Evaluation of Photosynthetic Performance and Adaptability of Grape Varieties in Arid Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Plant Materials and Cultivation
2.3. Determination of PN-PAR Response Curve
2.3.1. Chlorophyll Fluorescence Parameters Measurement
2.3.2. Method of SPAD Values and Leaf Phenotypes
2.4. Data Processing
3. Results
3.1. Leaf Phenotypes and SPAD Values
3.2. Photosynthetic Parameters of Leaves
3.3. Chlorophyll Fluorescence Parameters and Performance Index of Leaves
3.4. Blade JIP-Test Parameters
3.5. Energy Flux Parameter per Unit Area of Leaf Photosynthetic Object
3.6. Correlation Analysis of Photosynthetic Fluorescence Parameters
3.7. Comprehensive Evaluation of Photosynthetic Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, S.; Zhang, L.; Salahou, M.K.; Jiao, X.; Sang, H. Assessing the Spatial Pattern of Irrigation Demand under Climate Change in Arid Area. ISPRS Int. J. Geo-Inf. 2020, 9, 506. [Google Scholar] [CrossRef]
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of Photosynthesis in Non-Leaf Tissues. Biology 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yadav, V.; Bai, S.; Wu, J.; Zhou, X.; Zhang, W.; Han, S.; Wang, M.; Zeng, B.; Wu, X.; et al. Performance Evaluation of New Table Grape Varieties under High Light Intensity Conditions Based on the Photosynthetic and Chlorophyll Fluorescence Characteristics. Horticulturae 2023, 9, 1035. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Bernardo, S.; Correia, C.; Moutinho-Pereira, J. A comparative physiological study of three red varieties in the Demarcated Douro Region. Sci. Hortic. 2024, 327, 112873. [Google Scholar] [CrossRef]
- Zsófi, Z.; Tóth, E.; Rusjan, D.; Bálo, B. Terroir aspects of grape quality in a cool climate wine region: Relationship between water deficit, vegetative growth and berry sugar concentration. Sci. Hortic. 2010, 127, 494–499. [Google Scholar] [CrossRef]
- Kira, O.; Wen, J.; Han, J.; McDonald, A.J.; Barrett, C.B.; Ortiz-Bobea, A.; Liu, Y.; You, L.; Mueller, N.D.; Sun, Y. A scalable crop yield estimation framework based on remote sensing of solar-induced chlorophyll fluorescence (SIF). Environ. Res. Lett. 2024, 19, 044071. [Google Scholar] [CrossRef]
- Oxborongh, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficience into photochemical and non-photochamical components-calculation of qP and Fv-/Fm- without measuring Fo. Photosyn. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Liu, M. Response of photosynthesis and chlorophyll fluorescence to drought stress in two maize cultivars. Afr. J. Agric. Res. 2012, 7, 4751–4760. [Google Scholar] [CrossRef]
- Tanase, A.; Sumedrea, D.; Florea, A.; Onache, A.; Negru, M.; Oprea, M.; Asănică, A. Estimating the Tolerance of Three Table Grape Varieties to Water Stress by Chlorophyll Fluorescence Analysis. Sci. Papers Ser. B Hortic. 2023, 1, 319–326. [Google Scholar]
- Liu, Q.Q.; Huang, Z.J.; Wang, Z.N.; Chen, Y.F.; Wen, Z.M.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef]
- You, Y.; Lei, J.Q.; Wang, Y.D.; Xu, X.W. Comparative study of the implementation of environmental policies to combat desertification in Kuwait and the Hotan Region of China. Arab. J. Geosci. 2019, 12, 751. [Google Scholar] [CrossRef]
- Liu, L.N.; Wang, F.X. Drying Performance of 13 Grape Varieties in Hotan Prefecture, Xinjiang. Xinjiang Agric. Reclam. Sci. Technol. 2024, 47, 46–49. [Google Scholar]
- Fan, W.; Zhou, J.L.; Zheng, J.H.; Guo, Y.H.; Lina Hu, L.N.; Shan, R.Q. Hydrochemical characteristics, control factors and health risk assessment of groundwater in typical arid region Hotan Area, Chinese Xinjiang. Environ. Pollut. 2024, 363, 125301. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.B.; Zhang, G.C.; Wang, R.R.; Zhang, S.Y. Effect of soil water availability on photosynthesis in Ziziphus jujuba var. spinosus in a sand habitat formed from seashells: Comparison of four models. Photosynthetica 2014, 52, 253–261. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, X.G.; Hao, K.; Yang, Q.L.; Yang, X.Q.; Zhang, W.H.; Cong, Y. Light-response curve of photosynthesis and model fitting in leavesof Mangifera indica under different soil water conditions. Photosynthetica 2019, 57, 796–803. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, K.; Yan, S.; Wang, R.; Wang, H.; Zhao, H.; Zhao, F.; Qi, Y.; Yang, Y.; Wei, X.; et al. Response of photosynthesis to light and CO2 concentration in spring wheat under progressive drought stress. BMC Plant Biol. 2025, 25, 324. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Bykova, N.V.; Keerberg, O.; Pärnik, T.; Bauwe, H.; Gardeström, P. Interaction between Photorespiration and Respiration in Transgenic Potato Plants with Antisense Reduction in Glycine Decarboxylase. Planta 2005, 222, 130–140. [Google Scholar] [CrossRef]
- Wu, J.; Abudureheman, R.; Zhong, H.; Yadav, V.; Zhang, C.; Ma, Y.; Liu, X.; Zhang, F.; Zha, Q.; Wang, X. The Impact of High Temperatures in the Field on Leaf Tissue Structure in Different Grape Cultivars. Horticulturae 2023, 9, 731. [Google Scholar] [CrossRef]
- Khan, N.; Choi, S.-H.; Lee, C.-H.; Qu, M.; Jeon, J.-S. Photosynthesis: Genetic Strategies Adopted to Gain Higher Efficiency. Int. J. Mol. Sci. 2024, 25, 8933. [Google Scholar] [CrossRef] [PubMed]
- Korsakova, S.; Plugatar, Y.; Ilnitsky, O.; Karpukhin, M. A research on models of the photosynthetic light response curves on the example of evergreen types of plants. Agron. Res. 2019, 17, 518–539. [Google Scholar]
- Zhang, Y.L.; Xu, R.; Wang, Z.Z.; Zhang, J.; Tang, X.H.; Chen, Y.; Yan, X.L.; Qu, L.P. Photosynthetic characteristics of Paris polyphylla var. chinensis in response to different light intensities and soil water contents. Front. Plant Sci. 2025, 15, 1521714. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Wang, M.; Liu, H. Green light enhances growth, photosynthetic pigments and CO2 assimilation efficiency of lettuce as revealed by ‘knock out’ of the 480–560 nm spectral waveband. Photosynthetica 2017, 55, 144–152. [Google Scholar] [CrossRef]
- Zhu, T.T.; Li, J.; Liu, Y.Y.; Tong, X.J.; Yu, Q. Leaf photosynthetic light response of summer maize: Comparison of models and analysis of parameters. Photosynthetica 2020, 58, 19–28. [Google Scholar] [CrossRef]
- Gunasekera, H.; De Costa, W.; Nugawela, A. Canopy photosynthetic capacity and light response parameters of rubber (Hevea brasiliensis) with reference to exploitation. Curr. Agr. Res. 2013, 1, 1–12. [Google Scholar] [CrossRef]
- Asadi, M.; Rasouli, F.; Amini, T.; Hassanpouraghdam, M.B.; Souri, S.; Skrovankova, S.; Mlcek, J.; Ercisli, S. Improvement of Photosynthetic Pigment Characteristics, Mineral Content, and Antioxidant Activity of Lettuce (Lactuca sativa L.) by Arbuscular Mycorrhizal Fungus and Seaweed Extract Foliar Application. Agronomy 2022, 12, 1943. [Google Scholar] [CrossRef]
- Ma, X.H.; Zhou, Q.; Hu, Q.D.; Zhang, X.L.; Zheng, J.; Qian, R.J. Effects of Different Irradiance Conditions on Photosynthetic Activity, Photosystem II, Rubisco Enzyme Activity, Chloroplast Ultrastructure, and Chloroplast-Related Gene Expression in Clematis tientaiensis Leaves. Horticulturae 2023, 9, 118. [Google Scholar] [CrossRef]
- Neil, R.B. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Iakovleva, O.V.; Talipova, E.V.; Kukarskikh, G.P.; Krendeleeva, T.E.; Rubin, A.B. Fluorescence parameters of chlorophyll in leaves of caules plants in different environmental conditions. Biofizika 2005, 50, 1112–1119. [Google Scholar]
- Nielsen, S.L.; Simonsen, A.M. Photosynthesis and photoinhibition in two differently coloured varieties of Oxalis triangularis—The effect of anthocyanin content. Photosynthetica 2011, 49, 346–352. [Google Scholar] [CrossRef]
- Sato, R.; Ito, H.; Tanaka, A. Chlorophyll B Degradation by Chlorophyll B Reductase under High-Light Conditions. Photosynth. Res. 2015, 126, 249–259. [Google Scholar] [CrossRef]
- Ma, P.F.; Li, H.; Yang, Y.J.; Zhao, H.J.; Fu, X.J.; Zhang, C.N. Effects of salicylic acid on Chloroplast D1 protein phosphorylation and photosystem Ⅱ function of wheat under high temperature and strong Light stress. Chin. J. Appl. Ecol. 2008, 19, 2632–2636. [Google Scholar]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Larkum, A.W.D. Chlorophyll a Fluorescence A Signature of Photosynthesis. Phycologia 2006, 45, 478–479. [Google Scholar] [CrossRef]
- Yan, W.; Lu, Y.; Guo, L.; Liu, Y.; Li, M.; Zhang, B.; Zhang, B.; Zhang, L.; Qin, D.; Huo, J. Effects of Drought Stress on Photosynthesis and Chlorophyll Fluorescence in Blue Honeysuckle. Plants 2024, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, E.G.; Keller, M.; Sadras, V.; Roig, F.A.; Peña, J.P. High temperature during the budswell phase of grapevines increases shoot water transport capacity. Agric. For. Meteorol. 2020, 295, 108173. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Civardi, S. The effect of early leaf removal on whole-canopy gas exchange and vine performance of Vitis vinifera L. ‘Sangiovese’. Vitis 2008, 47, 1–6. [Google Scholar]
- Patakas, A.; Noitsakis, B.; Chartzoulakis, K. Changes in W.U.E. in Vitis Vinifera as Affected by Leaf Age. Acta Hortic. 1997, 449, 457–460. [Google Scholar] [CrossRef]
- Bertamini, M.; Nedunchezhian, N. Photoinhibition of photosynthesis in mature and young leaves of grapevine (Vitis vinifera L.). Plant Sci. 2003, 164, 635–644. [Google Scholar] [CrossRef]
- Hirano, K.; Noda, M.; Hasegawa, S.; Okamoto, G. Contribution of lateral and primary leaves to the development and quality of’Kyoho’grape berry. Jpn. Soc. Hortic. Sci. 1994, 63, 515–521. [Google Scholar] [CrossRef][Green Version]
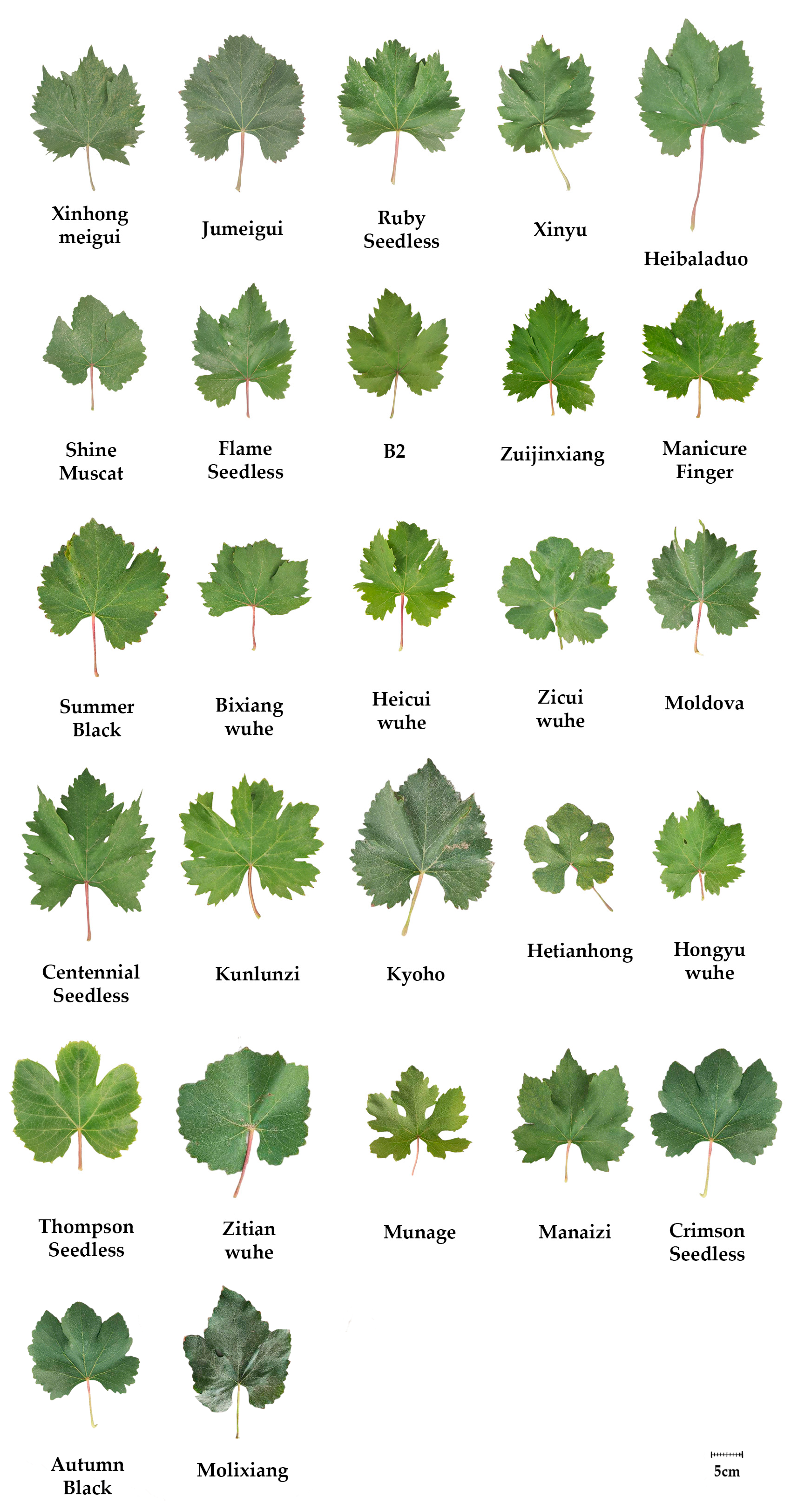


| Code | Cultivar | Parents | Type |
|---|---|---|---|
| 1 | Xinhongmeigui | Unknow | Eurasian hybrid |
| 2 | Jumeigui | Shenyangmeigui × Kyoho | Euro-American hybrid |
| 3 | Ruby Seedless | Emperor × Pirovan075 | Eurasian hybrid |
| 4 | Xinyu | E42-6 × Rizamat | Eurasian hybrid |
| 5 | Heibaladuo | Beni Balad × Yoneyama | Eurasian hybrid |
| 6 | Shine Muscat | Akitsu-21 × Hakunan | Euro-American hybrid |
| 7 | Flame Seedless | Unknow | Eurasian hybrid |
| 8 | B2 | Eurasian hybrid | |
| 9 | Zuijinxiang | 7601 × Kyoho | Euro-American hybrid |
| 10 | Manicure Finger | Unicorn × Baladi No.2 | Eurasian hybrid |
| 11 | Summer Black | Kyoho × Thompson Seedless | Euro-American hybrid |
| 12 | Bixiangwuhe | 1851 × Pearl of Csaba | Eurasian hybrid |
| 13 | Heicuiwuhe | Unknow | Eurasian hybrid |
| 14 | Zicuiwuhe | Niunai × Autumn Royal | Eurasian hybrid |
| 15 | Moldova | Guzali Kala × SV12375 | Euro-American hybrid |
| 16 | Centennial Seedless | Gold × Q25-6 | Eurasian hybrid |
| 17 | Kunlunzi | Eurasian hybrid | |
| 18 | Kyoho | Ishihara Wase × Centennial | Euro-American hybrid |
| 19 | Hetianhong | Eurasian hybrid | |
| 20 | Hongyuwuhe | Eurasian hybrid | |
| 21 | Thompson Seedless | Eurasian hybrid | |
| 22 | Zitianwuhe | Niunai × Autumn Royal | Eurasian hybrid |
| 23 | Munage | Eurasian hybrid | |
| 24 | Manaizi | Eurasian hybrid | |
| 25 | Crimson Seedless | Emperor × C33-199 | Eurasian hybrid |
| 26 | Autumn Black | Manicure Finger × Black Rose | Eurasian hybrid |
| 27 | Molixiang | Delaware × Royal Rose | Euro-American hybrid |
| Cultivar | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | SPAD |
|---|---|---|---|---|
| Xinhong meigui | 11.76 ± 1.62 cdefgh | 15.8 ± 0.79 def | 175.19 ± 0.24 cdefghi | 35.83 ± 3.3 cdef |
| Jumeigui | 12.14 ± 1.47 cdefgh | 15.32 ± 1.58 defg | 191.64 ± 13.29 bcdef | 41.62 ± 3.08 bcd |
| Ruby Seedless | 13.96 ± 1.79 b | 15.72 ± 1.93 def | 178.21 ± 9.56 cdefgh | 35.41 ± 2.18 ijk |
| Xinyu | 12.46 ± 0.78 bcdefg | 16.26 ± 0.96 bcde | 149.27 ± 14.44 fghijkl | 38.79 ± 1.64 fgh |
| Heibaladuo | 12.76 ± 0.74 bcdef | 16.4 ± 1.03 bcde | 163.96 ± 10.32 cdefghi | 39.09 ± 2.92 efg |
| Shine Muscat | 11 ± 0.31 fghij | 14.6 ± 1.75 efgh | 138.14 ± 14.2 ghijkl | 41.17 ± 2.21 bcde |
| Flame Seedless | 12.2 ± 0.83 bcdefgh | 15 ± 1.45 efgh | 169.44 ± 14.48 cdefghi | 41.96 ± 3.44 bc |
| B2 | 11.7 ± 0.7 defghi | 15.76 ± 0.91 def | 157.79 ± 14.98 efghij | 32.05 ± 1.74 l |
| Zuijinxiang | 12.46 ± 0.88 bcdefg | 14.02 ± 0.37 fghi | 153.79 ± 7.02 fghijkl | 33.59 ± 3.07 kl |
| Manicure Finger | 11.68 ± 1.06 defghi | 14.7 ± 1.53 efgh | 168.65 ± 33.8 cdefghi | 28.57 ± 1.35 m |
| Summer Black | 12.62 ± 0.86 bcdef | 16.74 ± 0.88 bcde | 197.55 ± 12.17 bcde | 36.83 ± 4.69 hij |
| Bixiang wuhe | 9.8 ± 0.99 j | 15.34 ± 1.65 defg | 132.91 ± 31.59 ijkl | 34.9 ± 1.85 jk |
| Heicui wuhe | 10.7 ± 1.31 ghij | 15.04 ± 1.76 efgh | 136.34 ± 1.31 hijkl | 32.89 ± 1.01 l |
| Zicui wuhe | 10.58 ± 0.63 hij | 15.9 ± 1.87 cdef | 164.4 ± 17.57 cdefghi | 37.46 ± 5.18 ghi |
| Moldova | 11.02 ± 0.99 fghij | 13.52 ± 1.33 ghi | 154.33 ± 16.39 fghijk | 40.41 ± 2.19 cdef |
| Centennial Seedless | 13.48 ± 1.04 bcd | 18.06 ± 1.44 ab | 219.42 ± 26.78 ab | 41.27 ± 2.36 ij |
| Kunlunzi | 12.9 ± 1.22 bcde | 18.08 ± 1.73 ab | 202.71 ± 27.01 bcd | 37.37 ± 2.36 ghi |
| Kyoho | 15.56 ± 1.6 a | 19.44 ± 1.92 a | 254.34 ± 49.06 a | 43.22 ± 2.35 ab |
| Hetianhong | 9.96 ± 1.14 ij | 12.42 ± 1.24 i | 113.33 ± 20.21 kl | 41.72 ± 2.24 bcd |
| Hongyu wuhe | 11.08 ± 0.87 efghij | 13.18 ± 0.54 hi | 118.52 ± 5.68 jkl | 34.95 ± 2.94 jk |
| Thompson Seedless | 13.54 ± 1.12 bc | 15.72 ± 1.8 def | 180.75 ± 13.77 bcdefg | 35.99 ± 1.68 ij |
| Zitian wuhe | 12.02 ± 2.05 cdefgh | 17.9 ± 1.8 abc | 205.52 ± 38.24 bc | 39.55 ± 2.99 def |
| Munage | 9.38 ± 1.23 j | 12.24 ± 1.22 i | 112.43 ± 4.67 l | 39.93 ± 2.81 bcde |
| Manaizi | 11.98 ± 1.89 cdefgh | 15.12 ± 1.79 defgh | 161.65 ± 36.38 defghi | 44.21 ± 3.04 a |
| Crimson Seedless | 12.42 ± 0.86 bcdefg | 17.26 ± 1.04 bcd | 180.69 ± 30.58 bcdefg | 36.47 ± 2.68 ij |
| Autumn Black | 11.54 ± 0.91 efghi | 13.76 ± 0.88 fghi | 136.77 ± 11.21 hijkl | 39.88 ± 1.67 cdef |
| Molixiang | 12.46 ± 1.39 bcdefg | 15.88 ± 1.82 cdef | 164.78 ± 12.06 cdefghi | 40.86 ± 1.04 cdef |
| Cultivar | RD (μmol·m−2·s−1) | PNmax (μmol·m−2·s−1) | Isat (μmol·m−2·s−1) | IC (μmol·m−2·s−1) | AQY |
|---|---|---|---|---|---|
| Xinhong meigui | 1.83 ± 0.13 def | 15.45 ± 4 ab | 1676.63 ± 189.78 c | 45.09 ± 20.95 def | 0.029 ± 0.015 abcde |
| Jumeigui | 3.29 ± 1 abc | 6.98 ± 2.1 fg | 1875.15 ± 375.45 abc | 101.46 ± 43.34 ab | 0.03 ± 0.003 abcde |
| Ruby Seedless | 2.09 ± 0.32 bcdef | 10.64 ± 0.61 bcdefg | 2379.14 ± 688.67 abc | 55.08 ± 5.39 cdef | 0.028 ± 0.003 bcdef |
| Xinyu | 1.49 ± 0.67 ef | 8.33 ± 2.03 efg | 1575.42 ± 274.7 c | 30.51 ± 13.19 ef | 0.027 ± 0.002 bcdef |
| Heibaladuo | 1.48 ± 1.18 ef | 6.3 ± 0.95 g | 1777.02 ± 143.65 bc | 46.59 ± 44.62 cdef | 0.018 ± 0.002 f |
| Shine Muscat | 2.13 ± 0.29 bcdef | 10.56 ± 1.58 bcdefg | 1681.86 ± 194.98 c | 44.91 ± 9.06 def | 0.033 ± 0.003 abcde |
| Flame Seedless | 2.69 ± 0.93 abcde | 16.23 ± 5.44 a | 2350.07 ± 898.15 abc | 58.4 ± 31.97 bcdef | 0.034 ± 0.009 abcd |
| B2 | 3.38 ± 0.13 ab | 8.01 ± 3.16 fg | 2317.52 ± 754.79 abc | 112.52 ± 8.31 a | 0.0267 ± 0.003 cdef |
| Zuijinxiang | 2.05 ± 0.9 bcdef | 9.97 ± 1.91 cdefg | 2370.9 ± 793.13 abc | 53.51 ± 17.6 cdef | 0.028 ± 0.003 bcdef |
| Manicure Finger | 1.82 ± 0.76 def | 7.86 ± 2.45 fg | 1982.48 ± 94.07 abc | 44.54 ± 20.59 def | 0.027 ± 0.002 bcdef |
| Summer Black | 1.58 ± 0.71 ef | 7.21 ± 1.03 fg | 1876.66 ± 303.06 abc | 42.36 ± 16.33 def | 0.022 ± 0.003 ef |
| Bixiang wuhe | 2.53 ± 0.86 abcdef | 8.78 ± 1.77 efg | 2427.97 ± 117.17 abc | 70.78 ± 22.65 abcde | 0.027 ± 0.005 cdef |
| Heicui wuhe | 1.56 ± 0.56 ef | 9.78 ± 1.96 cdefg | 1753.81 ± 589.13 bc | 32.85 ± 15.34 ef | 0.031 ± 0.011 abcde |
| Zicui wuhe | 3.2 ± 0.73 abcd | 11.27 ± 2.02 abcdefg | 2063.92 ± 493.08 abc | 70.39 ± 18.29 abcde | 0.028 ± 0.005 bcdef |
| Moldova | 2.83 ± 0.21 abcde | 14.68 ± 3.75 abc | 2144.93 ± 323.82 abc | 58.68 ± 10.66 bcdef | 0.035 ± 0.006 abcd |
| Centennial Seedless | 2.68 ± 0.6 abcde | 9.26 ± 1.33 defg | 1747.48 ± 219.88 bc | 62.54 ± 21.94 bcdef | 0.035 ± 0.001 abcd |
| Kunlunzi | 2.72 ± 0.39 abcde | 6.95 ± 1.4 fg | 1818.08 ± 422.82 abc | 82.66 ± 39.06 abcd | 0.025 ± 0.004 def |
| Kyoho | 3.66 ± 1.12 a | 11.95 ± 4.16 abcdef | 1857.32 ± 136.86 abc | 92.2 ± 42.97 abc | 0.032 ± 0.0093 abcde |
| Hetianhong | 2.51 ± 0.75 abcdef | 11.23 ± 1.51 abcdefg | 1554.89 ± 98.29 c | 48.94 ± 14.21 cdef | 0.037 ± 0.0018 abc |
| Hongyu wuhe | 2.54 ± 1.1 abcdef | 9.41 ± 2.05 defg | 1934.38 ± 561.64 abc | 67.21 ± 40.43 bcdef | 0.037 ± 0.0046 abc |
| Thompson Seedless | 1.15 ± 0.91 f | 11.91 ± 2.05 abcdef | 1776.84 ± 97.32 bc | 22.41 ± 15.69 f | 0.034 ± 0.0074 abcd |
| Zitian wuhe | 1.55 ± 0.58 ef | 12.1 ± 1.84 abcdef | 2047.34 ± 679.49 abc | 33.17 ± 13.57 ef | 0.034 ± 0.0022 abcd |
| Munage | 2.16 ± 0.48 bcdef | 16.24 ± 5.22 a | 2672.87 ± 811.76 ab | 49.73 ± 5.36 cdef | 0.031 ± 0.008 abcde |
| Manaizi | 1.83 ± 1.11 def | 10.39 ± 2.36 bcdefg | 1820.84 ± 105.01 abc | 34.56 ± 18.31 ef | 0.033 ± 0.005 abcde |
| Crimson Seedless | 1.56 ± 0.39 ef | 11.44 ± 1.83 abcdefg | 2745.15 ± 50.69 a | 34.64 ± 4.66 ef | 0.031 ± 0.004 abcde |
| Autumn Black | 2.55 ± 0.4 abcdef | 14.4 ± 3.47 abcd | 1909.5 ± 391.59 abc | 48.34 ± 10.31 cdef | 0.039 ± 0.007 ab |
| Molixiang | 1.88 ± 0.57 cdef | 13.45 ± 0.22 abcde | 2237.81 ± 870.03 abc | 37.7 ± 11.27 def | 0.041 ± 0.004 a |
| Cultivar | Fv/Fm | Fv/FO | PIabs | PItotal |
|---|---|---|---|---|
| Xinhong meigui | 0.76 ± 0.02 ghi | 3.22 ± 0.38 ghi | 0.66 ± 0.23 gh | 0.43 ± 0.16 g |
| Jumeigui | 0.8 ± 0.05 abcdefg | 4.31 ± 1.08 abcde | 1.36 ± 0.59 abcdef | 0.97 ± 0.55 bcdefg |
| Ruby Seedless | 0.79 ± 0.04 bcdefgh | 3.89 ± 0.81 cdefgh | 1.05 ± 0.64 bcdefgh | 1.12 ± 0.65 abcdef |
| Xinyu | 0.81 ± 0.03 abcde | 4.31 ± 0.88 abcde | 1.45 ± 0.71 abc | 1.5 ± 0.46 abcd |
| Heibaladuo | 0.78 ± 0.02 cdefgh | 3.66 ± 0.54 cdefghi | 0.78 ± 0.38 efgh | 0.75 ± 0.39 efg |
| Shine Muscat | 0.77 ± 0.01 efgh | 3.33 ± 0.18 fghi | 0.67 ± 0.08 gh | 0.91 ± 0.17 cdefg |
| Flame Seedless | 0.81 ± 0.02 abcd | 4.46 ± 0.67 abcd | 1.11 ± 0.37 bcdefgh | 0.83 ± 0.11 efg |
| B2 | 0.83 ± 0.02 ab | 4.9 ± 0.62 ab | 1.65 ± 0.65 ab | 1.22 ± 0.3 abcde |
| Zuijinxiang | 0.77 ± 0.03 efgh | 3.41 ± 0.6 fghi | 0.78 ± 0.42 efgh | 0.85 ± 0.34 efg |
| Manicure Finger | 0.8 ± 0.01 abcdefg | 4.08 ± 0.22 bcdefg | 1.04 ± 0.12 bcdefgh | 0.9 ± 0.13 defg |
| Summer Black | 0.72 ± 0.08 i | 2.82 ± 1 i | 0.58 ± 0.53 h | 1.1 ± 0.49 abcdef |
| Bixiang wuhe | 0.76 ± 0.02 hi | 3.15 ± 0.37 hi | 0.81 ± 0.23 defgh | 1.06 ± 0.32 abcdefg |
| Heicui wuhe | 0.76 ± 0.05 hi | 3.23 ± 0.74 ghi | 1.01 ± 0.56 cdefgh | 0.97 ± 0.4 bcdefg |
| Zicui wuhe | 0.82 ± 0.02 abcd | 4.46 ± 0.49 abcd | 1.78 ± 0.31 a | 1.66 ± 0.25 a |
| Moldova | 0.79 ± 0.03 bcdefgh | 3.83 ± 0.58 cdefgh | 0.74 ± 0.26 fgh | 1.37 ± 0.51 abcde |
| Centennial Seedless | 0.81 ± 0.03 abcd | 4.48 ± 0.79 abcd | 1.3 ± 0.63 abcdefg | 1.1 ± 0.54 abcdef |
| Kunlunzi | 0.77 ± 0.03 defgh | 3.46 ± 0.52 efghi | 0.69 ± 0.26 gh | 1.16 ± 0.3 abcdef |
| Kyoho | 0.76 ± 0.05 fgh | 3.38 ± 0.91 fghi | 1.04 ± 0.62 bcdefgh | 1.59 ± 1.05 ab |
| Hetianhong | 0.83 ± 0.01 ab | 5.02 ± 0.3 a | 1.43 ± 0.24 abcd | 1.55 ± 0.16 abc |
| Hongyu wuhe | 0.79 ± 0.01 cdefgh | 3.67 ± 0.15 cdefghi | 0.84 ± 0.17 cdefgh | 1.03 ± 0.36 abcdefg |
| Thompson Seedless | 0.78 ± 0.02 cdefgh | 3.63 ± 0.31 defghi | 0.95 ± 0.27 cdefgh | 1.51 ± 0.32 abcd |
| Zitian wuhe | 0.81 ± 0.01 abcdef | 4.15 ± 0.31 bcdef | 1.12 ± 0.22 bcdefgh | 1.35 ± 0.25 abcde |
| Munage | 0.8 ± 0.01 abcdefg | 4.14 ± 0.29 bcdef | 1.13 ± 0.24 bcdefgh | 1.05 ± 0.26 abcdefg |
| Manaizi | 0.77 ± 0.01 efgh | 3.31 ± 0.17 fghi | 0.86 ± 0.24 cdefgh | 0.55 ± 0.48 fg |
| Crimson Seedless | 0.82 ± 0.02 abc | 4.52 ± 0.55 abc | 1.41 ± 0.44 abcde | 1.04 ± 0.58 abcdefg |
| Autumn Black | 0.84 ± 0.01 a | 5.12 ± 0.23 a | 1.42 ± 0.41 abcd | 0.8 ± 0.33 efg |
| Molixiang | 0.81 ± 0.01 abcd | 4.36 ± 0.17 abcd | 1.4 ± 0.15 abcde | 1.39 ± 0.21 abcde |
| Cultivar | VJ | VI | φEo | dV/dto |
|---|---|---|---|---|
| Xinhong meigui | 0.62 ± 0.07 abcde | 0.86 ± 0.03 a | 0.29 ± 0.06 bcdefg | 1.44 ± 0.13 ab |
| Jumeigui | 0.57 ± 0.04 cdef | 0.83 ± 0.05 abcde | 0.35 ± 0.05 abcd | 1.16 ± 0.12 cde |
| Ruby Seedless | 0.62 ± 0.1 abcde | 0.8 ± 0.05 cdefgh | 0.3 ± 0.09 abcdefg | 1.29 ± 0.23 abcd |
| Xinyu | 0.56 ± 0.06 def | 0.78 ± 0.03 efgh | 0.35 ± 0.06 abc | 1.13 ± 0.14 cde |
| Heibaladuo | 0.66 ± 0.08 abc | 0.84 ± 0.05 abcd | 0.27 ± 0.07 defg | 1.36 ± 0.19 abcd |
| Shine Muscat | 0.66 ± 0.01 abc | 0.81 ± 0.02 abcdefg | 0.26 ± 0.01 efg | 1.31 ± 0.08 abcd |
| Flame Seedless | 0.63 ± 0.04 abcde | 0.84 ± 0.01 abcd | 0.31 ± 0.04 abcdefg | 1.27 ± 0.09 abcd |
| B2 | 0.55 ± 0.04 ef | 0.81 ± 0.01 abcdefg | 0.37 ± 0.04 a | 1.17 ± 0.16 cde |
| Zuijinxiang | 0.65 ± 0.08 abcd | 0.82 ± 0.03 abcdef | 0.27 ± 0.07 cdefg | 1.31 ± 0.2 abcd |
| Manicure Finger | 0.58 ± 0.01 cdef | 0.81 ± 0.02 bcdefg | 0.34 ± 0.01 abcde | 1.34 ± 0.06 abcd |
| Summer Black | 0.69 ± 0.12 a | 0.8 ± 0.04 cdefg | 0.23 ± 0.11 g | 1.47 ± 0.29 a |
| Bixiang wuhe | 0.6 ± 0.02 bcdef | 0.77 ± 0.01 fgh | 0.31 ± 0.02 abcdefg | 1.22 ± 0.09 bcd |
| Heicui wuhe | 0.59 ± 0.12 bcdef | 0.8 ± 0.04 defgh | 0.31 ± 0.1 abcdefg | 1.18 ± 0.25 cde |
| Zicui wuhe | 0.53 ± 0.01 f | 0.77 ± 0.03 fgh | 0.39 ± 0.01 a | 0.98 ± 0.05 e |
| Moldova | 0.68 ± 0.04 ab | 0.79 ± 0.03 defgh | 0.25 ± 0.04 fg | 1.37 ± 0.13 abc |
| Centennial Seedless | 0.6 ± 0.08 bcdef | 0.82 ± 0.03 abcdef | 0.33 ± 0.07 abcdef | 1.25 ± 0.22 abcd |
| Kunlunzi | 0.64 ± 0.04 abcde | 0.78 ± 0.02 efgh | 0.27 ± 0.04 bcdefg | 1.46 ± 0.14 a |
| Kyoho | 0.6 ± 0.08 bcdef | 0.76 ± 0.06 gh | 0.31 ± 0.08 abcdefg | 1.18 ± 0.24 cde |
| Hetianhong | 0.6 ± 0.03 bcdef | 0.79 ± 0.01 defgh | 0.34 ± 0.03 abcdef | 1.2 ± 0.05 cde |
| Hongyu wuhe | 0.64 ± 0.04 abcde | 0.81 ± 0.04 abcdefg | 0.27 ± 0.03 bcdefg | 1.24 ± 0.09 abcd |
| Thompson Seedless | 0.59 ± 0.04 bcdef | 0.75 ± 0.02 h | 0.32 ± 0.04 abcdef | 1.26 ± 0.13 abcd |
| Zitian wuhe | 0.61 ± 0.03 abcdef | 0.79 ± 0.02 defgh | 0.31 ± 0.03 abcdefg | 1.18 ± 0.08 cde |
| Munage | 0.61 ± 0.03 abcdef | 0.81 ± 0.02 abcdefg | 0.31 ± 0.03 abcdefg | 1.16 ± 0.1 cde |
| Manaizi | 0.56 ± 0.07 def | 0.86 ± 0.08 ab | 0.34 ± 0.05 abcdef | 1.32 ± 0.13 abcd |
| Crimson Seedless | 0.57 ± 0.05 cdef | 0.82 ± 0.05 abcdef | 0.35 ± 0.05 abcd | 1.18 ± 0.15 cde |
| Autumn Black | 0.59 ± 0.04 bcdef | 0.85 ± 0.02 abc | 0.34 ± 0.04 abcde | 1.29 ± 0.19 abcd |
| Molixiang | 0.56 ± 0.01 def | 0.78 ± 0.01 efgh | 0.36 ± 0.01 ab | 1.12 ± 0.07 de |
| Cultivar | ABS/CSm | DIo/CSm | TRo/CSm | ETo/CSm | REo/CSm |
|---|---|---|---|---|---|
| Xinhong meigui | 1810.6 ± 103.09 e | 430 ± 23.49 bcd | 1380.6 ± 114.25 f | 523 ± 121.7 fg | 194 ± 40.4 f |
| Jumeigui | 2422.6 ± 350.18 a | 463.2 ± 51.66 bcd | 1959.4 ± 378.27 a | 852.2 ± 236.02 ab | 344 ± 146.2 abcd |
| Ruby Seedless | 2357.8 ± 171.1 ab | 491 ± 69.84 b | 1866.8 ± 205.02 ab | 722.4 ± 256.95 abcdefg | 375 ± 123.65 abcd |
| Xinyu | 2313.8 ± 272.56 ab | 438.8 ± 30.01 bcd | 1875 ± 297.45 ab | 830 ± 238.97 abc | 418.8 ± 80.28 ab |
| Heibaladuo | 2241.4 ± 173.81 abc | 483.8 ± 47.71 b | 1757.6 ± 171.69 abcde | 601.4 ± 168.84 cdefg | 284.8 ± 88.02 def |
| Shine Muscat | 2019.4 ± 106.3 cde | 466.2 ± 18.78 bcd | 1553.2 ± 95.42 def | 524.6 ± 39.51 efg | 301.2 ± 30.41 cdef |
| Flame Seedless | 2449.2 ± 310.09 a | 448.2 ± 19.27 bcd | 2001 ± 304.56 a | 757 ± 183.16 abcde | 324.8 ± 48.11 abcd |
| B2 | 2314.6 ± 164.12 ab | 393.8 ± 27.62 d | 1920.8 ± 166.75 a | 864.6 ± 136.94 a | 371.4 ± 40.96 abcd |
| Zuijinxiang | 2003.8 ± 139.94 cde | 460.6 ± 62.52 bcd | 1543.2 ± 142.54 def | 543.8 ± 156.18 efg | 284.8 ± 66.15 def |
| Manicure Finger | 2236.2 ± 155.94 abc | 440 ± 24.77 bcd | 1796.2 ± 137.81 abcde | 755.6 ± 68.42 abcdef | 348.6 ± 21.05 abcd |
| Summer Black | 2091.5 ± 231.49 bcd | 565.5 ± 94.7 a | 1526 ± 316.89 ef | 504 ± 277.48 g | 308.5 ± 125.73 bcde |
| Bixiang wuhe | 1929.8 ± 145.69 de | 466.2 ± 22.4 bcd | 1463.6 ± 145.86 f | 591.6 ± 81.14 defg | 334.8 ± 44.3 abcd |
| Heicui wuhe | 2020.33 ± 239.49 cde | 482.67 ± 37.43 b | 1537.67 ± 270.1 def | 654.83 ± 261.96 abcdefg | 320.33 ± 97.27 abcde |
| Zicui wuhe | 2218.2 ± 219.76 abc | 406.4 ± 10.85 cd | 1811.8 ± 215.4 abcd | 858.8 ± 115.92 a | 411.6 ± 27.77 abc |
| Moldova | 2272.6 ± 130.57 abc | 476.2 ± 66.04 bc | 1796.4 ± 126.79 abcde | 574.8 ± 88.13 efg | 371 ± 67.19 abcd |
| Centennial Seedless | 2268.2 ± 158.97 abc | 419.4 ± 52.19 bcd | 1848.8 ± 173.55 abc | 750.6 ± 187.76 abcdef | 343 ± 75.61 abcd |
| Kunlunzi | 2021.4 ± 124.44 cde | 455.4 ± 27.99 bcd | 1566 ± 147.3 def | 569 ± 114.99 efg | 349.6 ± 43.18 abcd |
| Kyoho | 2323.4 ± 162.36 ab | 553.8 ± 144.63 a | 1769.6 ± 112.33 abcde | 716.4 ± 166.41 abcdefg | 425.4 ± 118.85 ab |
| Hetianhong | 2404.8 ± 38.17 a | 400.2 ± 15.39 d | 2004.6 ± 50.61 a | 811.6 ± 72.54 abcd | 420.6 ± 21.3 ab |
| Hongyu wuhe | 2046 ± 110.83 cde | 438.6 ± 37.35 bcd | 1607.4 ± 74.05 bcdef | 571.2 ± 31.99 efg | 308.8 ± 50.02 bcde |
| Thompson Seedless | 2029 ± 109.57 cde | 439 ± 17.07 bcd | 1590 ± 111.31 cdef | 650 ± 99.68 abcdefg | 396.6 ± 38.2 abcd |
| Zitian wuhe | 2372.4 ± 179.23 a | 460.6 ± 17.99 bcd | 1911.8 ± 168.49 a | 748 ± 107.23 abcdef | 404.2 ± 32.21 abc |
| Munage | 2215.2 ± 143.99 abc | 431.6 ± 22.76 bcd | 1783.6 ± 133.4 abcde | 692 ± 82.91 abcdefg | 330 ± 41.59 abcd |
| Manaizi | 1861.6 ± 171.22 de | 432.6 ± 43.26 bcd | 1429 ± 131.94 f | 622.2 ± 65.5 bcdefg | 210.8 ± 142.57 ef |
| Crimson Seedless | 2389.4 ± 160.49 a | 436.4 ± 50.01 bcd | 1953 ± 151.48 a | 840.6 ± 125.56 ab | 342.6 ± 82.52 abcd |
| Autumn Black | 2434.4 ± 104.65 a | 397.6 ± 7.06 d | 2036.8 ± 101.69 a | 837.6 ± 103.7 ab | 298.8 ± 52.25 cdef |
| Molixiang | 2440.8 ± 98.85 a | 455.8 ± 19.56 bcd | 1985 ± 86.09 a | 874 ± 40 a | 435.2 ± 39.95 a |
| Indicator | Factor Loading Coefficient | ||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| RD | −0.368 | −0.274 | 0.208 | 0.683 | 0.38 |
| PNmax | 0.2 | −0.378 | 0.659 | −0.041 | −0.157 |
| Isat | 0.208 | −0.096 | −0.014 | −0.088 | 0.477 |
| IC | −0.212 | −0.343 | 0.481 | 0.678 | 0.191 |
| AQY | 0.455 | −0.265 | 0.568 | 0.06 | −0.413 |
| SPAD | 0.177 | 0.008 | 0.571 | −0.389 | −0.409 |
| Fv/Fm | 0.894 | −0.322 | 0.031 | −0.059 | 0.116 |
| Fv/FO | 0.902 | −0.263 | 0.039 | −0.149 | 0.155 |
| PIabs | 0.956 | −0.043 | −0.185 | 0.052 | 0 |
| PItotal | 0.532 | 0.718 | 0.201 | 0.277 | −0.097 |
| dV/dto | 0.79 | 0.138 | −0.089 | 0.268 | −0.177 |
| ABS/CSm | 0.774 | 0.137 | 0.284 | −0.298 | 0.412 |
| DIo/CSm | 0.544 | −0.63 | −0.225 | 0.147 | −0.204 |
| TRo/CSm | 0.852 | 0.004 | 0.227 | −0.257 | 0.354 |
| ETo/CSm | 0.958 | −0.022 | −0.098 | −0.005 | 0.111 |
| REo/CSm | 0.663 | 0.659 | 0.196 | 0.216 | 0.088 |
| Leaf area | −0.047 | 0.535 | 0.004 | −0.342 | 0.015 |
| VJ | 0.725 | −0.112 | −0.405 | 0.292 | −0.253 |
| VI | 0.2 | 0.806 | 0.107 | 0.444 | −0.102 |
| φEo | 0.843 | −0.146 | −0.34 | 0.186 | −0.179 |
| Eigenvalue | 8.194 | 2.945 | 1.965 | 1.925 | 1.313 |
| Variance contribution rate (%) | 40.972 | 14.726 | 9.823 | 9.627 | 6.563 |
| Cumulative contribution rate (%) | 40.972 | 55.698 | 65.521 | 75.148 | 81.71 |
| Rank | Cultivar | Comprehensive Score | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|---|---|
| 1 | Molixiang | 0.97 | 1.33 | 0.21 | 1.33 | 0.89 | −0.04 |
| 2 | Zicuiwuhe | 0.79 | 1.63 | 0.71 | −1.22 | 0.73 | −1.18 |
| 3 | Hetianhong | 0.73 | 1.31 | −0.16 | 0.91 | 0.38 | −0.68 |
| 4 | Xinyu | 0.72 | 0.89 | 0.47 | −0.16 | 1.66 | 0.16 |
| 5 | Zitianwuhe | 0.6 | 0.5 | 0.47 | 1.25 | 0.54 | 0.7 |
| 6 | Crimson Seedless | 0.51 | 0.89 | −0.72 | −0.05 | 0.42 | 1.84 |
| 7 | Kyoho | 0.38 | 0.16 | 2.74 | 0.66 | −1.44 | −1.38 |
| 8 | B2 | 0.33 | 1.4 | 0.03 | −2.49 | −0.89 | 0.39 |
| 9 | Thompson Seedless | 0.33 | −0.19 | 0.67 | 0.68 | 2.29 | −0.6 |
| 10 | Ruby Seedless | 0.21 | 0 | 0.52 | 0.13 | −0.2 | 1.63 |
| 11 | Autumn Black | 0.16 | 1.07 | −1.95 | 0.71 | −0.91 | 0.02 |
| 12 | Munage | 0.12 | 0.28 | −0.91 | 0.76 | 0.17 | 0.38 |
| 13 | Jumeigui | 0.09 | 0.84 | 0.36 | −1.17 | −1.87 | −0.41 |
| 14 | Centennial Seedless | 0.09 | 0.54 | −0.07 | −0.01 | −0.92 | −0.72 |
| 15 | Flame Seedless | 0.09 | 0.47 | −0.93 | 1.27 | −1.59 | 0.63 |
| 16 | Moldova | 0.01 | −0.42 | 0.52 | 1.89 | −0.88 | 0.05 |
| 17 | Manicure Finger | 0 | 0.02 | −0.31 | −1.33 | 0.77 | 1.43 |
| 18 | Heicuiwuhe | −0.23 | −0.65 | −0.08 | −0.5 | 1.63 | −0.29 |
| 19 | Bixiangwuhe | −0.35 | −0.73 | 0.59 | −1.18 | 0.68 | −0.44 |
| 20 | Hongyuwuhe | −0.41 | −0.53 | −0.4 | −0.14 | 0.07 | −0.77 |
| 21 | Kunlunzi | −0.5 | −0.98 | 1.15 | −0.83 | −0.74 | −0.35 |
| 22 | Heibaladuo | −0.56 | −1.08 | −0.18 | −0.5 | −0.57 | 1.71 |
| 23 | Shine Muscat | −0.59 | −1.13 | −0.24 | 0.8 | −0.02 | −0.94 |
| 24 | Zuijinxiang | −0.61 | −1.09 | −0.38 | −0.41 | 0.05 | 0.63 |
| 25 | Summer Black | −0.65 | −2.04 | 1.41 | 0.32 | −0.16 | 1.3 |
| 26 | Manaizi | −0.95 | −0.88 | −1.72 | −0.43 | 0.12 | −1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhong, H.; Zhang, F.; Zhou, X.; Cheng, M.; Liu, H.; Lin, S.; Wang, L.; Wu, X.; Liu, L. Evaluation of Photosynthetic Performance and Adaptability of Grape Varieties in Arid Regions. Horticulturae 2025, 11, 1041. https://doi.org/10.3390/horticulturae11091041
Wang R, Zhong H, Zhang F, Zhou X, Cheng M, Liu H, Lin S, Wang L, Wu X, Liu L. Evaluation of Photosynthetic Performance and Adaptability of Grape Varieties in Arid Regions. Horticulturae. 2025; 11(9):1041. https://doi.org/10.3390/horticulturae11091041
Chicago/Turabian StyleWang, Runze, Haixia Zhong, Fuchun Zhang, Xiaoming Zhou, Meijuan Cheng, Hengde Liu, Shuping Lin, Liping Wang, Xinyu Wu, and Liqiang Liu. 2025. "Evaluation of Photosynthetic Performance and Adaptability of Grape Varieties in Arid Regions" Horticulturae 11, no. 9: 1041. https://doi.org/10.3390/horticulturae11091041
APA StyleWang, R., Zhong, H., Zhang, F., Zhou, X., Cheng, M., Liu, H., Lin, S., Wang, L., Wu, X., & Liu, L. (2025). Evaluation of Photosynthetic Performance and Adaptability of Grape Varieties in Arid Regions. Horticulturae, 11(9), 1041. https://doi.org/10.3390/horticulturae11091041







