Clonal Micropropagation of Promising Genotypes of Amygdalus communis L. for Population Restoration and Gene Pool Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Culture Initiation
2.3. Sterilization of Plant Tissues
2.4. Latent Infection Screening
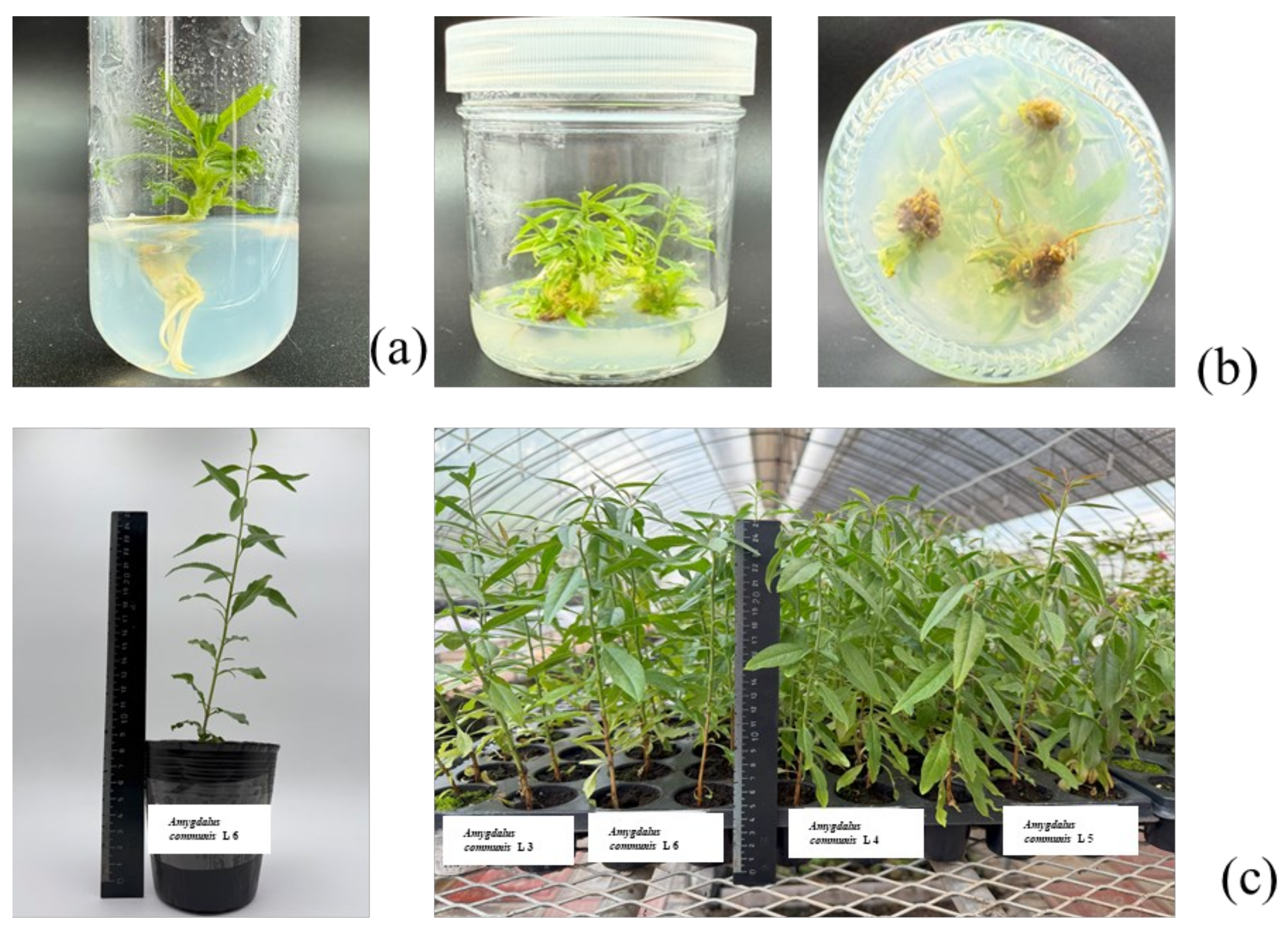
2.5. Microclonal Propagation
2.6. Data Analysis
3. Results
3.1. Mapping the Distribution and Selection of Amygdalus communis L. Genotypes
3.2. Microclonal Propagation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bairamov, L.A. Study of almond (Prunus dulcis) cultivars and forms cultivated in the Ordubad region, and the study of agroecological characteristics. Nauchnye Tr. Nakhchyvanskogo Otd. NANA 2022, 18, 145–151. (In Azerbaijani) [Google Scholar]
- Chernobay, I.G. The gene-pool collection of almond. Sci. Notes Cape Martyan Nat. Reserve 2015, 6, 229–234. (In Russian) [Google Scholar]
- Sinskaya, E.N. Historical Geography of Cultivated Flora; Kolos Publishing: Leningrad, Russia, 1969; pp. 160–161. (In Russian) [Google Scholar]
- Bairamov, L.A. Study and agrobiological characterization of almond cultivars and forms grown in the Julfa district of Nakhchivan. Bull. Sci. Pract. 2023, 9, 139–143. (In Russian) [Google Scholar] [CrossRef]
- Butkov, E.A.; Khamzaev, A.K.; Eshankulov, B.I. Conservation of wild almond in Uzbekistan. Proc. Introd. Acclim. Plants 2021, 1, 31–36. (In Russian) [Google Scholar]
- Ben Khadher, T.; Sassi-Aydi, S.; Aydi, S.; Mars, M.; Bouajila, J. Phytochemical profiling and biological potential of Prunus dulcis shell extracts. Plants 2023, 12, 2733. [Google Scholar] [CrossRef]
- Bolotova, A.S. Chemical composition of nuts of introduced sweet almond cultivars in southern Kyrgyzstan. Sci. Eur. 2017, 13, 8–12. (In Russian) [Google Scholar]
- Bolotov, S.; Kenzhebaev, S.K.; Bolotova, A.S. Development of almond cultivars in southern Kyrgyzstan. For. For. Cult. Res. Kyrg. 2006, 19, 76–82. (In Russian) [Google Scholar]
- Dejampour, J.; Majidi, I.; Khosravi, S.; Farhadi, S.; Shadmehr, A. In Vitro propagation of HS314 rootstock (Prunus amygdalus × P. persica). HortScience 2011, 46, 928–931. [Google Scholar] [CrossRef]
- Khalimova, M.R.; Tashpulatova, D.S. Range of common almond (Amygdalus communis) and its use in medicine. Future Sci. 2017, 2017, 345–346. (In Russian) [Google Scholar]
- Kairova, M.Z. Introduced nut species and their populations in Kazakhstan. Scientific Works of the Cheboksary Branch of the Main Botanical Garden named after N.V. Tsitsin RAS 2019, 13, 115–121. (In Russian) [Google Scholar]
- Zverev, N.E. Dynamics of growth of true pistachio and common almond in Turkmenistan and Kazakhstan. Probl. Desert Dev. 2018, 1, 60–62. (In Russian) [Google Scholar]
- Shalakhanova, A. Kazakhstan’s Sixth National Report on Biological Diversity. 2018. Available online: https://coilink.org/20.500.12592/khhwkp (accessed on 14 August 2025).
- Dzhuleva, V.; Atanasov, A. Micro-propagation of Platanus acerifolia In Vitro. Silvae Genet. 1994, 43, 215–218. (In Russian) [Google Scholar]
- Suleimanova, S.D. Microclonal propagation of fruit crops. East. Eur. Sci. J. 2016, 11, 47–54. (In Russian) [Google Scholar]
- Turdiyev, T. Microclonal propagation of common almond (Prunus dulcis Mill.) for restoration of regressing populations. Izdenister Natigeler 2024, 3, 178–187. (In Russian) [Google Scholar] [CrossRef]
- Arzumanov, V.A.; Butkov, E.A.; Turdieva, M.K.; Baimetov, K.I.; Yushev, A.A. Plant Resources of Fruit and Nut Species of Central Asia and Their Role in Forming Local Assortments; Bioversity International: Rome, Italy, 2015; p. 106. (In Russian) [Google Scholar]
- Vinter, M.A.; Sherbakov, N.A. Clonale Micropropogation and Sanitation of Prunus Domestica from the Plum Pox Potyvirus (PPV). 2018. Available online: https://ej.kubagro.ru/2018/09/pdf/13.pdf (accessed on 14 August 2025).
- Gülcan, R. Descriptors List for Almond (Prunus amygdalus) (Revised); International Board for Plant Genetic Resources (IBPGR): Rome, Italy, 1985; p. 30. [Google Scholar]
- Viss, P.R.; Brooks, E.M.; Driver, J.A. A simplified method for the control of bacterial contamination in woody plant tissue culture. Vitr. Cell. Dev. Biol. 1991, 27, 42. [Google Scholar] [CrossRef]
- Onay, A. Micropropagation of Pistachio. In Micropropagation of Woody Trees and Fruits; Springer: Dordrekht, the Netherlands, 2003; pp. 565–588. [Google Scholar]
- Dzhangaliev, A.D. Reproduction by seeds. In Wild Fruit Plants of Kazakhstan; Wiley: Hoboken, NJ, USA, 2001; pp. 82–87. (In Russian) [Google Scholar] [CrossRef]
- Besedina, E.N.; Buntsevich, L.L. Usovershenstvovaniya tekhnologii klonal’nogo mikrorazmnozheniya podvoev yabloni na ehtape vvedeniya v kul’turu in vitro. Politematicheskij Setevoj Ehlektronnyj Nauchnyj Zhurnal Kuban. Gos. Agrar. Univ. 2015, 111, pp. 1716–1734. Available online: https://cyberleninka.ru/article/n/usovershenstvovaniya-tehnologii-klonalnogo-mikrorazmnozheniya-podvoev-yabloni-na-etape-vvedeniya-v-kulturu-in-vitro?ysclid=mel01tbrh0124779078 (accessed on 14 August 2025). (In Russian).
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Filipova, L.M.; Matskevich, V.V.; Matskevich, O.V. Perspektivi rozmnozhennya migdalyu in vitro. 2020. Available online: https://rep.btsau.edu.ua/bitstream/BNAU/5519/1/%D0%9F%D0%B5%D1%80%D1%81%D0%BF%D0%B5%D0%BA%D1%82%D0%B8%D0%B2%D0%B8%20%D1%80%D0%BE%D0%B7%D0%BC%D0%BD%D0%BE%D0%B6%D0%B5%D0%BD%D0%BD%D1%8F%20%D0%BC%D0%B8%D0%B3%D0%B4%D0%B0%D0%BB%D1%8E%20in%20vitro.pdf (accessed on 14 August 2025).
- Orazov, A.; Myrzagaliyeva, A.; Mukhitdinov, N.; Tustubayeva, S. Callus induction with 6-BAP and IBA as a way to preserve Prunus ledebouriana (Rosaceae), an endemic plant of Altai and Tarbagatai, East Kazakhstan. Biodiversitas 2022, 23, 3178–3184. [Google Scholar] [CrossRef]
- Serafimovich, M.V.; Kirillov, V.Y.; Stikhareva, T.N. Effect of nutrient medium composition on viability of explants of Amygdalus ledebouriana in culture In Vitro. Vestn. Shakarim State Univ. 2020, 3, 289–293. (In Russian) [Google Scholar]
- Gürel, S.; Gülşen, Y. The effects of different sucrose, agar and pH levels on in vitro shoot production of almond (Amygdalus communis L.). Turk. J. Bot. 1998, 22, 363–373. [Google Scholar]
- Hisajıma, S. Multiple shoot formation from almond seeds and an excised single shoot. Agric. Biol. Chem. 1982, 46, 1091–1093. [Google Scholar] [CrossRef][Green Version]
- Kester, D.E.; Tabachnik, L.; Negueroles, J. Use of micropropagation and tissue culture to investigate genetic disorders in almond cultivars. In Symposium on Tissue Culture for Horticultural Purposes; The International Society for Horticultural Science, Section for Ornamental Plants: Leuven, Belgium, 1977; p. 95. [Google Scholar] [CrossRef]
- Isikalan, C.; Adıyaman Akbaş, F.; Namlı, S.; Tilkat, E.; Başaran, D. In Vitro micropropagation of almond (Amygdalus communis L. cv. Nonpareil). Afr. J. Biotechnol. 2008, 7, 1875–1880. [Google Scholar]
- Zmushko, A.A.; Rundya, A.P. Razmnozhenie gretskogo orekha (Juglans regia L.) in vitro. Plodovodstvo 2022, 27, 404–410. [Google Scholar]
- Shadenova, E.; Kaigermazova, M.; Sembekov, M.; Oshakbay, U.; Dzhangalina, E. Effects of various stimulants on the callusogenesis of jugnalis regia effigia. Izdenister Natigeler 2023, 2, 336–344. [Google Scholar]
- Türemis, N.; Çömlekçioglu, N. Identify the most suitable explants for walnut micro-propagation. V Temp. Fruit Belt Trop. Subtrop. 1996, 441, 379–382. [Google Scholar]
- Driver, D.A.; Kuniyuki, A.H. In Vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-Feasible Micropropagation of Mountain Laurel, Kalmia Latifolia, by Use of Shoot-Tip Culture; Combined Proceedings; International Plant Propagators’ Society: Cleveland, OH, USA, 1980; pp. 421–426. [Google Scholar]
- Nas, M.N.; Reed, E. Micro-propagation of hybrid hazelnuts: The composition of the medium, the physical state and the source of iron affect the morphogenesis of shoots, reproduction and viability of the explant. Acta Hortic. 2001, 556, 251–258. [Google Scholar] [CrossRef]
- Gulboev, D.T. Jptimal nutrient content and nutrient influence on germination of germ cells of bitter almond seeds (Amygdalus communis). Bull. Sci. 2020, 1, 90–95. [Google Scholar]
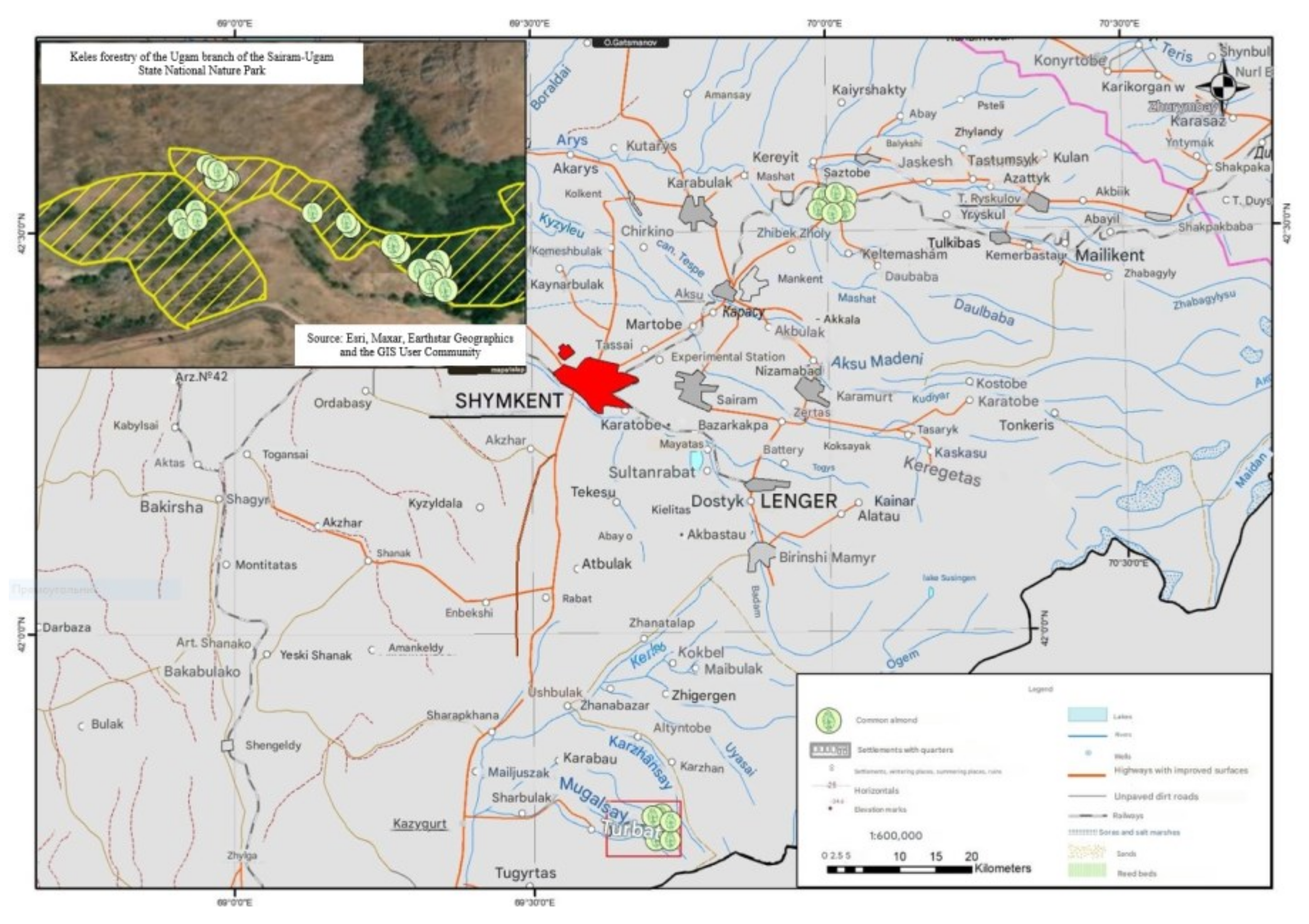

| Sample | Latitude | Longitude | Elevation | Collection Site |
|---|---|---|---|---|
| A 1 | 41°44′53.16″ | 069°42′13.26″ | 1352 | Keles Forestry of the Ugam Branch, Sairam-Ugam State National Nature Park, 3 km from the village of Turbat |
| A 2 | 41°44′53.52″ | 069°42′13.14″ | 1355 | |
| A 3 | 41°44′53.04″ | 069°42′12.90″ | 1340 | |
| A 4 | 41°44′52.98″ | 069°42′12.96″ | 1344 | |
| A 5 | 41°44′52.80″ | 069°42′12.78″ | 1346 | |
| A 6 | 41°44′52.50″ | 069°42′12.84″ | 1352 | |
| A 7 | 41°44′53.10″ | 069°42′12.36″ | 1341 | |
| A 8 | 41°44′52.62″ | 069°42′12.84″ | 1339 | |
| A 9 | 41°44′52.26″ | 069°42′13.50″ | 1349 | |
| A 10 | 41°44′54.00″ | 069°42′11.40″ | 1347 | |
| A 11 | 41°44′54.24″ | 069°42′11.16″ | 1348 | |
| A 12 | 41°44′55.05″ | 069°42′09.25″ | 1343 | |
| A 13 | 41°44′55.28″ | 069°42′09.05″ | 1345 | |
| A 14 | 41°44′55.71″ | 069°42′07.54″ | 1344 | |
| A 15 | 41°44′57.21″ | 069°42′03.76″ | 1342 | |
| A 16 | 41°44′57.20″ | 069°42′03.66″ | 1342 | |
| A 17 | 41°44′57.08″ | 069°42′03.50″ | 1342 | |
| A 18 | 41°44′57.24″ | 069°42′03.10″ | 1340 | |
| A 19 | 41°44′57.28″ | 069°42′03.14″ | 1341 | |
| A 20 | 41°44′57.20″ | 069°42′03.14″ | 1337 | |
| A 21 | 41°44′57.86″ | 069°42′02.76″ | 1337 | |
| A 22 | 41°44′57.72″ | 069°42′03.02″ | 1338 | |
| A 23 | 41°44′57.59″ | 069°42′03.29″ | 1336 | |
| A 24 | 41°44′55.45″ | 069°42′01.49″ | 1336 | |
| A 25 | 41°44′55.83″ | 069°42′02.29″ | 1338 | |
| A 26 | 42°32′02.81″ | 070°01′19.46″ | 603 | Village of Sastobe, Turkistan Region, 45 km from the city of Shymkent |
| A 27 | 42°32′03.15″ | 070°01′18.39″ | 602 | |
| A 28 | 42°32′03.04″ | 070°01′17.56″ | 600 | |
| A 29 | 42°32′02.12″ | 070°01′17.35″ | 599 | |
| A 30 | 42°32′01.69″ | 070°01′17.01″ | 597 | |
| A 31 | 42°32′01.54″ | 070°01′17.00″ | 597 | |
| A 32 | 42°32′01.48″ | 070°01′16.66″ | 598 | |
| A 33 | 42°32′04.71″ | 070°01′18.38″ | 600 | |
| A 34 | 42°32′04.73″ | 070°01′18.46″ | 601 | |
| A 35 | 42°32′04.88″ | 070°01′18.57″ | 601 | |
| A 36 | 42°32′05.12″ | 070°01′18.66″ | 601 | |
| A 37 | 42°32′02.30″ | 070°01′17.50″ | 608 | |
| A 38 | 42°32′03.17″ | 070°01′18.23″ | 606 | |
| A 39 | 42°32′03.11″ | 070°01′18.69″ | 606 | |
| A 40 | 42°32′05.43″ | 070°01′18.65″ | 603 | |
| A 41 | 42°32′05.09″ | 070°01′19.03″ | 604 | |
| A 42 | 42°32′04.90″ | 070°01′20.75″ | 606 | |
| A 43 | 42°32′03.66″ | 070°01′19.67″ | 608 | |
| A 44 | 42°32′02.91″ | 070°01′19.45″ | 607 | |
| A 45 | 42°32′02.50″ | 070°01′18.69″ | 606 | |
| A 46 | 42°32′01.45″ | 070°01′17.09″ | 599 | |
| A 47 | 42°32′01.14″ | 070°01′16.26″ | 596 | |
| A 48 | 42°32′03.92″ | 070°01′18.80″ | 615 | |
| A 49 | 42°32′03.41″ | 070°01′19.00″ | 614 | |
| A 50 | 42°32′02.95″ | 070°01′19.43″ | 613 | |
| A 51 | 42°32′04.58″ | 070°01′18.93″ | 609 | |
| A 52 | 42°32′04.83″ | 070°01′19.29″ | 608 | |
| A 53 | 42°32′05.14″ | 070°01′21.03″ | 609 | |
| A 54 | 42°32′05.43″ | 070°01′21.10″ | 608 |
| №. of Sample | Kernel Taste | Nut Shape | Nut Size | Ease of Husk Removal | Shell Hardness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sweet | Bitter | Round | Ovate | Elongated | Sweet | Bitter | Round | Ovate | Elongated | Sweet | Bitter | Round | Ovate | Elongated | Sweet | |
| A 1 | + | + | + | + | + | |||||||||||
| A 2 | + | + | + | + | + | |||||||||||
| A 3 | + | + | + | + | + | |||||||||||
| A 4 | + | + | + | + | + | |||||||||||
| A 5 | + | + | + | + | + | |||||||||||
| A 6 | + | + | + | + | + | |||||||||||
| A 7 | + | + | + | + | + | |||||||||||
| A 8 | + | + | + | + | + | |||||||||||
| A 9 | + | + | + | + | + | |||||||||||
| A 10 | + | + | + | + | + | |||||||||||
| A 11 | + | + | + | + | + | |||||||||||
| A 12 | + | + | + | + | + | |||||||||||
| A 13 | + | + | + | + | + | |||||||||||
| A 14 | + | + | + | + | + | |||||||||||
| A 15 | + | + | + | + | + | |||||||||||
| A 16 | + | + | + | + | + | |||||||||||
| A 17 | + | + | + | + | + | |||||||||||
| A 18 | + | + | + | + | + | |||||||||||
| A 19 | + | + | + | + | + | |||||||||||
| A 20 | + | + | + | + | + | |||||||||||
| A 21 | + | + | + | + | + | |||||||||||
| A 22 | + | + | + | + | + | |||||||||||
| A 23 | + | + | + | + | + | |||||||||||
| A 24 | + | + | + | + | + | |||||||||||
| A 25 | + | + | + | + | + | |||||||||||
| A 26 | + | + | + | + | + | |||||||||||
| A 27 | + | + | + | + | + | |||||||||||
| A 28 | + | + | + | + | + | |||||||||||
| A 29 | + | + | + | + | + | |||||||||||
| A 30 | + | + | + | + | + | |||||||||||
| A 31 | + | + | + | + | + | |||||||||||
| A 32 | + | + | + | + | + | |||||||||||
| A 33 | + | + | + | + | + | |||||||||||
| A 34 | + | + | + | + | + | |||||||||||
| Sample | Nuts | Kernel | Embryonic Axis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Thickness | Length | Width | Thickness | Length | Width | Thickness | |
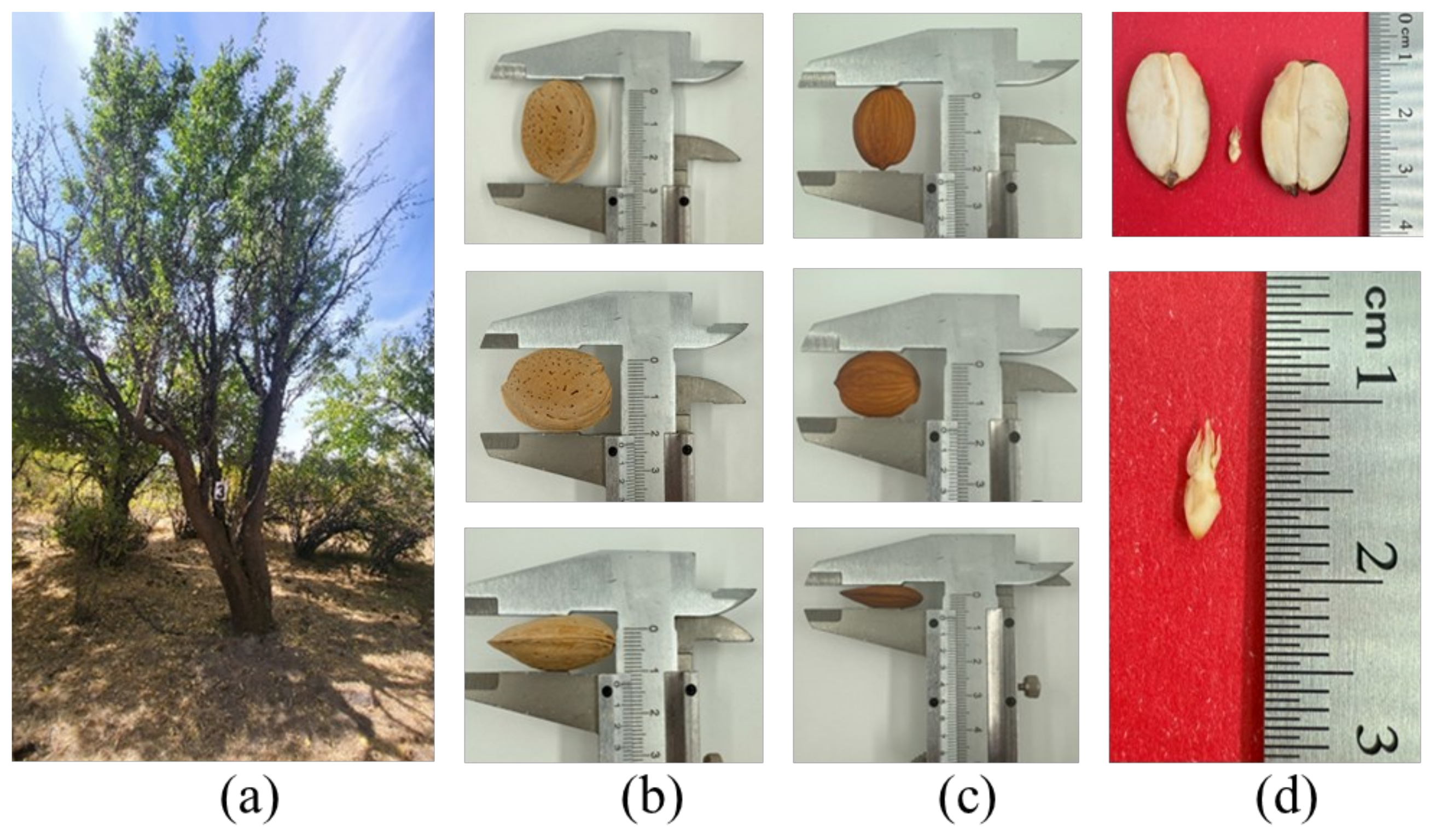
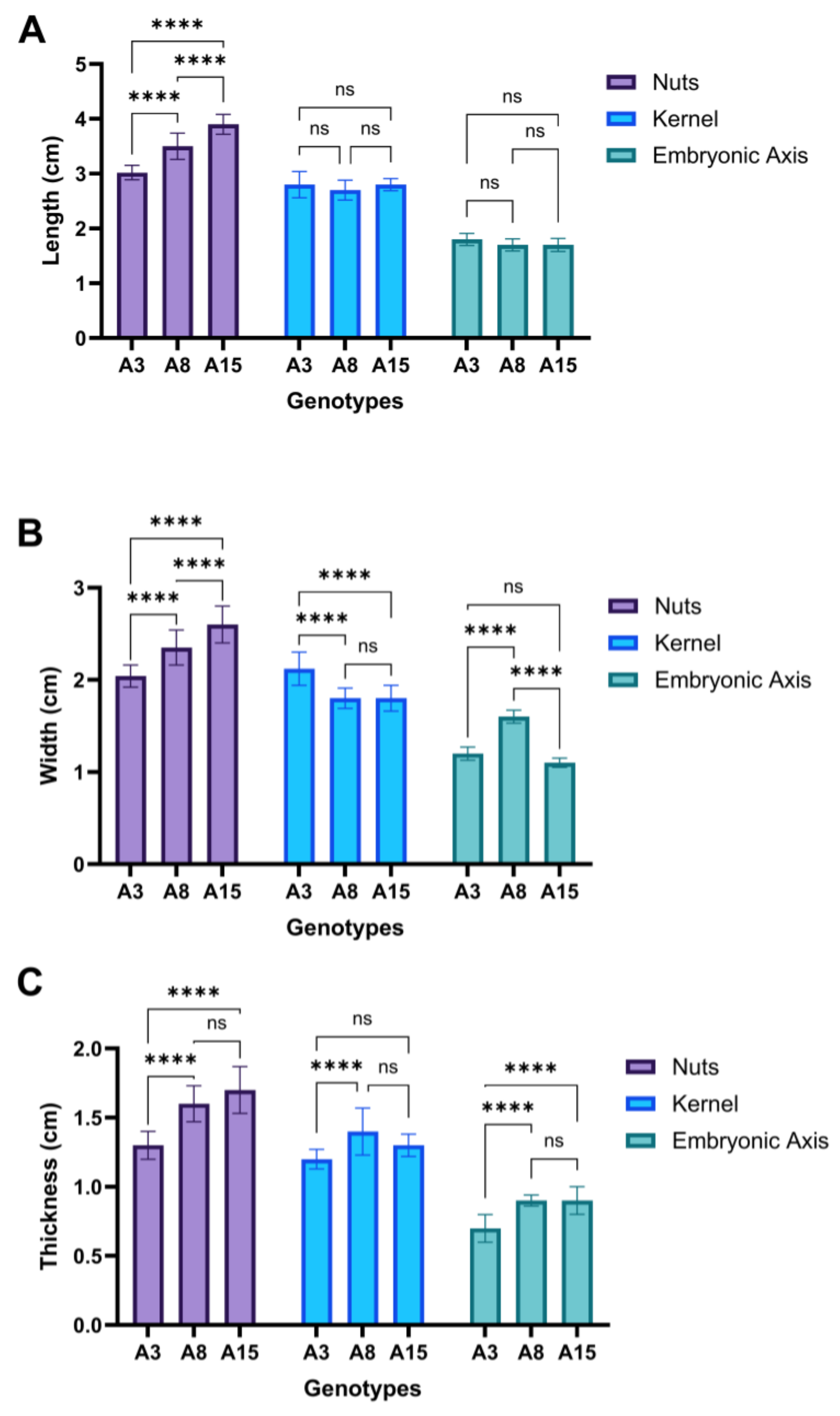
| A 3 | 3.02 ± 0.13 a | 2.04 ± 0.12 a | 1.3 ± 0.1 a | 2.8 ± 0.24 abc | 2.12 ± 0.18 a | 1.2 ± 0.07 ac | 1.8 ± 0.11 abc | 1.2 ± 0.07 ac | 0.7 ± 0.1 a |
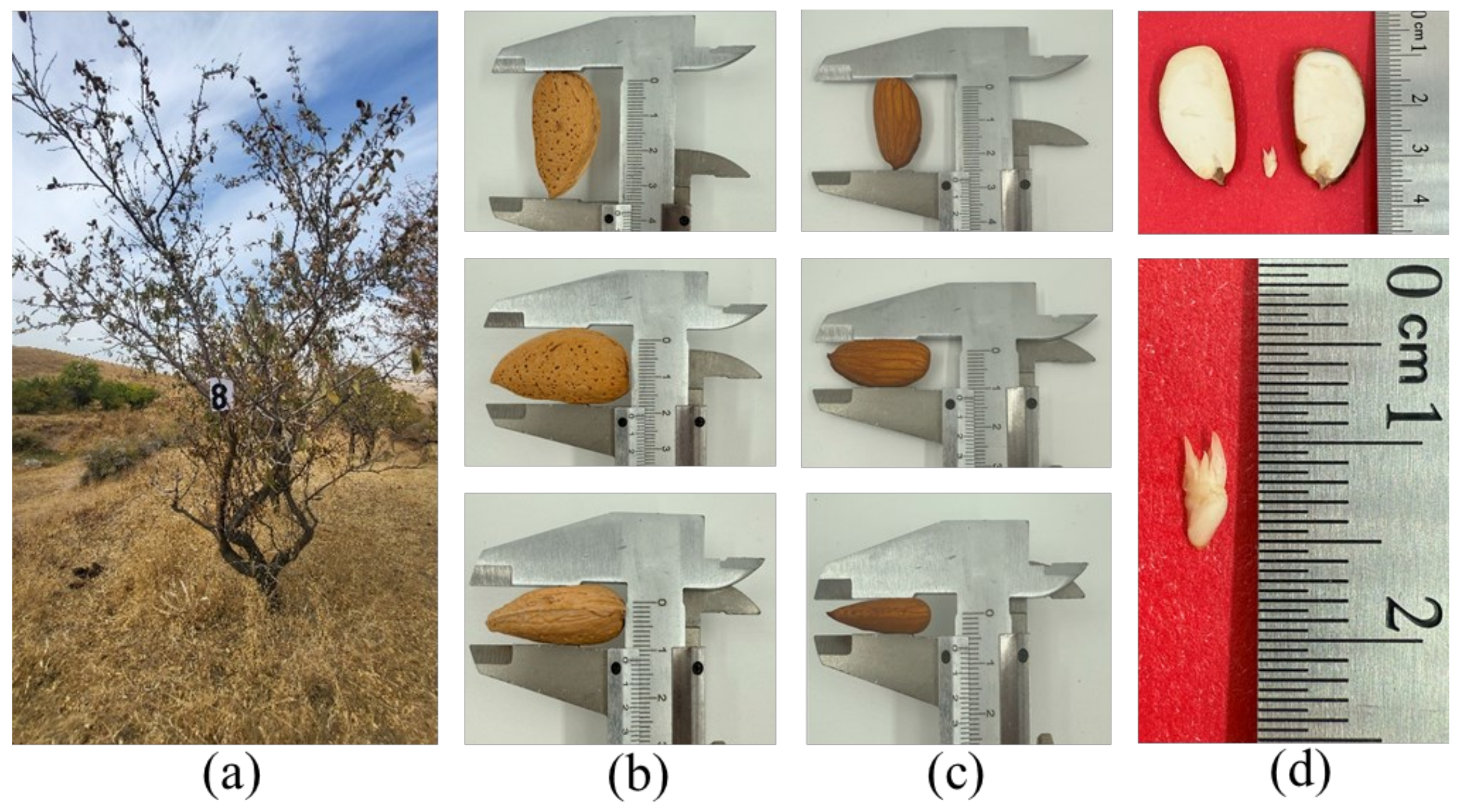
| A 8 | 3.5 ± 0.24 b | 2.35 ± 0.19 b | 1.6 ± 0.13 bc | 2.7 ± 0.18 abc | 1.8 ± 0.11 bc | 1.4 ± 0.17 bc | 1.7 ± 0.11 abc | 1.6 ± 0.07 b | 0.9 ± 0.04 bc |
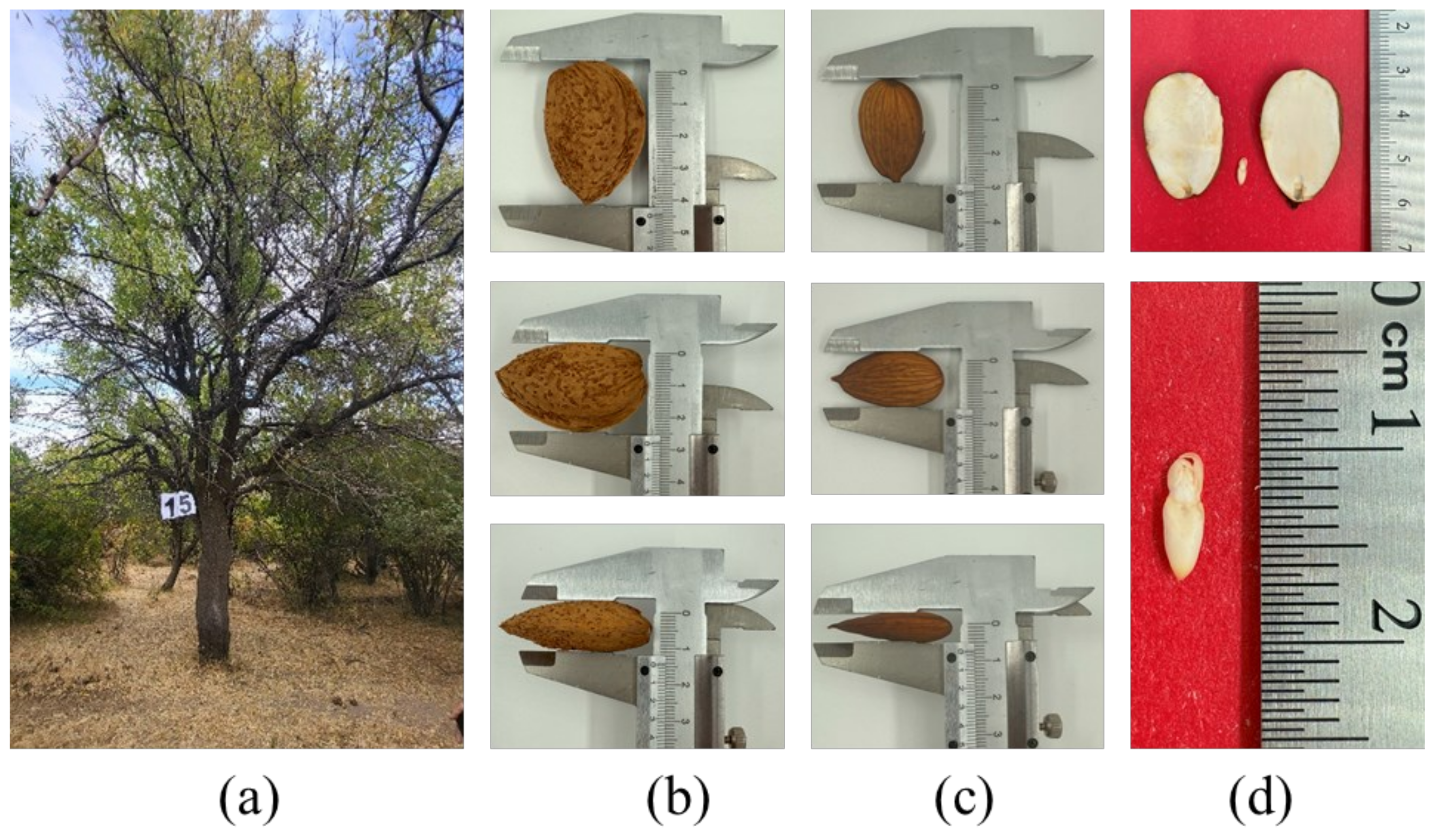
| A 15 | 3.9 ± 0.18 c | 2.6 ± 0.2 c | 1.7 ± 0.17 bc | 2.8 ± 0.11 abc | 1.8 ± 0.14 bc | 1.3 ± 0.08 abc | 1.7 ± 0.12 abc | 1.1 ± 0.05 ac | 0.9 ± 0.1 bc |
| Nutrient Medium | Callus Formation, pcs * | Average Number of Shoots (pcs ± SD) | Average Multiplication Coefficient | Average Shoot Length (cm ± SD) | Leaf Color ** |
|---|---|---|---|---|---|
| Genotype A3 | |||||
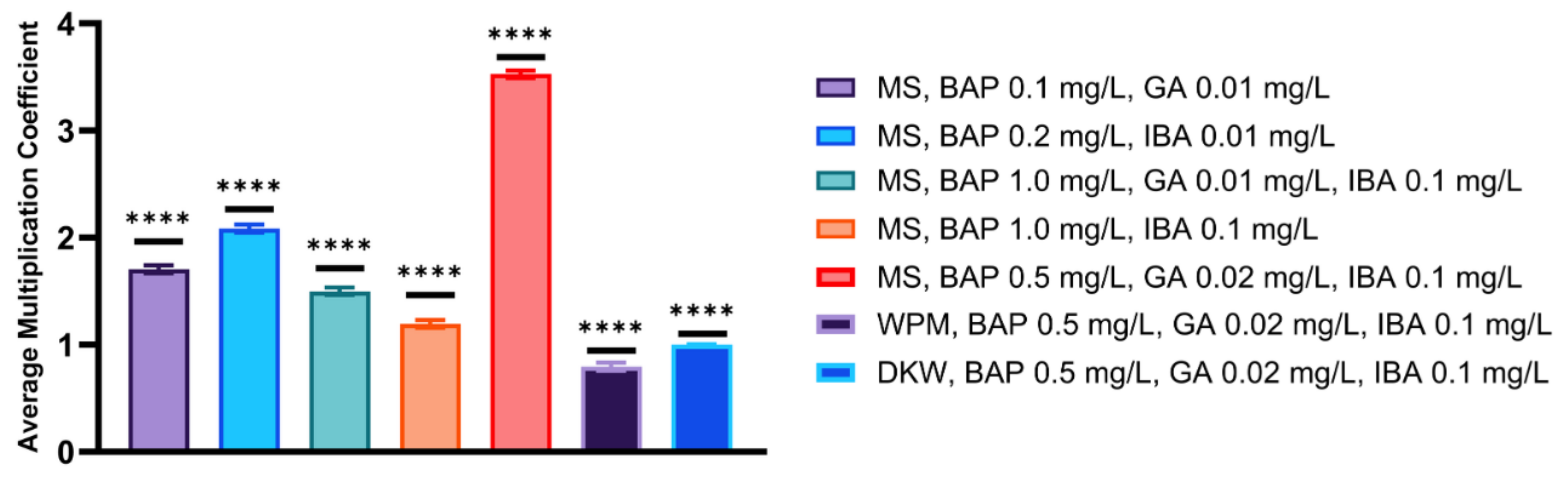
| MS, BAP 0.1 mg/L, GA 0.01 mg/L | 5 | 77 ± 1.58 a | 1.71 | 2.6 ± 0.5 abcd | 1 |
| MS, BAP 0.2 mg/L, IBA 0.01 mg/L | 8 | 94 ± 1.58 b | 2.08 | 2.4 ± 0.6 abcd | 4 |
| MS, BAP 1.0 mg/L, GA 0.01 mg/L, IBA 0.1 mg/L | 13 | 68 ± 1.00 c | 1.51 | 1.9 ± 0.5 abcdfg | 3 |
| MS, BAP 1.0 mg/L, IBA 0.1 mg/L | 11 | 55 ± 1.58 d | 1.22 | 1.7 ± 0.4 abcdfg | 4 |
| MS, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 9 | 158 ± 1.58 e | 3.51 | 5.7 ± 0.3 e | 5 |
| WPM, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 14 | 36 ± 1.00 f | 0.8 | 1.1 ± 0.3 cdfg | 2 |
| DKW, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 17 | 45 ± 0.00 g | 1 | 0.9 ± 0.2 cdfg | 3 |
| Genotype A8 | |||||
| MS, BAP 0.1 mg/L, GA 0.01 mg/L | 10 | 76 ± 1.00 a | 1.68 | 2.7 ± 0.6 abcd | 3 |
| MS, BAP 0.2 mg/L, IBA 0.01 mg/L | 9 | 96 ± 1.00 b | 2.13 | 2.5 ± 0.4 abcd | 3 |
| MS, BAP 1.0 mg/L, GA 0.01 mg/L, IBA 0.1 mg/L | 16 | 69 ± 1.00 c | 1.5 | 2.1 ± 0.5 abcdfg | 3 |
| MS, BAP 1.0 mg/L, IBA 0.1 mg/L | 14 | 54 ± 1.00 d | 1.2 | 1.9 ± 0.5 abcdfg | 4 |
| MS, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 10 | 159 ± 1.00 e | 3.53 | 5.6 ± 0.4 e | 4 |
| WPM, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 12 | 35 ± 1.00 f | 0.77 | 1.2 ± 0.3 cdfg | 2 |
| DKW, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 15 | 45 ± 0.00 g | 1 | 1.0 ± 0.3 cdfg | 1 |
| Genotype A15 | |||||
| MS, BAP 0.1 mg/L, GA 0.01 mg/L | 7 | 75 ± 1.58 a | 1.66 | 2.8 ± 0.4 abcd | 2 |
| MS, BAP 0.2 mg/L, IBA 0.01 mg/L | 5 | 95 ± 1.00 b | 2.11 | 2.5 ± 0.6 abcd | 4 |
| MS, BAP 1.0 mg/L, GA 0.01 mg/L, IBA 0.1 mg/L | 12 | 70 ± 1.00 c | 1.55 | 2.0 ± 0.5 abcdfg | 3 |
| MS, BAP 1.0 mg/L, IBA 0.1 mg/L | 10 | 56 ± 1.00 d | 1.24 | 1.8 ± 0.4 abcdfg | 4 |
| MS, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 13 | 160 ± 1.00 e | 3.55 | 5.9 ± 0.4 e | 5 |
| WPM, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 17 | 38 ± 1.41 f | 0.84 | 1.2 ± 0.3 cdfg | 3 |
| DKW, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 21 | 45 ± 0.00 g | 1 | 1.1 ± 0.3 cdfg | 2 |
| Genotype A28 | |||||
| MS, BAP 0.1 mg/L, GA 0.01 mg/L | 6 | 79 ± 2.00 a | 1.75 | 2.8 ± 0.6 abcd | 2 |
| MS, BAP 0.2 mg/L, IBA 0.01 mg/L | 7 | 93 ± 1.58 b | 2.06 | 2.6 ± 0.5 abcd | 2 |
| MS, BAP 1.0 mg/L, GA 0.01 mg/L, IBA 0.1 mg/L | 10 | 67 ± 1.00 c | 1.48 | 2.0 ± 0.6 abcdfg | 4 |
| MS, BAP 1.0 mg/L, IBA 0.1 mg/L | 12 | 53 ± 1.00 d | 1.17 | 1.9 ± 0.5 abcdfg | 4 |
| MS, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 10 | 157 ± 1.00 e | 3.48 | 5.6 ± 0.4 e | 5 |
| WPM, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 18 | 37 ± 1.00 f | 0.82 | 1.2 ± 0.3 cdfg | 2 |
| DKW, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 8 | 45 ± 0.00 g | 1 | 0.9 ± 0.3 cdfg | 1 |
| Genotype A30 | |||||
| MS, BAP 0.1 mg/L, GA 0.01 mg/L | 5 | 78 ± 1.58 a | 1.73 | 2.7 ± 0.5 abcd | 2 |
| MS, BAP 0.2 mg/L, IBA 0.01 mg/L | 8 | 92 ± 1.58 b | 2.04 | 2.5 ± 0.4 abcd | 2 |
| MS, BAP 1.0 mg/L, GA 0.01 mg/L, IBA 0.1 mg/L | 11 | 66 ± 1.00 c | 1.46 | 2.1 ± 0.5 abcdfg | 3 |
| MS, BAP 1.0 mg/L, IBA 0.1 mg/L | 13 | 52 ± 1.00 d | 1.15 | 1.8 ± 0.4 abcdfg | 4 |
| MS, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 9 | 161 ± 1.58 e | 3.57 | 5.8 ± 0.3 e | 5 |
| WPM, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 12 | 34 ± 1.58 f | 0.75 | 1.3 ± 0.3 cdfg | 2 |
| DKW, BAP 0.5 mg/L, GA 0.02 mg/L, IBA 0.1 mg/L | 7 | 45 ± 0.00 g | 1 | 1.0 ± 0.3 cdfg | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turdiyev, T.; Duisenova, K.; Kovalchuk, I.; Madenova, A.; Baizhumanova, S.; Yemesheva, K.; Mikhailenko, N.; Tuigunov, Z. Clonal Micropropagation of Promising Genotypes of Amygdalus communis L. for Population Restoration and Gene Pool Conservation. Horticulturae 2025, 11, 999. https://doi.org/10.3390/horticulturae11090999
Turdiyev T, Duisenova K, Kovalchuk I, Madenova A, Baizhumanova S, Yemesheva K, Mikhailenko N, Tuigunov Z. Clonal Micropropagation of Promising Genotypes of Amygdalus communis L. for Population Restoration and Gene Pool Conservation. Horticulturae. 2025; 11(9):999. https://doi.org/10.3390/horticulturae11090999
Chicago/Turabian StyleTurdiyev, Timur, Kumissay Duisenova, Irina Kovalchuk, Aigul Madenova, Saule Baizhumanova, Kamila Yemesheva, Natalya Mikhailenko, and Zakir Tuigunov. 2025. "Clonal Micropropagation of Promising Genotypes of Amygdalus communis L. for Population Restoration and Gene Pool Conservation" Horticulturae 11, no. 9: 999. https://doi.org/10.3390/horticulturae11090999
APA StyleTurdiyev, T., Duisenova, K., Kovalchuk, I., Madenova, A., Baizhumanova, S., Yemesheva, K., Mikhailenko, N., & Tuigunov, Z. (2025). Clonal Micropropagation of Promising Genotypes of Amygdalus communis L. for Population Restoration and Gene Pool Conservation. Horticulturae, 11(9), 999. https://doi.org/10.3390/horticulturae11090999







