Abstract
To optimize the quality and quantity of basil cultivars, this study investigated four varieties of nutrient-rich growing media compared with chemical fertilizers at the recommended dose in the soil-grown system, and commercial growing media (control) for producing holy basil and Genovese basil under greenhouse conditions. The experiment used a completely randomized design (CRD) with six treatments and five replications. With greater levels of chlorophyll, T3 and T4 growing media, consisting of top soil, filter cake, long-term/short-term composted chicken manure, coconut coir dust, and rice husk ash at a ratio of 3:2:2:1.5:1.5 v/v, produced the largest fresh yield when used for holy basil and Genovese basil productions, respectively. However, the net profit margin showed no discernible variations from T3–T5 and T2–T6 growing media, respectively. Nevertheless, T3 or T4 and T4 growing media were recommended for holy basil and Genovese basil production, respectively, based on highest productivity and intricacy of preparation, while also ensuring that the product retains its quality in terms of antioxidant bioactive components. In addition to maintaining the biomass of basil plants’ productivity even when they are cultivated in appropriate growing media, Genovese basil needs to be fertilized with organic fertilizer, like chicken manure, following the fifth or sixth harvesting period. Holy basil should be harvested after the fourth harvest period.
1. Introduction
Organic vegetable consumption has increased recently as consumers have become more conscious of the risks associated with chemical residues in agricultural products [1,2]. These residues are caused by both the issue of excessive pesticide use and the ongoing use of chemical fertilizers, which deplete soil fertility and hurt both human health and the environment [3,4]. Cultivating crops in fertile soil is one of the crucial concepts that can significantly reduce the need for chemical fertilizers and pesticides [5]. To maintain soil fertility, appropriate management is needed to achieve agricultural productivity and reverse soil degradation [6]. Organic amendments like agricultural residues (e.g., rice husk, coconut dust, and manure) have been shown to significantly improve the physical, chemical, and biological properties of soil [7,8,9]. The benefits of applying organic amendments have been reported, including lowering heavy metal levels, increasing nutrient uptake, and preventing the growth of crop pathogens [10,11].
Ocimum L., a large genus belonging to the Lamiaceae family, is rich in aromatic species that are widely distributed in Africa, Asia, and Central and South America [12,13]. Within this genus, O. tenuiflorum L. (holy basil) and O. basilicum L. (sweet basil) are popular culinary herbs in Thailand, providing flavor and fragrance to food [14]. Genovese basil, also known as “Italian basil or Italian large leaf” in Thailand, is a specific cultivar of sweet basil. It is indigenous to Italy’s Liguria region and has developed over time into a unique cultivar with an intense fragrance and unmistakable flavor that is renowned worldwide as the main component of pesto sauce [15,16]. According to several studies, holy basil and Genovese basil’s aboveground plant parts are typically used to extract the volatile oils and compounds that constitute multiple applications in food, cosmetics, and pharmaceutical industries [17,18,19]. Furthermore, some of the literature has demonstrated the antioxidant and anti-inflammatory properties of these plants performed by their phytochemical compounds, such as phenolic acids (ferulic, cinnamic, ursolic), flavonoids (apigenin), and eugenol as a main active substance in their volatile oils [20,21,22,23]. Most Ocimum L. plants have the ability to adapt to both abiotic (such as drought, salinity, and high temperature) and biotic (such as pathogens and pests) stressors through morphological, physiological, and biochemical changes, which accounts for their capacity for avoidance or tolerance [24]. The pesticide-resistant development of pathogens and pests, coupled with soil degradation, means that outdoor basil production requires a lot of pesticides and fertilizers to maintain target yields. This situation was confirmed continuously from the finding of pesticide residues exceeding acceptable limits in holy basil and sweet basil [25,26]. To avoid excess pesticide contamination in the final products and soil problem issues on a land-scale, greenhouse basil production with nutrient-rich growing media can represent an alternative to the conventional methods, ensuring the safety and sustainability of food production.
Although commercial growing media production for vegetable plants often relies on peat moss, perlite, and vermiculite, there is numerous research exploring alternative sustainable options derived from agricultural and local wastes, which show a significant interest in enhancing plant production and minimizing the depletion of natural resources [27,28]. For instance, coconut coir dust, sugarcane filter cake, rice husk, and rice husk ash are frequently employed as substitutes for conventional peat-based or soil-based media in Southeast Asia, with potential advantages for plant development and sustainability [29,30,31]. Additionally, rather than being utilized separately, these components are frequently mixed to provide growing media with optimal qualities for specific plants or growing conditions [30,32]. To supply the nutrient-rich growing media, however, organic fertilizer, including livestock manure and farmyard manure, is a popular organic addition used to provide nutrient sources for plant production [33,34]. Chicken manure, a major co-product of layer and broiler production, is typically cycled back into crop production as a nutrient-rich fertilizer. Chicken manure may improve the physical, chemical, and biological characteristics of soil, thereby increasing crop yields [35,36,37,38]. Several studies have shown that chicken manure can significantly increase fresh vegetable yield, with results comparable to those obtained from using synthetic fertilizers at recommended doses [39,40]. However, the plant nutritive composition, as well as the moisture content, pH, and soluble salt level in chicken manure has been shown to vary widely as a function of dietary supplement, litter storage (e.g., bedding materials, feathers) [41], and handling conditions (composting and drying process) [38,42].
In reference to our earlier study that showed the optimal temperature control system for growing lettuce in the same greenhouse, the growing medium consisting of topsoil, composted rain tree leaves, filtrate cake, long-term composted chicken manure, coconut coir dust and chopped husk, and rice husk ash was utilized during the three harvesting periods. It produced a high yield over the average standard size (>80 g plant−1) under the proper temperature-controlling conditions [43]. In Thailand, composted rain tree leaves are a valuable soil amendment known to improve the soil’s nutritional content, drainage, and structure in gardens and agriculture [44]. The addition of rain tree leaves, which have the lowest C:N ratio and a high N content, also provides nutrients when they break down and encourages microbial activity, which promotes organic matter turnover and nutrient cycling [45,46]. However, the current use of rain tree leaves as raw materials in the growing media still has many limitations, including the decomposition duration and heavy metal contamination. It is well known as a heavy metal accumulating plant from the atmosphere and has been used for monitoring air pollution levels [47,48]. Finding other growing media components with comparable physical characteristics to replace rain tree leaves is still an issue that requires research. Additionally, a significant portion of the plant nutrients needed for the growing media for producing vegetables that will last six months or longer, like basil, without the need for fertilizer additives and that prevent excessive vegetative growth and/or the effects of salinity, have not yet been thoroughly studied. Therefore, this study’s goal is to determine the best kind and amount of chicken manure as a source of plant nutrients appropriate for growing basil in greenhouses, and to assess the ideal growing media compositions that can minimize the usage of rain tree leaves.
2. Materials and Methods
2.1. Plant Materials and Experimental Design
The experiment was carried out in a completely randomized design (CRD) with five replicates inside the greenhouse at the Agricultural Technology Farming Center greenhouse, Thammasat University, Thailand (Latitude: 14.074191, Longitude: 100.609026). The experimental plants were the holy basil (Ocimum tenuiflorum L.) and Genovese basil (Ocimum basilicum L.). Seeds were sown in 104-cell polystyrene trays (1 seed/cell) using commercial peat moss as the planting medium. After fifteen days, uniformized seedlings with four true leaves were subsequently transplanted into individual plastic pots (D: 26 cm × H: 20 cm) filled with six different growing media consisting of T1 = Commercial soil mixed-medium (Control); T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v; T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v; T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v; T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v; and T6 = Topsoil with recommended fertilizer doses (Table 1). Each treatment in an experiment had five replicates, and within each replicate, there were three plant samples (pots).

Table 1.
Different growing media for holy basil and Genovese basil production.
T1, or commercial soil-mixed media, was the most widely used and cost-effective growing medium among Thai customers. It comprised topsoil, sand, and organic fertilizer in a 1:1:2 v/v ratio. However, this growing medium was employed as a controlled growing medium because it required extra nutrients for the best possible plant growth.
The chicken manure used in T2–T5 was made from laying hen manure and rice husk bedding material. It was divided into two groups: short-term composted chicken manure (SM), which had a composting period of roughly three months, and long-term composted chicken manure (LM), which had a composting period of approximately twelve months.
The recommended fertilizer dosages in T6, 5 t ha−1 of cow manure and 187.50 kg ha−1 of a 15-15-15 formula (N-P-K) fertilizer, were used as a basal application, while 187.50 kg ha−1 of a 15-15-15 formula (N-P-K) and urea (46-0-0) were applied as a top dressing after each harvesting time for holy basil and Genovese basil, respectively. Nevertheless, no fertilizer was applied to the plants cultivated under treatments T1–T5 until the last harvest.
2.2. Planting Management in Greenhouse
Kumsong et al. [43] detailed the greenhouse used in this experiment and its construction and controlling systems. To keep the temperature differential between the greenhouse’s interior and exterior below 2 °C, fogging and ventilation fans were run in the greenhouse from transplanting until the end of harvest. From 10:00 to 15:00 h, the fogging system was electrically operated for 10 min each hour, ensuring that the greenhouse’s average relative humidity was between 60% and 80%. For the ventilation fan system, two ventilation fans were operated from 10:00 to 16:00 h for air ventilation inside the greenhouse. Trail pots were set up on greenhouse benches and given drip irrigation twice daily at 8:00 and 16:00 h as required to maintain vigorous growth until harvesting. Insects and diseases were eradicated by spraying natural extracts, such as wood vinegar and neem extract, while weeds were removed mechanically. Environmental parameters, including temperature, humidity, and light intensity, were continuously measured by sensors outside and inside the greenhouse linked to the IoT system. Throughout the six harvesting periods (one crop), the average hourly values during the daytime (10:00–16:00 h) of relative humidity, temperature, and light intensity both inside and outside of the greenhouse were recorded (Figure 1). The relative humidity value in the greenhouse was 11.47% lower than the outside conditions, whereas a higher temperature was recorded inside the greenhouse, which increased by 1.32% compared to the outside conditions. In terms of average light intensity, it was discovered that the light intensity in the greenhouse decreased by 56.72% compared to the outside conditions during the determined periods (Figure 1).
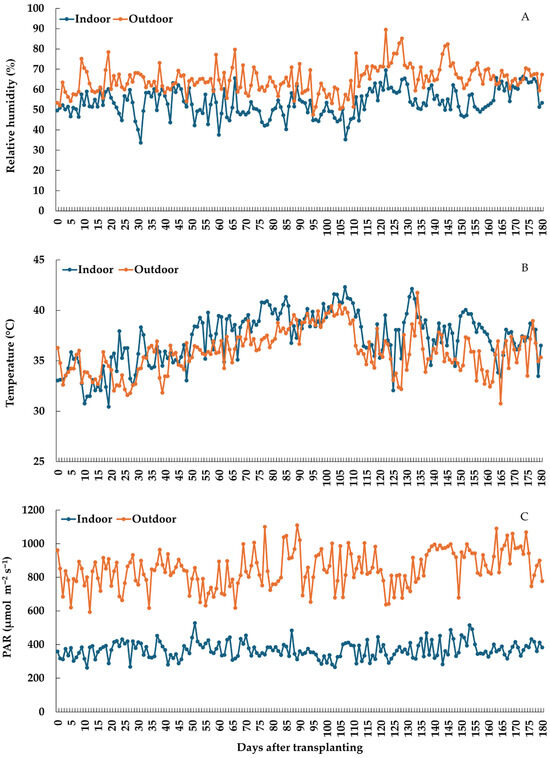
Figure 1.
Average micro-environmental conditions between indoors and outdoors during the experiment: (A) Relative humidity; (B) Temperature; (C) Photosynthetically active radiation.
2.3. Data Collection and Analysis
2.3.1. Physicochemical Properties of Growing Media
Growing media samples before treatments were air-dried, crushed, and sieved through a 2 mm sieve to analyze specific soil chemical properties. The pH and EC of each growing medium sample were measured in water at a 1:10 w/v ratio determined using a pH-EC meter (SciberScanPC510, EUTEC, Singapore). The organic matter was calculated using the Walkley and Black [49] method, while the total N concentration was determined by using the Kjeldahl method. The total P and K level in the media sample was measured using a modified version of the standard protocol of the Association of Official Analytical Chemists (AOAC). In brief, 1.0 g of media sample was mixed with a solution of nitric-perchloric acid (HNO3:HClO4 in a ratio of 2:1 v/v) and digested. After digestion, the samples were diluted with distilled water to a final volume of 50 mL and stored in plastic tubes at room temperature. Total P in distilled samples was determined using a spectrophotometer (UV-1280, Shimadzu, Kyoto, Japan) at 420 nm. In contrast, total K concentration was analyzed by an atomic absorption spectrometer (PinAAcle900F, Perkin-Elmer, Waltham, MA, USA).
For the physical properties, analysis of each growing medium, bulk density and total pore space were determined following the modified method described by Di Gioia et al. [50] and Qin et al. [51]. The growing media were dried at 105 ± 1 °C and transferred to known-volume cylinders. Then, the bulk density of the growing media samples was calculated, which was defined as the dry mass in a given volume. To investigate each growing media sample’s total pore space, 50 mL of each sample was saturated with distilled water for 6 h. The excess water was allowed to drain for 2 min by gravity. The weight of saturated growing media was recorded, and the total pore space was determined as follows [51]:
Total pore space (%) = [(Saturated sample weight/Dry sample weight) × 100]
2.3.2. Growth and Yield Determination
Three plant samples of holy basil and Genovese basil were randomly selected from each replication in different treatments of growing media. The plant height, width, stem diameter, and number of branches per plant were recorded before each harvesting at 30, 60, 90, 120, 150, and 180 days after transplanting, referring to the 1st harvesting to the 6th harvesting, respectively. The plant height was measured using the ruler, from the growing media level at the base of the plant to the terminal bud, while the plant width was collected as the average width measured at the widest point 90° to the plant. The stem diameter was measured at 10 cm above the growing media surface level.
Leaf morphological traits at the fourth expanded leaf from the top of the stem of each plot sample were measured, including leaf length, width, and area. Leaf length was measured from the lamina tip to the point of intersection of the lamina and stem, and leaf width was measured from tip to tip between the widest lamina with a simple ruler, as referenced in Bazaz et al. [52]. The leaf area of each leaf was measured using an area meter (CI-202 portable laser leaf area meter, CID Bio-Science, Inc., Washington, DC, USA) calibrated to 1.00 cm2.
To determine each plant’s fresh weight, a section 15 cm above the ground was harvested at each harvesting period. The leaves and stem were then separated and weighed on a digital scale. The sample dry weights were measured after oven-drying at 50 ± 3 °C for 72 h or until a stable dry weight was achieved.
2.3.3. Photosynthetic Pigments
The chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid were extracted from 0.5 g of holy basil and Genovese basil fresh leaf sample using 10 mL of 80% acetone as a solvent. After incubation at 4 °C for 72 h in the dark, the supernatant was measured at 645, 663, and 470 nm using an ultraviolet spectrophotometer (UV-1280, Shimadzu, Japan). The absorbances were used to calculate the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid, expressed as mg g−1 fresh weight by using the formula as described by Mackinney [53] and Yu et al. [54].
2.3.4. Phytochemical Analysis
The total phenolics, flavonoids, and antioxidant activity in dry aboveground samples of holy basil and Genovese basil were determined according to the method of Chutimanukul et al. [22], with some modifications. In brief, the total phenolic content was determined by the Folin–Ciocalteu spectrophotometric method. The result was calculated from the calibration curve of gallic acid and expressed as mg gallic acid equivalent (GAE) g−1 dry weight. The quantity of total flavonoid content was determined using the colorimetric method, based on the standard curve of rutin solution dissolved in dimethyl sulfoxide. The concentration was stated as mg rutin equivalent (RE) g−1 dry weight. For the free radical scavenging activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) was employed as a free radical. Trolox was used as a reference antioxidant, and the antioxidant activity was shown as the inhibition percentage of DPPH absorbance using the following formula [22]
where Ac = control reaction absorbance; As = sample reaction absorbance.
DPPH radical scavenging (%) = [(Ac − As)/Ac] × 100
Anthocyanin content was determined only in dry aboveground samples of holy basil following the procedure described by Chutimanukul et al. [22]. The absorbance (A) of the reaction mixture was measured at 530 and 675 nm using a spectrophotometer, and the anthocyanin content was calculated using the following formula:
Anthocyanin content (μg g−1 dry weight) = A530 − (0.33 × A657)
2.3.5. Nutrient Accumulation in Plant Tissues
The dried shoot samples were ground and then sieved through a 2 mm sieve to analyze N, P, and K contents on a % dry weight basis. With certain adjustments as outlined in Section 2.3.1, the standard methodology of the Association of Official Analytical Chemists (AOAC) was followed to determine the P and K contents in the plant tissues and the total N content, which was determined using the Kjeldahl method.
2.3.6. Yield Estimation and Economic Benefit Analysis
One of the study’s objectives was to provide producers with practical enterprise budgets to help them determine the most profitable ways to manage the growing media for basil production in the greenhouse. The economic tool known as partial budgeting is widely used to evaluate the economic profitability of different production methods and to illustrate the impact of modifications to production operations. Focusing exclusively on changes in income flows and/or expenses, such as increased income, decreased costs, and reduced income, is a fundamental tenet of partial budgeting [55]. This study focused on changes in production costs from different growing media as a benchmark case.
Both the production cost and the annual total income were evaluated for the economic benefit analysis. The impact of differences in production costs from using different growing media and fertilizer applications on overall financial performance was also investigated. Three categories were used to classify the production costs: (1) greenhouse construction cost, (2) direct cost, and (3) labor cost, of which (2) and (3) are related to daily operations. The greenhouse construction cost was calculated based on the standard price of a smart greenhouse, mainly composed of steel structure parts, plastic films, energy sources supplied from solar panels, controlling systems, irrigation, ventilation, etc. The study’s greenhouse cost was 40 USD per square meter or 2880.00 USD per 6 × 12 m greenhouse. Direct costs were considered for seedlings, pots, growing media, fertilizer, and other chemicals that control fungi and insects. Considering the cost of growth material for the six treatments, the costs for planting 800 plants in one greenhouse were 240.43, 192.80, 129.29, 111.14, 120.22, and 108.85 USD, respectively. Labor costs for plot construction, irrigation monitoring, weeding, and harvesting were computed using the Ministry of Labor of Thailand’s minimum daily wage rate of 10.55 USD in the study area (Pathum Thani province, Thailand) (Table 2). Throughout the study period, the labor cost for the greenhouse production was precisely calculated in hours per 6 × 12 m greenhouse. However, the pots in the direct costs and the greenhouse construction cost will be subtracted from the second crop’s direct and total costs to calculate the annual costs for the first and second crops (each of which runs for six months).

Table 2.
Cost structure for basil production with different growing media in 6 × 12 m greenhouse.
The fresh weight of harvested whole plants, including stem and leaves, of holy basil and Genovese basil produced under various growing media was measured monthly during the study period. All the harvested whole plants were characterized with no physical damage to leaves, referring to marketable yield. The net marketable yield (kg) per 6 × 12 m greenhouse was calculated from planting with 30 × 30 cm spacing between plants and rows in the 6 × 12 m greenhouse, namely 800 plants per greenhouse. The net annual yield of holy basil and Genovese basil was estimated for each of the first and second crops over six months. Total sales were calculated from the net annual yield, based on the GAP (good agricultural practice) market price of 9.92 and 3.69 USD kg−1 for the fresh marketable yield of Genovese basil and holy basil, respectively. The actual value analysis of revenue and production cost was converted into USD (USD 1 = THB 35.27) using prevailing rates during the study period. The gross margin, net income, and profit margin were calculated by the following formula [55]:
where Total sales were calculated as unit market price × net crop yield (6 months harvesting) × the shrinkage rate (uncertainly yield loss ≈ 8%) [56].
Gross margin = [(Total sales − Total direct costs)/Total sales] × 100
Net income = Total sales − Total costs
Net profit margin (%) = (Net income/Total sales) × 100
2.4. Statistical Analysis
Experimental treatment effects were analyzed using a randomized complete block design with five replications, and the statistical analysis of data collected was carried out using standard analysis of variance (ANOVA) by using IBM SPSS Statistics, Version 26.0 software (IBM Crop., Armonk, NY, USA). To determine the significance of the difference between the means, Duncan’s multiple range test was computed at the 0.05 probability level (p ≤ 0.05). A correlation heatmap was generated using GraphPad® version 10.2.0 [57] to facilitate visual inspection and evaluate the relationships between different parameters for two basil types.
3. Results
3.1. Physical and Chemical Properties of Growing Media
The highest bulk density (1.22 kg m−3) and lowest total pore space (30.86%) were observed in T3. The different proportions and types of organic waste in the growing media significantly affected the chemical properties. T3 and T5 had the significantly highest pH, with averages of 8.59, whereas T2 had the significantly highest EC value, which was 2.49 dS m−1. However, the lowest organic matter resulted from T6 (3.81%), followed by T2 (11.47%), whereas T1, T3, T4, and T5 had the highest organic matter between 15.42 and 17.77% (Table 2). The highest total Nitrogen (N) was obtained for T3 (0.89%), without any significant difference between values resulting from T4 (0.82%) and T5 (0.78%). In addition, the highest total phosphorus (P) resulted from T3 (0.30%), whereas the total potassium (K) was significantly the greatest in T1 (9.01%) (Table 3).

Table 3.
Selected physicochemical properties of different growing media for holy basil and Genovese basil production.
3.2. Plant Growth Under Different Growing Media Treatments
The morphological traits (Figures S1 and S2) and all plant growth indicators at various ages of holy basil and Genovese basil production under greenhouse conditions were significantly impacted by the growing media (Figure 2A–H). Despite being harvested every 30 days, it seems that the canopy characteristics of holy basil and Genovese basil tended to be greater as plant age increased. In the case of holy basil, different growing media treatments were shown to affect traits related to plant growth, recorded at 30–180 days after transplanting. The highest plant height at 30, 60, 90, 120, 150, and 180 days after transplanting was observed in T2, T6, T4 T2, T3, and T5, respectively (Figure 2A). In terms of plant width, the higher plant width at 30 and 60 days after transplanting was observed in T6, which was not significantly different from that resulting from T2 and T3 at 30 days after transplanting (Figure 2B). In addition, the highest plant width at 90, 120, 150, and 180 days after transplanting was observed in T4, T5, T3, and T5–T6, respectively. The greater number of branches of holy basil was observed at 90 days after transplanting in T3 and T4. In addition, the greatest number of branches at 120, 150, and 180 days after transplanting was clearly observed in T5, which was not significantly different from that resulting from T3 at 180 days after transplanting (Figure 2C). Furthermore, the stem diameter of holy basil gradually increased with the plant age, a similar trend to Genovese basil. The lowest stem diameters were also observed in the control treatment. The stem diameter of T3 was significantly larger at all plant ages, while it was not statistically different from that of T2 and T4–T6 at some point in the days after transplanting (Figure 2D).
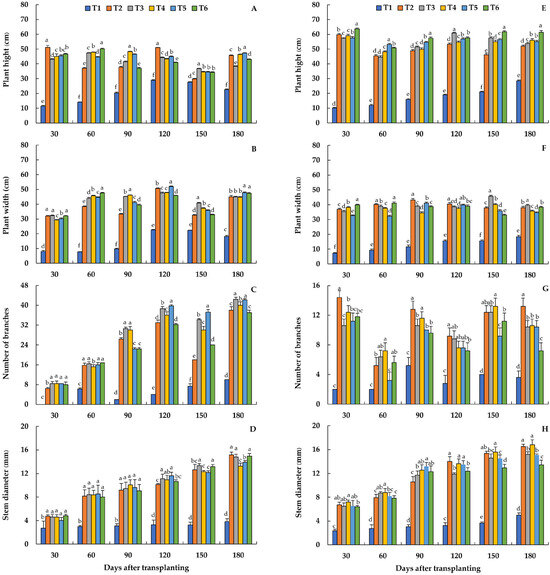
Figure 2.
Growth response of holy basil (A–D) and Genovese basil (E–H) at 30–180 days after transplanting under different growing media. T1 = Commercial soil mixed-medium (Control), T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v, T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v, T6 = Topsoil with recommended fertilizer doses.
In the case of Genovese basil, the plants from T6 had significantly greater height at 30, 90, 150, and 180 days after transplanting than those from the other treatments. At 60 and 120 days after transplanting, however, significantly higher heights were observed with the plants from T5 and T3, respectively (Figure 2E). For the plant width, T6 resulted in the greatest plant width at 30 and 60 days after transplanting, which was not significantly different from that resulting from T2 at 60 days after transplanting. At 90 and 120 days after transplanting, T2 resulted in significantly greatest plant width, which was not significantly different from that resulting from T5 at 120 days after transplanting. At 150 and 180 days after transplanting, T3 resulted in the greatest plant width, with the average values of 45.64 and 39.60 cm, respectively (Figure 2F). For the number of branches, T2 had the significantly greater number of branches at 30, 90, 120, and 180 days after transplanting, which were not significantly different from that resulting from T4 at 90 days after transplanting, T3–T5 at 120 days after transplanting, and T3 and T4 at 150 days after transplanting, respectively. At 60 days after transplanting, however, the greater number of branches resulted from T4, which was not significantly different from that resulting from T3 and T6 (Figure 2G). The stem diameter of Genovese basil gradually increased with the plant age, with the lowest stem diameters observed in the control treatment (T1) at all the plant ages. However, the greater stem diameters of Genovese basil at 120–180 days after transplanting were clearly observed in T2 and T4, which were not significantly different from that resulting from T5 at 120 days after transplanting (Figure 2H).
Leaf characteristics, including leaf width, leaf length, and leaf area per leaf of the fourth expanded leaf from the top of the stem at 180 days after transplanting, were significantly affected by different growing media treatments (Table 4). In the case of holy basil, the lowest leaf width and leaf length were observed only in T1, while T2–T6 resulted in the greater leaf length and leaf width, which were in a range of 2.44–2.80 and 5.14–5.62 cm, respectively. However, T3 and T4 resulted in the greatest single leaf area (12.53 and 12.59 cm2, respectively), which was not significantly different from that resulting from T2 and T5 (12.12 and 11.99 cm2, respectively). In the case of Genovese basil, the significantly greater leaf width was observed in T3, T6, T2, and T4, respectively, which was shown to be between 3.66 and 3.90 cm. In addition, T2, T3, and T4 also resulted in significantly greater leaf length, with average values of 7.90, 7.56, and 7.22 cm, respectively. For the single leaf area, however, only T4 resulted in the highest single leaf area (34.17 cm2), which was significantly higher than those resulting from the other treatments (Table 4).

Table 4.
Leaf characteristics of holy basil and Genovese basil 180 days after transplanting under different growing media.
3.3. Yield Characteristics Under Different Growing Media Treatments
Different growing media had an influence (p ≤ 0.05) on the yield components of both holy basil and Genovese basil at all harvesting periods, as shown in Figure 3. Among the yield components, the average leaf to total fresh (aboveground) yield ratio at all the harvesting periods for holy basil and Genovese basil was 72.26% and 75.82%, respectively. The stem-to-total fresh yield ratio for holy basil and Genovese basil was an average of 27.74% and 24.18%, respectively. In the case of holy basil, the higher fresh yield recording at the first, fourth, and sixth harvesting periods resulted from T4 (37.96, 76.61, and 51.70 g plant−1, respectively), which was not significantly different from that resulting from T3 at the fourth harvesting period (76.94 g plant−1). At the second harvesting period, T5 and T6 resulted in the highest fresh yield, with the average of 46.73 and 46.94 g plant−1, respectively (Figure 3).
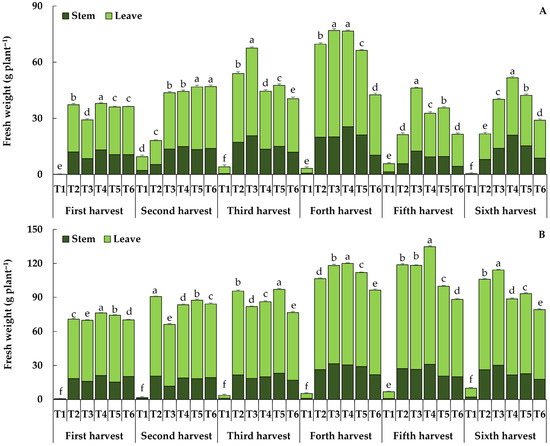
Figure 3.
Impact of different growing media on the fresh yield of holy basil (A) and Genovese basil (B) across six harvest periods. T1 = Commercial soil mixed-medium (Control), T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v, T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v, T6 = Topsoil with recommended fertilizer doses.
During the other two times measured, the third and fifth harvesting periods, the highest fresh yield resulted from T3, which was 67.50 and 46.14 g plant−1, respectively. It seems that the total fresh yield of holy basil tended to increase in the fourth harvesting period and then gradually declined in the fifth. Nevertheless, the highest total fresh and dry yields throughout six harvest periods were observed, resulting from T3 (303.30 and 35.31 g plant−1, respectively), where the total dry yield was not significantly different from that resulting from T4 (34.97 g plant−1) (Table 5).

Table 5.
Influence of different growing media on total fresh and dry yields of holy basil and Genovese basil throughout the six harvest periods.
In the case of Genovese basil, T4 resulted in the highest fresh yield at the first, fourth, and fifth harvesting periods, with an average value of 76.19, 120.01, and 134.68 g plant−1, respectively. During the other three times measured, the second, third, and sixth harvesting period, the highest fresh yield resulted from T2 (90.66 g plant−1), T5 (97.03 g plant−1), and T3 (114.27 g plant−1), respectively. It appears that the total fresh yield tended to rise during the fifth harvesting period before progressively declining during the sixth. However, the greater total fresh and dry weight of Genovese basil throughout six harvest periods was observed in T4 and T2. The total fresh yield of Genovese basil resulting from T4 and T2 was shown to be 589.15 and 588.23 g plant−1, respectively, while the total dry yield of those treatments was shown to be 49.42 and 49.02 g plant−1, respectively (Table 5).
3.4. Photosynthetic Pigments Under Different Growing Media Treatments
The ANOVA results demonstrated that variations in growing media had an influence (p ≤ 0.05) on some photosynthetic pigments of both holy basil and Genovese basil (Table 6 and Table 7). For holy basil, the chlorophyll a and carotenoid contents were revealed to not be influenced significantly by the different growing media, which were shown to be between 0.46 and 0.49 mg g−1 FW, and 11.19 and 12.39 mg g−1 FW, respectively. Nevertheless, the highest chlorophyll b and total chlorophyll contents were observed in T3 and T6, both with the average values of 0.62 and 1.10 mg g−1 FW, respectively (Table 6).

Table 6.
Influence of different growing media on photosynthetic pigments of holy basil at 180 days after transplanting.

Table 7.
Influence of different growing media on photosynthetic pigments of Genovese basil at 180 days after transplanting.
In the case of Genovese basil, the highest chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents were observed resulting from T4 and T6, where the chlorophyll a content was not significantly different from that resulting from T2, T3, and T5 (Table 7).
3.5. Phytochemical Composition Under Different Growing Media Treatments
Significant effects of growing media on the secondary metabolite content and antioxidation capacity of holy basil and Genovese basil were observed (Table 8 and Table 9). For holy basil, the highest total phenolic and total flavonoid contents, as well as DPPH radicle scavenging, were observed resulting from T1 (18.77 mg GAE g−1 DW, 17.63 mg RE g−1 DW, and 90.73%, respectively), but the total phenolic content was not significantly different resulting from T2 (10.66 mg GAE g−1 DW). For the total anthocyanin content, however, T6 resulted in the highest total anthocyanin content (1.88 μg g−1 DW), which caused significantly greater total anthocyanin content than all other treatments (Table 8).

Table 8.
Influence of different growing media on phytochemical contents of holy basil at 180 days after transplanting.

Table 9.
Influence of different growing media on phytochemical contents of Genovese basil at 180 days after transplanting.
The same tendency was also observed in Genovese basil, which found that the total phenolics and total flavonoids contents were significantly highest in the plant resulting from T1, with the average values of 20.64 mg GAE g−1 DW and 19.69 mg RE g−1 DW, respectively. In addition, the greatest DPPH radical scavenging was observed resulting from T1 (82.06%) and was significantly greater than that resulting from all the other treatments (Table 9).
3.6. Nutrient Accumulation in Plant Tissues Under Different Growing Media Treatments
The significant effects of growing media on the macronutrients in the aboveground (leaf + stem) and root tissues of holy basil and Genovese basil grown under greenhouse conditions are shown in Table 10 and Table 11, respectively. In the case of holy basil, the highest total N concentrations in aboveground tissues resulted from T2 and T3, while the highest total N concentrations in root tissues were observed in T2 (1.90%). The total P concentrations in aboveground tissues resulting from T1 (0.13%) were significantly greater than those resulting from all other treatments, whereas the highest total P concentrations resulted from T3 (0.09%). Total K concentrations in aboveground tissues were highest in plants resulting from T6 (1.49%), although the highest total K concentrations in root tissues resulted from T3 (4.11%) (Table 10).

Table 10.
Influence of different growing media on macronutrient concentrations in plant tissues of holy basil 180 days after transplanting.

Table 11.
Influence of different growing media on macronutrient concentrations in plant tissues of Genovese basil at 180 days after transplanting.
In the case of Genovese basil, the highest total N concentrations in aboveground tissues resulted from T6 (3.51%). In root tissues, the total N concentrations were highest in plants resulting from T2–T6 growing media. Total P concentrations in aboveground and root tissues were highest in plants resulting from T3 and T5, with averages of 0.16% and 0.18% for aboveground tissues, and 0.12% and 0.14% for root tissues, respectively. In addition, the T2–T5 growing media resulted in a higher total K concentration in the aboveground tissues, without any significant difference from each other. However, the significantly greatest total K concentrations in root tissues resulted from T1 (3.64%) (Table 11).
3.7. Economic Profitability
To account for the possible risks in the year-round greenhouse production of holy basil and Genovese basil, the primary economic performance indicators (total sales, gross margin, net income, and net profit margin) were computed, as indicated in Table 12 and Table 13.

Table 12.
Economic performance indicators for holy basil grown under different growing media.

Table 13.
Economic performance indicators for Genovese basil production under different growing media in one year-round.
In the case of holy basil, the total sales from growing holy basil in T3 were substantially higher than those from other growing media during the first crop period. Additionally, T3, T4, and T5 growing media provided the highest gross margin values, ranging from 27.55 to 33.28%. In comparison to the other growth media, T3 produced the best net income, even though the net income under all growing media treatments was negative. Furthermore, because net income is negative for all growing media treatments, the net profit margin, which varies from −384.45 to −6402.83%, is likewise negative. Nevertheless, as compared to the other growing media, T3–T5 growing media still had the highest net profit margin (Table 12). During the second crop period, holy basil grown in T3 growing medium had the most significant total sales and net profits, at 822.69 and 12.82 USD, respectively. Even during the second crop period, T1 growing media also produced a negative gross margin (−407.68 USD). When holy basil was grown on T3–T5 growing media, the net profit margin was comparable, with the highest values being seen in comparison to the other growing media. Nevertheless, only T3 gave the positive net profit margin (1.56%), compared with the other growing media (Table 12).
In the case of the first crop period’s production of Genovese basil, T3 yielded the highest total sales and net income, whereas T2 did not significantly alter total sales. The production of Genovese basil in T1 (commercial media) yielded the lowest gross margin and net profit margin when compared to T2-T6 growing media, which had relatively high gross margins (85.11–87.66%) and net profit margins (−10.90–7.80%), both of which were not statistically significant (Table 13). However, T6 produced a negative profit margin, indicating that it is not a sustainable production model because the production expenditures under this treatment exceeded the total revenue for a given period. For the second crop period, continuously for one year-round, producing Genovese basil in T3 obtained the significantly highest in the total sales, whereas the highest net income was observed in T4. The treatment of T2-T5 growing media, however, produced the noticeably largest gross margin and net profit margin, which ranged from 93.66 to 95.57% and 79.67 to 63.43%, respectively. Nonetheless, there was no discernible difference in their net profit margin between T6 growth media (77.01%). However, T1’s net profit margin for the year-round greenhouse production of Genovese basil remained negative (Table 13).
3.8. Data Correlation Analysis
To understand the relationships between the different traits recorded under different growing media treatments for holy basil and Genovese basil production in the greenhouse, the correlations between the collected parameters were investigated and are shown in Figure 4 and Figure 5. A positive relationship between parameters was assumed when the correlation coefficient (r2) was 0.70 or higher.
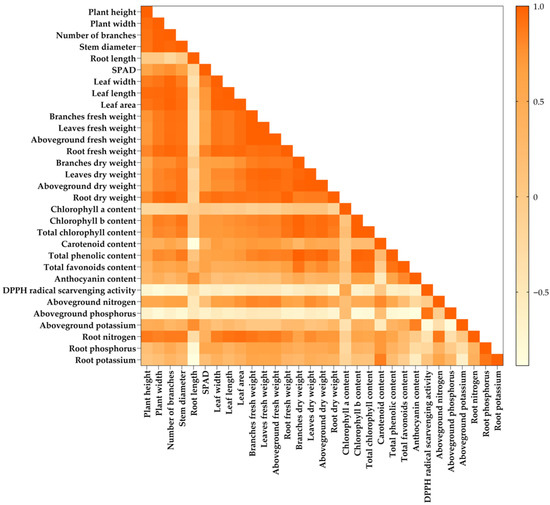
Figure 4.
A correlational plot from 31 parameters recorded from holy basil at 180 days after transplanting, grown under different growing media. T1 = Commercial soil mixed-medium (Control), T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v, T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v, T6 = Topsoil with recommended fertilizer doses.
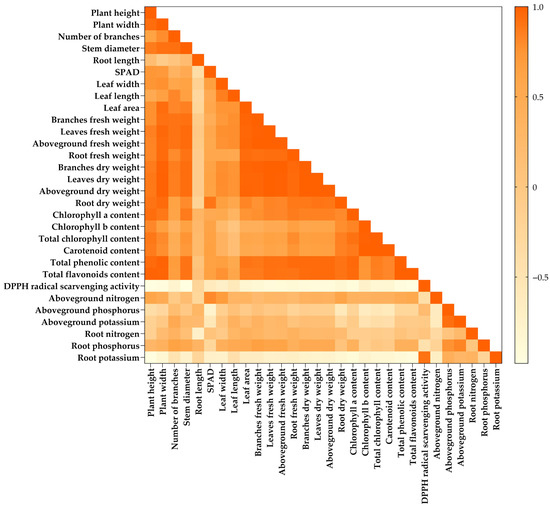
Figure 5.
A correlational plot from 30 parameters recorded from Genovese basil at 180 days after transplanting, grown under different growing media. T1 = Commercial soil mixed-medium (Control), T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v, T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v, T6 = Topsoil with recommended fertilizer doses.
For the correlation between the collected parameters of holy basil at 180 days after transplanting, it was found that the growth parameters, including plant width, leaf number, and stem diameter, displayed a higher positive correlation with chlorophyll b, total chlorophyll, and total phenolics, as in a range of r2 = 0.778–0.893. However, these growth parameters were negatively correlated with the DPPH radical scavenging (r2 > −0.729). Among the correlation between growth parameters and macronutrients in different plant parts, the plant width (r2 = −0.799) and stem diameter (r2 = −0.770) displayed a higher negative correlation with the aboveground P. Whereas, the plant height, leaf number and stem diameter showed a strong positive correlation with the root N (r2 > 0.866). In addition, the root length displayed a higher positive correlation with the anthocyanin (r2 = 0.764) and the aboveground K (r2 = 0.782), although a negative correlation was found between the root length and root P and K (r2 > −0.768). For the correlations between phytochemical contents and macronutrients in plant tissues, the highest negative correlation (r2 > −0.773) was observed between the aboveground P and phytochemical contents, including total phenolics, total flavonoids, and anthocyanin, whereas the aboveground P displayed a higher positive correlation with DPPH radical scavenging (r2 = 0.907). Contrarily, the aboveground K displayed a positive correlation with the anthocyanin (r2 = 0.846) and a negative correlation with DPPH radical scavenging (r2 = 0.830). Nevertheless, the aboveground fresh weight displayed a strong positive correlation with total N in aboveground (r2 = 0.807) and root (r2 = 0.869) tissues, as well as with chlorophyll b (r2 = 0.827), total chlorophyll (r2 = 0.805), total carotenoid (r2 = 0.718) and total phenolics (r2 = 0.843) (Figure 4).
In the case of Genovese basil (at 180 days after transplanting), the growth parameters, including plant height, plant width, and stem diameter, displayed a higher positive correlation with total chlorophyll, carotenoid, total phenolics, and total flavonoids, as in a range of r2 = 0.708–0.979. However, these growth parameters were negatively correlated with the DPPH radical scavenging (r2 > −0.951) and the root K (r2 > −0.951). Of the macronutrients found in the Genovese basil’s aboveground and root tissues, only the root K showed this strong correlation. In addition, the aboveground N was positively correlated with the SPAD value (r2 = 0.800) and leaf width (r2 = 0.718). Contrarily, the aboveground P (r2 = −0.781) and root K (r2 = −0.730) displayed a higher negative correlation with the SPAD value. Nevertheless, the root N also displayed a negative correlation with the root length (r2 = −0.751). Whereas both the aboveground and root P have a higher positive correlation with the aboveground K (r2 = 0.926 and 0.830, respectively). Furthermore, the higher negative correlation was observed between the root K and the photosynthetic pigments and phytochemical contents, including total chlorophyll, carotenoid, total phenolics, and total flavonoids (r2 > −0.839), whereas the root K displayed a higher positive correlation with DPPH radical scavenging (r2 = 0.908). Nevertheless, the aboveground fresh weight, which represents the yield, displayed a strong positive correlation with chlorophyll a (r2 = 0.810), total phenolics (r2 = 0.916), and total flavonoids (r2 = 0.908), whereas the strong negative correlation was found with the root K (r2 = −0.717) and DPPH radical scavenging (r2 = −0.909) (Figure 5).
4. Discussion
The need for sustainable horticulture is growing as a result of population growth, resource constraints, and climate change, which are all contributing to the issues facing food security [58,59], as traditional farming practices for vegetable production in Thailand are frequently hampered by nutrient depletion, soil degradation, and water utilization inefficiencies, as well as the problems of weed control, and disease and insect pest outbreaks [60]. Greenhouse vegetable production with suitable growing media, especially those that contain rich plant nutrition obtained from organic materials, presents a viable way to increase crop yields and quality (no use of chemical substances) while reducing their adverse effects on the environment in this regard. According to yield study results for both Genovese and holy basil at six harvesting intervals (30–180 days after transplanting), the number of branches and leaf area had a strong positive correlation with the total fresh weight yield, which T3 for holy basil, and T2 and T4 for Genovese basil tended to obtain the significantly higher values of leaf area and number of branches in almost all the harvesting periods. Interestingly, the fresh yield of Genovese basil increased steadily until the fifth harvesting (120–150 days after transplanting) and only slightly decreased in the sixth harvesting periods, whereas the fresh yield of holy basil increased gradually until the fourth harvesting (90–120 days after transplanting) in all treatments and then decreased by nearly half (40–69%). Although both holy basil and Genovese basil can be harvested multiple times over a one to two-year period, these results showed that, even though they were planted in the best growing media (T3 and T4, respectively), the production of holy basil should receive organic fertilizer, such as chicken manure, after the fourth harvesting period. In contrast, the production of Genovese basil should receive it after the fifth or sixth harvesting period to maintain the yield biomass.
In the current study, studies in many plants, including holy basil and Genovese basil, have shown a strongly positive correlation between the total chlorophyll content and plant growth and biomass. This relationship is attributed to chlorophyll being crucial for photosynthesis, the process by which plants convert light energy into chemical energy, ultimately fueling plant growth and biomass accumulation. Higher chlorophyll levels generally lead to more efficient photosynthesis, producing greater biomass [61,62]. In general, leaf chlorophyll content and changes in content are controlled and affected by both the surrounding environment (e.g., temperature, soil nutrients, rainfall, and sunlight) and plant morphological and physiological factors, including leaf area index (LAI) and nutrient availability [63,64]. A vast literature has been conducted on the importance of nitrogen in the structure of chlorophyll in plants, as higher N content in leaves is strongly associated with higher chlorophyll content and increased chloroplast activity and thus increased photosynthetic productivity [65,66,67]. The present study did not find a significantly positive correlation between the aboveground N and total chlorophyll content in both basil types.
Additionally, our study found a negative correlation between total chlorophyll content and certain nutrient levels in basil plants. Specifically, there was a negative correlation between chlorophyll and phosphorus (P) in the aboveground tissues of holy basil, and between chlorophyll and potassium (K) in the root tissues of Genovese basil. Although phosphorus is an essential nutrient for plants, playing a vital role in various metabolic processes, including chlorophyll synthesis, the negative correlation between aboveground P and chlorophyll content in this study may suggest that when plants have high chlorophyll content (implying active photosynthesis and potentially high growth rates), they might have a lower demand for P in their shoots, or they might be utilizing P more efficiently. This was consistent with the previous research that found the amount of chlorophyll a and b in Nufar basil (Ocimum basilicum ‘Nufar’) was approximately 0.004 mg g−1 FW [68], and the total chlorophyll content was an average of 0.79 mg g−1 FW [69], which was 100 and 1.2 times lower than the current study of holy basil, respectively. Additionally, the amount of P accumulated in the shoot of the basil plant (Ocimum basilicum L.) from the findings of Lima et al. [70] was observed around 3.0 g kg−1, which was 3 times higher than the current study. Conversely, Genovese basil’s negative correlation between chlorophyll content and root K may suggest that high chlorophyll levels (and possibly higher growth) are associated with either a decreased need for K in root tissues or a more effective transfer of K from roots to other plant parts [71]. Since the aboveground K was higher than the root K, our findings supported the hypothesis that plants with higher levels of chlorophyll and growth may have evolved more effective systems for moving K from the roots to the leaves and stems, where it is required for photosynthesis and other metabolic functions [72,73]. Further analysis, such as nutrient ratios and other physiological parameters, such as photosynthetic ability, would be needed to understand these correlations’ implications fully.
In holy basil, plant tissue nitrogen (N) accumulation is positively correlated with total anthocyanin content. The highest concentration of nitrogen in the plant tissues and the highest anthocyanin content were specifically found in the T6 growth media, which supplied a high concentration of nitrogen released rapidly from chemical fertilizer. Although several studies have demonstrated that nitrogen deficiency can sometimes trigger anthocyanin synthesis as a stress response [74,75], recent studies have shown that adequate nitrogen levels generally lead to higher anthocyanin production [76,77], which agrees with the current study. Utasee et al. [76] revealed that N application can improve anthocyanin synthesis by promoting its biosynthesis pathway by using phenylalanine as a precursor. However, many environmental factors often affect anthocyanin biosynthesis, including light, temperature, water supply, and soil nitrogen content [78].
Based on the relationship between the primary metabolites, such as carotenoid and chlorophyll, and the secondary metabolites, such as flavonoids and phenolics compounds of holy basil and Genovese basil in this study, it was found that flavonoids and phenolics compounds have a strong positive correlation with both chlorophyll b and total chlorophyll contents (r2 > 0.738). Some studies suggest that these flavonoids and phenolics compounds may prevent damage to chlorophyll or control its production, particularly under stress conditions. Furthermore, these compounds might contribute to the preservation or improvement of photosynthetic capacity, or they might be generated in response to comparable environmental conditions that affect chlorophyll levels [79,80,81].
Studies of a wide range of plant species have shown more secondary metabolites are frequently produced by stressed plants (such as nutrient deficiencies, water scarcity, or pathogen attacks), which these compounds play a major role in the adaptation of plants to the environment and in overcoming stress conditions [82,83]. These agreed with our findings of holy basil and Genovese basil, which observed that plants cultivated in the T1 growing media exhibited the slowest development but accumulated the highest amounts of phytochemical contents, including phenolics and flavonoids. Although the optimal growing media for maximum yield of holy basil and Genovese basil, namely T3 and T4, did not produce the highest phytochemical contents, compared to T1, where plants did not grow but yielded the highest phytochemical contents, which is consistent with the findings of Ren et al. [84]. The phytochemical contents recorded from all growing media treatments in this investigation align with the typical ranges reported in previous studies. For instance, the total phenolics content in this study were observed with values between 7.37 and 18.77 mg GAE g−1 DW for holy basil, and between 7.15 and 20.64 mg GAE g−1 DW for Genovese basil, while the total phenolics content in previous studies for various holy basil (Ocimum tenuiforum L.) and sweet basil (Ocimum basilicum L.) cultivars spanned a range of 6.91–45.96 [85] and 3.47–17.58 mg GAE g−1 DW [86], respectively. Additionally, the previous studies showed the DPPH assay for measuring the antioxidant activity, revealing an average antioxidant activity of around 70.96% in the Thai holy basil cultivars [85] and 68.17% in Genovese basil [87], while the DPPH antioxidant activity recorded from this study were in a range of 70.06–90.73% for holy basil, and 67.97–82.06% for Genovese basil, respectively.
According to our study’s yield and economic performance indicators, Genovese basil produced more than holy basil, as evidenced by the latter’s twice as large leaf area (Table 4). Furthermore, Genovese basil’s greater selling price is another element that contributes to its larger profitability than holy basil. Nevertheless, increasing total sales and revenue does not always result in higher profitability; net profit (in absolute monetary terms) and net profit margin must be introduced to gauge profitability. In the case of holy basil, only T3 growth media, however, produced a positive net profit margin for the second crop. It is likely that T3 growing media, which consists of topsoil, filter cake, long-term composted chicken manure, coconut coir dust, and rice husk ash at a ratio of 3:2:2:1.5:1.5 v/v, was the optimal growing media for holy basil production in a green-house while also having the highest fresh weight yield and producing higher levels of chlorophyll and flavonoids in plant tissues. Although, T4 growing media, which consisted of topsoil, filter cake, short-term composted chicken manure, coconut coir dust, and rice husk ash at a ratio of 3:2:2:1.5:1.5 v/v, continued to yield negative net profit margin even during the second crop period, it is also a good choice for holy basil pro-duction because it is less expensive than T3 growing media, produces higher phytochemical contents, both total phenolics and total flavonoids, and offers higher DPPH radical scavenging and total anthocyanin than T3 growing media. In the case of Genovese basil, T2-T5 growing media produced a highly positive net profit margin since the first crop. Nonetheless, T4 growing media (topsoil, filter cake, short-term composted chicken manure, coconut coir dust, and rice husk ash at a ratio of 3:2:2:1.5:1.5 v/v) was marketed as the best growing media for Genovese basil production in greenhouse conditions due to its highest fresh yield, lowest cost of growing medium and higher obtaining of total chlorophyll, carotenoid, total phenolics, and total flavonoids.
According to our estimate of economic return, which is calculated as the net profit margin, 800 basil plants grown in a 6 × 12 m greenhouse should produce 80–100 g per plant, or 66–79 kg per greenhouse, per month to achieve a positive net profit margin from the first crop that can be deducted from all costs, including direct costs, labor costs, and greenhouse construction costs. Additionally, the fresh yield of basil must be generated at a minimum price per unit of 9.9 USD, which the non-fertilizer and chemical-free products, as in the management of the T2–T5 growing media treatments, should be able to provide a guideline for increasing the value of the products from being sold in the form of safe products. The previous research revealed that organic fresh vegetables generally cost more than conventional ones, with price premiums ranging from 5 to 10% [88,89]. Therefore, our investigation could serve as a reference point and can assist producers with reducing costs in specific areas, and aid in selecting and adjusting growing media to maximize potential profits.
5. Conclusions
This research further demonstrated that nutrient-rich growing media with beneficial physicochemical properties significantly affect the yield and quality of holy basil and Genovese basil, including economic return as described by the net profit margin. Considering the above-mentioned findings, T4 growing media, which was composed of topsoil, filter cake, short-term composted chicken manure, coconut coir dust, and rice husk ash at a ratio of 3:2:2:1.5:1.5 v/v, was the most effective for vegetative growth, yield quality (phytochemical content), and net profit margin of Genovese basil production in the greenhouse. Regarding holy basil, T3 (consists of topsoil, filter cake, long-term composted chicken manure, coconut coir dust, and rice husk ash at a ratio of 3:2:2:1.5:1.5 v/v) and T4 growth media outperform other growing media regarding net profit margin and fresh weight yield. Nonetheless, T4 growing media was the least expensive, which might be more advantageous for future basil production. In addition to sustaining the yield biomass of basil plants even when grown under suitable growing media, Genovese basil production should receive organic fertilizer, such as chicken manure, after the fifth or sixth harvesting period. In the holy basil production, it should be received after the fourth harvest period.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11091040/s1, Figure S1. Growth appearance of holy basil at 30–180 days after transplanting under different growing media. T1 = Commercial soil mixed-medium (Control), T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v, T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v, T6 = Topsoil with recommended fertilizer doses; Figure S2. Growth appearance of Genovese basil at 30–180 days after transplanting under different growing media. T1 = Commercial soil mixed-medium (Control), T2 = Topsoil (T):composted rain tree leaves (CL):filter cake (FC):long-term composted chicken manure (LM):coconut coir dust (CD):rice husk ash (RHA) at a ratio of 3:1:2:2:1:1 v/v, T3 = T:FC:LM:CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T4 = T:FC:short-term composted chicken manure (SM):CD:RHA at a ratio of 3:2:2:1.5:1.5 v/v, T5 = T:FC:LM:SM:CD:RHA at a ratio of 3:2:1:1:1.5:1.5 v/v, T6 = Topsoil with recommended fertilizer doses.
Author Contributions
Conceptualization, O.T. (Ornprapa Thepsilvisut); methodology, O.T. (Ornprapa Thepsilvisut); formal analysis, O.T. (Ornprapa Thepsilvisut); investigation, O.T. (Ornprapa Thepsilvisut), O.T. (Opas Trithaveesak), P.I. and S.B.; resources, O.T. (Ornprapa Thepsilvisut); data curation, O.T. (Ornprapa Thepsilvisut), O.T. (Opas Trithaveesak), P.I. and S.B.; writing—original draft preparation, O.T. (Ornprapa Thepsilvisut); writing—review and editing, O.T. (Ornprapa Thepsilvisut), P.C. (Preuk Chutimanukul), and P.C. (Panita Chutimanukul); supervision, H.E.; funding acquisition, O.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by Thailand Science Research and Innovation (TSRI) Fundamental Fund (TUFF09/2568), fiscal year 2025.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the Department of Agricultural Technology, Faculty of Science and Technology, Thammasat University for providing experimental and laboratory facilities.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hansmann, R.; Baur, I.; Binder, C.R. Increasing organic food consumption: An integrating model of drivers and barriers. J. Clean. Prod. 2020, 275, 123058. [Google Scholar] [CrossRef]
- Javanmardi, J.; Goli, I.; Choobchian, S.; Varnik, R.; Ghazali, S.; Miceikiene, A.; Pour, M.; Mar’echal, K.; Azadi, H. Public preferences and attitudes toward organic vegetables: The case of Iranian consumers. Int. J. Gastron. Food Sci. 2025, 39, 101094. [Google Scholar] [CrossRef]
- Cecílio Filho, A.B.; Nascimento, C.S.; de Jesus Pereira, B.; Nascimento, C.S. Nitrogen fertilisation impacts greenhouse gas emissions, carbon footprint, and agronomic responses of beet intercropped with arugula. J. Environ. Manag. 2022, 307, 114568. [Google Scholar] [CrossRef]
- Chikte, T.; Kopta, T.; Psota, V.; Arizmendi, J.; Chwil, M. A comprehensive review of low- and zero-residue pesticide methods in vegetable production. Agronomy 2024, 14, 2745. [Google Scholar] [CrossRef]
- Khan, M.T.; Aleinikovienė, J.; Butkevičienė, L.M. Innovative organic fertilizers and cover crops: Perspectives for sustainable agriculture in the era of climate change and organic agriculture. Agronomy 2024, 14, 2871. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Purohit, S.; Alam, E.; Islam, M.K. Advancements in soil management: Optimizing crop production through interdisciplinary approaches. J. Agric. Food Res. 2024, 18, 101528. [Google Scholar] [CrossRef]
- Varela Milla, O.; Rivera, E.B.; Huang, W.J.; Chien, C.C.; Wang, Y.M. Agronomic properties and characterization of rice husk and wood biochars and their effect on the growth of water spinach in a field test. J. Soil Sci. Plant Nutr. 2013, 13, 251–266. [Google Scholar] [CrossRef]
- Kumari, K.; Prasad, J.; Solanki, S.; Chaudhary, R. Long-term effect of crop residues incorporation on yield and soil physical properties under rice—Wheat cropping system in calcareous soil. J. Soil Sci. Plant Nutr. 2018, 18, 27–40. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, A. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Zeeshan Manzoor, M.; Sarwar, G.; Ibrahim, M.; Rehan, S.S.; Hasnain, Z.; Rais, A.; Gul, S.; Alfagham, A.T.; Manono, B.O.; Mehmood, K.; et al. Remediation quantum of organic amendments to immobilize potentially toxic heavy metals in wastewater-contaminated soils through maize cultivation. Front. Environ. Sci. 2024, 12, 1420705. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Kalita, M.; Devi, N. A taxonomic review of the genus Ocimum L. (Ocimeae, Lamiaceae). Plant Sci. Today 2023, 10, 126–137. [Google Scholar] [CrossRef]
- Lekhapan, P.; Anamthawat-Jónsson, K.; Chokchaichamnankit, P. Comparative karyotype analysis and chromosome evolution in the genus Ocimum L. from Thailand. Trop. Nat. Hist. 2021, 21, 27–40. [Google Scholar] [CrossRef]
- Kandil, M.A.M.; Khatab, M.E.; Ahmed, S.S.; Schnug, E. Herbal and essential oil yield of Genovese basil (Ocimum basilicum L.) grown with mineral and organic fertilizer sources in Egypt. J. Kult. 2009, 61, 443–449. [Google Scholar] [CrossRef]
- Betuzzi, F.; Campioli, D.; Malaspina, P.; Rapallo, F.; Bottino, G.; Scrigna, G.; Minuto, G.; Cornara, L. Morphological and phytochemical characterization of old Ligurian basil accessions: Recovery of old biodiversity for future exploitation. Plants 2025, 14, 553. [Google Scholar] [CrossRef]
- Ngamakeue, N.; Chitprasert, P. Encapsulation of holy basil essential oil in gelatin: Effects of palmitic acid in carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food Bioprocess Technol. 2016, 9, 1735–1745. [Google Scholar] [CrossRef]
- Chinedu, E.; Ofili, C.C. Ocimum species: Ethnomedicinal uses, phytochemistry and pharmacological importance. Int. J. Curr. Res. Physiol. Pharmacol. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Spence, C. Sweet basil: An increasingly popular culinary herb. Int. J. Gastron. Food Sci. 2024, 36, 100927. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Corrado, G.; Pannico, A.; De Pascale, S.; Rouphael, Y. Morpho-physiological responses and secondary metabolites modulation by preharvest factors of three hydroponically grown Genovese basil cultivars. Front. Plant Sci. 2021, 12, 671026. [Google Scholar] [CrossRef]
- Rusu, T.; Cowden, R.J.; Moraru, P.I.; Maxim, M.A.; Ghaley, B.B. Overview of multiple applications of basil species and cultivars and the effects of production environmental parameters on yields and secondary metabolites in hydroponic systems. Sustainability 2021, 13, 11332. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Jindamol, H.; Thongtip, A.; Korinsak, S.; Romyanon, K.; Toojinda, T.; Darwell, C.T.; Wanichananan, P.; Panya, A.; Kaewsri, W.; et al. Physiological responses and variation in secondary metabolite content among Thai holy basil cultivars (Ocimum tenuiflorum L.) grown under controlled environmental conditions in a plant factory. Front. Plant Sci. 2022, 13, 1008917. [Google Scholar] [CrossRef] [PubMed]
- Azizah, N.S.; Irawan, B.; Kusmoro, J.; Safriansyah, W.; Farabi, K.; Oktavia, D.; Doni, F.; Miranti, M. Sweet basil (Ocimum basilicum L.)—A review of its botany, phytochemistry, pharmacological activities, and biotechnological development. Plants 2023, 12, 4148. [Google Scholar] [CrossRef] [PubMed]
- Avasiloaiei, D.I.; Calara, M.; Brezeanu, P.M.; Murariu, O.C.; Brezeanu, C. On the future perspectives of some medicinal plants within Lamiaceae botanic family regarding their comprehensive properties and resistance against biotic and abiotic stresses. Genes 2023, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Namvong, U.; Chongrattanameteekul, W. Pesticide residues on sweet basil, Ocimum basilicum L. (Labiatae) under different production systems from Central Thailand. Kasetsart J. 2013, 47, 695–703. Available online: https://li01.tci-thaijo.org/index.php/anres/article/view/243119 (accessed on 21 January 2025).
- Prokchon U-sap. Statistics on Pesticide Residues in Vegetables and Fruits 2024. Thai-PAN. Available online: https://thaipan.org/conference/2025 (accessed on 3 July 2025).
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Litterick, A.M.; Holmes, S.; Frederickson-Matika, D.E.; Green, S. How safe are peat-free growing media? An exploration of plant pathogen risks to the horticultural industry and recommendations for risk mitigation. Plant People Planet 2025, 1–16, early view. [Google Scholar] [CrossRef]
- Asadathorn, P.; Setthapun, W.; Rakwichian, W.; Kusolsatit, T. Development of growing media from sugar industrial waste. J. Appl. Res. Sci. Technol. 2016, 15, 14–21. [Google Scholar]
- Ritthidechrat, K.; Anuwong, C. Effects of different potting media on the growth of commercial cacti. ASEAN J. Sci. Technol. Rep. 2022, 25, 59–67. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Muchjajib, U.; Muchjajib, S.; Suknikom, S.; Butsai, J. Evaluation of organic media alternatives for the production of microgreens in Thailand. Acta Hortic. 2015, 1102, 157–162. [Google Scholar] [CrossRef]
- Badagliacca, G.; Testa, G.; La Malfa, S.G.; Cafaro, V.; Lo Presti, E.; Monti, M. Organic fertilizers and bio-waste for sustainable soil management to support crops and control greenhouse gas emissions in mediterranean agroecosystems: A review. Horticulturae 2024, 10, 427. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Salas, R.A.; Godoy, R.M.R.; Salas, F.M.; Harper, N.M.S.; Asio, V.B. Yield and postharvest qualities of two genotypes of eggplant (Solanum melongena L.) applied with different levels of chicken dung. Environ. Asia 2020, 13, 81–86. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock manure and the impacts on soil health: A review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Salamandane, A.; Muetanene, B.A.; Ismael, F.; Vintuar, P. Application of chicken manure and organic compost to produce onion (Allium cepa L.) and turnip (Brassica rapa L.) in greenhouse. Eur. J. Agric. Food Sci. 2022, 4, 557. [Google Scholar] [CrossRef]
- Manogaran, M.D.; Shamsuddin, R.; Yusoff, M.H.M.; Lay, M.; Siyal, A.A. A review on treatment processes of chicken manure. Clean. Circ. Bioecon. 2022, 2, 100013. [Google Scholar] [CrossRef]
- Paudel, K.P.; Sukprakarn, S.; Sidathani, K.; Osotsapar, Y. Effects of organic manures on production of lettuce (Lactuca sativa L.) in reference to chemical fertilizer. Kasetsart J. 2004, 38, 31–37. [Google Scholar]
- Khalid, K.A.; Shafei, A.M. Productivity of dill (Anethum graveolens L.) as influenced by different organic manure rates and sources. Arab Univ. J. Agric. Sci. 2005, 13, 901–913. [Google Scholar] [CrossRef]
- Ball, M.E.E.; Wright, L.P.; Wilson, K.; Richmond, H.; Cummings, R.; Smyth, S.; Davison, M.; Forbes, K.; Thompson, J.; Bryson, P. The nutrient content of litter and manure from different poultry systems—Updating and establishing the nutrient profile. Sustainability 2024, 16, 6633. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.; Xi, L.; Dou, Q.; Shi, Z.; Liu, T.; Wang, L. Drying characteristics of chicken manure under a variable temperature process. Appl. Sci. 2025, 15, 4093. [Google Scholar] [CrossRef]
- Kumsong, N.; Thepsilvisut, O.; Imorachorn, P.; Chutimanukul, P.; Pimpha, N.; Toojinda, T.; Trithaveesak, O.; Ratanaudomphisut, E.; Poyai, A.; Hruanun, C.; et al. Comparison of different temperature control systems in tropical-adapted greenhouses for green romaine lettuce production. Horticulturae 2023, 9, 1255. [Google Scholar] [CrossRef]
- Kudreaung, P.; Chromkaew, Y.; Chinachanta, K.; Chaiwan, F.; Shutsrirung, A. Microbial decomposition of longan leaf: I. physico-chemical and biological changes during composting. Asia Pac. J. Sci. Technol. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- Charoenchang, N.; Pinphanichakarn, P.; Pattaragulwanit, K.; Thaniyavarn, S.; Juntongjin, K. Utilization of agricultural materials to enhance microbial degradation of polycyclic aromatic hydrocarbons in soil. J. Sci. Res. Chula. Univ. 2003, 28, 1–13. [Google Scholar]
- Ahmad, M.R.; Uddin, M.A.; Juwel, M.A.I.; Sultana, T.; Hashem, M.A.; Moslehuddin, A.Z.M. Integrated use of raintree leaves with urea on BRRI dhan 41 rice. Int. J. Nat. Soc. Sci. 2015, 2, 66–75. [Google Scholar]
- Rahman, M.M.; Adzkia, U.; Nandika, D.; Siregar, I.Z.; Karlinasari, L. Urban tree bark analysis for monitoring of air pollution level in Jakarta business district. IOP Conf. Ser. Earth Environ. Sci. 2022, 1109, 012052. [Google Scholar] [CrossRef]
- Wakano, D.; Sahertian, D.E. Heavy metal lead (Pb) accumulation in Trembesi leaves (Samanea saman (Jacq.) Merr.) on Dr. J. Leimena and Jenderal Sudirman street, Ambon (Case study: Pandemic period). AIP Conf. Proc. 2023, 2588, 030015. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining organic carbon in soils: Effect of variationsindigestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251c263. [Google Scholar] [CrossRef]
- Di Gioia, F.; Bellis, P.D.; Mininni, C.; Santamaria, P.; Serio, F. Physicochemical, agronomical and microbiological evaluation ofalternative growing media for the production of rapini (Brassica rapa L.) microgreens. J. Sci. Food Agric. 2017, 97, 1212–1219. [Google Scholar] [CrossRef]
- Qin, D.; He, Q.; Mousavi, S.M.N.; Abbey, L. Evaluation of aging methods on the surface characteristics of hydrochar and germination indices for kale seeds. Horticulturae 2023, 9, 545. [Google Scholar] [CrossRef]
- Bazaz, A.M.; Karimian, Z.; Bannayan, M. Modeling individual leaf area of basil (Ocimum basilicum) using different methods. Int. J. Plant Prod. 2011, 5, 439–447. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Yu, X.L.; Hu, Q.L.; Huang, Y. Study on extracting methods and characteristics of chlorophyll in peony. J. Luoyang Norm. Univ. 2005, 5, 113–115. [Google Scholar] [CrossRef]
- Wei, X.; Khachatryan, H.; Rihn, A. Production costs and profitability for selected greenhouse grown annual and perennial crops: Partial enterprise budgeting and sensitivity analysis. HortScience 2020, 55, 637–646. [Google Scholar] [CrossRef]
- Fisher, P.; Hodges, A.; Swanekamp, B.; Hall, C.; The New Economics of Greenhouse Production. Floriculture Research Alliance. Available online: http://ellisonchair.tamu.edu/files/2013/09/Combined-costing-series.pdf (accessed on 8 January 2024).
- Motulsky, H.J. GraphPad Statistics Guide. 2024. Available online: http://www.graphpad.com/guides/prism/10/statistics/index.htm (accessed on 10 February 2025).
- Pawlak, K.; Kołodziejczak, M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Saleem, A.; Anwar, S.; Nawaz, T.; Fahad, S.; Saud, S.; Rahman, T.U.; Khan, M.N.R.; Nawaz, T. Securing a sustainable future: The climate change threat to agriculture, food security, and sustainable development goals. J. Umm Al-Qura Univ. Appl. Sci. 2024, 11, 595–611. [Google Scholar] [CrossRef]
- Norsuwan, T.; Panyasai, T.; Utasuk, K.; Saltikulnukarn, T.; Thippachote, K. Effect of climatic conditions and pest constraints on seasonal yield gaps in pesticide-free vegetable production under integrated pest management in Chiang Mai province, Thailand. Agric. Nat. Resour. 2021, 55, 139–146. [Google Scholar]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef]
- He, H.; Yu, L.; Yang, X.; Luo, L.; Liu, J.; Chen, J.; Kou, Y.; Zhao, W.; Liu, Q. Effects of different soils on the biomass and photosynthesis of Rumex nepalensis in subalpine region of southwestern China. Forests 2022, 13, 73. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using nondestructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Talebzadeh, F.; Valeo, C. Evaluating the effects of environmental stress on leaf chlorophyll content as an index for tree health. IOP Conf. Ser. Earth Environ. Sci. 2021, 1006, 012007. [Google Scholar] [CrossRef]
- Fathi, H. Role of nitrogen (N) in plant growth, photosynthesis pigments, and N use efficiency: A review. Agrisost 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The utilization and roles of nitrogen in plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Litvin, A.G.; Currey, C.J.; Wilson, L.A. Effects of supplemental light source on basil, dill, and parsley growth, morphology, aroma, and flavor. J. Am. Soc. Hortic. Sci. 2020, 145, 18–29. [Google Scholar] [CrossRef]
- Crișan, I.; Bunea, A.; Vârban, D.; Cordea, M.I.; Horga, V.; Vînătoru, C.; Stoie, A.; Vârban, R. Variation in the photosynthetic leaf pigments of different basil (Ocimum spp.) genotypes under varying conditions at the flowering stage. Horticulturae 2024, 10, 740. [Google Scholar] [CrossRef]
- Lima, J.C.; do Nascimento, M.N.; de Oliveira, U.C.; dos Santos, A.R.; Brito, G.S.; da Silva Santos, J. Nutritional diagnosis of nitrogen and phosphorus in Ocimum basilicum L. plants grown under macronutrient applications. Comun. Sci. 2023, 14, e3867. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Ladikou, E.V.; Oikonomou, A.; Chatzistathis, T.; Chatziperou, G. Exploring the impact of potassium on growth, photosynthetic performance, and nutritional status of lemon trees (cv. Adamopoulou) grafted onto sour orange and Volkamer lemon rootstocks. Sustainability 2023, 15, 15858. [Google Scholar] [CrossRef]
- Becker, C.; Urlić, B.; Jukić Špika, M.; Kläring, H.P.; Krumbein, A.; Baldermann, S.; Goreta Ban, S.; Perica, S.; Schwarz, D. Nitrogen limited red and green leaf lettuce accumulate flavonoid glycosides, caffeic acid derivatives, and sucrose while losing chlorophylls, B-carotene and xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Cui, J. Increased sucrose in the hypocotyls of radish sprouts contributes to nitrogen deficiency-induced anthocyanin accumulation. Front. Plant Sci. 2016, 7, 1976. [Google Scholar] [CrossRef]
- Utasee, S.; Jamjod, S.; Lordkaew, S.; Prom-U-Thai, C. Improve anthocyanin and zinc concentration in purple rice by nitrogen and zinc fertilizer application. Rice Sci. 2022, 29, 435–450. [Google Scholar] [CrossRef]
- Feng, W.; Xue, W.; Zhao, Z.; Wang, H.; Shi, Z.; Wang, W.; Chen, B.; Qiu, P.; Xue, J.; Sun, M. Nitrogen level impacts the dynamic changes in nitrogen metabolism, and carbohydrate and anthocyanin biosynthesis improves the kernel nutritional quality of purple waxy maize. Plants 2024, 13, 2882. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand, E.; Basteau, C.; Hilbert, C.; van Leeuwen, C.; Delrot, S.; Gomès, E. Nitrogen supply affects anthocyanin biosynthetic and regulatory genes in grapevine cv. Cabernet-Sauvignon berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. The relationship between phenolics and flavonoids production with total non-structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2011, 16, 162–174. [Google Scholar] [CrossRef]
- Jakovljevic, D.; Momcilovic, J.; Bojovic, B.; Stankovic, M. The short-term metabolic modulation of basil (Ocimum basilicum L. cv. ‘Genovese’) after exposure to cold or heat. Plants 2021, 10, 590. [Google Scholar] [CrossRef]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Balancing yield and antioxidant capacity in basil microgreens: An exploration of nutrient solution concentrations in a floating system. Agriculture 2023, 13, 1691. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Gaude, A.A.; Jalmi, S.K. Environmental stress induced biosynthesis of plant secondary metabolites transcriptional regulation as a key. Crop Des. 2025, 4, 100100. [Google Scholar] [CrossRef]
- Ren, X.; Lu, N.; Xu, W.; Zhuang, Y.; Takagaki, M. Optimization of the yield, total phenolic content, and antioxidant capacity of basil by controlling the electrical conductivity of the nutrient solution. Horticulturae 2022, 8, 216. [Google Scholar] [CrossRef]
- Saelao, T.; Chutimanukul, P.; Suratanee, A.; Plaimas, K. Analysis of antioxidant capacity variation among thai holy basil cultivars (Ocimum tenuiflorum L.) using density-based clustering algorithm. Horticulturae 2023, 9, 1094. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Nin, S.; Bini, L.; Antonetti, M.; Manzi, D.; Bonetti, D. Growing ‘Genovese’ and ‘Valentino’ basil in pots using peat substrate combined with phytoremediated sediment: Effects on yield and nutraceutical content. Sustainability 2023, 15, 7314. [Google Scholar] [CrossRef]
- Alberto de Morais Watanabe, E.; Alfinito, S.; Castelo Branco, T.V.; Felix Raposo, C.; Athayde Barros, M. The consumption of fresh organic food: Premium pricing and the predictors of willingness to pay. J. Food Prod. Mark. 2023, 29, 41–55. [Google Scholar] [CrossRef]
- Smoluk-Sikorska, J. Differences between prices of organic and conventional food in Poland. Agriculture 2024, 14, 2308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).