Transcriptome Analysis Reveals Key Genes Involved in Fruit Length Trait Formation in Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
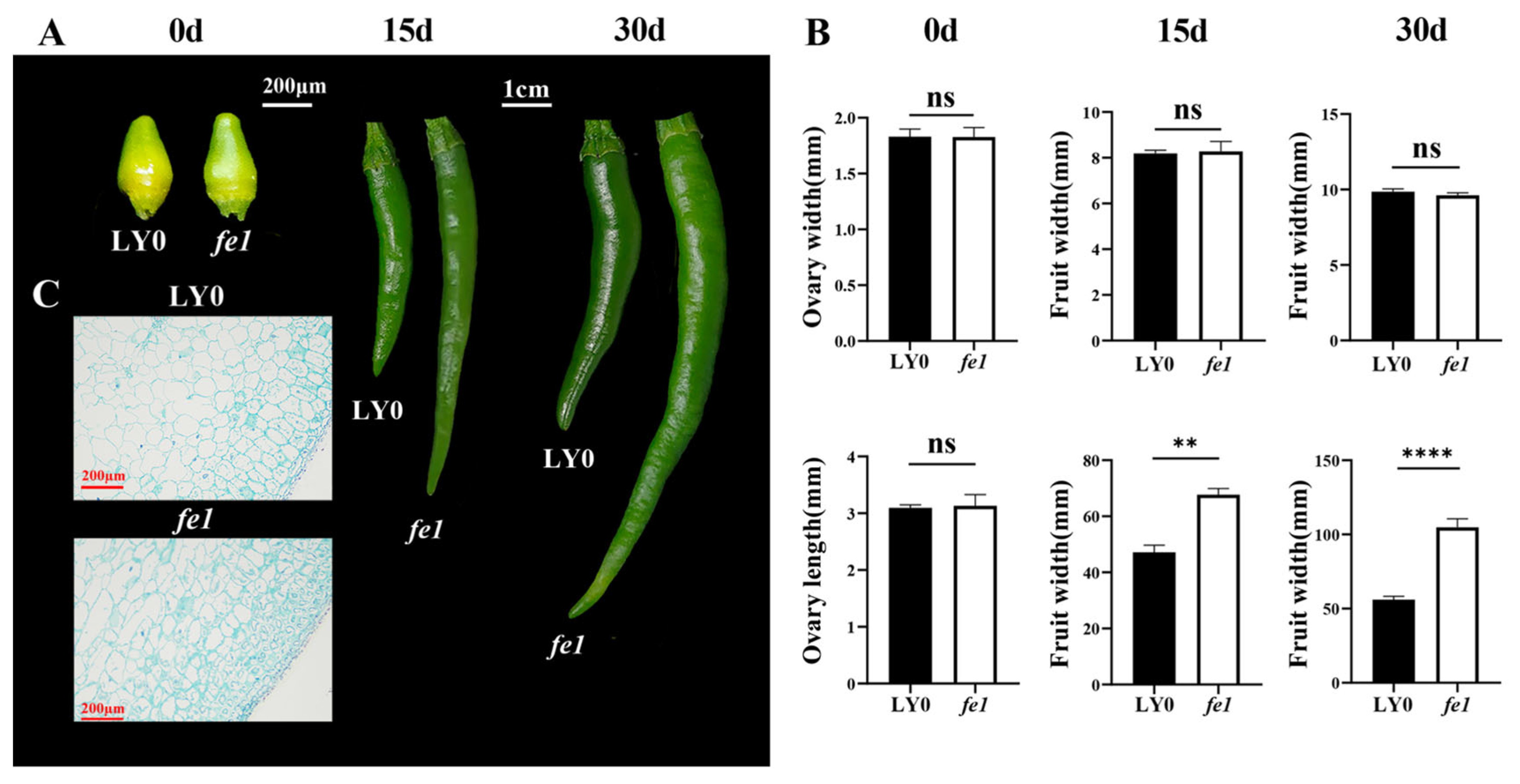
2.2. Morphological Trait Measurement
2.3. Cytological Observation of Fruits
2.4. RNA Extraction, Library Preparation, and Sequencing
2.5. RNA-Seq and Differentially Expressed Gene (DEG) Analysis
2.6. GO and KEGG Pathway Enrichment Pathway Analysis
2.7. Co-Expression Network Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Data Analysis
3. Results
3.1. Phenotypic Observation
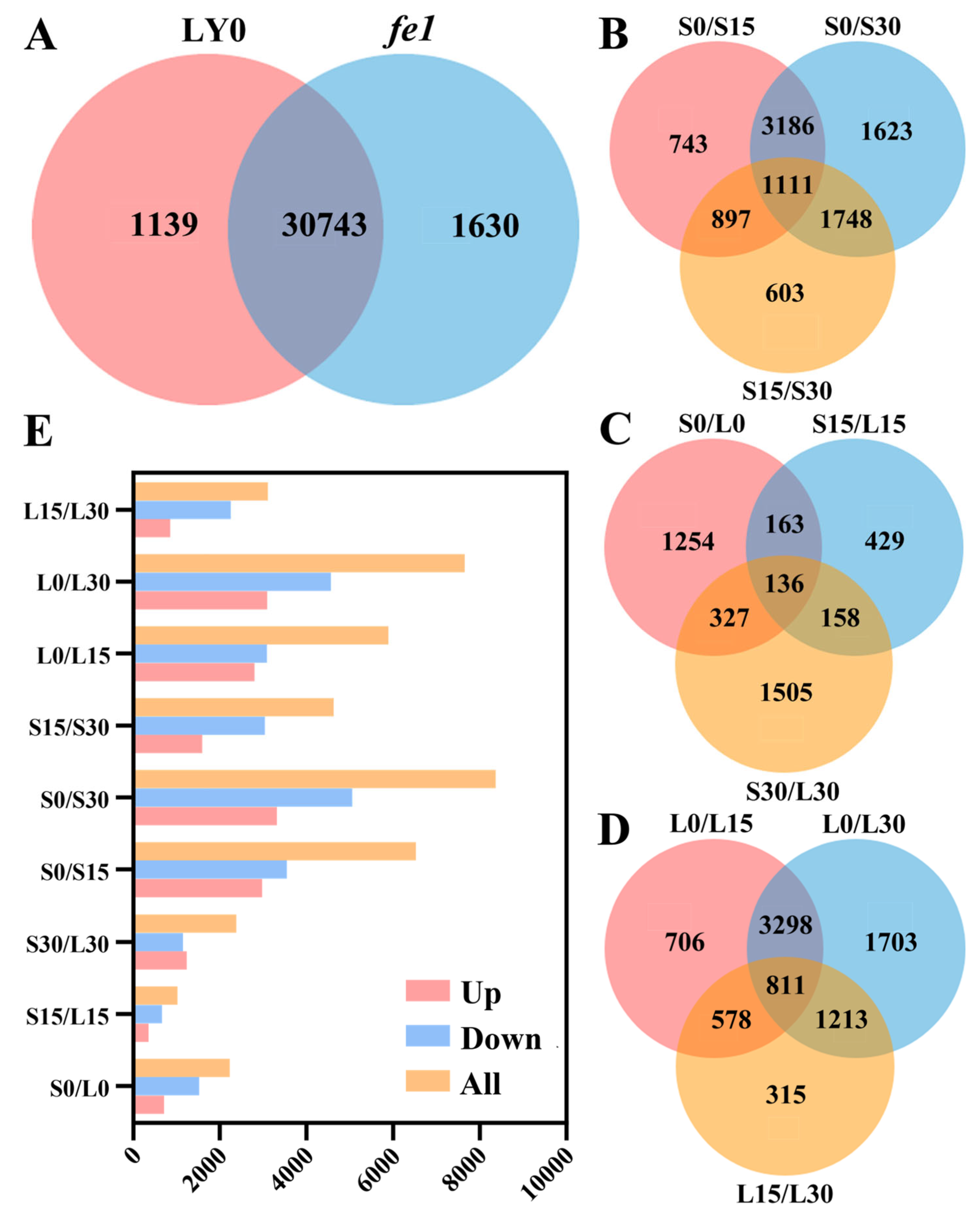
3.2. RNA-Seq and Annotation of DEGs
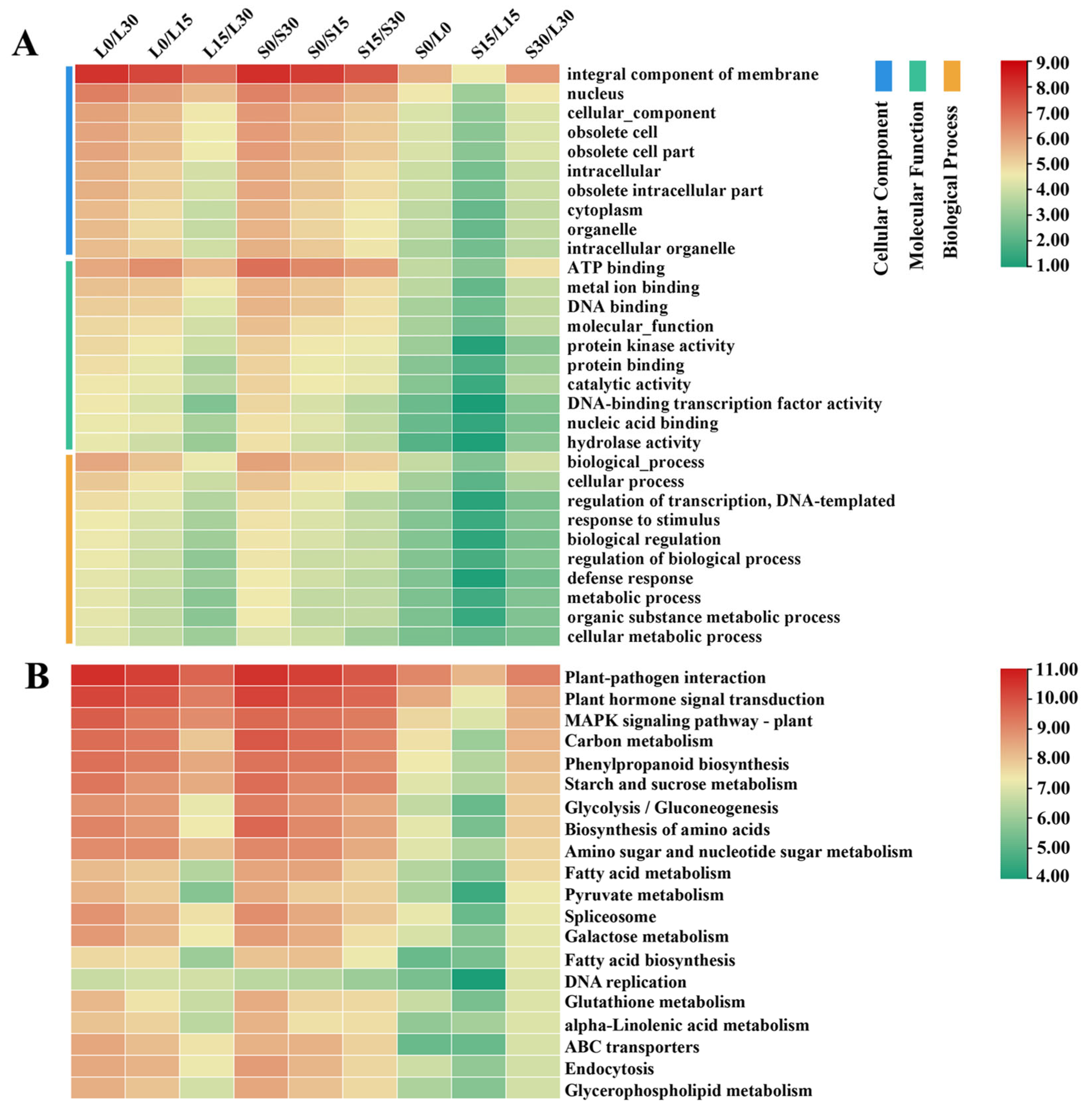
3.3. GO Enrichment Analysis
3.4. KEGG Pathway Analysis
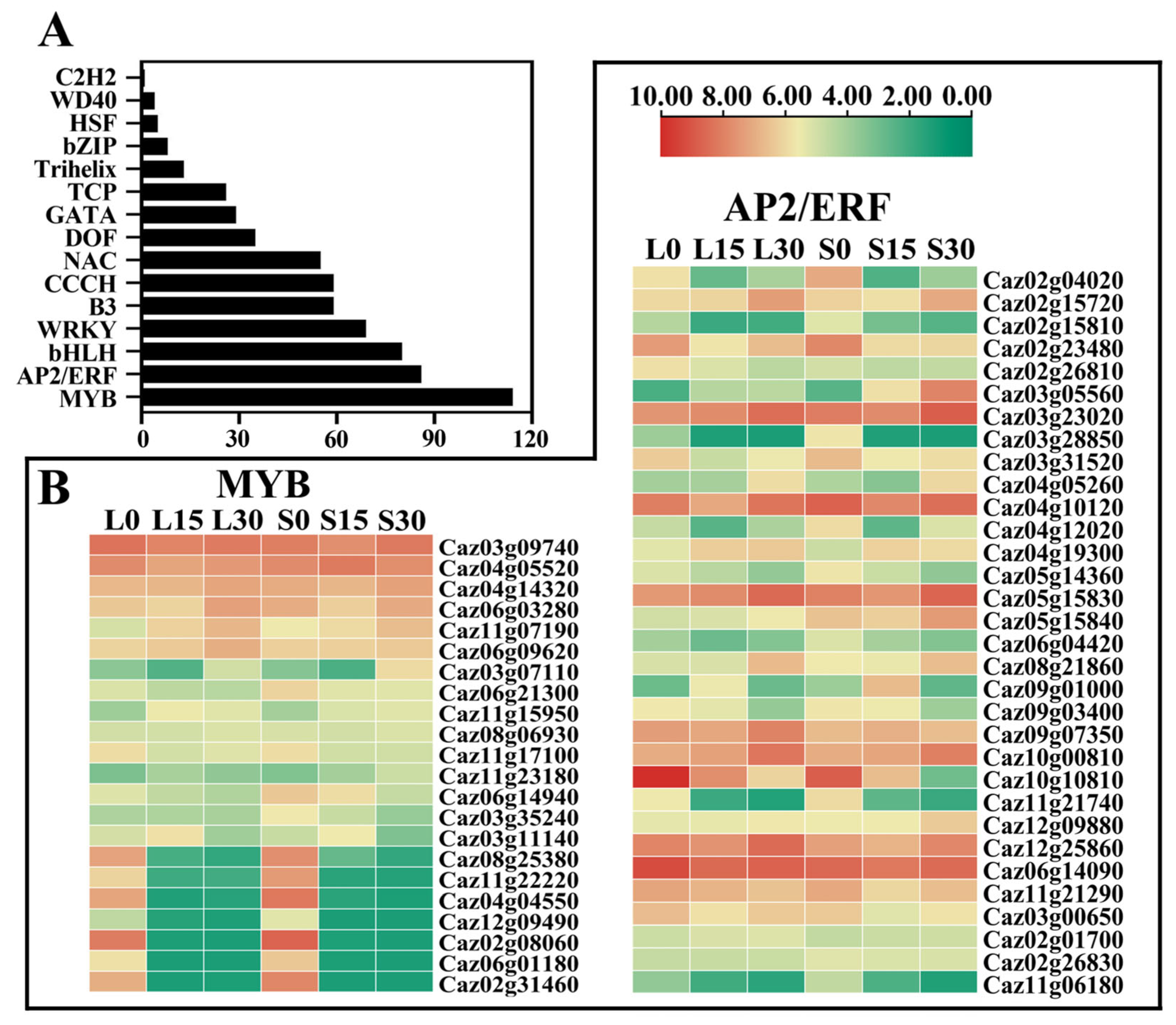
3.5. Analysis of Transcription Factor (TF)-Related DEGs
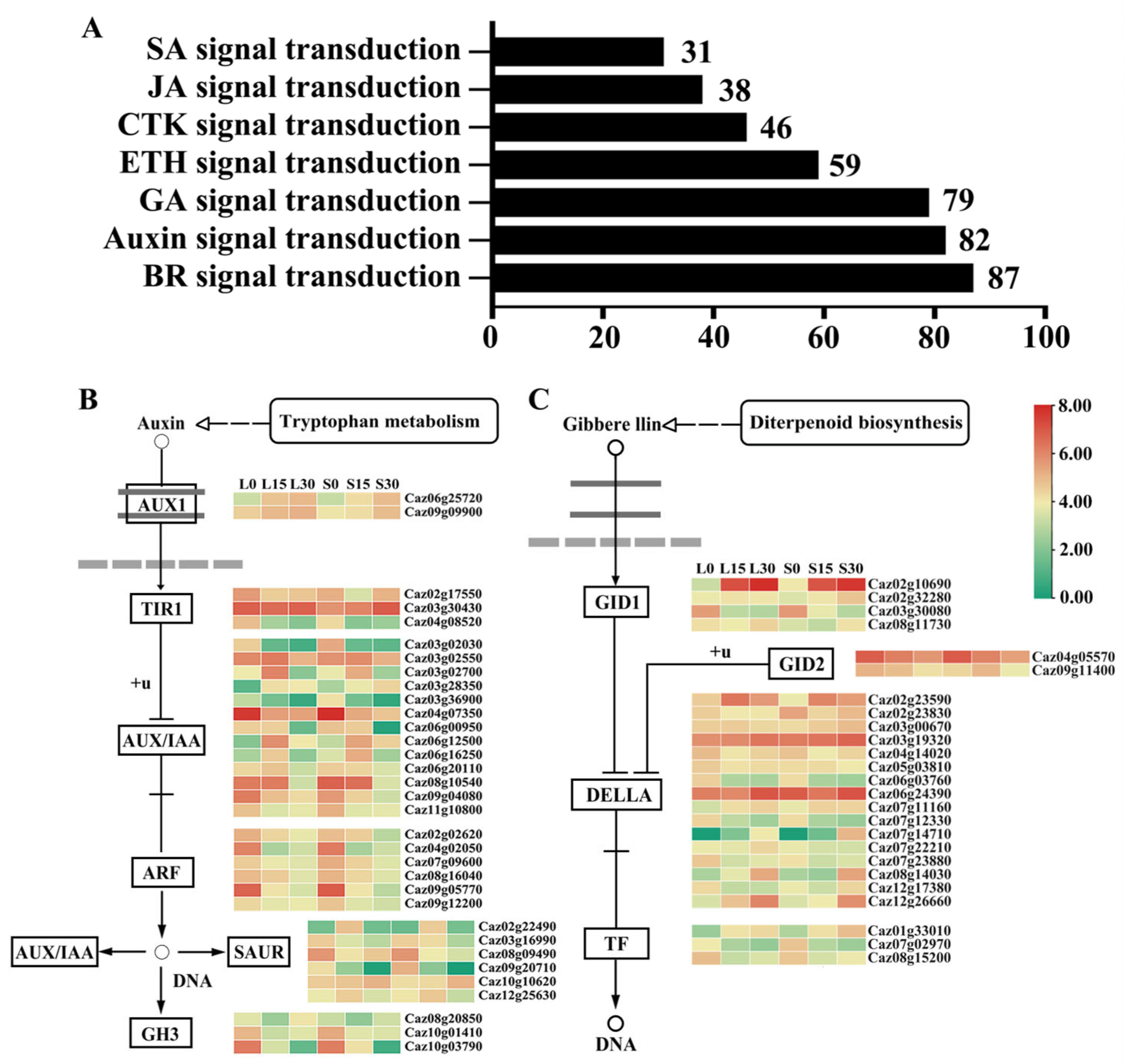
3.6. Analysis of DEGs Related to Auxin and Gibberellin (GA) Signal Transduction Pathways
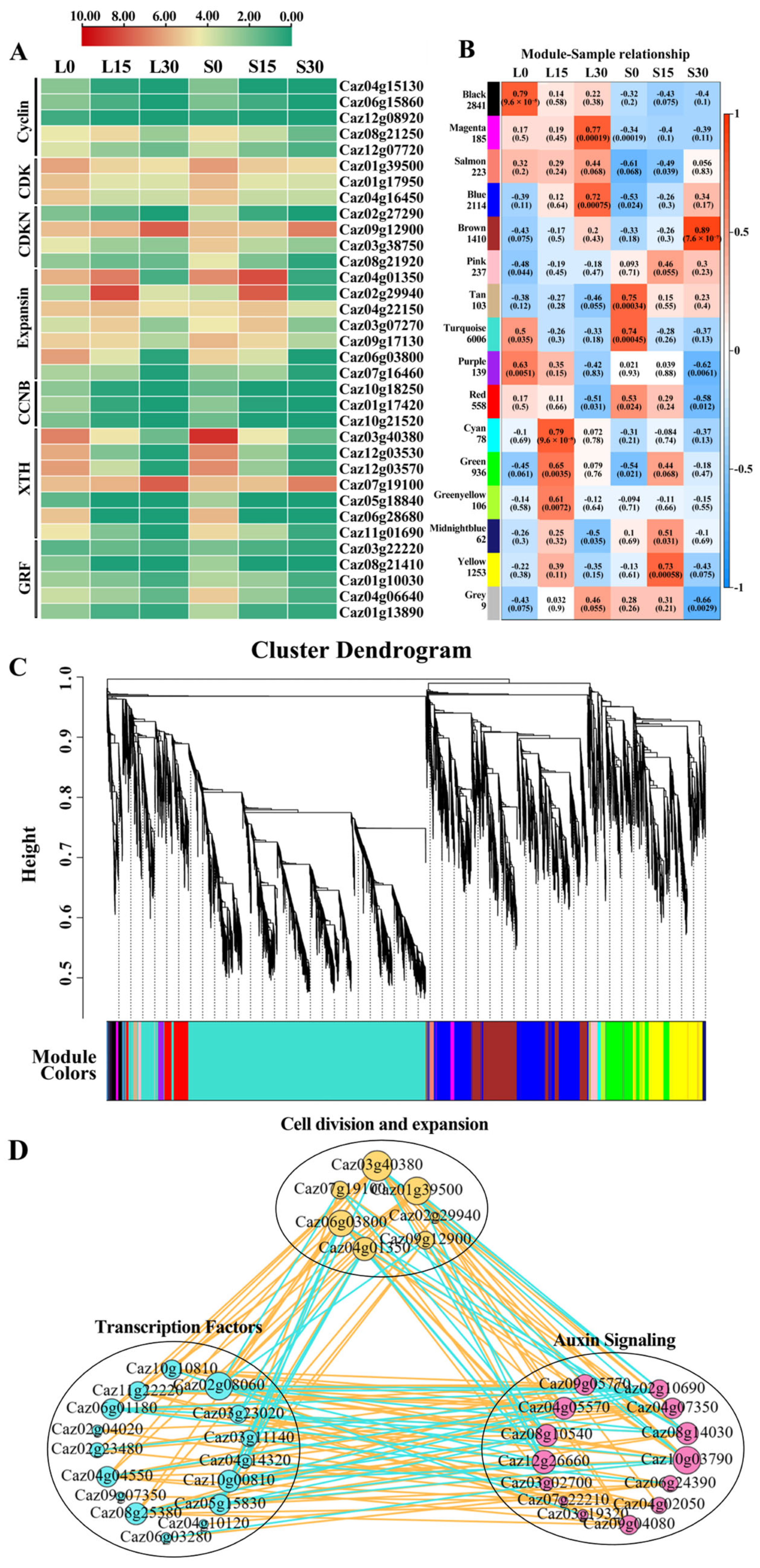
3.7. Expression Analysis of DEGs Related to Cell Division and Expansion
3.8. Co-Expression Network Analysis of DEGs Related to Pepper Fruit Length
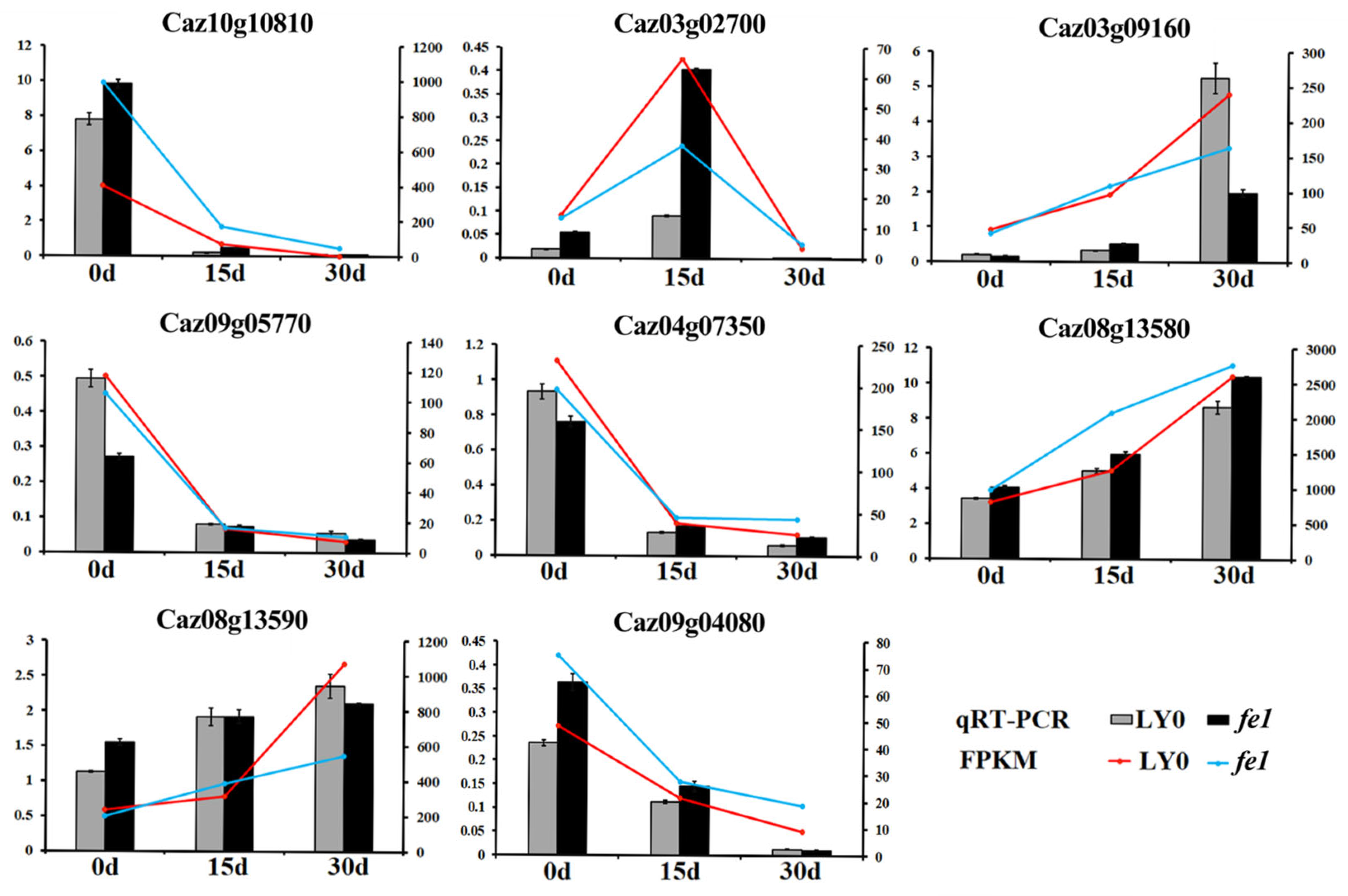
3.9. Validation of RNA-Seq Data Using qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMS | Ethyl methylsulfone |
| DAF | DNA amplification fingerprinting |
| RNA-Seq | RNA sequencing |
| WGCNA | Weighted gene co-expression network analysis |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Haralayya, B.; Asha, I.S. Molecular Marker Application in Capsicum spp: A Supplement to Conventional Plant Breeding. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3840–3855. [Google Scholar] [CrossRef]
- Zou, Z.; Zou, X. Geographical and Ecological Differences in Pepper Cultivation and Consumption in China. Front. Nutr. 2021, 8, 718517. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.U.; Ben Chaim, A.; Borovsky, Y.; Paran, I. Mapping of yield-related QTLs in pepper in an interspecific cross of Capsicum annuum and C. frutescens. Theor. Appl. Genet. 2003, 106, 1457–1466. [Google Scholar] [CrossRef]
- Yu, T.; Ai, G.; Xie, Q.; Wang, W.; Song, J.; Wang, J.; Tao, J.; Zhang, X.; Hong, Z.; Lu, Y.; et al. Regulation of tomato fruit elongation by transcription factor BZR1.7 through promotion of SUN gene expression. Hortic. Res. 2022, 9, uhac121. [Google Scholar] [CrossRef]
- Ali, Q. Morphological and agronomic characterization of tomato under field conditions. Pure Appl. Biol. 2017, 6, 1021–1029. [Google Scholar] [CrossRef]
- Tswanya, M.; Kyuka, C. Correlation Analysis on Growth and Yield Parameters of Tomato Varieties as Influenced by Pinching, Mineral Fertilizers and Mulching in Three Seasons. J. Biol. Agric. Healthc. 2023, 13, 6. [Google Scholar]
- Che, G.; Pan, Y.; Liu, X.; Li, M.; Zhao, J.; Yan, S.; He, Y.; Wang, Z.; Cheng, Z.; Song, W.; et al. Natural variation in CRABS CLAW contributes to fruit length divergence in cucumber. Plant Cell 2023, 35, 738–755. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; et al. A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nat. Commun. 2018, 9, 4734. [Google Scholar] [CrossRef]
- Xin, T.; Zhang, Z.; Li, S.; Zhang, S.; Li, Q.; Zhang, Z.-H.; Huang, S.; Yang, X. Genetic Regulation of Ethylene Dosage for Cucumber Fruit Elongation. Plant Cell 2019, 31, 1063–1076. [Google Scholar] [CrossRef]
- Chaim, A.B.; Paran, I.; Grube, R.C.; Jahn, M.; van Wijk, R.; Peleman, J. QTL mapping of fruit-related traits in pepper (Capsicum annuum). Theor. Appl. Genet. 2001, 102, 1016–1028. [Google Scholar] [CrossRef]
- Han, K.; Jeong, H.-J.; Yang, H.-B.; Kang, S.-M.; Kwon, J.-K.; Kim, S.; Choi, D.; Kang, B.-C. An ultra-high-density bin map facilitates high-throughput QTL mapping of horticultural traits in pepper (Capsicum annuum). DNA Res. 2016, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Ro, N.-Y.; Patil, A.; Lee, J.-H.; Kwon, J.-K.; Kang, B.-C. Uncovering Candidate Genes Controlling Major Fruit-Related Traits in Pepper via Genotype-by-Sequencing Based QTL Mapping and Genome-Wide Association Study. Front. Plant Sci. 2020, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Arjun, K.; Dhaliwal, M.S.; Jindal, S.K.; Fakrudin, B. Mapping of fruit length related QTLs in interspecific cross (Capsicum annuum L. × Capsicum galapagoense Hunz.) of chilli. Breed. Sci. 2018, 68, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qiao, Y.-M.; Li, Y.; Yu, Y.-N.; Gong, Z.-H. Identification of Fruit Traits Related QTLs and a Candidate Gene, CaBRX, Controlling Locule Number in Pepper (Capsicum annuum L.). Horticulturae 2022, 8, 146. [Google Scholar] [CrossRef]
- Borovsky, Y.; Paran, I. Characterization of fs10.1, a major QTL controlling fruit elongation in Capsicum. Theor. Appl. Genet. 2011, 123, 657–665. [Google Scholar] [CrossRef]
- Mao, L.; Shen, Y.; Cui, Q.; Huang, Y.; Zhang, X.; Lv, J.; Xing, W.; Zhang, D.; Fang, N.; Chen, D.; et al. The IQ67-domain protein IQD1 regulates fruit shape through complex multiprotein interactions in pepper (Capsicum annuum L.). Plant Biotechnol. J. 2025, 23, 2651–2666. [Google Scholar] [CrossRef]
- Quan, G.; Xia, P.; Lian, S.; Wu, Y.; Zhu, G. Zinc uptake system ZnuACB is essential for maintaining pathogenic phenotype of F4ac+ enterotoxigenic E. coli (ETEC) under a zinc restricted environment. Vet. Res. 2020, 51, 127. [Google Scholar] [CrossRef]
- Shen, Y.; Mao, L.; Zhou, Y.; Sun, Y.; Lv, J.; Deng, M.; Liu, Z.; Yang, B. Transcriptome Analysis Reveals Key Genes Involved in Trichome Formation in Pepper (Capsicum annuum L.). Plants 2024, 13, 1090. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, Y.; Zhang, B.; He, M.; Wang, Z.; Lu, C.; Kang, Y. Analysis of Methylome, Transcriptome, and Lipid Metabolites to Understand the Molecular Abnormalities in Polycystic Ovary Syndrome. Diabetes Metab. Syndr. Obes. 2023, 16, 2745–2763. [Google Scholar] [CrossRef]
- Amit, K.; Ahad, I.; Kumar, V.; Thakur, S. Genetic variability and correlation studies for growth and yield characters in chilli (Capsicum annuum L.). J. Spices Aromat. Crops 2014, 23, 170–177. [Google Scholar]
- Patel, D.; Patel, B.; Patel, J.R.; Kuchhadiya, G.V. Genetic variability and character association studies for green fruit yield and quality component traits in chilli (capsicum annuum var. longum (dc.) sendt.). Electron. J. Plant Breed. 2015, 6, 472–478. [Google Scholar]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription. Plant Cell 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Abel, S.; Theologis, A. Early Genes and Auxin Action. Plant Physiol. 1996, 111, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, F.; Friml, J.; Ding, Z. Auxin signaling: Research advances over the past 30 years. J. Integr. Plant Biol. 2022, 64, 371–392. [Google Scholar] [CrossRef]
- Piya, S.; Shrestha, S.K.; Binder, B.; Stewart, C.N.; Hewezi, T. Protein-protein interaction and gene co-expression maps of ARFs and Aux/IAAs in Arabidopsis. Front. Plant Sci. 2014, 5, 744. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latché, A.; Pech, J.-C.; Bouzayen, M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef]
- Chaabouni, S.; Jones, B.; Delalande, C.; Wang, H.; Li, Z.; Mila, I.; Frasse, P.; Latché, A.; Pech, J.-C.; Bouzayen, M. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J. Exp. Bot. 2009, 60, 1349–1362. [Google Scholar] [CrossRef]
- Deng, W.; Yan, F.; Liu, M.; Wang, X.; Li, Z. Down-regulation of SlIAA15 in tomato altered stem xylem development and production of volatile compounds in leaf exudates. Plant Signal Behav. 2012, 7, 911–913. [Google Scholar] [CrossRef]
- Overvoorde, P.J.; Okushima, Y.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Liu, A.; Onodera, C.; Quach, H.; et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 2005, 17, 3282–3300. [Google Scholar] [CrossRef]
- Razo-Mendivil, F.G.; Hernandez-Godínez, F.; Hayano-Kanashiro, C.; Martínez, O. Transcriptomic analysis of a wild and a cultivated varieties of Capsicum annuum over fruit development and ripening. PLoS ONE 2021, 16, e0256319. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Shi, J.; Scheben, A.; Zhan, J.; Wang, X.; Liu, G.; Yan, G.; King, G.J.; Edwards, D.; Wang, H. Genetic and signalling pathways of dry fruit size: Targets for genome editing-based crop improvement. Plant Biotechnol. J. 2020, 18, 1124–1140. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yang, R.; Wang, Y.; Ma, Y.; Lin, Y.; Li, Z.; Song, Y.; Feng, X.; Li, L. Candidate gene mining of GA-mediated regulation of pear fruit shape. Hortic. Environ. Biotechnol. 2024, 65, 403–412. [Google Scholar] [CrossRef]
- de Jong, M.; Wolters-Arts, M.; Feron, R.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009, 57, 160–170. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16 (Suppl. S1), S181–S189. [Google Scholar] [CrossRef]
- Martí, C.; Orzáez, D.; Ellul, P.; Moreno, V.; Carbonell, J.; Granell, A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007, 52, 865–876. [Google Scholar] [CrossRef]
- Tsaballa, A.; Pasentsis, K.; Darzentas, N.; Tsaftaris, A.S. Multiple evidence for the role of an Ovate-like gene in determining fruit shape in pepper. BMC Plant Biol. 2011, 11, 46. [Google Scholar] [CrossRef]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. An R2R3-MYB Transcription Factor Regulates Capsaicinoid Biosynthesis. Plant Physiol. 2017, 174, 1359–1370. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Wu, Y.-W.; Wang, Y.; Zhang, J.; Zheng, Y.; Li, Y.; Li, X.-B. bHLH transcription factors LP1 and LP2 regulate longitudinal cell elongation. Plant Physiol. 2021, 187, 2577–2591. [Google Scholar] [CrossRef]
- Liu, R.; Song, J.; Liu, S.; Chen, C.; Zhang, S.; Wang, J.; Xiao, Y.; Cao, B.; Lei, J.; Zhu, Z. Genome-wide identification of the Capsicum bHLH transcription factor family: Discovery of a candidate regulator involved in the regulation of species-specific bioactive metabolites. BMC Plant Biol. 2021, 21, 262. [Google Scholar] [CrossRef]
- Wang, F.; Yang, F.; Zhu, D.; Saniboere, B.; Zhou, B.; Peng, D. MYB44 plays key roles in regulating plant responses to abiotic and biotic stress, metabolism, and development. J. Plant Biochem. Biotechnol. 2024, 33, 462–473. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, J.; Zhang, M.; Huang, S.; Chen, X. Comparative Transcriptomic Analysis of Two Bottle Gourd Accessions Differing in Fruit Size. Genes 2020, 11, 359. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, F.; Deng, Y.; Zhong, F.; Tian, P.; Lin, D.; Deng, J.; Zhang, Y.; Huang, T. Sly-miR159 regulates fruit morphology by modulating GA biosynthesis in tomato. Plant Biotechnol. J. 2022, 20, 833–845. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, H.; Cabrera, A.; Meulia, T.; van der Knaap, E. SUN Regulates Vegetative and Reproductive Organ Shape by Changing Cell Division Patterns. Plant Physiol. 2011, 157, 1175–1186. [Google Scholar] [CrossRef]
- Muñoz-Bertomeu, J.; Miedes, E.; Lorences, E.P. Expression of xyloglucan endotransglucosylase/hydrolase (XTH) genes and XET activity in ethylene treated apple and tomato fruits. J. Plant Physiol. 2013, 170, 1194–1201. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, Q.; Zhang, Z.; Meng, K.; Hou, Y.; Ban, Q.; Suo, J.; Rao, J. Analysis of Xyloglucan Endotransglycosylase/Hydrolase (XTH) Genes and Diverse Roles of Isoenzymes during Persimmon Fruit Development and Postharvest Softening. PLoS ONE 2015, 10, e0123668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Li, P.; Duan, J.; Huang, F.; Hou, J.; Zou, X.; Ou, L.; Liu, Z.; Yang, S. Transcriptome Analysis Reveals Key Genes Involved in Fruit Length Trait Formation in Pepper (Capsicum annuum L.). Horticulturae 2025, 11, 1025. https://doi.org/10.3390/horticulturae11091025
Zeng J, Li P, Duan J, Huang F, Hou J, Zou X, Ou L, Liu Z, Yang S. Transcriptome Analysis Reveals Key Genes Involved in Fruit Length Trait Formation in Pepper (Capsicum annuum L.). Horticulturae. 2025; 11(9):1025. https://doi.org/10.3390/horticulturae11091025
Chicago/Turabian StyleZeng, Jie, Peiru Li, Jingwei Duan, Fei Huang, Jinqi Hou, Xuexiao Zou, Lijun Ou, Zhoubin Liu, and Sha Yang. 2025. "Transcriptome Analysis Reveals Key Genes Involved in Fruit Length Trait Formation in Pepper (Capsicum annuum L.)" Horticulturae 11, no. 9: 1025. https://doi.org/10.3390/horticulturae11091025
APA StyleZeng, J., Li, P., Duan, J., Huang, F., Hou, J., Zou, X., Ou, L., Liu, Z., & Yang, S. (2025). Transcriptome Analysis Reveals Key Genes Involved in Fruit Length Trait Formation in Pepper (Capsicum annuum L.). Horticulturae, 11(9), 1025. https://doi.org/10.3390/horticulturae11091025






