Molecular Cascades of Heat Stress Responses in Solanaceae with Emphasis on Capsicum annuum L., Integrating Heat Shock Transcription Factors and Proteins
Abstract
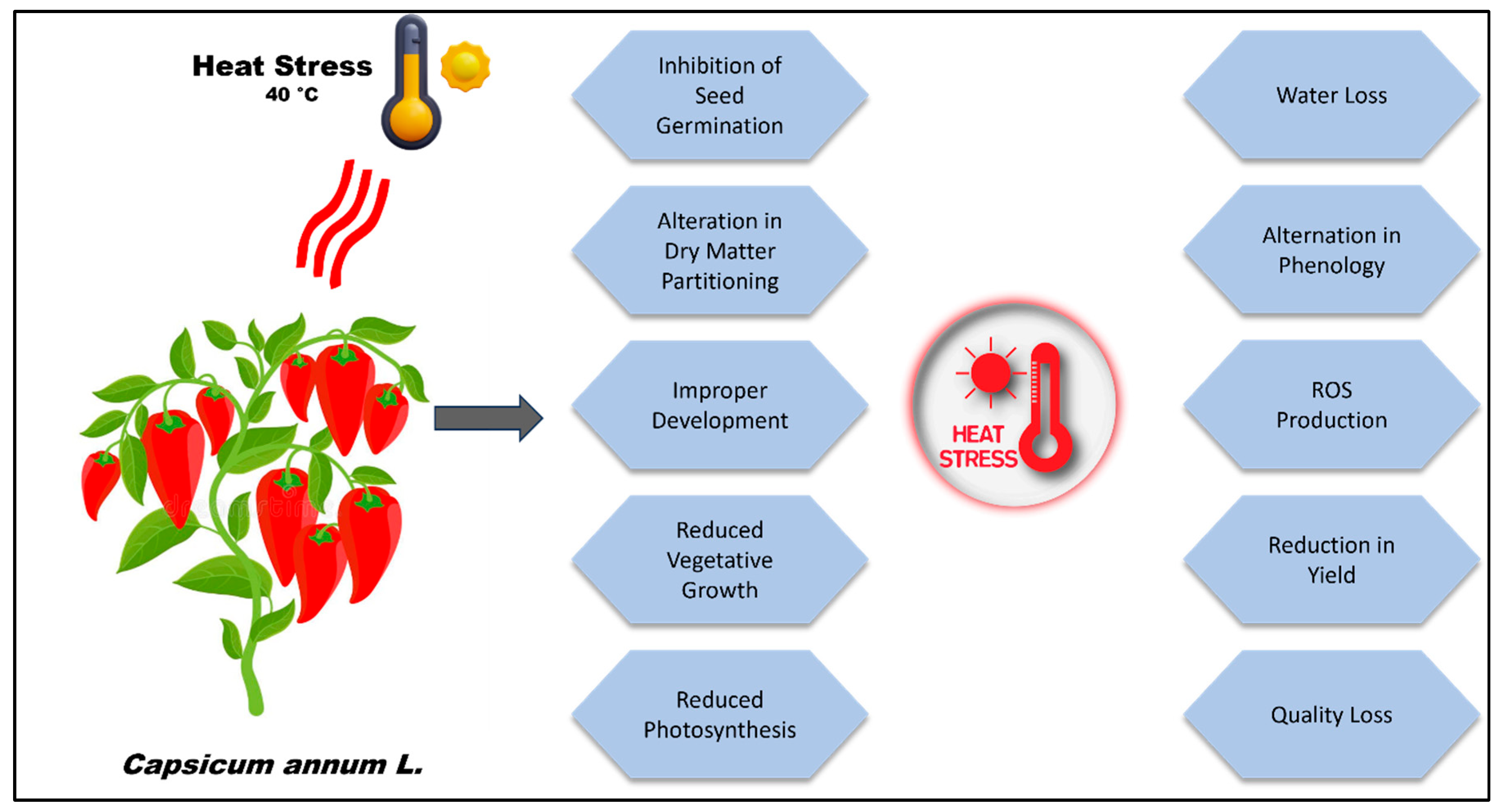
1. Introduction
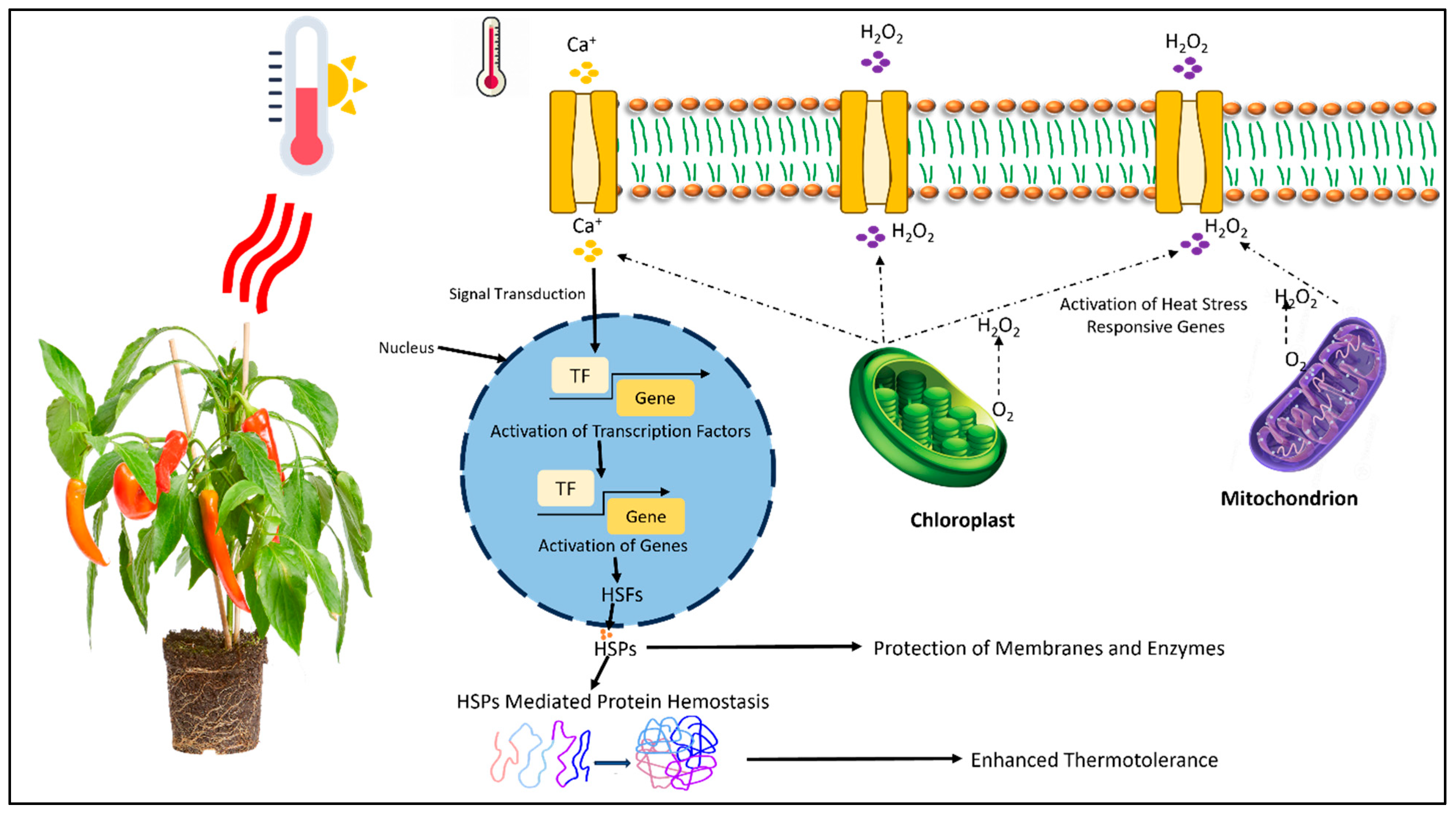
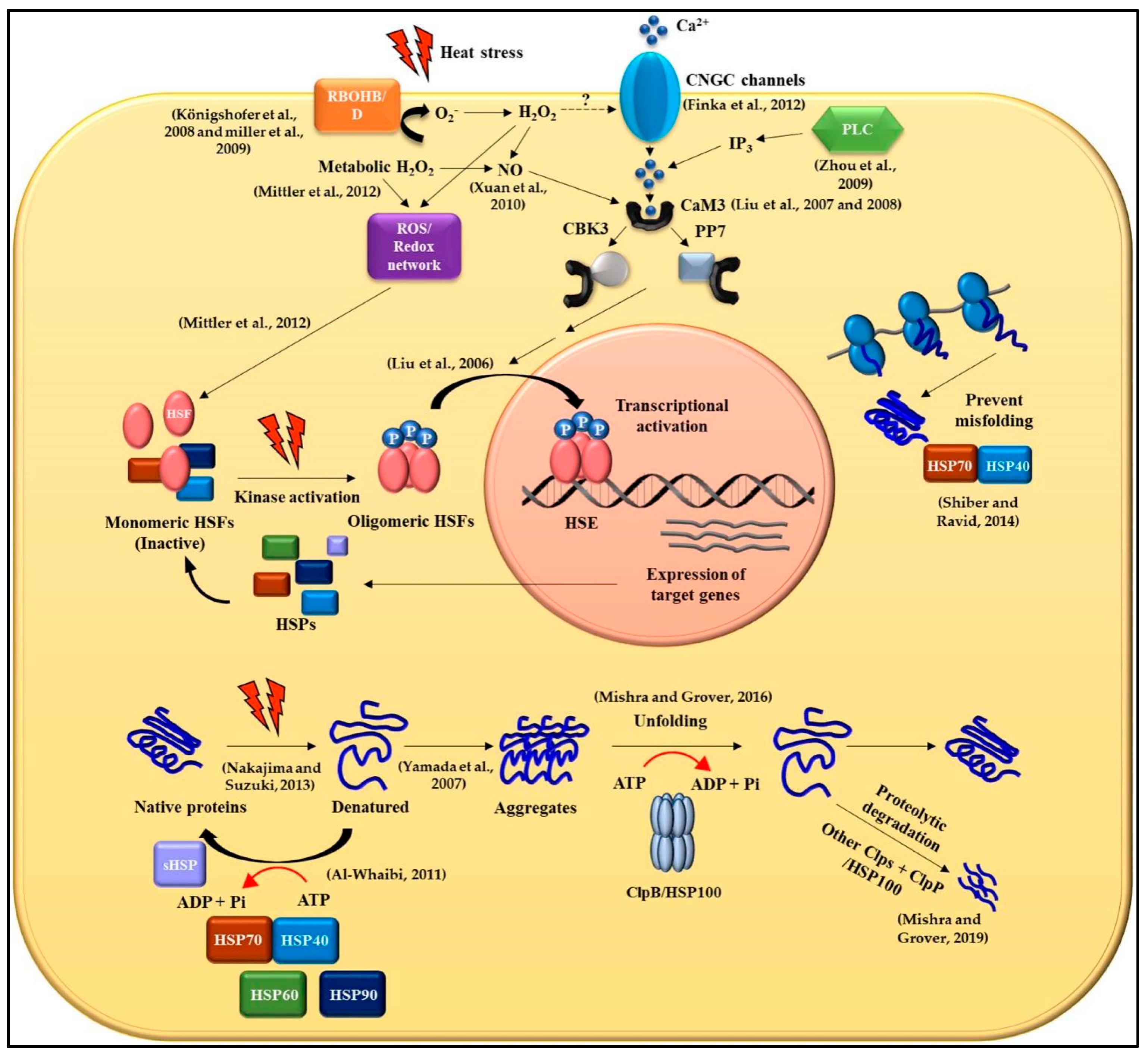
2. Mechanisms Involved in the Perception and Response to HS
3. Genetic Regulation of HS Response in C. annuum L
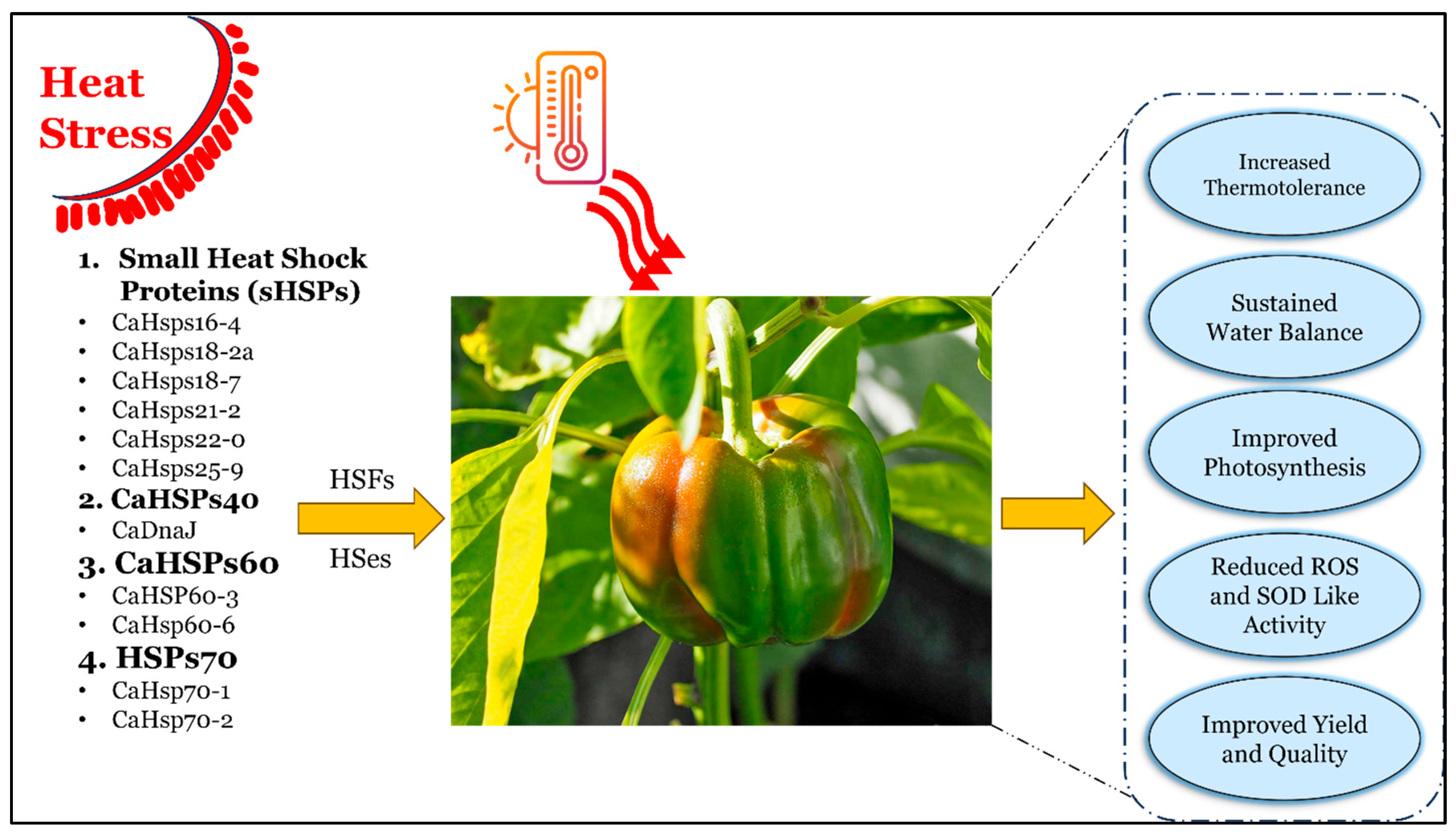
3.1. Heat Shock Proteins (HSPs)
3.1.1. Role of sHSPs in HS Responses
3.1.2. Role of CaHSP60s in HS Responses
3.1.3. Role of CaHSP70s in HS Responses
3.1.4. Role of CaHSP90s in HS Responses
3.1.5. Role of HSP100s in HS Responses
3.1.6. Functional Hierarchy of HSPs
3.2. Heat Shock Factors (HSFs)
3.2.1. CaHSFsA
3.2.2. CaHSFsB
3.2.3. CaHSFs
3.3. Genome-Wide Survey of HSPs/HSFs in C. annuum
3.4. Comparative Molecular Cascades in Other Solanaceous Plants
3.4.1. Molecular Basis of HSR in Potato
3.4.2. Molecular Basis of HSR in Tomato
3.4.3. Molecular Basis of HSR in Eggplant
3.5. Molecular Mechanisms of HS Response (HSR)
3.6. Understanding the HSR in Pepper: The Role of HSFs
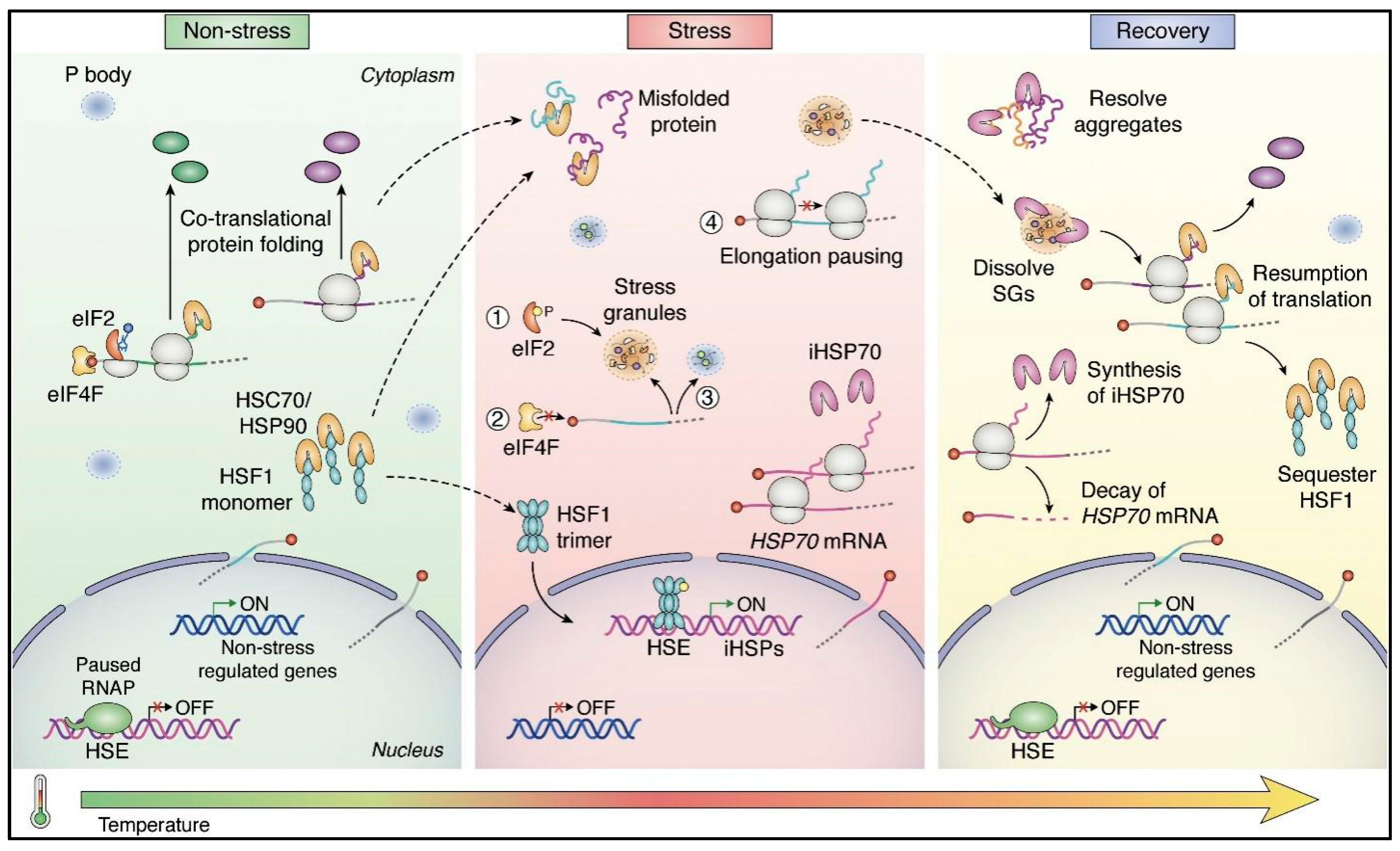
3.6.1. The Interplay of Translation Factors and Stress Granules
3.6.2. Epigenetic Regulation in the HSR of Pepper
3.6.3. Recovery Mechanisms and the Return to Homeostasis
4. Conclusions and Future Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Heat Stress |
| HSPs | Heat shock proteins |
| HSFs | Heat shock factors |
| HSEs | Heat shock elements |
| HSR | Heat shock response |
| HDACs | Histone deacetylases |
| RD | Repressor domain |
| CBK3 | Calmodulin-binding protein kinase 3 |
| CNGCs | Cyclic nucleotide-gated calcium channels |
| HTHH | High temperature–high humidity |
| VIGS | Virus-induced gene silencing |
| PCR | Polymerase chain reaction |
| DEGs | Differentially expressed genes |
| TF | Transcription factor |
References
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Chapter 4—Climate Change and Future of Agri-Food Production. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 49–79. ISBN 978-0-323-91001-9. [Google Scholar]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s Contribution to Climate Change and Role in Mitigation Is Distinct from Predominantly Fossil CO2-Emitting Sectors. Front. Sustain. Food Syst. 2021, 4, 518039. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.-Z.; et al. Vitamin Variation in Capsicum Spp. Provides Opportunities to Improve Nutritional Value of Human Diets. PLoS ONE 2016, 11, e0161464. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Martínez, E.; García-Martínez, M.D.; Adalid-Martínez, A.M.; Pereira-Dias, L.; Casanova, C.; Soler, E.; Figàs, M.R.; Raigón, M.D.; Plazas, M.; Soler, S.; et al. Fruit Composition Profile of Pepper, Tomato and Eggplant Varieties Grown under Uniform Conditions. Food Res. Int. 2021, 147, 110531. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular Mechanisms of Plant Tolerance to Heat Stress: Current Landscape and Future Perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef]
- Lin, T.H.; Lin, S.W.; Wang, Y.W.; van Zonneveld, M.; Barchenger, D.W. Growing Environment and Heat Treatment Effects on Intra- and Interspecific Pollination in Chile Pepper (Capsicum Spp.). Agronomy 2021, 11, 1275. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-Y.; Wang, K.-X.; Yan, M.-Y.; Kanwar, M.K.; Li, D.-Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin Alleviates High Temperature-Induced Pollen Abortion in Solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef]
- Grant, R.F.; Kimball, B.A.; Conley, M.M.; White, J.W.; Wall, G.W.; Ottman, M.J. Controlled Warming Effects on Wheat Growth and Yield: Field Measurements and Modeling. Agron. J. 2011, 103, 1742–1754. [Google Scholar] [CrossRef]
- Ahmad, M.; Imtiaz, M.; Shoib Nawaz, M.; Mubeen, F.; Imran, A. What Did We Learn From Current Progress in Heat Stress Tolerance in Plants? Can Microbes Be a Solution? Front. Plant Sci. 2022, 13, 794782. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Yang, E.Y.; Cho, M.C.; Chae, S.Y.; Jeong, H.B.; Chae, W.B. Heat-Tolerant Hot Pepper Exhibits Constant Photosynthesis via Increased Transpiration Rate, High Proline Content and Fast Recovery in Heat Stress Condition. Sci. Rep. 2021, 11, 14328. [Google Scholar] [CrossRef]
- Rosmaina; Utami, D.; Aryanti, E. Zulfahmi Impact of Heat Stress on Germination and Seedling Growth of Chili Pepper (C. annuum L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012032. [Google Scholar] [CrossRef]
- Locharoen, S.; Chulaka, P. Response of ‘Hua-Ruea’ Chili Pepper (C. annuum L.) to Salicylic Acid under Heat Stress. Sci. Technol. Asia 2021, 26, 142–151. [Google Scholar]
- Gajanayake, B.; Trader, B.W.; Reddy, K.R.; Harkess, R.L. Screening Ornamental Pepper Cultivars for Temperature Tolerance Using Pollen and Physiological Parameters. HortScience 2011, 46, 878–884. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, K.; Khar, A.; Parihar, B.R.; Tomar, B.S.; Mangal, M. Morphological, Biochemical and Molecular Insights on Responses to Heat Stress in Chilli. Indian J. Hortic. 2022, 79, 15–22. [Google Scholar] [CrossRef]
- Miao, W.; Song, J.; Huang, Y.; Liu, R.; Zou, G.; Ou, L.; Liu, Z. Comparative Transcriptomics for Pepper (C. annuum L.) under Cold Stress and after Rewarming. Appl. Sci. 2021, 11, 10204. [Google Scholar] [CrossRef]
- Qian, D.; Wang, M.; Niu, Y.; Yang, Y.; Xiang, Y. Sexual Reproduction in Plants under High Temperature and Drought Stress. Cell Rep. 2025, 44, 115390. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, L.; Agronomy, A.S. Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; ASA-CSSA-SSSA; American Society of Agronomy: Madison, WI, USA, 2008; ISBN 978-0-89118-167-5. [Google Scholar]
- Kim, M.K.; Jeong, H.B.; Yu, N.; Park, B.M.; Chae, W.B.; Lee, O.J.; Lee, H.E.; Kim, S. Comparative Heat Stress Responses of Three Hot Pepper (C. annuum L.) Genotypes Differing Temperature Sensitivity. Sci. Rep. 2023, 13, 14203. [Google Scholar] [CrossRef]
- Saha, S.R.; Hossain, M.M.; Rahman, M.M.; Kuo, C.G.; Abdullah, S. Effect of High Temperature Stress on the Performance of Twelve Sweet Pepper Genotypes. Bangladesh J. Agric. Res. 2010, 35, 525–534. [Google Scholar] [CrossRef]
- Bello, A.S.; Ahmed, T.; Saadaoui, I.; Ben-Hamadou, R.; Hamdi, H. Heat-Stress-Induced Changes in Enzymatic Antioxidant Activities and Biochemical Processes in Bell Pepper (C. annuum L.) Seedlings. Turk. J. Agric. For. 2023, 47, 1165–1173. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.L.; Murakami, K. Effect of High Temperature on Fruit Productivity and Seed-Set of Sweet Pepper (C. annuum L.) in the Field Condition. JAST-A 2015, 5, 515–520. [Google Scholar] [CrossRef]
- Huang, L.-J.; Cheng, G.-X.; Khan, A.; Wei, A.-M.; Yu, Q.-H.; Yang, S.-B.; Luo, D.-X.; Gong, Z.-H. CaHSP16.4, a Small Heat Shock Protein Gene in Pepper, Is Involved in Heat and Drought Tolerance. Protoplasma 2018, 256, 39–51. [Google Scholar] [CrossRef]
- Liu, C. Multiomics Analyses Reveal High Temperature-Induced Molecular Regulation of Ascorbic Acid and Capsaicin Biosynthesis in Pepper Fruits. Environ. Exp. Bot. 2022, 201, 104941. [Google Scholar] [CrossRef]
- Shaked, R.; Rosenfeld, K.; Pressman, E. The Effect of Low Night Temperatures on Carbohydrates Metabolism in Developing Pollen Grains of Pepper in Relation to Their Number and Functioning. Sci. Hortic. 2004, 102, 29–36. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Shi, F.; Liu, C.; Liu, X.; Ji, S. Intermittent Warming Improves Postharvest Quality of Bell Peppers and Reduces Chilling Injury. Postharvest Biol. Technol. 2015, 101, 18–25. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid Alleviates Chilling-Induced Oxidative Stress in Pepper by Enhancing Antioxidation Systems and Maintenance of Photosystem II. Acta Physiol. Plant. 2015, 37, 222. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, M.; Han, X.; Wu, J.; Wang-Pruski, G. Response of Ornamental Pepper to High-Temperature Stress and Role of Exogenous Salicylic Acid in Mitigating High Temperature. J. Plant Growth Regul. 2019, 39, 133–146. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Boyle, D.L.; Welti, R.; Jagadish, S.V.K.; Prasad, P.V.V. Decreased Photosynthetic Rate under High Temperature in Wheat Is Due to Lipid Desaturation, Oxidation, Acylation, and Damage of Organelles. BMC Plant Biol. 2018, 18, 55. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, H.; Liang, M.; Lu, M. Both Silencing- and over-Expression of Pepper CaATG8c Gene Compromise Plant Tolerance to Heat and Salt Stress. Environ. Exp. Bot. 2017, 141, 10–18. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Hu, W.; Xiang, Y.; Yan, M.; Wang, J. Overexpressing Heat-Shock Protein OsHSP50.2 Improves Drought Tolerance in Rice. Plant Cell Rep. 2018, 37, 1585–1595. [Google Scholar] [CrossRef]
- Wang, L.-J.; Li, S.-H. Salicylic Acid-Induced Heat or Cold Tolerance in Relation to Ca2+ Homeostasis and Antioxidant Systems in Young Grape Plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.-H.; Ma, X.; Zhai, Y.-F.; Gong, Z.-H.; Lu, M.-H. Genome-Wide Analysis of the Hsp70 Family Genes in Pepper (C. annuum L.) and Functional Identification of CaHsp70-2 Involvement in Heat Stress. Plant Sci. 2016, 252, 246–256. [Google Scholar] [CrossRef]
- Singh, R.K.; Jaishankar, J.; Muthamilarasan, M.; Shweta, S.; Dangi, A.; Prasad, M. Genome-Wide Analysis of Heat Shock Proteins in C4 Model, Foxtail Millet Identifies Potential Candidates for Crop Improvement under Abiotic Stress. Sci. Rep. 2016, 6, 32641. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant Molecular Stress Responses Face Climate Change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Kim, Y.-K.; Grover, A. Rice sHsp Genes: Genomic Organization and Expression Profiling under Stress and Development. BMC Genom. 2009, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.R. The Evolution, Function, Structure, and Expression of the Plant sHSPs. J. Exp. Bot. 2013, 64, 391–403. [Google Scholar] [CrossRef]
- Haslbeck, M.; Vierling, E. A First Line of Stress Defense: Small Heat Shock Proteins and Their Function in Protein Homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef]
- Jaya, N.; Garcia, V.; Vierling, E. Substrate Binding Site Flexibility of the Small Heat Shock Protein Molecular Chaperones. Proc. Natl. Acad. Sci. USA 2009, 106, 15604–15609. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-H.; Zhang, H.-X.; Ali, M.; Gai, W.-X.; Cheng, G.-X.; Yu, Q.-H.; Yang, S.-B.; Li, X.-X.; Gong, Z.-H. A Small Heat Shock Protein CaHsp25.9 Positively Regulates Heat, Salt, and Drought Stress Tolerance in Pepper (C. annuum L.). Plant Physiol. Biochem. 2019, 142, 151–162. [Google Scholar] [CrossRef]
- Guo, M.; Zhai, Y.-F.; Lu, J.-P.; Chai, L.; Chai, W.-G.; Gong, Z.-H.; Lu, M.-H. Characterization of CaHsp70-1, a Pepper Heat-Shock Protein Gene in Response to Heat Stress and Some Regulation Exogenous Substances in C. annuum L. Int. J. Mol. Sci. 2014, 15, 19741–19759. [Google Scholar] [CrossRef]
- González-Gordo, S.; Palma, J.M.; Corpas, F.J. Small Heat Shock Protein (sHSP) Gene Family from Sweet Pepper (C. annuum L.) Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO). Plants 2023, 12, 389. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Z.; Liu, K.; Zhao, Y.; Nie, Z.; Zhang, L.; Muhammad Fahim, A.; Yang, X. Comparative Transcriptome Analysis Reveals Differential Gene Expression Pattern Associated with Heat Tolerance in Pepper (C. annuum L.). Horticulturae 2023, 9, 801. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Y.; Yu, C.; Li, N.; Shen, S.; Liu, Y.; Gao, S.; Jiao, C.; Yao, M. Transcriptomics Analysis of Heat Stress-Induced Genes in Pepper (C. annuum L.) Seedlings. Horticulturae 2021, 7, 339. [Google Scholar] [CrossRef]
- Fan, F.; Yang, X.; Cheng, Y.; Kang, Y.; Chai, X. The DnaJ Gene Family in Pepper (C. annuum L.): Comprehensive Identification, Characterization and Expression Profiles. Front. Plant Sci. 2017, 8, 689. [Google Scholar] [CrossRef]
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The Plant Heat Stress Transcription Factor (HSF) Family: Structure, Function and Evolution. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Sebastian, J. Plants and Global Warming: Challenges and Strategies for a Warming World. Plant Cell Rep. 2024, 43, 27. [Google Scholar] [CrossRef]
- Xiao, J.-J.; Zhang, R.-X.; Khan, A.; ul Haq, S.; Gai, W.-X.; Gong, Z.-H. CaFtsH06, A Novel Filamentous Thermosensitive Protease Gene, Is Involved in Heat, Salt, and Drought Stress Tolerance of Pepper (C. annuum L.). Int. J. Mol. Sci. 2021, 22, 6953. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.-H.; Lu, J.-P.; Zhai, Y.-F.; Wang, H.; Gong, Z.-H.; Wang, S.-B.; Lu, M.-H. Genome-Wide Analysis of the CaHsp20 Gene Family in Pepper: Comprehensive Sequence and Expression Profile Analysis under Heat Stress. Front. Plant Sci. 2015, 6, 806. [Google Scholar] [CrossRef]
- Sun, J.-T.; Cheng, G.-X.; Huang, L.-J.; Liu, S.; Ali, M.; Khan, A.; Yu, Q.-H.; Yang, S.-B.; Luo, D.-X.; Gong, Z.-H. Modified Expression of a Heat Shock Protein Gene, CaHSP22.0, Results in High Sensitivity to Heat and Salt Stress in Pepper (C. annuum L.). Sci. Hortic. 2019, 249, 364–373. [Google Scholar] [CrossRef]
- Haq, S.; Khan, A.; Ali, M.; Gai, W.-X.; Zhang, H.-X.; Yu, Q.-H.; Yang, S.-B.; Wei, A.-M.; Gong, Z.-H. Knockdown of CaHSP60-6 Confers Enhanced Sensitivity to Heat Stress in Pepper (C. annuum L.). Planta 2019, 250, 2127–2145. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In Vivo Aspects of Protein Folding and Quality Control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Saibil, H.R.; Fenton, W.A.; Clare, D.K.; Horwich, A.L. Structure and Allostery of the Chaperonin GroEL. J. Mol. Biol. 2013, 425, 1476–1487. [Google Scholar] [CrossRef]
- Renner, T.; Waters, E.R. Comparative Genomic Analysis of the Hsp70s from Five Diverse Photosynthetic Eukaryotes. Cell Stress Chaperones 2007, 12, 172–185. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Kundnani, P.; Grover, A. Functional Analysis of Hsp70 Superfamily Proteins of Rice (Oryza sativa). Cell Stress Chaperones 2013, 18, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.-Y.; Kaplan, F.; Guy, C.L. Plant Hsp70 Molecular Chaperones: Protein Structure, Gene Family, Expression and Function. Physiol. Plant. 2001, 113, 443–451. [Google Scholar] [CrossRef]
- Sung, D.-Y.; Kaplan, F.; Lee, K.-J.; Guy, C.L. Acquired Tolerance to Temperature Extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Expression of Target Gene Hsp70 and Membrane Stability Determine Heat Tolerance in Chili Pepper. J. Am. Soc. Hortic. Sci. 2015, 140, 144–150. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.-S.; Yi, C.-Y.; Wang, F.; Zhou, J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Hydrogen Peroxide Mediates Abscisic Acid-Induced HSP70 Accumulation and Heat Tolerance in Grafted Cucumber Plants. Plant Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef]
- Shinozaki, F.; Minami, M.; Chiba, T.; Suzuki, M.; Yoshimatsu, K.; Ichikawa, Y.; Terasawa, K.; Emori, Y.; Matsumoto, K.; Kurosaki, T.; et al. Depletion of Hsp90β Induces Multiple Defects in B Cell Receptor Signaling. Trends Plant Sci. 2006, 22, 53–65. [Google Scholar] [CrossRef]
- Yurina, N. Heat Shock Proteins in Plant Protection from Oxidative Stress. Mol. Biol. 2023, 57, 951–964. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Wang, P.; Li, Y.; Wang, M.; Zhu, B.; Liao, D.; Yun, T.; Huang, W.; Chen, Y.; et al. Genome-Wide Characterization of HSP90 Gene Family in Chinese Pumpkin (Cucurbita moschata Duch.) and Their Expression Patterns in Response to Heat and Cold Stresses. Agronomy 2023, 13, 430. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, K.; Hoshikawa, K.; Kang, M.; Jang, S. Frontiers | Molecular Bases of Heat Stress Responses in Vegetable Crops With Focusing on Heat Shock Factors and Heat Shock Proteins. Front. Plant Sci. 2022, 13, 837152. [Google Scholar] [CrossRef]
- Burton, B.M.; Baker, T.A. Remodeling Protein Complexes: Insights from the AAA+ Unfoldase ClpX and Mu Transposase. Protein Sci. 2005, 14, 1945–1954. [Google Scholar] [CrossRef]
- Mishra, R.; Grover, A. ClpB/Hsp100 Proteins and Heat Stress Tolerance in Plants. Crit. Rev. Biotechnol. 2016, 36, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Katikaridis, P.; Bohl, V.; Mogk, A. Resisting the Heat: Bacterial Disaggregases Rescue Cells From Devastating Protein Aggregation. Front. Mol. Biosci. 2021, 8, 681439. [Google Scholar] [CrossRef] [PubMed]
- Chennakesavulu, K.; Singh, H.; Trivedi, P.K.; Jain, M.; Yadav, S.R. State-of-the-Art in CRISPR Technology and Engineering Drought, Salinity, and Thermo-Tolerant Crop Plants. Plant Cell Rep. 2022, 41, 815–831. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Ruan, M.; Zhao, H.; Wen, Y.; Chen, H.; He, F.; Hou, X.; Song, X.; Jiang, H.; Ruan, Y.-L.; Wu, L. The Complex Transcriptional Regulation of Heat Stress Response in Maize. Stress Biol. 2024, 4, 24. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.-P.; Zhai, Y.-F.; Chai, W.-G.; Gong, Z.-H.; Lu, M.-H. Genome-Wide Analysis, Expression Profile of Heat Shock Factor Gene Family (CaHSFs) and Characterisation of CaHSFA2 in Pepper (C. annuum L.). BMC Plant Biol. 2015, 15, 151. [Google Scholar] [CrossRef]
- Nover, L.; Bharti, K.; Döring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.-D. Arabidopsis and the Heat Stress Transcription Factor World: How Many Heat Stress Transcription Factors Do We Need? Cell Stress Chaperones 2001, 6, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Zhang, H.; Zhang, Y.; Zhao, L.; Liu, Z.; Guo, X. Characteristics and Regulating Role in Thermotolerance of the Heat Shock Transcription Factor ZmHSF12 from Zea mays L. J. Plant Biol. 2019, 62, 329–341. [Google Scholar] [CrossRef]
- Xue, G.-P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The Heat Shock Factor Family from Triticum Aestivum in Response to Heat and Other Major Abiotic Stresses and Their Role in Regulation of Heat Shock Protein Genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Cao, W.; Wang, J.; Yu, J.; Yang, Z.; Huang, B. Characterization and Functional Analysis of FaHSFC1b from Festuca Arundinacea Conferring Heat Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2702. [Google Scholar] [CrossRef]
- Gai, W.-X.; Yang, F.; Ali, M.; Ahmad, A.; Gong, Z.-H. Pepper Heat Shock Transcription Factor A1d Contributes to Seed Thermotolerance and Germination Vigor. Sci. Hortic. 2023, 311, 111786. [Google Scholar] [CrossRef]
- Kotak, S.; Port, M.; Ganguli, A.; Bicker, F.; von Koskull-Döring, P. Characterization of C-Terminal Domains of Arabidopsis Heat Stress Transcription Factors (HSFs) and Identification of a New Signature Combination of Plant Class A HSFs with AHA and NES Motifs Essential for Activator Function and Intracellular Localization. Plant J. 2004, 39, 98–112. [Google Scholar] [CrossRef]
- Schramm, F.; Larkindale, J.; Kiehlmann, E.; Ganguli, A.; Englich, G.; Vierling, E.; Koskull-Döring, P.V. A Cascade of Transcription Factor DREB2A and Heat Stress Transcription Factor HSFA3 Regulates the Heat Stress Response of Arabidopsis. Plant J. 2008, 53, 264–274. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Yang, S.; Wu, R.; Wang, Y.; Hussain, A.; Noman, A.; Khan, M.I.; Liu, Z.; Qiu, A.; Guan, D.; et al. C. annuum HSFB2a Positively Regulates the Response to Ralstonia Solanacearum Infection or High Temperature and High Humidity Forming Transcriptional Cascade with CaWRKY6 and CaWRKY40. Plant Cell Physiol. 2018, 59, 2608–2623. [Google Scholar] [CrossRef]
- Bakery, A.; Vraggalas, S.; Shalha, B.; Chauhan, H.; Benhamed, M.; Fragkostefanakis, S. Heat Stress Transcription Factors as the Central Molecular Rheostat to Optimize Plant Survival and Recovery from Heat Stress. New Phytol. 2024, 244, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, X.; Zhang, X.; Yin, Q.; Xie, L.; Zou, X.; Liu, F.; Dai, X. Transcriptome Data Reveal Gene Clusters and Key Genes in Pepper Response to Heat Shock. Front. Plant Sci. 2022, 13, 946475. [Google Scholar] [CrossRef]
- Gai, W.-X.; Ma, X.; Li, Y.; Xiao, J.-J.; Khan, A.; Li, Q.-H.; Gong, Z.-H. CaHSFA1d Improves Plant Thermotolerance via Regulating the Expression of Stress- and Antioxidant-Related Genes. Int. J. Mol. Sci. 2020, 21, 8374. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Y.X.; Ji, J.J.; Ma, B.P.; Lu, M.H.; Gong, Z.H. Cloning and Expression Analysis of Heat-Shock Transcription Factor Gene CaHSFA2 from Pepper (C. annuum L.). Genet. Mol. Res. 2014, 17, 1865–1875. [Google Scholar] [CrossRef]
- Guo, J.; Wu, J.; Ji, Q.; Wang, C.; Luo, L.; Yuan, Y.; Wang, Y.; Wang, J. Genome-Wide Analysis of Heat Shock Transcription Factor Families in Rice and Arabidopsis. J. Genet. Genom. 2008, 35, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Liang, Y.; Wang, Q.; Li, W.; Khan, A.; Li, B.; Wang, Y.; Su, H.; Zhang, R.; Guo, C.; et al. Genome-Wide Identification of FCS-Like Zinc Finger (FLZ) Genes in Four Solanaceae Plant Species and Functional Characterization of SlFLZ2 and SlFLZ18 in Tomato under Heat Stress. Sci. Hortic. 2023, 317, 112015. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, R.; Niu, S.; Si, H.; Yang, Q.; Rajora, O.P.; Li, X.-Q. Heat-Stress-Induced Sprouting and Differential Gene Expression in Growing Potato Tubers: Comparative Transcriptomics with That Induced by Postharvest Sprouting. Hortic. Res. 2021, 8, 226. [Google Scholar] [CrossRef]
- Taher, D.; Solberg, S.Ø.; Prohens, J.; Chou, Y.; Rakha, M.; Wu, T. World Vegetable Center Eggplant Collection: Origin, Composition, Seed Dissemination and Utilization in Breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef]
- Ahn, G.; Jeong, S.Y.; Khan, H.A.; Aulia, A.C.; Shin, G.-I.; Ji, M.G.; Sultana Chowdhury, M.S.; Kim, D.Y.; Lee, S.Y.; Yun, D.J.; et al. FAD and NADPH Binding Sites of YUCCA6 Are Essential for Chaperone Activity and Oxidative Stress Tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. 2025, 218, 109335. [Google Scholar] [CrossRef]
- Rykaczewska, K. The Impact of High Temperature during Growing Season on Potato Cultivars with Different Response to Environmental Stresses. Am. J. Plant Sci. 2013, 4, 2386–2393. [Google Scholar] [CrossRef]
- Herman, D.J.; Knowles, L.O.; Knowles, N.R. Heat Stress Affects Carbohydrate Metabolism during Cold-Induced Sweetening of Potato (Solanum tuberosum L.). Planta 2017, 245, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Gupta, S.K.; Niu, S.; Li, X.-Q.; Yang, Q.; Chen, G.; Zhu, W.; Haroon, M. Transcriptome Analysis of Heat Stress Response Genes in Potato Leaves. Mol. Biol. Rep. 2020, 47, 4311–4321. [Google Scholar] [CrossRef]
- Ginzberg, I.; Barel, G.; Ophir, R.; Tzin, E.; Tanami, Z.; Muddarangappa, T.; de Jong, W.; Fogelman, E. Transcriptomic Profiling of Heat-Stress Response in Potato Periderm. J. Exp. Bot. 2009, 60, 4411–4421. [Google Scholar] [CrossRef]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Alpuche-Solís, Á.G.; Juárez-Maldonado, A. Impact of Carbon Nanomaterials on the Antioxidant System of Tomato Seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef]
- Graci, S.; Cigliano, R.A.; Barone, A. Exploring the Gene Expression Network Involved in the Heat Stress Response of a Thermotolerant Tomato Genotype. BMC Genom. 2024, 25, 509. [Google Scholar] [CrossRef]
- Lin, H.-H.; Lin, K.-H.; Syu, J.-Y.; Tang, S.-Y.; Lo, H.-F. Physiological and Proteomic Analysis in Two Wild Tomato Lines under Waterlogging and High Temperature Stress. J. Plant Biochem. Biotechnol. 2016, 25, 87–96. [Google Scholar] [CrossRef]
- Su, H.-Z.; Ma, S.-Y.; Ma, X.-H.; Song, Y.; Wang, X.-M.; Cheng, G.-X. Transcriptome Analyses Show Changes in Heat-Stress Related Gene Expression in Tomato Cultivar ‘Moneymaker’ under High Temperature. J. Plant Biochem. Biotechnol. 2023, 32, 328–337. [Google Scholar] [CrossRef]
- Liu, R.; Shu, B.; Wang, Y.; Yu, B.; Wang, Y.; Gan, Y.; Liang, Y.; Qiu, Z.; Yang, J.; Yan, S.; et al. Transcriptome Analysis Reveals Key Genes Involved in the Eggplant Response to High-Temperature Stress. Environ. Exp. Bot. 2023, 211, 105369. [Google Scholar] [CrossRef]
- Zhang, A.; Zhu, Z.; Shang, J.; Zhang, S.; Shen, H.; Wu, X.; Zha, D. Transcriptome Profiling and Gene Expression Analyses of Eggplant (Solanum melongena L.) under Heat Stress. PLoS ONE 2020, 15, e0236980. [Google Scholar] [CrossRef] [PubMed]
- van Oosten-Hawle, P.; Morimoto, R.I. Organismal Proteostasis: Role of Cell-Nonautonomous Regulation and Transcellular Chaperone Signaling. Genes Dev. 2014, 28, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Cano-Ramirez, D.L.; Carmona-Salazar, L.; Morales-Cedillo, F.; Ramírez-Salcedo, J.; Cahoon, E.B.; Gavilanes-Ruíz, M. Plasma Membrane Fluidity: An Environment Thermal Detector in Plants. Cells 2021, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, K.L.; Means, A.R.; York, B. The Ca2+/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft. Trends Endocrinol. Metab. 2016, 27, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xu, C. Identification and Characterization of Proteins Associated with Plant Tolerance to Heat Stress. J. Integr. Plant Biol. 2008, 50, 1230–1237. [Google Scholar] [CrossRef]
- Liu, B.; Han, Y.; Qian, S.-B. Co-Translational Response to Proteotoxic Stress by Elongation Pausing of Ribosomes. Mol. Cell 2013, 49, 453–463. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Fan, S.; Jin, Z.; Huang, C. Targeting Stress Granules: A Novel Therapeutic Strategy for Human Diseases. Pharmacol. Res. 2020, 161, 105143. [Google Scholar] [CrossRef]
- Dang, F.; Lin, J.; Xue, B.; Chen, Y.; Guan, D.; Wang, Y.; He, S. Frontiers | CaWRKY27 Negatively Regulates H2O2-Mediated Thermotolerance in Pepper (C. annuum). Front. Plant Sci. 2018, 9, 1633. [Google Scholar] [CrossRef]
- Boopathy, L.R.A.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms Tailoring the Expression of Heat Shock Proteins to Proteostasis Challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar] [CrossRef]
- Sterner, D.E.; Berger, S.L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone Deacetylases and Mechanisms of Regulation of Gene Expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
| HSP Family | Symbol | Expression | Description | Reference |
|---|---|---|---|---|
| sHSPs | CaHSP16-4 | Upregulated | A lessened production of reactive oxygen species (ROS) was associated with CaHSP16.4, which is produced during the HS and drought stress in C. annuum | [42] |
| CaHSP18-2a | Upregulated | Interacted with heat stress-related genes to mitigate salt and heat-induced stresses. | [53] | |
| CaHSP18-7 | Upregulated | Induces the ROS scavenging potentials of the C. annuum plants, which result in the alleviation of ROS production by interacting with antioxidant enzymes | [45] | |
| CaHSP21-2 | Upregulated | Overexpression resulted in increased sensitivity to heat stress in leaves and roots. | [53] | |
| CaHSP22-0 | Up/ Downregulated | Upstream and downstream regulations of CaHSP22 played a crucial role in acquiring thermosensitivity and salt stress tolerance by inducing their relative expressions | [54] | |
| CaHSP25-9 | Upregulated | In transgenic Arabidopsis, the upregulation of CaHSP25.9 strengthened the HS tolerance to salt- and drought-related stresses. | [40] | |
| CaHSP40s | CaDnaJ | Upregulated | The response of the genes was upregulated threefold, which resulted in a heat stress response. | [49] |
| CaHSP60s | CaHSP60-3 | Downregulated | Under HS, the downstream expression was observed in heat-tolerant B6 and heat-sensitive R9 lines, which resulted in a profound HSR in pepper lines. | [55] |
| CaHSP60-6 | Knockdown | Knockdown of CaHSP60-6 resulted in an increase in acquired thermosensitivity when plants were exposed to HS. | [55] | |
| CaHSP70s | CaHSP70-1 | Upregulated | CaHsp70-1, as a member of the cytosolic Hsp70 subgroup, may be involved in HS defense response via a signal transduction pathway containing Ca2+, H2O2, and putrescine. | [46] |
| CaHSP70-2 | Upregulated | Slightly lowered expression of CaHSP70sgenes was observed under optimal conditions, but HS increased their expression by many folds, which indicated their profound impacts in combating heat-induced changes and their efficiency in acquiring thermotolerance in pepper. | [37] |
| HSF Family | Symbol | Expression | Description | Reference |
|---|---|---|---|---|
| CaHSFA | CaHSFA1d | Up-/ Downregulated | CaHSFA1d silencing in pepper lines resulted in reduced thermotolerance. CaHSFA1d overexpression led to an increased insensitivity to the elevated temperatures in Arabidopsis. | [85] |
| CaHSFA2 | Upregulated | CaHSFA2 activates the heat stress response pathway, leading to the induction of heat shock proteins (HSPs) that help maintain protein homeostasis and prevent protein aggregation. | [74,86] | |
| CaHSFB | CaHSFB2a | Upregulated | CaHSFB2a overexpression positively regulates the response to Ralstonia solanacearum infection or high temperature and high humidity, forming a transcriptional cascade with CaWRKY6 and CaWRKY40. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajjad, N.; Kang, Y.; Khattak, M.; Lu, M. Molecular Cascades of Heat Stress Responses in Solanaceae with Emphasis on Capsicum annuum L., Integrating Heat Shock Transcription Factors and Proteins. Horticulturae 2025, 11, 1038. https://doi.org/10.3390/horticulturae11091038
Sajjad N, Kang Y, Khattak M, Lu M. Molecular Cascades of Heat Stress Responses in Solanaceae with Emphasis on Capsicum annuum L., Integrating Heat Shock Transcription Factors and Proteins. Horticulturae. 2025; 11(9):1038. https://doi.org/10.3390/horticulturae11091038
Chicago/Turabian StyleSajjad, Nadia, Yong Kang, Mahnoor Khattak, and Minghui Lu. 2025. "Molecular Cascades of Heat Stress Responses in Solanaceae with Emphasis on Capsicum annuum L., Integrating Heat Shock Transcription Factors and Proteins" Horticulturae 11, no. 9: 1038. https://doi.org/10.3390/horticulturae11091038
APA StyleSajjad, N., Kang, Y., Khattak, M., & Lu, M. (2025). Molecular Cascades of Heat Stress Responses in Solanaceae with Emphasis on Capsicum annuum L., Integrating Heat Shock Transcription Factors and Proteins. Horticulturae, 11(9), 1038. https://doi.org/10.3390/horticulturae11091038






