, and coccinellids such as Coccinella septempunctata and Cheilomenes sexmaculata. In candytuft IS, a strong tri-trophic interaction between the flower and D. rapae significantly reduced aphid populations, for each additional D. rapae, aphid numbers decreased by 48.53 in 2018. The marigold IS recorded the highest Shannon diversity index in 2021–22. The longest adult survival of C. septempunctata (8.67 ± 3.35 days), in the absence of aphids was recorded on candytuft flowers. The total sugars and protein in flowers positively influenced the longevity of the adult coccinellid beetles (R2-40.42 and 20.79%, respectively). Calendula intercropping yielded the highest revenue return of Indian rupee (₹) 11.33 per INR 1 invested, compared to the cauliflower monocrop (1.58). These findings demonstrate that, intercropping and habitat manipulation can enhance ecological pest control and reduce the dependence on synthetic chemicals.
1. Introduction
Cauliflower (Brassica oleracea L. ssp. botrytis) is a widely cultivated vegetable crop valued for its edible curd. However, its production, along with other cole crops such as cabbage, faces continuous threats from emerging insect pests [1,2]. Unfortunately, the incidence of these pests has been on the rise in recent years, causing significant financial losses for farmers. For instance, Krishnamoorthy [3] reported that the diamondback moth (DBM), Plutella xylostella L. (Lepidoptera: Plutellidae) could cause up to 52% yield loss in cauliflower, while infestation of the cabbage aphid, Brevicoryne brassicae (L.) (Homoptera: Aphididae) was associated with yield losses of up to 41.9% [4]. To mitigate these threats, farmers often apply insecticides, sometimes twice a week to control pests such as P. xylostella [5]. The repeated use of insecticides, either individually or in combination, had adverse effects on non-target arthropods, including natural enemies [6,7,8], and may contribute to the selection of insect resistance [9]. In addition, insecticide exposure has health implications, affecting the skin, gastrointestinal, nervous, respiratory, reproductive, and endocrine systems [10]. Occupational, unintentional, or intentional exposure to high levels of insecticides can lead to hospitalization and even death [11]. Consequently, the need for eco-friendly pest management techniques has become imperative and urgent.
Ecological approaches such as habitat management or Ecological Engineering (EE) are gaining recognition as integral to sustainable pest control strategies [12]. These approaches rely on an in-depth understanding of pest behavior, bio-ecology, and the natural regulation. Habitat management can be implemented at various levels, including within a specific crop, throughout the farm, or even at the landscape scale. One effective strategy for habitat management is intercropping, which helps manage pests and contributes significantly to conservation of natural enemies. By increasing the diversity of the ecosystem, intercropping reduces insect pests [13], enhances survival of natural enemies [14], deters pests from host plants, and ultimately enhances crop yield. Ecological engineering has emerged as a promising approach for pest management, focusing on cultural strategies to manipulate habitats and improve biological control of key pests [15]. This method involves ecologically conscious habitat manipulation to favor natural enemies and strengthen biological control in agricultural systems. It aligns with the principles of conservation biological control (CBC) [16,17], providing an effective alternative to chemical dependency. Though both CBC and habitat manipulation aim to improve conditions for natural enemies (“the enemy hypothesis”), their specific strategies may differ [18].
Studies have shown that intercropping can significantly reduce pest damage while enhancing natural enemy populations [19,20]. Therefore, it is of utmost importance to implement habitat manipulation and diversify farming practices to establish an ecological infrastructure that caters to the needs of natural enemies, providing them with suitable food sources like pollen and nectar, alternate prey, and shelter [12,20]. Intercropping horticulture crops with floriculture crops has emerged as a novel approach, as it not only increases profits for farmers but also ensures financial stability and higher returns. In such cases, farmers can offset potential losses with monocropping during low market value. To test this hypothesis, our study assessed five winter season flowering crops in both lab and field settings, aiming to answer the following questions:
- How effectively do the intercrops attract natural enemies, and how do they impact pest population dynamics?
- Can floral resources sustain natural enemies during periods of low pest density?
- What is the economic return from the selected intercrops, particularly during periods of low market value for cauliflower?
We hypothesized that floral intercropping would interfere with pest host-finding behavior and simultaneously enhance natural enemy performance, resulting in improved yield and profitability in cauliflower cultivation.
2. Materials and Methods
2.1. Study Location and Details of Intercrops
The field experiments were conducted at the Division of Entomology, ICAR- Indian Agricultural Research Institute (IARI), Pusa campus, New Delhi (28°38′ N; 77°09′ E; alt. 228.61 m). The climate in the region is classified under monsoon influenced humid subtropical. The average temperature remains 25.09 °C annually, with an average annual precipitation of 797.3 mm. The topography of the field was fairly uniform, with a gentle slope. The soil was sandy loam in texture with a crumby structure. The main crop cauliflower and selected flower crops viz., candytuft, Iberis sempervirens L. (Brassicaceae); calendula (pot marigold), Calendula officinalis L. (Asteraceae); marigold, Tagetes erecta L. (Asteraceae); white daisy, Bellis perennis L. (Asteraceae) and cineraria, Pericallis × hybrida (Asteraceae) seeds were purchased from Punjab Beej Company, Chowk Baraf Khana, New Delhi 110007, India and were sown in a raised nursery bed. Sowing dates were synchronized to ensure that flower blooming coincided with peak pest incidence in cauliflower. The field trial was laid out in a Randomized Complete Block Design with seven treatments; each replicated three times (Figure 1). One-month-old cauliflower seedlings were transplanted at a spacing of 60 × 45 cm. Flower strips were planted in a 7:1 crop-to-flower row ratio, with the fourth row in each plot designated for flowers.
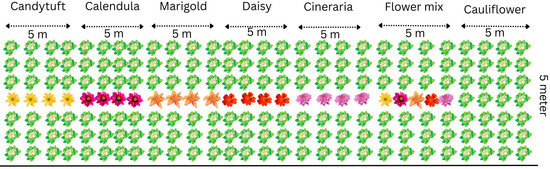
Figure 1.
Layout plan of the field experimentation.
In the sixth treatment, a flower mix was created using ten plants of each flower species grown together in a single plot. Each replication plot measured 5 × 5 m, hence the total area of replicated trial was 15 × 15 m, giving a total experimental area of 15 × 15 m. The experiments were conducted during the winter seasons of 2017–18 and 2021–22. All recommended agronomic practices were followed, except for insecticide application [19].
2.2. Field Observations on Insect Pests and Their Natural Enemies
The abundance of insect pests and their natural enemies were determined for each intercropping system. Insect pest populations were recorded from 10 randomly tagged cauliflower plants in each replication at weekly intervals and the observations continued till harvest. In the case of lepidopteran pests, the plants were observed for total larval and pupal populations per plant. In the case of aphids, all the leaves were observed for the population of aphids (both nymphs and adults), and damage incidence > 30 aphids/plant was taken as a benchmark. Predators were grouped into coccinellids and syrphids, and their abundance was recorded for all strips. A non-destructive method was used to collect data on insect pests. The abundance of parasitoids associated with Lipaphis erysimi (Kaltenbach) (Homoptera: Aphididae), P. xylostella, and Pieris brassicae (L.) (Lepidoptera: Pieridae) was monitored weekly through visual counts and by sampling pest populations exhibiting signs of parasitism. Aphid mummies and parasitized larvae were brought to the laboratory for observation of parasitoid emergence. In addition, the parasitized insects were also observed for the possible emergence of hyperparasitoids. In addition to direct visual count, 5 yellow pan traps filled with soap water were placed in each intercropped cauliflower plot (15 traps/each treatment) during peak insect activity weeks and observed daily for the presence of arthropods and the insects trap data was used for estimating biodiversity indices. Finally, the yield of cauliflower from each replication was recorded and converted to a per-hectare basis. Similarly, seeds harvested from flower crops in each plot were also converted to yield per hectare. The monetary value of the yields was calculated in Indian rupees, and the benefit–cost ratio was estimated.
Effect of Floral Diet on the Longevity of Adult Coccinellid, Coccinella septempunctata L. (Coccinellidae: Coleoptera)
The effect of five different floral diets viz., C. officinalis (Figure 2a), Pericallis × hybrida (Figure 2b), T. erecta (Figure 2c), B. perennis (Figure 2d), and I. sempervirens (Figure 2e), on the survival of adults of coccinellid predator C. septempunctata was assessed in the laboratory conditions. To conduct the experiment, the pupae of C. septempunctata collected from the cauliflower field were kept in Petri plates (90 mm) until adult emergence. For each treatment, 15 newly emerged adults were provided with a single flower type. A control group received only cauliflower leaves. Each treatment was replicated four times. To maintain the prolonged freshness of leaves, 5 mL of Hoagland’s media (1.5%) was poured into each Petri plate at one side in a slant position and kept for solidifying. The cut end of the petiole of the flower diet was inserted into Hoagland’s media. The agar supported flowers and provided a water source, kept the flowers fresh. The survival of C. septempunctata adults was monitored daily. The average longevity of adult beetles was recorded. The fresh flowers (same numbers used for longevity studies) were brought from the field for estimation of total sugar and total soluble protein. The total sugar content in the nectar of flowers was estimated by the Dubois method [21] using glucose as standard (D-(+)-Glucose monohydrate, Hi-LR™), and the total soluble protein content in the flowers was estimated by the Bradford method [22] using Bovine Serum Albumin as standard. The total sugar and total soluble protein content in the flowers was correlated with predator longevity.
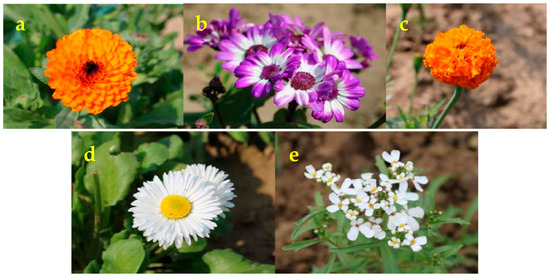
Figure 2.
Flowers of different intercrops used in the field studies (a) Calendula; (b) Cineraria; (c) Marigold; (d) Daisy; (e) Candytuft.
2.3. Identification of Natural Enemies and Their Characterization
The parasitoids that emerged from the field-collected insect pests were preserved in 70% alcohol and sent for identification at ICAR- NBAIR, Bengaluru, India. Further, the identity was confirmed through molecular approaches, i.e., sequencing of COX1. The genomic DNA was isolated using a DNA extraction kit (QIAGEN DNeasy, Hilden, Germany) following the manufacturer’s protocols. The whole individual of each parasitoid was placed in 1.5 mL micro-centrifuge tubes separately. DNA thus obtained was subjected to PCR amplification of a 658 bp region near the 5′ terminus of the COX1 gene following the standard protocol [23]. The following primers were used for the amplification of the COX1 gene, i.e., forward primer (LCO14905′-GGTCAACAAATCATAAAGATATTGG-3′) and reverse primer (HCO 21985′-TAAACTTCAGGGTGACCAAAAAATCA-3′) [24]. Polymerase Chain Reaction (BioRad C1000™) was carried out in flat capped 200 μL PCR tubes (Tarsons, Kolkata, India). An aliquot of 50 μL reaction volume contained: 5 μL Taq buffer, 1 μL 10 mMdNTP mixes, 1 μL (20 pmol/μL) forward primer, 1 μL (20 pmol/μL) reverse primer, 1 μL Taq DNA polymerase (1 U/μL), 5 μL DNA (50 ng/μL), and 36 μL sterile water. Thermo cycling consisted of an initial denaturation of 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 45 °C for 1 min, and extension at 72 °C for 1 min. As standard protocol, the amplified products were analyzed on 1.5% agarose gel electrophoresis [25]. M/s Chromous, Bengaluru, sequenced the amplified products. The samples were bi-directionally sequenced and checked for homology, insertions, deletions, and stop codons and frameshifts using NCBI-BLAST and ORF finder. The accession number has been obtained for all the natural enemies.
2.4. Data Analysis
The weekly data on insect count collected from all sampling methods were averaged for the experimental plot. The data from field experiments were subjected to analysis of variance using the Web Agri Stat Package (WASP Version 2.0) software developed by ICAR—Central Coastal Agricultural Research Institute, Goa, India [26]. The laboratory data on the longevity of C. septempunctata adults was analyzed in CRD using WASP software. One-way analysis of variance (ANOVA) was used to test the mean differences among treatments at 1% and 5% levels of significance, using Duncan’s multiple range test (DMRT) for post hoc comparisons. The biodiversity indices viz., Shannon–Weiner diversity index and Simpson index were calculated based on the mean insect count data using PAST 4.03 Software [27]. To analyze the tri-trophic interactions, we used a dummy variable regression model implemented in STATA software, version 15.1 [28]. In case of Aphids, the regression model with seven treatments, three independent variables, and their interactions with treatments involved setting up dummy variables to represent the treatments and their interactions. The treatments are considered as T1, T2, …, T7 and the independent variables as X1, X2, X3.
The model equation would take the following form:
where,
- Y is the dependent variable.
- X1, X2, X3 are the continuous independent variables.
- Di is dummy variables for the treatments T1, T2… T7
- β1, β2, β3 are the coefficients for X1, X2, X3 respectively.
- βi+3 are the coefficients for the treatment dummy variables Di
- βi+9, βi+15, βi+21 are the coefficients for the interaction terms between X1, X2, X3 and the treatment dummy variables Di respectively.
- ϵ is the error term.
Some of the variables are omitted because of multicollinearity issues. The choice of reference treatment for interpreting the coefficients of the dummy variables was control treatment. Similar dummy variable regression models were fitted for DBM and cabbage butterfly considering the number of independent variables and their interaction with the treatments.
3. Results
3.1. Identification of Natural Enemies
The identification report of primary and secondary parasitoids was received from ICAR- NBAIR, Bengaluru, namely C. septempunctata (ON705842; generalist predator in cauliflower ecosystem), Cheilomenes sexmaculata (Coccinellidae: Coleoptera) (ON797332; generalist predator in cauliflower ecosystem); Diaeretiella rapae (Braconidae: Hymenoptera) (ON797330; Primary solitary parasitoid parasitizing the aphids infesting cauliflower), Cotesia vestalis (Braconidae: Hymenoptera) (ON797332; solitary larval parasitoid emerged from P. xylostella). Secondary parasitoids included Tetrastichus sp. (Eulophidae: Hymenoptera) (ON803454; from pupae of coccinellids), and Pachyneuron aphidis (Pteromalidae: Hymenoptera) (ON803455; from aphid mummies of D. rapae).
3.2. Effect of Habitat Manipulations on the Incidence of Insect Pests
A total of nine insect pests belonging to six families of three orders were recorded. An aphid complex comprising L. erysimi, Myzus persicae (Sulzer), and B. brassicae was observed during the study period. The diamondback moth, P. xylostella, and the Cabbage butterfly, P. brassicae, were the major pests, and the cabbage looper, Trichoplusia ni (H.; Noctuidae: Lepidoptera), and painted bug, Bagrada hilaris Burmeister (Pentatomidae: Hemiptera), were the minor pests observed. Aphid incidence varied significantly across treatments. The lowest aphid populations were recorded in the cineraria (103.27 ± 20.91) and candytuft (99.28 ± 14.88) intercropping systems during 2017–18 and 2021–22, respectively (Table 1). The severity of P. xylostella infestation was lower during 2017–18 compared to 2021–22, with the lowest number of larvae/plants recorded in calendula (2.56 ± 0.38 and 6.58 ± 1.84, respectively) IS during both the year of study. The incidence of P. brassicae was lowest in calendula (11.25 ± 0.25 and 10.89 ± 0.69 larvae/plant) IS, respectively, during 2017–18 and 2021–22. The incidence of other defoliators was minor during both years of study; however, the incidence varied significantly between the intercrops.

Table 1.
Effect of intercropping on the incidence of insects pests (mean ± SE) on cauliflower during Rabi season 2017–18 and 2021–22.
3.3. Effect of Habitat Manipulations on the Incidence of Natural Enemies
A total of six groups of natural enemies consisting of three predators and three parasitoids were recorded to feed on the various stages of insect pests (Table 2). All the predators (syrphids, coccinellids, and spiders) were the generalist, while the parasitoids (C. vestalis, C. glomerata, and D. rapae) were specialists of the cole crop pests. Syrphid activity varied significantly between the intercrops, and the maximum numbers was observed in cineraria (4.00 ± 1.11/plant) and calendula (5.75 ± 0.50/plant) IS. The activity of coccinellid beetles was dominant on candytuft (9.25 ± 1.73/plant) and flower mix (9.58 ± 2.25/plant) IS during 2021–22. The incidence of C. vestalis (parasitized cocoon) was non-significant between the different IS during 2017–18, while during 2021–22, the highest number of cocoons was collected from calendula IS (4.56 ± 0.19 cocoons/plant). Similarly, the maximum number of parasitized cocoons of C. glomerata (5.89 ± 1.07) was found on calendula IS during 2017–18, although this number was not significant in the 2021–22. The activity of D. rapae in terms of aphid mummies was highest in the calendula IS during both the years of study.

Table 2.
Effect of intercropping on the incidence of natural enemies (mean ± SE) on cauliflower during Rabi season 2017–18 and 2021–22.
3.4. Tritrophic Interaction and Their Effect on Pest Population Dynamics
Aphid complex: The dummy regression model results indicated that the individual effects of all intercropping treatments (T3 to T8) did not significantly impact aphid populations (Table 3). Likewise, the influence of natural enemies, such as syrphids and coccinellids, on the pest populations showed no significant effect in limiting aphid populations. However, the presence of D. rapae had a considerable impact, which demonstrated a noteworthy effect on population change (p < 0.0001). The coefficient value suggested that for every 90.34 increase in aphid count, there is was an increase in one D. rapae parasitoid.

Table 3.
Parameter estimates of dummy regression models with an interaction effect of intercrops and natural enemies on aphid complex.
The interaction effects revealed that planting intercrops did not influence aphid population change by attracting syrphid populations. However, when coccinellid beetles interacted with intercrops, only the combination of Cineraria × Coccinellids (T5; p < 0.021) significantly reduced the aphid populations. Notably, the interaction of all intercrops with D. rapae significantly influenced the decline in aphid populations. For instance, for every increase in one D. rapae parasitoid in candytuft intercrop (T1), there was a decrease of 48.53 aphids in 2018. Similar observations were noted in calendula (T2; −76.51), marigold (T3; −67.73), daisy (T4; −38.05), cineraria (T5; −57.05), and flower mix (T6; −42.72), all with respective p values of <0.0001, <0.0001, <0.021, <0.0001, and <0.010. However, in 2022, the dummy regression model against aphids in the cauliflower ecosystem demonstrated that neither intercrops, natural enemies, nor their interactions significantly influenced aphid population changes.
Diamondback moth, P. xylostella: Regarding P. xylostella populations, the interaction effect of intercrops with C. vestalis in 2018 showed that Calendula × C. vestalis (p < 0.004) had a significant impact in reducing pest populations, which resulted in a reduction in −2.20 P. xylostella larvae for every increase in one C. vestalis parasitoid. A similar pattern of individual effects were observed in 2022, but none of the interaction effects were significant (Table 4).

Table 4.
Parameter estimates of dummy regression models with an interaction effect of intercrops and natural enemies on P. xylostella.
Cabbage butterfly, P. brassicae: Regarding cabbage butterfly populations, the interaction effects of intercrops with C. glomerata in 2018 did not significantly impact population reduction (Table 5). However, the individual effect of C. glomerata on P. brassicae was significant (p < 0.047). Conversely, interaction effect was observed during 2022, except for the flower mix × C. glomerata interaction which had significant effect on reduction in population. The results showed reduction of 19.11 P. brassicae populations for every increase in the C. glomerata population.

Table 5.
Parameter estimates of dummy regression models with an interaction effect of intercrops and natural enemies on P. brassicae during 2018.
3.5. Effect of Habitat Manipulations on the Different Diversity Indices
During 2017–18, the maximum species richness was observed in calendula (12.00) IS, and candytuft IS recorded the highest Simpson index (D; 0.3291). The Shannon (H) index was the highest in calendula (1.89), whereas evenness was observed in candytuft (0.4765) (Table 6). However, during 2021–22, the maximum species richness was observed in marigold (10.00). However, calendula IS recorded the highest Simpson index (D; 0.8091). The Shannon (H) index was highest in the marigold (1.896) IS, whereas evenness was observed in the marigold (0.3728).

Table 6.
Diversity indices of insects in sole cropped and intercropped fields at IARI, New Delhi, India.
3.6. Effect of Habitat Manipulations on the Incidence of Hyperparasitoids
A total of three hyperparasitoids were recovered from the primary natural enemies. Tetrastichus sp (Eulophidae), a gregarious hyperparasitoid, emerged from the pupae of C. septempunctata. In addition, the Dinocampus coccinellae (Schrank) (Braconidae) cocoon was found attached to the adults of C. septempunctata. Interestingly, Pachyneuron aphidis Bouché (Pteromalidae) emerged from the field-collected aphid mummies parasitized by D. rapae.
3.7. Longevity of C. Septempunctata Adults on the Flowers of Intercrops and Its Relation with the Total Sugars and Total Protein
The longevity of the adults varied significantly on the flowers of the different intercrops used in the study (Table 7). The most prolonged adult survival was recorded on candytuft flowers (8.67 ± 3.35 days), followed by the marigold (8.1 ± 2.60 days). The longevity on calendula, cineraria, and daisy flowers was 5.9 ± 1.66, 4.63 ± 1.19, and 6 ± 2 days, respectively. Interestingly, the adults without food survived for 3.89 ± 1.05 days. The total soluble protein (TSP) and the total sugar (TS) content varied among the intercrops, and the results showed that marigold, calendula, cineraria, candytuft, and daisy contained 2.52 and 1.59; 6.44 and 1.62; 2.30 and 1.98; 5.90 and 2.62; 1.58 and 1.65 mg/g of flower, respectively.

Table 7.
Adult longevity of C. septempunctata on flowers of intercrop and respective sugar and protein concentration in different flowers.
The correlation between the longevity of adults on flowers with their respective TS and TSP. The correlation coefficient (r-value) showed that the value was non-significant (0.57: TS and 0.69: TSP) but positively influenced the longevity of adults. To test the hypothesis, the regression equation was drawn the R2 value showed that the TSP influenced 40.42% of the survival of adults, while TS influenced 20.79% of adult survival (Figure 3).
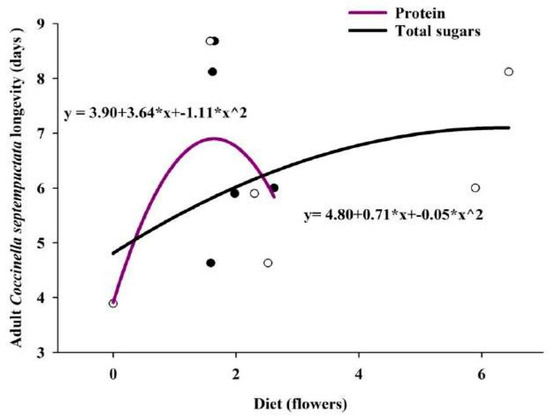
Figure 3.
Scatter plots of predator longevity vs. sugar/protein content with regression lines.
3.8. Effect of Habitat Manipulations on the Curd Weight of Cauliflower, Its Economics and Benefit Cost Ratio of Intercropping Systems
The average weight (g) of the harvested cauliflower heads revealed that the heads of intercropped system were substantially heavier than the heads harvested from monocrop plots (Table 8 and Figure 4).

Table 8.
Benefit cost ratio (BCR) in Indian rupees (₹) of different flower intercropping systems in cauliflower (mean of two seasons data).
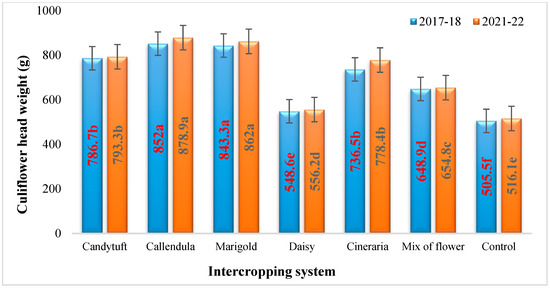
Figure 4.
Effect of flower intercropping on the mean cauliflower head weight (g) per plant. Values followed by different letters within the same-colored column are significantly different.
The results showed that the cauliflower intercropped with calendula (852 and 878.9 g) and marigold (843.3 and 862 g) had significantly higher head weights during both the years, respectively. The intercropping systems with candytuft (786.7 and 793.3 g) and cineraria (736.5 and 778.4 g) showed the second highest yields and were statistically equivalent. The monocrop of cauliflower had the lowest head weight during the study year (505.5 and 516.1 g), respectively.
The yield of calendula and marigold was 15.58 and 15.35 tonnes per hectare, with respective seed yields of 100 kg and 12 kg. The daisy intercropping system had the lowest yield of 9.94 tons with a respective seed yield of 60 kg per hectare. The percent increase in yield over control for the different intercropping systems was as follows: candytuft (39.14), calendula (52.44), marigold (50.21), cineraria (13.77), and flower mix (14.77). At the same time, the daisy intercropping system recorded a 2.74% lower cauliflower yield. The B: C ratio of different intercropping systems is arranged in descending order as follows: calendula (11.33) > candytuft (7.96) > mix of flower (6.80) > daisy (6.55) > cineraria (5.80) > marigold (4.89). The monocrop of cauliflower had the lowest B:C ratio of 1.58 per rupee invested.
4. Discussion
Deterrence of pest colonization through intra-field diversity is probably one of the more promising means of controlling insect pests. Diversity in the crop field may have a profound effect on insect colonization and has been well-documented in the case of intercropping [29]. Intercrops can enhance conservation biological control by providing food and shelter that attract and sustain natural enemies [30]. Intercrops also improve the structural complexity of the habitat below ground by augmenting organic matter and optimizing microclimates for soil-dwelling natural enemies [31]. The results on the effect of intercrops on the incidence of P. xylostella and P. brassicae showed that the planting of intercrops significantly reduced the pest incidence over the monocrop of cauliflower; it was mainly due to the diversity created by the intercrops. Smith and McSorley [32] reported similar results. Further, Ma et al. [33] reported that the cultural practices like intercropping or mixing crops of different types helped in prevention of pest infestation.
The host selection by any pest is influenced by several parameters, including non-host plants. Intercrops may deter pests by acting as physical barriers or through emission of non-specific volatiles [34]. The mean data of both years showed that the calendula intercropping system had the lower number of P. xylostella larvae and was most effective in reducing pest damage, followed by the marigold intercropping system. The P. xylostella had a narrow host range and preferred to feed on brassicaceous hosts. However, the incidence reduced when the cole crops were intercropped with the non-host crops [35]. Because, herbivore must expend more time and energy looking for the plants they can eat, when it encounters one that they cannot consume (non-host plant) [34]. The results are supported by the similar observations on its natural enemy, i.e., during both the years of study, the maximum larvae of P. xylostella parasitized by C. vestalis were recovered from the calendula intercropping system.
The pot marigold, Calendula officinalis is a winter annual known for its beautiful flowers and offers ample floral resources like nectar and pollen [36]. Moreover, the yellow flowers remained the most attractive to hymenopterans [37]. Similar observations were also observed on the incidence of P. brassicae, in which lower pest incidence and its parasitoid C. glomerata was observed in the calendula intercropping system. Furthermore, unlike most previous studies, we quantified both pest and natural enemy diversity across the two seasons, and also assessed hyperparasitoid incidence and predator longevity on floral resources. Our findings provided strong evidence that the observed changes in pest dynamics were primarily driven by the colonization of natural enemies.
The volatiles from host plants were believed to attract foraging phytophagous insect adults, while the volatiles from non-host plants repel them. The incidence of aphids varied significantly among the different intercropping systems. The lowest incidence of aphids was recorded in cineraria and calendula intercropping systems. Similar results have been documented earlier [38,39]. Organic compounds released by non-host plants may act as insect repellents contributing to low insect pest infestations [40]. The activity of natural enemies of aphids like coccinellids, syrphids, and the hymenopteran parasitoid (D. rapae) was highest in the cropping systems, like calendula, cineraria, and flower mix. No other invertebrate natural enemy was noticed on the cauliflower during routine sampling, which indicated the predation/parasitization by syrphids, coccinellids, and D. rapae might be a primary cause of the differences in aphid population [41,42].
The increased diversity of natural enemies can mainly be attributed to habitat manipulation by introducing intercrops. The same findings were also reported by Denno et al. [43]. Botanical diversity has been shown to increase ladybird beetle activity as studied by Elliott et al. [44]. Chemicals from non-host plants are believed to discourage foraging adults, inhibit egg-laying, and contribute to behavioral control of insect pests [45]. Due to the availability of flower resources as an extra source of food, the predators or parasitoids become more prevalent in cropping systems with enhanced crop diversification [46,47]. The enhanced longevity of coccinellids and its positive association with the sugar and protein concentration of flowers supported pest regulation. Hyperparasitoids are the new targets in biological control and potential disruptors of successful biological control [48]. The recovery of hyperparasitoids in the intercropped field in the later stages of crop growth showed the permanent establishment of primary natural enemies in the intercropping system. However, the role of hyperparasitoids and the extent of disruptions in natural biological control need to be studied in detail for the further discussion.
The results of the tri-trophic interaction between the host, pests, and their natural enemies are significantly influenced by intercropping systems. The interaction of calendula × D. rapae and cineraria × coccinellids significantly reduced the aphid populations. The results of one-way ANOVA also showed the lower pests and the highest natural enemies in the respective natural enemies’ double proof of the outcome of the interaction studies. We believe that the yellow flower color of calendula and dark green foliage with blue color might be the reason for more attraction of D. rapae and coccinellids beetles, respectively [49]. Herbivore-induced plant volatiles play a crucial role in these interactions, as they can attract predators and parasitoids to herbivore-attacked plants [50]. The results on the economic yield of cauliflower showed that the cauliflower intercropped with calendula (852 and 878.9 g) and marigold (843.3 and 862 g) had significantly higher head weights during both the years, respectively. Introducing flower crops as intercrops increase the ecosystem’s diversity, thereby increasing the action of natural enemies on insect pests, and ultimately contributes to more yield due to less damage from pests [29,30]. The diverse natural enemies observed in the calendula and marigold intercropping systems acted on the insect pest, which resulted in natural pest control and enhanced yield [51].
5. Conclusions
This study demonstrated that increasing floral diversity within cauliflower cropping systems can effectively reduce pest infestations by enhancing the abundance and activity of natural enemies. Among all treatments, the calendula intercropping system yielded the greatest benefits, with a 52.44% increase in cauliflower yield over the control and the highest economic return of INR 11.33 for every INR 1 invested. Candytuft intercropping also performed well, with a benefit–cost ratio of 7.96. The study suggested that the intercropping can be a practical ecological approach for pest management in cauliflower cultivation. It could help reduce insect pest damage, promote natural enemies’ presence, and provide farmers with financial benefits. Implementing intercropping practices and habitat manipulation can contribute to sustainable crop production and reduce the reliance on synthetic chemicals for pest management.
Author Contributions
Conceptualization, Material preparation: S.S.S.; Conduct of field studies, data collection and curation: K.M.C., G.R.H., R.K., K.G.N., S.K. and R.H.S.; Statistical analysis: K.M.C. and H.H.V.; The first draft of the manuscript was written by K.M.C., C.M. and S.S.S.; Revision and Review: S.C., E.V.M., J.S.R. and L.R. All authors have read and agreed to the published version of the manuscript.
Funding
The work is part of ICAR-IARI in-house project “Studies of population dynamics/epidemiology, host-plant relationship, tri-trophic interactions, and development of pest management strategies in relation to climate change and contemporary cropping systems” (CRSC-IARI-SIL-2014033265).
Data Availability Statement
All data available in the manuscript.
Acknowledgments
The first author thankful to DST, Gov. of India for Inspire fellowship (IF1607771) during the study period. The first author is thankful to the Director, ICAR-IGFRI, Jhansi for his encouragement and also Head, Division of Entomology, ICAR- IARI, New Delhi for providing the research facilities. The first author acknowledged Ankita Gupta for her assistance in identifying primary and secondary parasitoids.
Conflicts of Interest
The authors declare that they have no conflict of interest. The plant collection and use were in accordance with all the relevant guidelines.
Abbreviations
The following abbreviations are used in this manuscript:
| CBC | Conservation Biological Control |
| DBM | Diamondback moth |
| EE | Ecological Engineering |
| IARI | Indian Agricultural Research Institute |
| ICAR | Indian Council of Agricultural Research |
| IS | Intercropping System |
| NBAIR | National Bureau of Agricultural Insect Resources |
| WASP | Web Agri Stat Package |
References
- Keerthi, M.C.; Suroshe, S.S. Effect of host plants on the fitness and demographic parameters of the diamondback moth, Plutella xylostella (L.) using age-stage, two-sex life tables. J. Plant Dis. Prot. 2023, 131, 143–154. [Google Scholar] [CrossRef]
- Keerthi, M.C.; Suroshe, S.S.; Singh, P.K.; Chander, S.; Vinod Kumar, P. Life table and demographic parameters of mustard aphid, Lipaphis erysimi (Kaltenbach) (Hemiptera: Aphididae) on five brassicaceous host crops. Curr. Sci. 2024, 126, 77–84. [Google Scholar]
- Krishnamoorthy, A. Biological control of diamondback moth, Plutella xylostella (L.), an Indian scenario with reference to past and future strategies. In Proceedings of the International Symposium, Montpellier, France, 21–24 October 2002; Kirk, A.A., Bordat, D., Eds.; CIRAD: Montpellier, France, 2004; pp. 204–211. [Google Scholar]
- Singh, N.; Dhiman, S. Quality and quantity loss by aphid infestation in vegetables grown under protected cultivation in Ladakh region. Def. Life Sci. J. 2018, 1, 71–74. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Saeed, S.; Noor-Ul-Ane, M.; Crickmore, N. Genetic, biochemical, and physiological characterization of spinosad resistance in Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2008, 101, 1658–1666. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Singh, J.; Singh, S.; Kathpal, T.S. Monitoring of pesticidal contamination of farmgate vegetables from Hisar. Environ. Monit. Assess. 2004, 90, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Amarasekare, K.G.; Shearer, P.W.; Mills, N.J. Testing the selectivity of pesticide effects on natural enemies in laboratory bioassays. Biol. Control 2016, 102, 7–16. [Google Scholar] [CrossRef]
- Regan, K.; Ordosch, D.; Glover, K.D.; Tilmon, K.J.; Szczepaniec, A. Effects of a pyrethroid and two neonicotinoid insecticides on population dynamics of key pests of soybean and abundance of their natural enemies. Crop Prot. 2017, 98, 24–32. [Google Scholar] [CrossRef]
- Shad, S.A.; Sayyed, A.H.; Fazal, S.; Saleem, M.A.; Zaka, S.M.; Ali, M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). J. Pest Sci. 2012, 85, 153–162. [Google Scholar] [CrossRef]
- Sanborn, M.; Kerr, K.J.; Sanin, L.H.; Cole, D.C.; Bassil, K.L.; Vakil, C. Non-cancer health effects of pesticides: Systematic review and implications for family doctors. Can. Fam. Physician 2007, 53, 1712–1720. [Google Scholar]
- Gunnell, D.; Eddleston, M.; Phillips, M.R.; Konradsen, F. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health 2007, 7, 357. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Risch, S.J. Intercropping as cultural pest control: Prospects and limitations. Environ. Manag. 2005, 7, 9–14. [Google Scholar] [CrossRef]
- Cai, H.J.; You, M.S.; Lin, C. Effects of intercropping systems on community composition and diversity of predatory arthropods in vegetable fields. Acta Ecol. Sin. 2010, 30, 190–195. [Google Scholar] [CrossRef]
- Mitsch, W. Ecological engineering a cooperative role with the planetary life-support system. Environ. Sci. Technol. 1993, 27, 438–445. [Google Scholar] [CrossRef]
- Rusch, A.; Valantin-Morison, M.; Sarthou, J.P.; Roger-Estrade, J. Biological control of insect pests in agroecosystems: Effects of crop management, farming systems, and seminatural habitats at the landscape scale: A review. Adv. Agron. 2010, 109, 219–259. [Google Scholar]
- Gurr, G.M.; Scarratt, S.L.; Wratten, S.D.; Berndt, L.; Irvin, N. Ecological engineering, habitat manipulation and pest management. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; Cambridge University Press: Cambridge, UK, 2004; pp. 1–12. [Google Scholar]
- Mailafiya, D.M.; Degri, M.M. Stem borer’s species composition, abundance and infestation on maize and millet in Maiduguri, Nigeria. Arch. Phytopathol. Plant Prot. 2012, 45, 1286–1291. [Google Scholar] [CrossRef]
- Keerthi, M.C.; Sharma, R.K.; Suroshe, S.S.; Sinha, S.R. Ecological engineering in cauliflower for aphid management. Indian J. Agric. Sci. 2020, 90, 1356–1358. [Google Scholar] [CrossRef]
- Hertzog, L.R.; Ebeling, A.; Weisser, W.W.; Meyer, S.T. Plant diversity increases predation by ground-dwelling invertebrate predators. Ecosphere 2017, 8, e01990. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Jangam, A.K.; Thali, P. WASP—Web Agri Stat Package; ICAR Research Complex for Goa: Old Goa, India, 2002. [Google Scholar]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Hardy, M.A. Regression with Dummy Variables; Sage: Thousand Oaks, CA, USA, 1993; No. 93. [Google Scholar]
- Risch, S.J.; Andow, D.; Altieri, M.A. Agroecosystem diversity and pest control: Data, tentative conclusions, and new research directions. Environ. Entomol. 1983, 12, 625–629. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Rowen, E.; Tooker, J.F.; Blubaugh, C.K. Managing fertility with animal waste to promote arthropod pest suppression. Biol. Control 2019, 134, 130–140. [Google Scholar] [CrossRef]
- Smith, H.A.; McSorley, R. Intercropping and pest management: A review of major concepts. Am. Entomol. 2000, 46, 154–161. [Google Scholar] [CrossRef]
- Ma, X.M.; Liu, X.X.; Zhang, Q.W.; Zhao, J.Z.; Cai, Q.N.; Ma, Y.A.; Chen, D.M. Assessment of cotton aphids, Aphis gossypii, and their natural enemies on aphid-resistant and aphid-susceptible wheat varieties in a wheat–cotton relay intercropping system. Entomol. Exp. Appl. 2006, 121, 235–241. [Google Scholar] [CrossRef]
- Hooks, C.R.; Johnson, M.W. Impact of agricultural diversification on the insect community of cruciferous crops. Crop Prot. 2003, 22, 223–238. [Google Scholar] [CrossRef]
- Andow, D.A. Vegetational diversity arthropod population response. Annu. Rev. Entomol. 1991, 36, 561–566. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.; Tan, X.; Desneux, N.; Zappala, L.; Zhang, F.; Wang, S. Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sauteri (Hemiptera: Anthocoridae). Pest Manag. Sci. 2017, 73, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Papiorek, S.; Junker, R.R.; Alves-dos-Santos, I.; Melo, G.A.; Amaral-Neto, L.P.; Sazima, M.; Wolowski, M.; Freitas, L.; Lunau, K. Bees, birds and yellow flowers: Pollinator-dependent convergent evolution of UV patterns. Plant Biol. 2016, 18, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Laxman, G. Investigations on IPM Interventions for the Management of Major Insect Pests of Cabbage (Brassica oleraceae var. capitata). Ph.D. Thesis, Indian Agricultural Research Institute, New Delhi, India, 2018. [Google Scholar]
- Mahendran, B. Investigations on IPM Interventions for the Management of Major Insect Pests of Cabbage (Brassica oleraceae var. botrytis). Ph.D. Thesis, Indian Agricultural Research Institute, New Delhi, India, 2015. [Google Scholar]
- Sulvai, F.; Chauque, B.J.M.; Macuvele, D.L.P. Intercropping of lettuce and onion controls caterpillar thread, Agrotis ipsilon, major insect pest of lettuce. Chem. Biol. Technol. Agric. 2016, 3, 28. [Google Scholar] [CrossRef]
- Pfiffner, L.; Luka, H.; Schlatter, C.; Juen, A.; Traugott, M. Impact of wildflower strips on biological control of cabbage lepidopterans. Agric. Ecosyst. Environ. 2009, 129, 310–314. [Google Scholar] [CrossRef]
- Nelson, E.H.; Matthews, C.E.; Rosenheim, J.A. Predators reduce prey population growth by inducing changes in prey behavior. Ecology 2004, 85, 1853–1858. [Google Scholar] [CrossRef]
- Denno, R.F.; Finke, D.L.; Langelotto, G.A. Direct and indirect effects of vegetation structure and habitat complexity on predator-prey and predator-predator interactions. In Ecology of Predator–Prey Interactions; Barbosa, P., Castellanos, I., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 211–239. [Google Scholar]
- Elliott, N.C.; Kieckhefer, R.W.; Michels, G.J.; Giles, K.L. Predator abundance in alfalfa fields in relation to aphids, within-field vegetation, and landscape matrix. Environ. Entomol. 2002, 31, 253–260. [Google Scholar] [CrossRef]
- Jankowska, B.; Wilk, A. Effect of pot marigold (Calendula officinalis L.) and cypress spurge (Euphorbia cyparissias L.) plant water extracts on the occurrence of pest insects on white cabbage. Folia Hortic. 2011, 23, 21–28. [Google Scholar] [CrossRef]
- Haddad, N.M.; Tilman, D.; Haarstad, J.; Ritchie, M.; Knops, J.M. Contrasting effects of plant richness and composition on insect communities: A field experiment. Am. Nat. 2001, 158, 17–35. [Google Scholar] [CrossRef]
- Lavandero, B.; Wratten, S.; Shishehbor, P.; Worner, S. Enhancing the effectiveness of the parasitoid, Diadegma semiclausum (Helen): Movement after use of nectar in the field. Biol. Control 2005, 34, 152–158. [Google Scholar] [CrossRef]
- Tougeron, K.; Tena, A. Hyperparasitoids as new targets in biological control in a global change context. Biol. Control 2019, 130, 164–171. [Google Scholar] [CrossRef]
- Kemp, E.A.; Cottrell, T.E. Effect of lures and colors on capture of lady beetles (Coleoptera: Coccinellidae) in Tedders pyramidal traps. Environ. Entomol. 2015, 44, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Suroshe, S.S.; Keerthi, M.C.; Hithesh, G.R.; Yogesh, Y.; Rakesh Kumar, S. Chander. Ecological Engineering for Insect Pest Management. In Innovative Biotic Stress Management Strategies in Crops, 1st ed.; Singh, D., Pervez, R., Kumar, A., Eds.; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).