Evaluating Water Use Dynamics and Yield Responses in Capsicum chinense Cultivars Using Integrated Sensor-Based Irrigation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experiment Design
2.3. Sap Flux Measurements
2.4. Plant Physiological Measurements
2.5. Plant Morphological Measurements
2.6. Plant Yield
2.7. Phytochemical Analysis
2.8. Statistical Analysis
- Physiological traits: photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (E)
- Morphological traits: plant height, stem diameter
- Yield components: number of pods, average pod weight, total yield (Mg·ha−1)
- Phytochemical traits: capsaicin, dihydrocapsaicin, nordihydrocapsaicin concentrations, and total Scoville Heat Units (SHU)
- Water-use dynamics: sap flux (g·d−1)
3. Results
3.1. Greenhouse Conditions
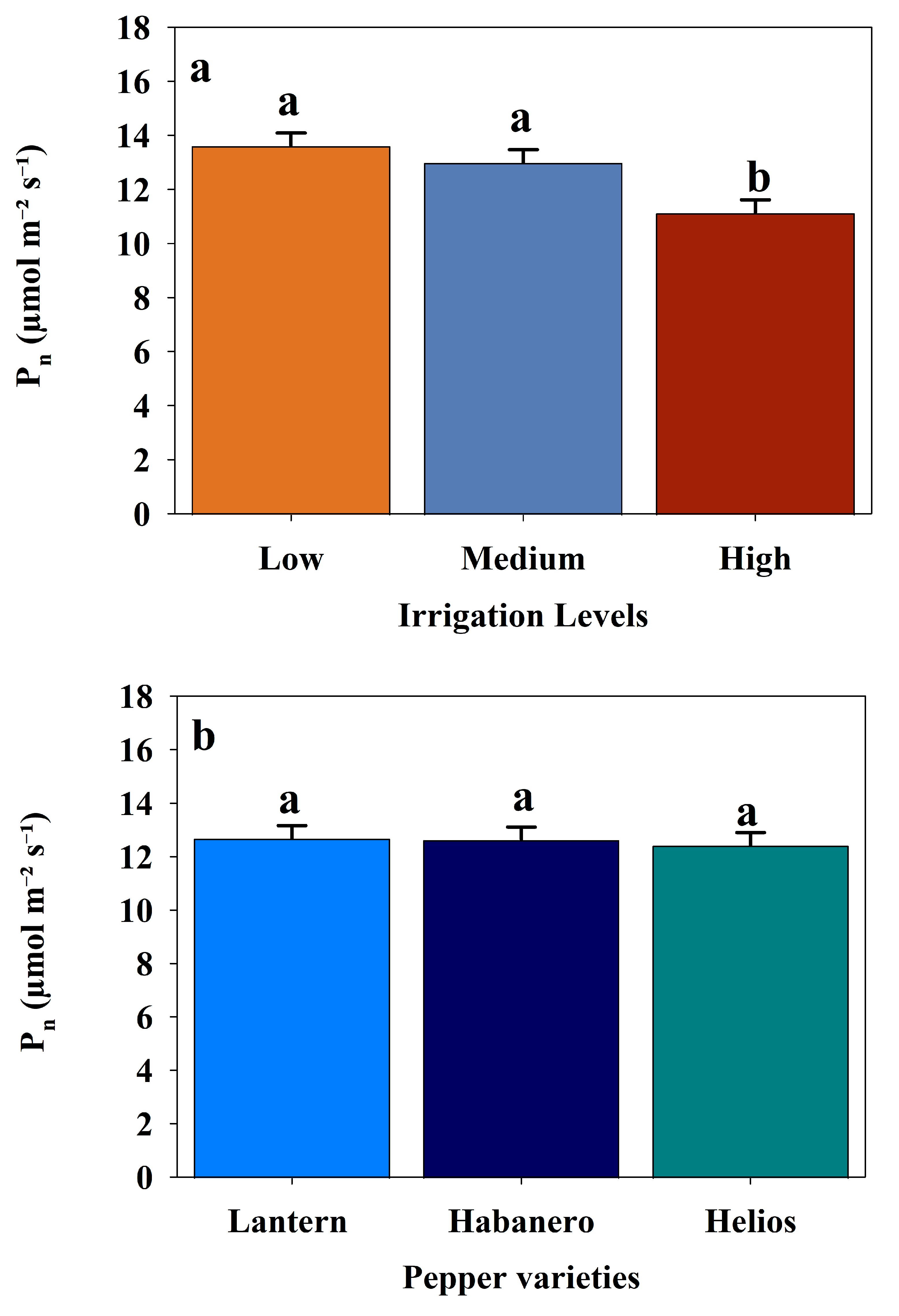
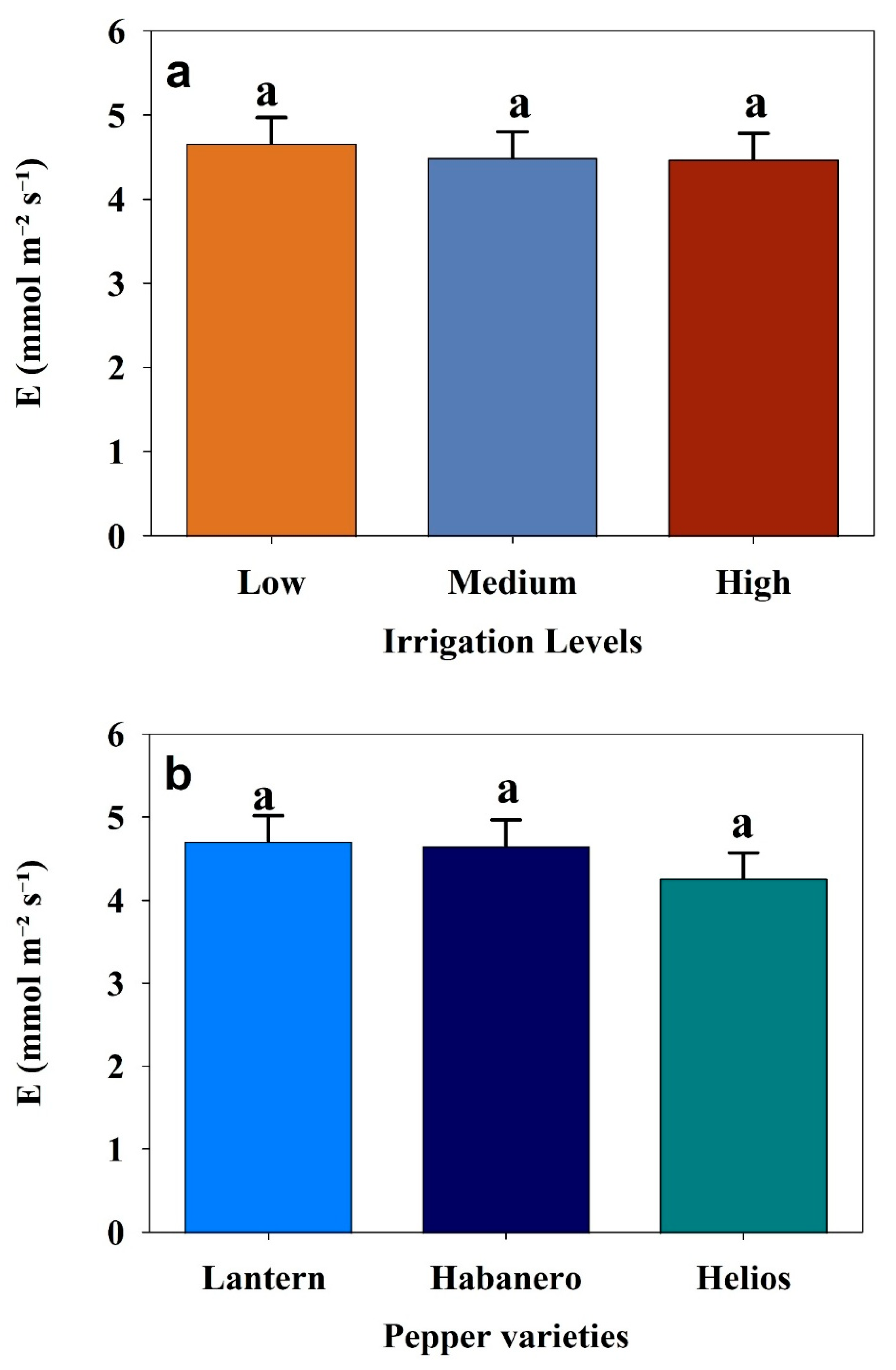
3.2. Effect of Different Irrigation Levels on the Physiological Responses of Three Pepper Cultivars
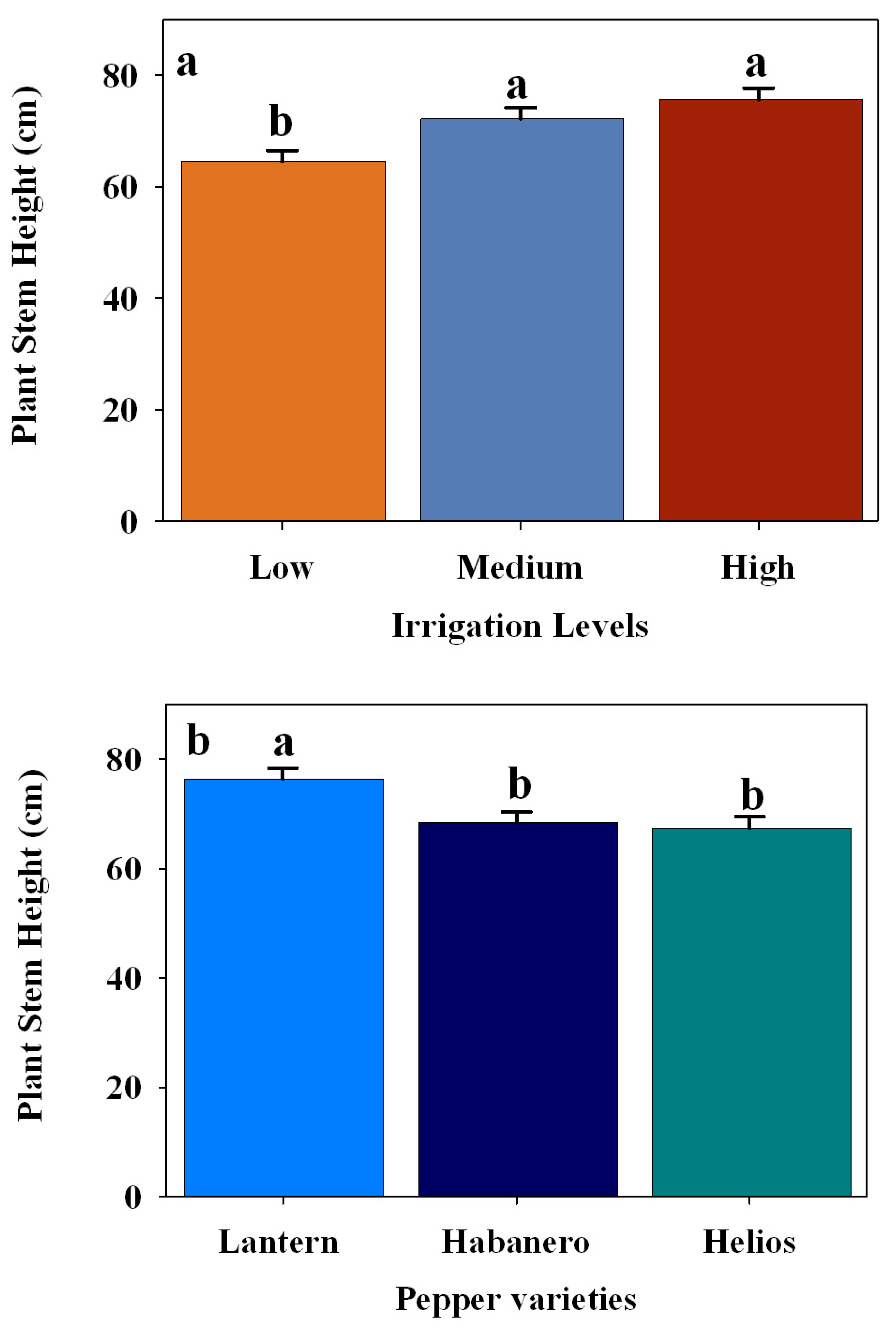
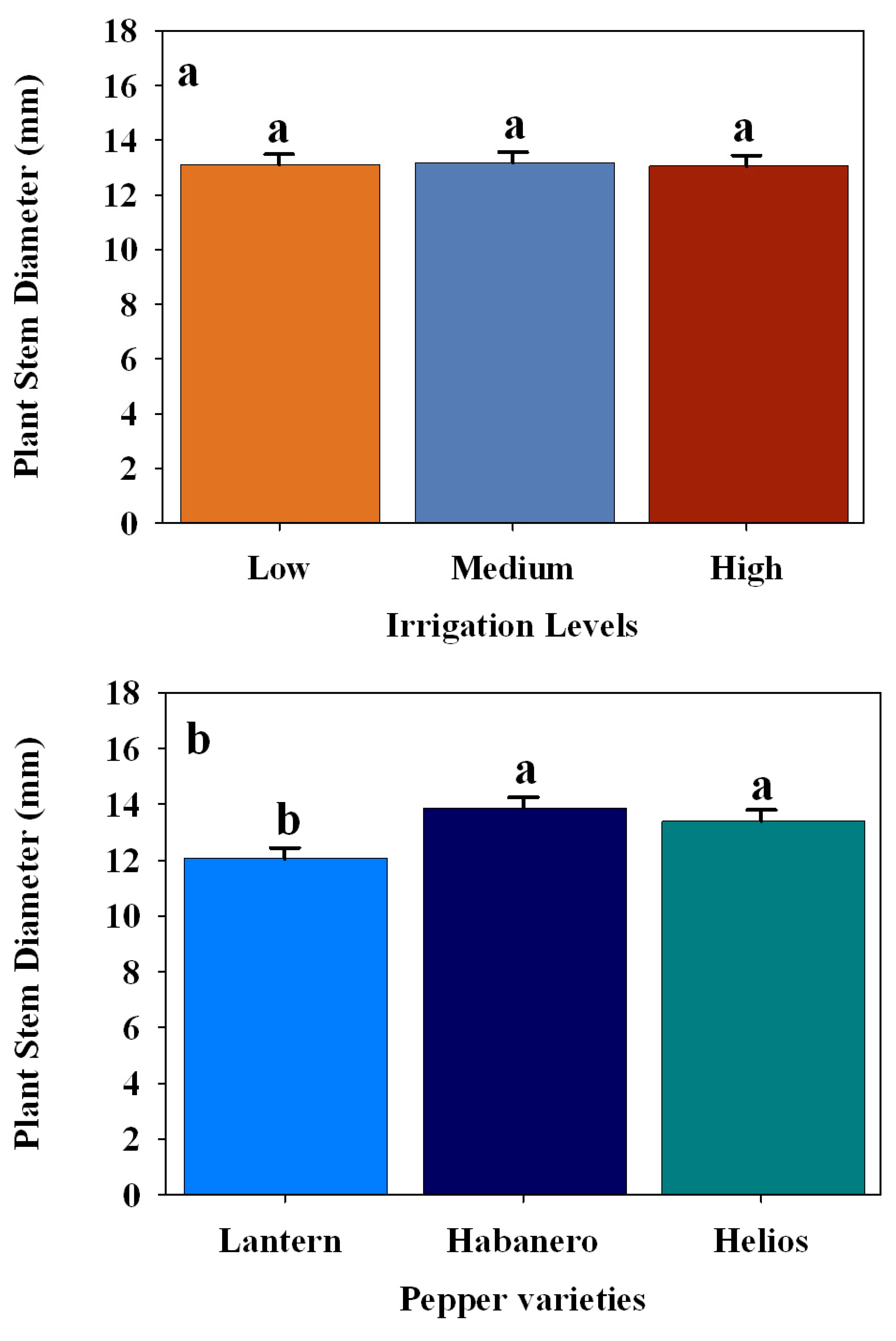
3.3. Effect of Different Irrigation Levels on the Plant Morphology in Three Pepper Cultivars
3.4. Effect of Different Irrigation Levels on Yield and Yield Components in Three Pepper Cultivars
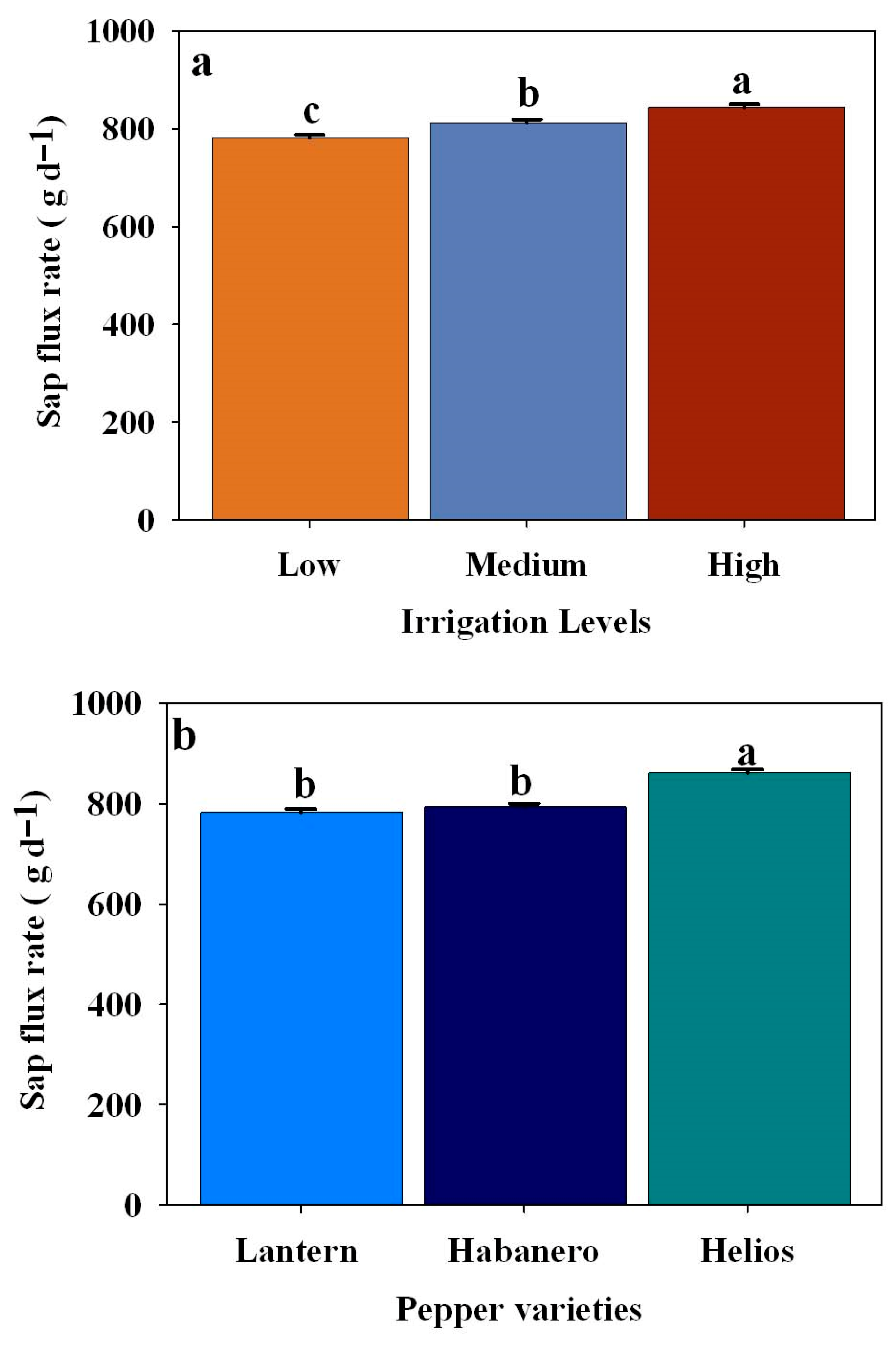
3.5. Effect of Different Irrigation Levels on Average Sap Flux Rate (g d−1) in Three Pepper Cultivars
3.6. Effect of Different Irrigation Levels on Capsaicinoid Content and Pungency in Three Hot Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barik, S.; Ponnam, N.; Reddy, A.C.; LakshmanaReddy, D.C.; Saha, K.; Acharya, G.C.; Reddy, M.K. Breeding peppers for industrial uses: Progress and prospects. Ind. Crops Prod. 2022, 178, 114626. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Setzer, W.N. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, anti-obesity, nutritional and other beneficial effects of different chili pepper: A review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gao, C.; Luo, S.; Yao, Z.; Ye, Q.; Wan, H.; Zhou, G.; Liu, C. Effects of storage temperature at the early postharvest stage on the firmness, bioactive substances, and amino acid compositions of chili pepper (Capsicum annuum L.). Metabolites 2023, 13, 820. [Google Scholar] [CrossRef]
- Emmanuel-Ikpeme, C. Comparative evaluation of the nutritional, phytochemical and microbiological quality of three pepper varieties. J. Food Nutr. Sci. 2014, 2, 74–80. [Google Scholar] [CrossRef]
- Shaha, R.K.; Rahman, S.; Asrul, A. Bioactive compounds in chili peppers (Capsicum annuum L.) at various ripening (green, yellow and red) stages. Ann. Biol. Res. 2013, 4, 27–34. [Google Scholar]
- Khaitov, B.; Umurzokov, M.; Cho, K.M.; Lee, Y.J.; Park, K.W.; Sung, J. Importance and production of chili pepper; heat tolerance and efficient nutrient use under climate change conditions. Korean J. Agric. Sci. 2019, 46, 769–779. [Google Scholar] [CrossRef]
- Variyar, P.S.; Singh, I.P.; Adiani, V.; Suprasanna, P. Peppers: Biological, Health, and Postharvest Perspectives, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-032-45353-8. [Google Scholar]
- Lozada, D.N.; Bosland, P.W.; Barchenger, D.W.; Haghshenas-Jaryani, M.; Sanogo, S.; Walker, S. Chile pepper (Capsicum) breeding and improvement in the “multi-omics” era. Front. Plant Sci. 2022, 13, 879182. [Google Scholar] [CrossRef]
- Madala, N.; Nutakki, M.K. Hot pepper-history-health and dietary benefits & production. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2532–2538. [Google Scholar]
- Hernández-Pérez, T.; Gómez-García, M.d.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef]
- Alonso-Villegas, R.; González-Amaro, R.M.; Figueroa-Hernández, C.Y.; Rodríguez-Buenfil, I.M. The genus Capsicum: A review of bioactive properties of its polyphenolic and capsaicinoid composition. Molecules 2023, 28, 4239. [Google Scholar] [CrossRef]
- Joshi, D.D.; Somkuwar, B.G.; Kharkwal, H.; Chander, S. Aroma based varieties of Capsicum chinense Jacq., geographical distribution and scope for expansion of the species. J. Appl. Res. Med. Aromat. Plants 2022, 29, 100379. [Google Scholar] [CrossRef]
- Ahmad, F.; Kusumiyati, K.; Soleh, M.A.; Khan, M.R.; Sundari, R.S. Chili crop innovation: Exploring enclosed growing designs for varied varieties—A review. Agrosyst. Geosci. Environ. 2024, 7, e20491. [Google Scholar] [CrossRef]
- Cantliffe, D.J.; Shaw, N.; Jovicich, E.; Rodriguez, J.C.; Secker, I.; Karchi, Z. Passive ventilated high-roof greenhouse production of vegetables in a humid, mild winter climate. Acta Hortic. 2001, 1, 195–202. [Google Scholar] [CrossRef]
- Zapata-García, S.; Temnani, A.; Berríos, P.; Espinosa, P.J.; Monllor, C.; Pérez-Pastor, A. Optimizing crop water productivity in greenhouse pepper. Agronomy 2024, 14, 902. [Google Scholar] [CrossRef]
- Alvarado, K.A.; Mill, A.; Pearce, J.M.; Vocaet, A.; Denkenberger, D. Scaling of greenhouse crop production in low sunlight scenarios. Sci. Total Environ. 2020, 707, 136012. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Erol, Ü.H. Morphological, phytochemical, biochemical, and enzymatic evaluation of pepper species (Capsicum annuum L. and Capsicum chinense Jacq.) at developmental stages under drought stress. Russ. J. Plant Physiol. 2023, 71, 106. [Google Scholar] [CrossRef]
- Wassie, W.A.; Andualem, A.M.; Molla, A.E.; Tarekegn, Z.G.; Aragaw, M.W.; Ayana, M.T. Growth, physiological, and biochemical responses of Ethiopian red pepper (Capsicum annuum L.) cultivars to drought stress. Sci. World J. 2023, 2023, 4374318. [Google Scholar] [CrossRef]
- Molla, A.E.; Andualem, A.M.; Ayana, M.T.; Zeru, M.A. Effects of drought stress on growth, physiological and biochemical parameters of two Ethiopian red pepper (Capsicum annuum L.) cultivars. J. Appl. Hortic. 2023, 25, 32–38. [Google Scholar] [CrossRef]
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Mahmood, T.; Rana, R.M.; Ahmar, S.; Saeed, S.; Gulzar, A.; Khan, M.A.; Wattoo, F.M.; Wang, X.; Branca, F.; Mora-Poblete, F.; et al. Effect of drought stress on capsaicin and antioxidant contents in pepper genotypes at reproductive stage. Plants 2021, 10, 1286. [Google Scholar] [CrossRef]
- Sahitya, U.L.; Krishna, M.S.R.; Deepthi, R.S. Biochemical and physiological changes induced by water stress in hot pepper (Capsicum annuum L.) genotypes. Plant Cell Biotech. Mol. Biol. 2018, 19, 179–195. [Google Scholar]
- Sun, Y.; Niu, G.; Osuna-Avila, P. Effects of substrate moisture content on growth and physiological response of chili pepper (Capsicum annuum L.). Agrociencia 2019, 53, 59–72. [Google Scholar]
- Pedroza-Sandoval, A.; Minjares-Fuentes, J.R.; Trejo-Calzada, R.; Gramillo-Avila, I. Physiological and productivity responses in two chili pepper morphotypes (Capsicum annuum L.) under different soil moisture contents. Horticulturae 2024, 10, 92. [Google Scholar] [CrossRef]
- Zhang, R.; Chai, Y.; Liang, X.; Liu, X.; Wang, X.; Hu, Z. A new plant-wearable sap flow sensor reveals the dynamic water distribution during watermelon fruit development. Horticulturae 2024, 10, 649. [Google Scholar] [CrossRef]
- Mancha, L.A.; Uriarte, D.; Prieto, M.D.H. Characterization of the transpiration of a vineyard under different irrigation strategies using sap flow sensors. Water 2021, 13, 2867. [Google Scholar] [CrossRef]
- Sharma, H.; Shukla, M.K.; Bosland, P.W.; Steiner, R. Soil moisture sensor calibration, actual evapotranspiration, and crop coefficients for drip irrigated greenhouse chile peppers. Agric. Water Manag. 2017, 179, 81–91. [Google Scholar] [CrossRef]
- Priandana, K.; Wahyu, R.A.F. Development of automatic plant irrigation system using soil moisture sensors for precision agriculture of chili. In Proceedings of the 2020 International Conference on Smart Technology and Applications (ICoSTA), Surabaya, Indonesia, 20–22 February 2020; IEEE: New York, NY, USA, 2020; pp. 1–4. [Google Scholar]
- Gultom, J.H.; Harsono, M.; Khameswara, T.D.; Santoso, H. Smart IoT water sprinkle and monitoring system for chili plant. In Proceedings of the 2017 International Conference on Electrical Engineering and Computer Science (ICECOS), Palembang, Indonesia, 22–23 August 2017; IEEE: New York, NY, USA, 2017; pp. 212–216. [Google Scholar]
- Ahmad, F.; Kusumiyati, K.; Arief Soleh, M.; Rabnawaz Khan, M.; Siti Sundari, R. Microclimates growing and watering volumes influences the physiological traits of chili pepper cultivars in combating abiotic stress. Sci. Rep. 2025, 15, 4183. [Google Scholar] [CrossRef]
- García-Rodríguez, L.D.C.; Morales-Viscaya, J.A.; Prado-Olivarez, J.; Barranco-Gutiérrez, A.I.; Padilla-Medina, J.A.; Espinosa-Calderón, A. Fuzzy mathematical model of photosynthesis in jalapeño pepper. Agriculture 2024, 14, 909. [Google Scholar] [CrossRef]
- Bachtiar, A.N.; Faisal, M.; Darwis, M. Development of smart plant watering system application based on internet of things. JISA (J. Inform. Dan Sains) 2025, 8, 74–80. [Google Scholar] [CrossRef]
- Kim, K.H.; Shawon, M.R.A.; An, J.H.; Lee, H.J.; Kwon, D.J.; Hwang, I.; Bae, J.H.; Choi, K.Y. Effect of shade screen on sap flow, chlorophyll fluorescence, NDVI, plant growth and fruit characteristics of cultivated paprika in greenhouse. Agriculture 2022, 12, 1405. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, C.; Wang, G.; He, B.; Hao, B.; Han, Y.; Wang, B.; Bao, R.; Syed, T.N. A review of precision irrigation water-saving technology under changing climate for enhancing water use efficiency, crop yield, and environmental footprints. Agriculture 2024, 14, 1141. [Google Scholar] [CrossRef]
- Patle, G.T.; Kumar, M.; Khanna, M. Climate-smart water technologies for sustainable agriculture: A review. J. Water Clim. Change 2020, 11, 1455–1466. [Google Scholar] [CrossRef]
- Toromade, A.S.; Soyombo, D.A.; Kupa, E.; Ijomah, T.I. Reviewing the impact of climate change on global food security: Challenges and solutions. Int. J. Appl. Res. Soc. Sci. 2024, 6, 1403–1416. [Google Scholar] [CrossRef]
- Atmos 41 Gen 2 Weather Station; METER Group. Available online: https://metergroup.com/products/atmos-41/ (accessed on 1 March 2025).
- Sharma, H.; Reinhardt, K.; Lohse, K.A. Fundamental intra-specific differences in plant–water relations in a widespread desert shrub (Artemisia tridentata). Plant Ecol. 2020, 221, 925–938. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, Y.; Cheng, R.; Li, T. Photosynthetic induction of the leaves varies among pepper cultivars due to stomatal oscillation. Sci. Hortic. 2023, 318, 112126. [Google Scholar] [CrossRef]
- Goo, H.; Roh, Y.; Lee, J.; Park, K.S. Analysis of bell pepper (Capsicum annuum L.) leaf spectral properties and photosynthesis according to growth period. Horticulturae 2024, 10, 646. [Google Scholar] [CrossRef]
- Sharma, H.; Shukla, M.K.; Bosland, P.W.; Steiner, R.L. Physiological responses of greenhouse-grown drip-irrigated chile pepper under partial root zone drying. HortScience 2015, 50, 1224–1229. [Google Scholar] [CrossRef]
- Marutani, M.; Clemente, S.; Bacalia, K.; Santos, E.; Kotwal, S.; Valerio, G.; Calalang, J.; Sala, N.; Delfin, M.; Desamito, C.; et al. Plant, Food, Natural Resources, and Sustainable Agriculture Factsheet 2021-2: Phytochemical Analyses by HPLC; University of Guam, College of Natural and Applied Sciences: Mangilao, Guam, 2021. [Google Scholar]
- Chang, L.; Liu, M.J.; Lyu, J.L.; Du, S. Characteristics of soil moisture limitation and non-limitation in the response of sap flow to transpiration driving factors. J. Appl. Ecol. 2024, 35, 1064–1072. [Google Scholar]
- Alongi, F.; Petek-Petrik, A.; Mukarram, M.; Torun, H.; Schuldt, B.; Petrík, P. Somatic drought stress memory affects leaf morpho-physiological traits of plants via epigenetic mechanisms and phytohormonal signalling. Plant Gene 2025, 42, 100509. [Google Scholar] [CrossRef]
- Lukić, N.; Kukavica, B.; Davidović-Plavšić, B.; Hasanagić, D.; Walter, J. Plant stress memory is linked to high levels of antioxidative enzymes over several weeks. Environ. Exp. Bot. 2020, 178, 104166. [Google Scholar] [CrossRef]
- Hussain, T.; Ayyub, C.M.; Amjad, M.; Hussain, M. Analysis of morpho-physiological changes occurring in chili genotypes (Capsicum spp.) under high temperature conditions. Pak. J. Agric. Sci. 2021, 58, 43–50. [Google Scholar]
- Nieto-Garibay, A.; Barraza, A.; Caamal-Chan, G.; Murillo-Amador, B.; Troyo-Diéguez, E.; Burgoa-Cruz, C.A.; Chaves, M. Habanero pepper (Capsicum chinense) adaptation to water-deficit stress in a protected agricultural system. Funct. Plant Biol. 2022, 49, 295–306. [Google Scholar] [CrossRef]
- Kulkarni, M.; Phalke, S. Evaluating variability of root size system and its constitutive traits in hot pepper (Capsicum annuum L.) under water stress. Sci. Hortic. 2009, 120, 159–166. [Google Scholar] [CrossRef]
- Goto, K.; Yabuta, S.; Ssenyonga, P.; Tamaru, S.; Sakagami, J.-I. Response of leaf water potential, stomatal conductance and chlorophyll content under different levels of soil water, air vapor pressure deficit and solar radiation in chili pepper (Capsicum chinense). Sci. Hortic. 2021, 281, 109943. [Google Scholar] [CrossRef]
- Ou, L.J.; Zou, X.X. The photosynthetic stress responses of five pepper species are consistent with their genetic variability. Photosynthetica 2012, 50, 49–55. [Google Scholar] [CrossRef]
- Erwin, J.; Hussein, T.; Baumler, D.J. Pepper photosynthesis, stomatal conductance, transpiration, and water use efficiency differ with variety, indigenous habitat, and species of origin. HortScience 2019, 54, 1662–1666. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ağar, G.; Ors, S.; Dursun, A.; Kul, R.; Akgül, G. Physiological and biochemical changes of pepper cultivars under combined salt and drought stress. Gesunde Pflanz. 2022, 74, 675–683. [Google Scholar] [CrossRef]
- Ferrara, A.; Lovelli, S.; Di Tommaso, T.; Perniola, M. Flowering, growth and fruit setting in greenhouse bell pepper under water stress. J. Agron. 2011, 10, 12–19. [Google Scholar] [CrossRef]
- Widuri, L.I.; Lakitan, B.; Sakagami, J.; Yabuta, S.; Kartika, K.; Siaga, E. Short-term drought exposure decelerated growth and photosynthetic activities in chili pepper (Capsicum annuum L.). Ann. Agric. Sci. 2020, 65, 149–158. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.H.; Park, K.S.; Son, J.E. Evaluating plant stress conditions in paprika by comparing internal electrical conductivity, photosynthetic response, and sap flow. Hortic. Environ. Biotechnol. 2019, 60, 41–48. [Google Scholar] [CrossRef]
- Stommel, J.R.; Camp, M.J.; Dumm, J.M.; Haynes, K.G.; Luo, Y.; Schoevaars, A.M. Heritability of fresh-cut fruit quality attributes in Capsicum. J. Am. Soc. Hortic. Sci. 2016, 141, 308–314. [Google Scholar] [CrossRef]
- Lozada, D.N.; Whelpley, M.; Acuña-Galindo, A. Genetic architecture of chile pepper (Capsicum spp.) QTLome revealed using meta-QTL analysis. Horticulturae 2021, 7, 227. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Pascual, B.; Nájera, I.; Domene, M.A.; Baixauli, C.; Pascual-Seva, N. Effects of deficit irrigation on the yield and irrigation water use efficiency of drip-irrigated sweet pepper (Capsicum annuum L.) under Mediterranean conditions. Irrig. Sci. 2020, 38, 89–104. [Google Scholar] [CrossRef]
- Macias-Bobadilla, I.; Vargas-Hernandez, M.; Guevara-Gonzalez, R.G.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Torres-Pacheco, I. Differential response to water deficit in chili pepper (Capsicum annuum L.) growing in two types of soil under different irrigation regimes. Agriculture 2020, 10, 381. [Google Scholar] [CrossRef]
- Mohammed, S.; Hussen, A. Influence of deficit irrigation levels on agronomic performance of pepper (Capsicum annuum L.) under drip at Alage, central rift valley of Ethiopia. PLoS ONE 2023, 18, e0280639. [Google Scholar] [CrossRef]
- Valiente-Banuet, J.I.; Gutiérrez-Ochoa, A. Effect of irrigation frequency and shade levels on vegetative growth, yield, and fruit quality of piquin pepper (Capsicum annuum L. var. glabriusculum). HortScience 2016, 51, 573–579. [Google Scholar] [CrossRef]
- Sahana, M.F.; Sugirtharan, M. Effect of different irrigation intervals on growth and yield of chili crop grown in sandy soil. AGRIEAST J. Agric. Sci. 2021, 15, 1–13. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Yu, H.; Yang, X.; Jiang, W. Deficit irrigation affects growth, yield, vitamin C content, and irrigation water use efficiency of hot pepper grown in soilless culture. HortScience 2014, 49, 722–728. [Google Scholar] [CrossRef]
- Morgan, E.I.; Zamora, E.; Nelson, S.D.; Donato-Molina, C.; Anoruo, A. Effects of deficit irrigation in pepper plants (Capsicum annuum) grown under greenhouse conditions. World J. Agric. Soil Sci. 2024, 9, 1–7. [Google Scholar]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lau, N.; Medina-Lara, F.; Minero-García, Y.; Zamudio-Moreno, E.; Guzmán-Antonio, A.; Echevarría-Machado, I.; Martínez-Estévez, M. Water deficit affects the accumulation of capsaicinoids in fruits of Capsicum chinense Jacq. HortScience 2011, 46, 487–492. [Google Scholar] [CrossRef]
- Khan, M.; Farooque, A.; Haque, M.; Rahim, M.; Hoque, M. Effects of water stress at various growth stages on the physio-morphological characters and yield in chili. Bangladesh J. Agric. Res. 1970, 33, 353–362. [Google Scholar] [CrossRef]
- Zamljen, T.; Jakopič, J.; Hudina, M.; Veberič, R.; Slatnar, A. Influence of intra- and interspecies variation in chilies (Capsicum spp.) on metabolite composition of three fruit segments. Sci. Rep. 2021, 11, 4932. [Google Scholar] [CrossRef]
- Phillips, N.; Bergh, J.; Oren, R.; Linder, S. Effects of nutrition and soil water availability on water use in a Norway spruce stand. Tree Physiol. 2001, 21, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Moreshet, S.; Takagaki, M.; Shinohara, Y.; Ito, T. Effects of drought stress on sap flow, stomatal conductance, and leaf water potential of pepper cultivars (Capsicum annuum L.). Jpn. J. Trop. Agric. 2003, 47, 61–69. [Google Scholar]
- Agele, S.; Cohen, S.S.; Assouline, S. Hydraulic characteristics and water relations of net house-grown bell pepper as affected by irrigation regimes in a Mediterranean climate. Environ. Exp. Bot. 2006, 57, 226–235. [Google Scholar] [CrossRef]
- Yao, C.; Moreshet, S.; Aloni, B. Water relations and hydraulic control of stomatal behaviour in bell pepper plant in partial soil drying. Plant Cell Environ. 2001, 24, 227–235. [Google Scholar] [CrossRef]
- Phimchan, P.; Techawongstien, S.; Chanthai, S.; Bosland, P.W. Impact of drought stress on the accumulation of capsaicinoids in Capsicum cultivars with different initial capsaicinoid levels. HortScience 2012, 47, 1204–1209. [Google Scholar] [CrossRef]
- Jinagool, W.; Arom, P. Influence of watering regimes on physiological traits, growth, yield, and capsaicin content of chilies. ASEAN J. Sci. Technol. Rep. 2023, 26, 20–31. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Adalid-Martínez, A.M.; Pires, C.K.; Ribes-Moya, A.M.; Fita, A.; Rodríguez-Burruezo, A. The effect of the varietal type, ripening stage, and growing conditions on the content and profile of sugars and capsaicinoids in Capsicum peppers. Plants 2023, 12, 231. [Google Scholar] [CrossRef]
- Phimchan, P.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in Capsicum under drought stress. J. Agric. Food Chem. 2014, 62, 7057–7062. [Google Scholar] [CrossRef] [PubMed]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Stability analysis of yield and capsaicinoids content in chili (Capsicum spp.) grown across six environments. Euphytica 2012, 187, 11–18. [Google Scholar] [CrossRef]
- RMSMB, R.; Minami, M.; Nemoto, K.; Prabandaka, S.S.; Matsushima, K. Relationship between water supply and sugar and capsaicinoids contents in fruit of chili pepper (Capsicum annuum L.). Hortic. J. 2021, 90, 58–67. [Google Scholar] [CrossRef]
- Flores, N.; Delgado, E.; Walker, S.; Rojas-Contreras, J.; Pámanes-Carrasco, G.A. Effect of water stress on functional and marketable properties of roasted Big Jim chile pepper (Capsicum annuum) in the southern USA. Acta Agric. Pecu. 2020, 6, 1–8. [Google Scholar]
- Mahendran, S.; Bandara, D.C. Effects of soil moisture stress at different growth stages on vitamin C, capsaicin and β-carotene contents of chili (Capsicum annuum L.) fruits and their impact on yield. Trop. Agric. Res. 2000, 12, 95–106. [Google Scholar]
- Zamljen, T.; Zupanc, V.; Slatnar, A. Influence of irrigation on yield and primary and secondary metabolites in two chilies species, Capsicum annuum L. and Capsicum chinense. Jacq. Agric. Water Manag. 2020, 234, 106104. [Google Scholar] [CrossRef]
- Haris, M.M.; Silva, T.M.; Gulub, G.; Terada, N.; Shinohara, T.; Sanada, A.; Koshio, K. Growth, quality and capsaicin concentration of hot pepper (Capsicum annuum) under drought conditions. J. ISSAAS 2020, 26, 100–110. [Google Scholar]
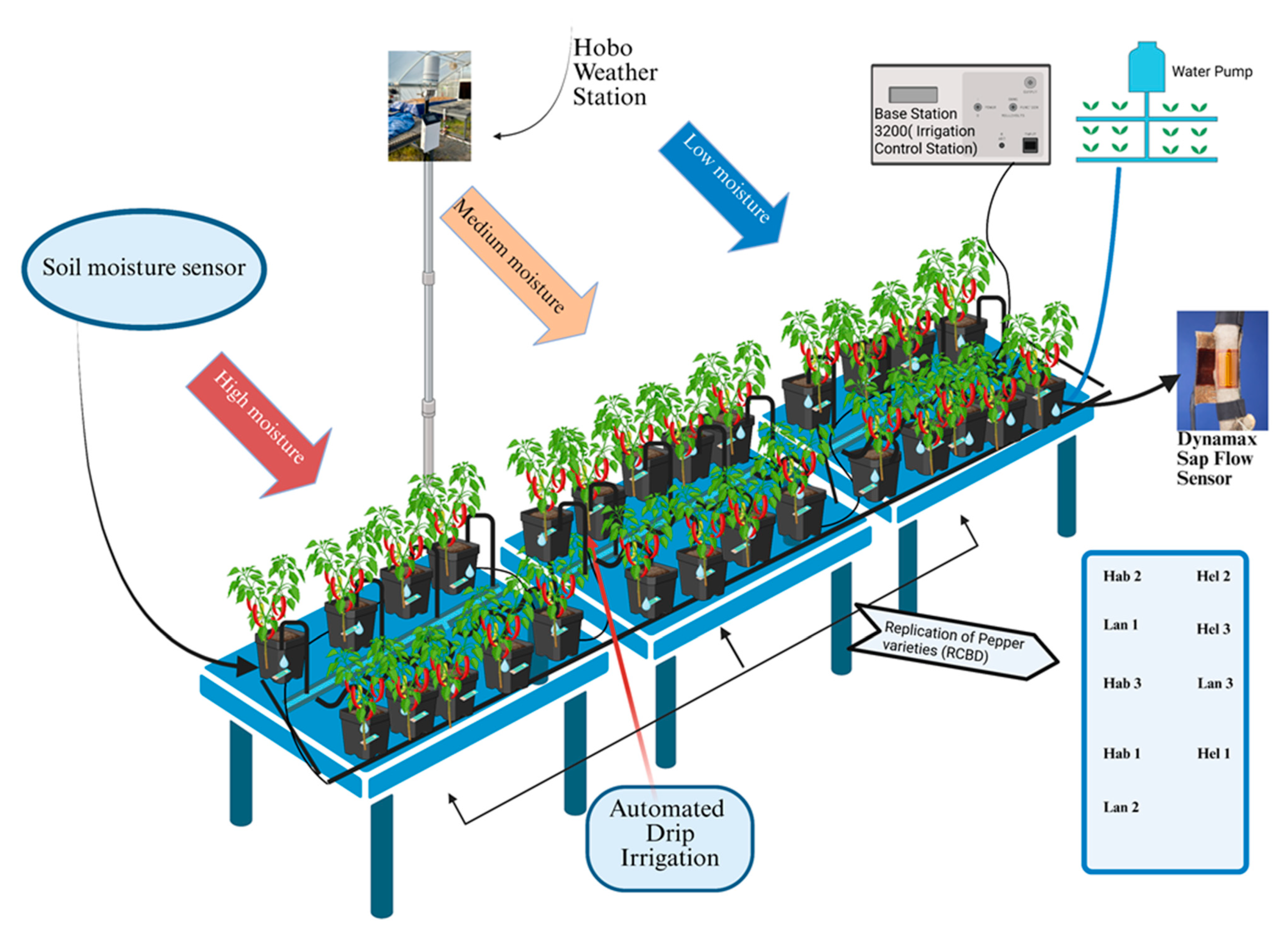
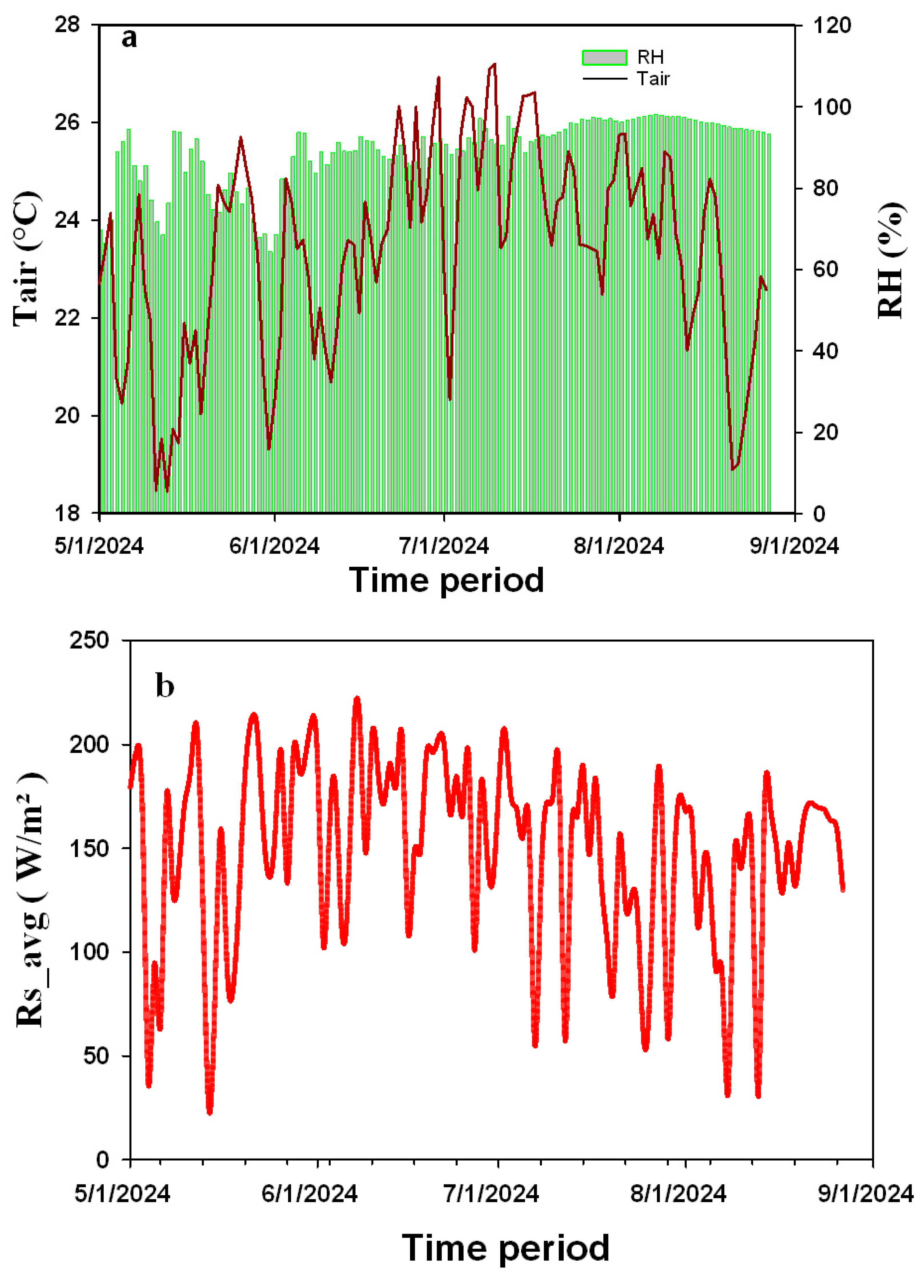





| Growth Stage | Fertilizer Type | Concentration | Dilution Rate | Volume Per Pot |
|---|---|---|---|---|
| Vegetative Phase | Alaska Fish (5-1-1) | 90 mL/2 gal water | Applied at 2- and 4-week intervals | 200 mL |
| Reproductive Phase | Tiger Bloom (2-8-4) | 100 mL/5 gal water | Applied biweekly | 500 mL initially, increased to 1000 mL |
| Reproductive Phase | Alaska Fish (5-1-1) | 90 mL/2 gal water | Alternated biweekly with Tiger Bloom | Increased to 500 mL |
| Measurements | Factors | dF | F-Values | p-Values |
|---|---|---|---|---|
| Photosynthetic rate (Pn) | Pepper varieties | 2 | 0.0848 | 0.9187 |
| Irrigation levels | 2 | 7.4670 | 0.0007 | |
| Pepper varieties × Irrigation levels | 4 | 0.2659 | 0.8998 | |
| Transpiration rate (E) | Pepper varieties | 2 | 0.6431 | 0.5263 |
| Irrigation levels | 2 | 0.1149 | 0.8915 | |
| Pepper varieties × Irrigation levels | 4 | 0.1437 | 0.9657 | |
| Stomatal Conductance rate (gs) | Pepper varieties | 2 | 0.0734 | 0.7789 |
| Irrigation levels | 2 | 0.2500 | 0.9293 | |
| Pepper varieties × Irrigation levels | 4 | 0.1656 | 0.9558 | |
| Height (cm) | Pepper varieties | 2 | 5.8541 | 0.0031 |
| Irrigation levels | 2 | 8.0941 | 0.0003 | |
| Pepper varieties × Irrigation levels | 4 | 0.0283 | 0.9985 | |
| Stem diameter (mm) | Pepper varieties | 2 | 5.9272 | 0.0028 |
| Irrigation levels | 2 | 0.0223 | 0.9779 | |
| Pepper varieties × Irrigation levels | 4 | 0.3810 | 0.8223 | |
| Fruit number (pods/plant) | Pepper varieties | 2 | 0.6935 | 0.5085 |
| Irrigation levels | 2 | 0.4288 | 0.6557 | |
| Pepper varieties × Irrigation levels | 4 | 0.2874 | 0.8835 | |
| Average fruit weight (g) | Pepper varieties | 2 | 6.0731 | 0.0066 |
| Irrigation levels | 2 | 3.1694 | 0.0580 | |
| Pepper varieties × Irrigation levels | 4 | 1.4556 | 0.2432 | |
| Total average Pod yield (g/plant) | Pepper varieties | 2 | 8.3747 | 0.0015 |
| Irrigation levels | 2 | 1.6319 | 0.2143 | |
| Pepper varieties × Irrigation levels | 4 | 0.4731 | 0.7550 | |
| Scoville Heat Unit (SHU) | Pepper varieties | 2 | 7.9134 | 0.0020 |
| Irrigation levels | 2 | 0.8017 | 0.4590 | |
| Pepper varieties × Irrigation levels | 4 | 0.6756 | 0.6147 | |
| Capsaicin (%) | Pepper varieties | 2 | 8.2576 | 0.0016 |
| Irrigation levels | 2 | 2.0375 | 0.1499 | |
| Pepper varieties × Irrigation levels | 4 | 1.7591 | 0.1663 | |
| Dihydrocapsaicin (%) | Pepper varieties | 2 | 6.8828 | 0.0038 |
| Irrigation levels | 2 | 2.1381 | 0.1374 | |
| Pepper varieties × Irrigation levels | 4 | 1.7186 | 0.1750 | |
| Nordihydrocapsaicin (%) | Pepper varieties | 2 | 17.7409 | <0.0001 |
| Irrigation levels | 2 | 0.2715 | 0.7643 | |
| Pepper varieties × Irrigation levels | 4 | 1.2146 | 0.3277 | |
| Sap flux rate (g d−1) | Pepper varieties | 2 | 24.5647 | <0.0001 |
| Irrigation levels | 2 | 45.0686 | <0.0001 | |
| Pepper varieties × Irrigation levels | 4 | 0.0892 | 0.9858 |
| Variety | Irrigation Treatment | Number of Pods (n) | Average Pod Weight (g) | Average Pod Yield (g/Plant) |
|---|---|---|---|---|
| Habanero | Low | 111.5 ± 11.46 (a) | 6.60 ± 0.51 (a) | 730.87 ± 57.03 (a) |
| Medium | 114.5 ± 9.43 (a) | 5.67 ± 0.27 (a) | 646.14 ± 50.25 (a) | |
| High | 103.5 ± 16.15 (a) | 7.48 ± 0.51 (a) | 774.06 ± 125.40 (a) | |
| Lantern | Low | 97 ± 10.67 (a) | 5.42 ± 0.51 (a) | 508.94 ± 68.87 (a) |
| Medium | 101.25 ± 25.63 (a) | 6.02 ± 0.96 (a) | 542.25 ± 63.62 (a) | |
| High | 95.75 ± 4.44 (a) | 5.76 ± 0.51 (a) | 557.84 ± 73.37 (a) | |
| Helios | Low | 95 ± 7.39 (a) | 7.42 ± 0.51 (a) | 704.93 ± 60.95 (a) |
| Medium | 117.5 ± 16.91 (a) | 6.26 ± 0.24 (a) | 731.72 ± 96.42 (a) | |
| High | 113 ± 4.85 (a) | 7.87 ± 0.51 (a) | 883.87 ± 28.13 (a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, H.; Kwekutsu, E.; Bhowmik, A.; Sharma, H. Evaluating Water Use Dynamics and Yield Responses in Capsicum chinense Cultivars Using Integrated Sensor-Based Irrigation System. Horticulturae 2025, 11, 978. https://doi.org/10.3390/horticulturae11080978
Sidhu H, Kwekutsu E, Bhowmik A, Sharma H. Evaluating Water Use Dynamics and Yield Responses in Capsicum chinense Cultivars Using Integrated Sensor-Based Irrigation System. Horticulturae. 2025; 11(8):978. https://doi.org/10.3390/horticulturae11080978
Chicago/Turabian StyleSidhu, Harjot, Edmond Kwekutsu, Arnab Bhowmik, and Harmandeep Sharma. 2025. "Evaluating Water Use Dynamics and Yield Responses in Capsicum chinense Cultivars Using Integrated Sensor-Based Irrigation System" Horticulturae 11, no. 8: 978. https://doi.org/10.3390/horticulturae11080978
APA StyleSidhu, H., Kwekutsu, E., Bhowmik, A., & Sharma, H. (2025). Evaluating Water Use Dynamics and Yield Responses in Capsicum chinense Cultivars Using Integrated Sensor-Based Irrigation System. Horticulturae, 11(8), 978. https://doi.org/10.3390/horticulturae11080978






