GC-MS Non-Target Metabolomics-Based Analysis of the Volatile Aroma in Cerasus humilis After Grafting with Different Rootstocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cultivation and Sampling
2.3. Chemical Reagents
2.4. Headspace Solid-Phase Microextraction (HS–SPME)
2.5. Determination of Volatile Compounds Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.6. Data Preprocessing and Annotation
3. Results
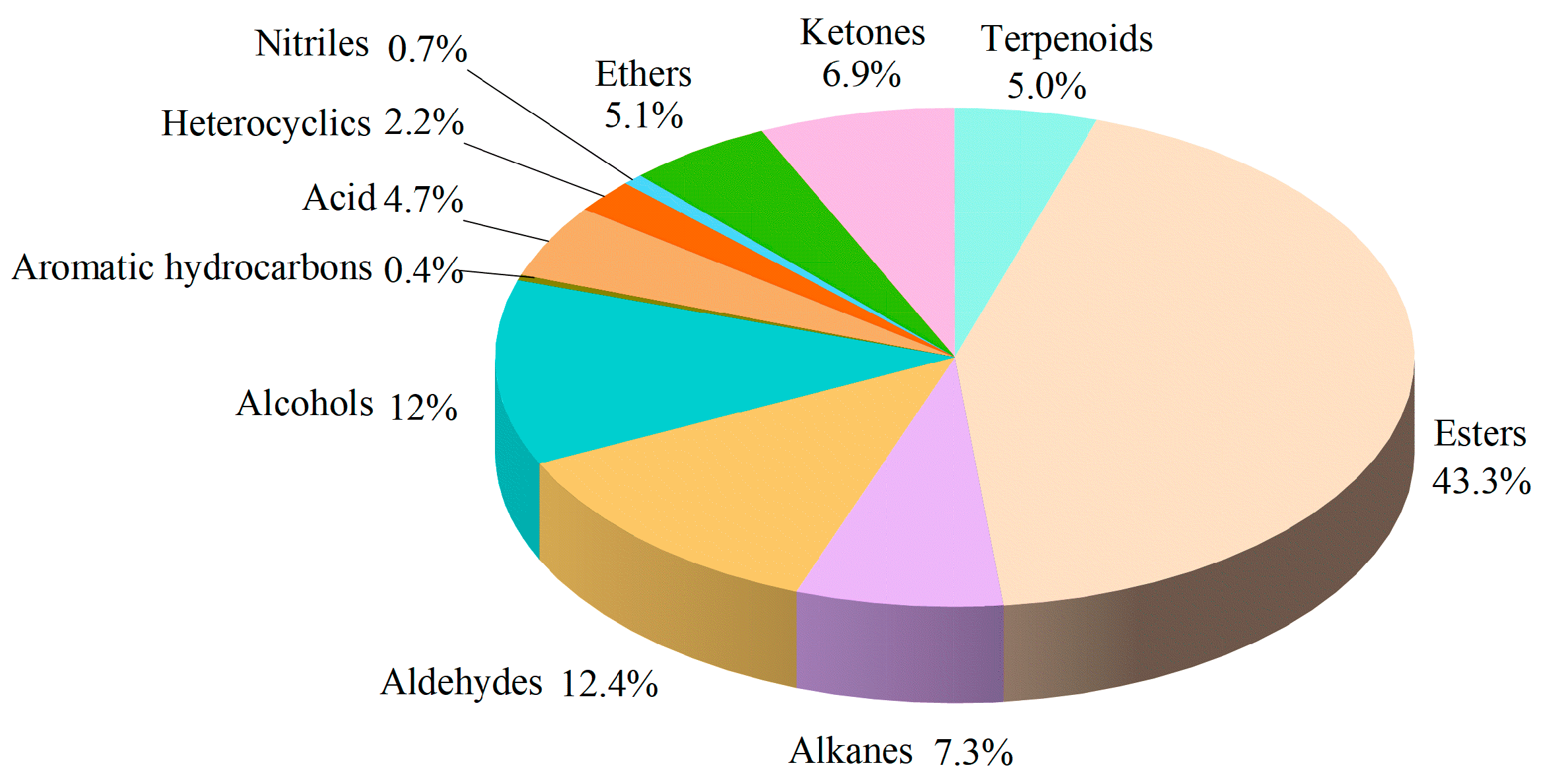
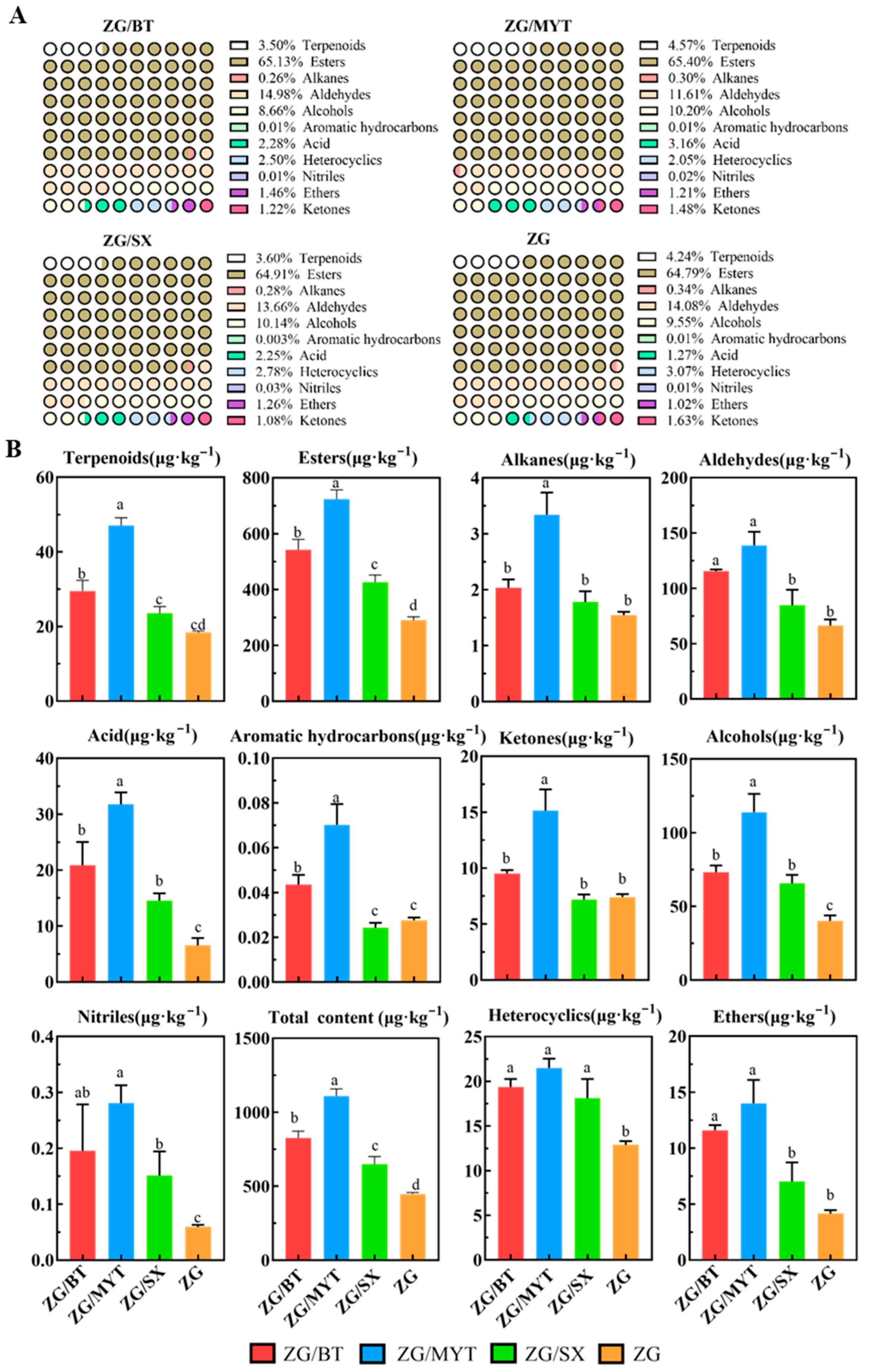
3.1. Characteristics of Volatile Compounds in Different Stock and Spike Combinations
3.2. Characteristic Volatile Compounds of Different Rootstock–Scion Combinations
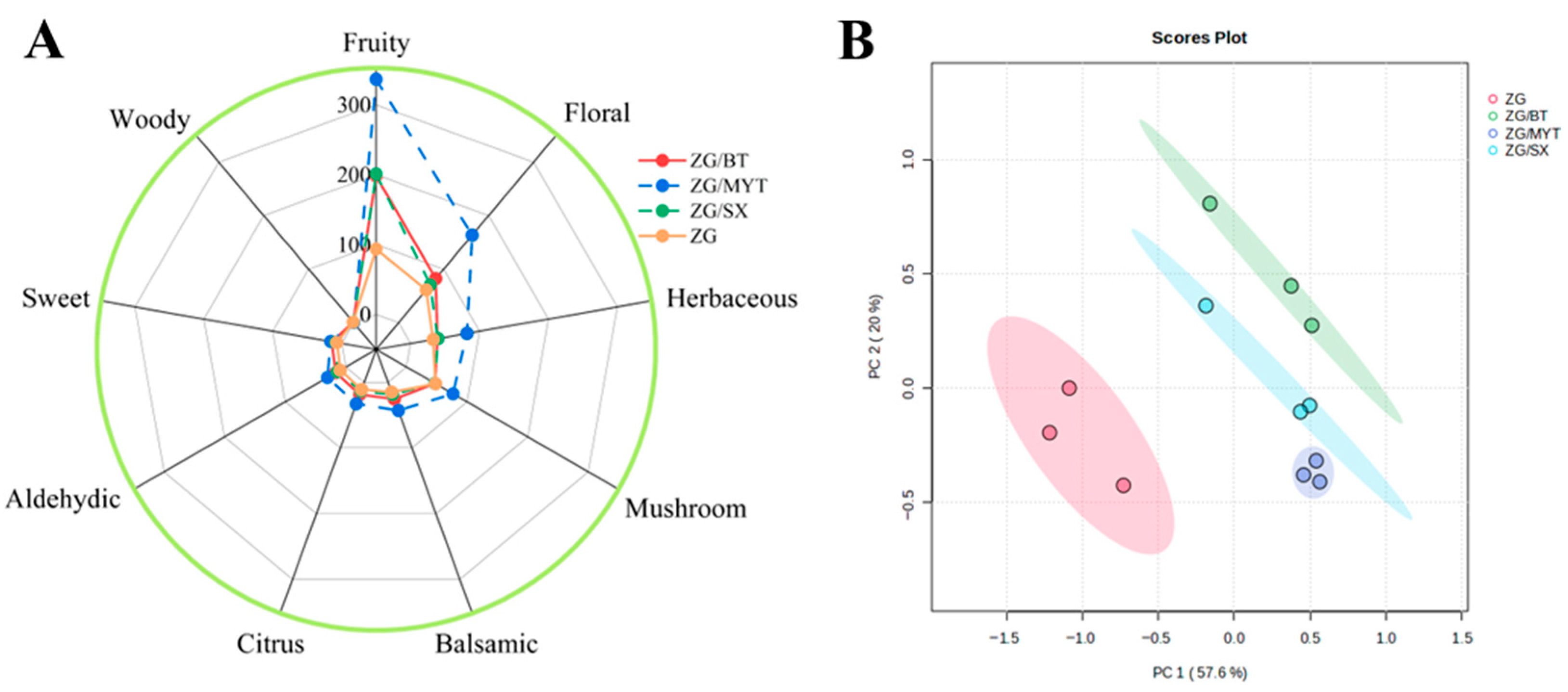
3.3. Analysis of Aromatic Series
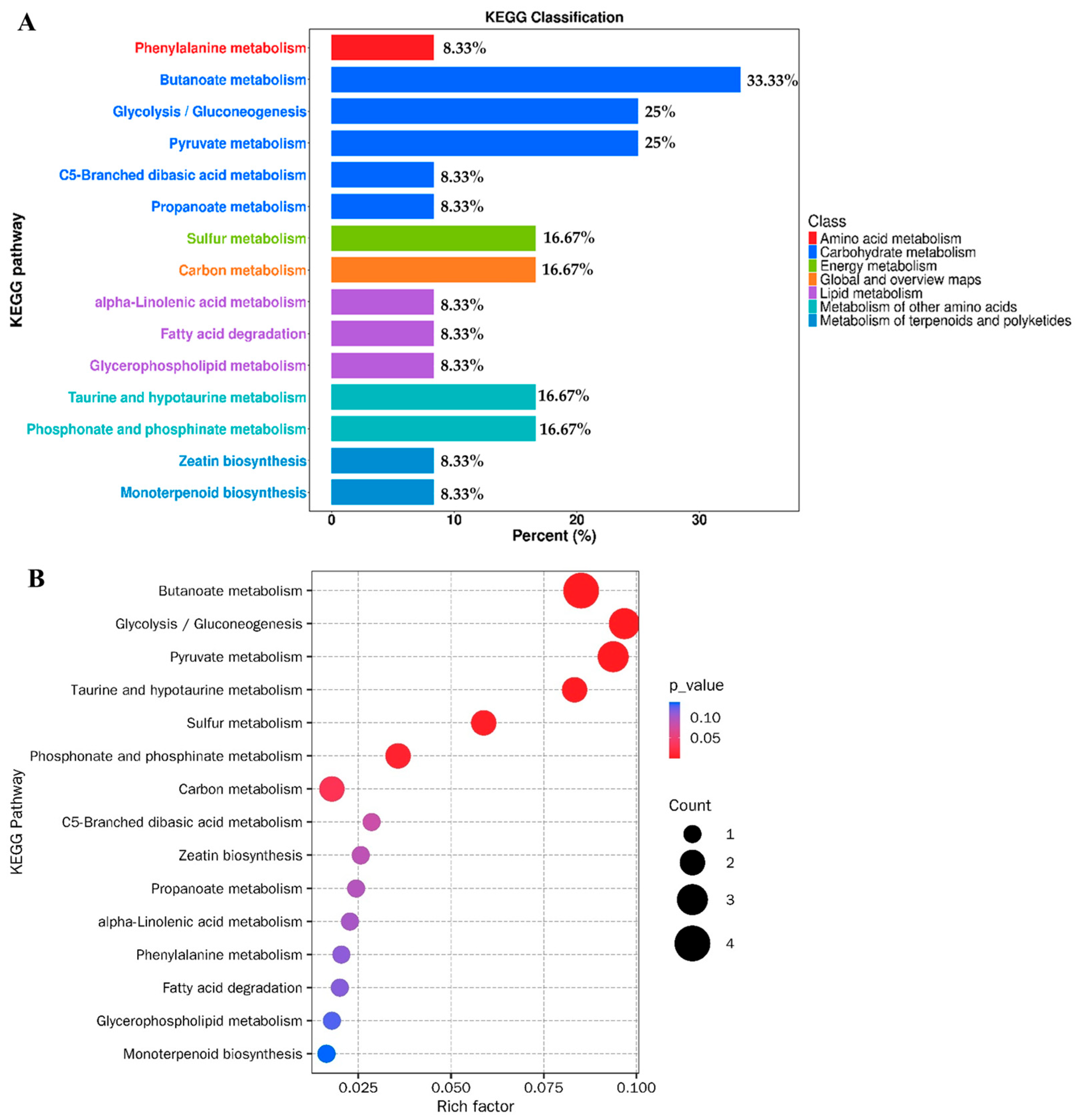
3.4. KEGG Classification and Enrichment Analysis of Differential Metabolites
4. Discussion
4.1. Regulatory Effect of Rootstocks on the Composition of Volatile Compounds in C. humilis Fruits
4.2. Differences in Key Aroma Compounds and Their Flavor Contributions
4.3. The Regulatory Mechanism of Metabolic Pathways on the Formation of Aroma Induced by Rootstocks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| OAV | Odor activity value |
| AAT | Amino acid transporter |
| MEP | 2-C-methyl-D-erythritol 4-phosphate pathway |
| MVA | Mevalonate pathway |
| IPP | Isopentenyl pyrophosphate |
| DMAPP | Dimethylallyl pyrophosphate |
| LOX | Lipoxygenase |
| ATP | Adenosine triphosphate |
| TPS | Terpene synthase |
References
- Hua, Q.Y.; Wei, T.; Han, W.; Wu, G.H.; Liu, Q. Progress in the study of composition, volatile flavor compounds and bioactivity of Chinese dwarf cherry. Food Mach. 2024, 40, 186–192. [Google Scholar]
- Liu, M.; Du, J.J. Distribution of European Plum germplasm resources and expression of variety Traits. Deciduous Fruit Tree 2010, 3, 18–21. [Google Scholar]
- Khoo, G.M.; Clausen, M.R.; Pedersen, B.H.; Larsen, E. Bioactivity and total phenolic content of 34 sour cherry cultivars. J. Food Compos. 2011, 24, 772–776. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Si, Q.; Zhai, R.; Zhou, Y.B.; Zhang, X. Comparison of nutrients in wild and cultivated Chinese dwarf cherry. Food Res. Dev. 2017, 38, 104–108. [Google Scholar]
- Liu, J.Y.; Xin, X.L.; Lan, R. Study on detection of amino acids in Chinese dwarf cherry juice with isotope internal standard method. Food Res. Dev. 2010, 31, 140–143. [Google Scholar]
- Xu, M.L.; Qiao, G.X.; Liu, Y.J. The effects of different rootstocks on the survival and fruit quality of European plum grafting. J. Nucl. Agric. Sci. 2023, 37, 2503–2509. [Google Scholar]
- Sun, L.; Wang, X.Y.; Wang, H.L.; Yan, A.L.; Zhang, G.J.; Ren, J.C.; Xu, H.Y. The Influence of Rootstocks on the Growth and Aromatic Quality of Two Table Grape Varieties. Sci. Agric. Sin. 2021, 54, 4405–4420. [Google Scholar]
- Han, X.; Yang, H.Y.; Chen, W.K. Comparison of Aroma Compounds in Fruits of Vitis vinifera cv. Danna Grafted on Different Rootstocks. Food Sci. 2022, 43, 223–231. [Google Scholar]
- Zhao, L.; Jiang, Z.; Song, L. Effects of Different Rootstocks on Fruit Quality and Aroma Compounds of Red Chief Apples. J. North China Agric. Sci. 2014, 29, 234–238. [Google Scholar]
- Selamat, J.; Rozani, N.A.A.; Murugesu, S. Application of the Metabolomics Approach in Food Authentication. Molecules 2021, 26, 7565. [Google Scholar] [CrossRef]
- Hew, P.S.; Jinap, S.; Jambari, N.N.; Murugesu, S.; Sanny, M.; Khatib, A.; Sukor, R. Quality and safety of food product—Current assessment, issues, and metabolomics as a way forward. Food Chem Adv. 2024, 4, 100–632. [Google Scholar] [CrossRef]
- Duan, W.; Guan, Q.; Zhang, H.L.; Wang, F.Z.; Lu, R.; Li, D.M.; Geng, Y.; Xu, Z.-H. Improving flavor, bioactivity, and changing metabolic profiles of goji juice by selected lactic acid bacteria fermentation. Food Chem. 2023, 408, 135–155. [Google Scholar] [CrossRef]
- He, W.; Pan, H.L.; Pan, T.F.; Tang, H.R.; Wang, X.R.; Pan, D.M. Research Progress on the Interaction Between Scion and Rootstock in Fruit Trees. Acta Hortic. Sin. 2017, 44, 1645–1657. [Google Scholar]
- Zhang, J.; Ai, D.; Meng, J.X.; Wei, Y.C.; Zhang, Y. Research Progress on the Interaction Mechanism between Rootstock and scion in Plant grafting. J. Northwest AF Univ. (Nat. Sci. Ed.) 2022, 50, 139–145. [Google Scholar]
- Zhao, Z.H.; Hao, Y.F.; Liu, Y.J.; Shi, Y.S.; Lin, X.; Wang, L.; Wen, P.; Hu, X.P.; Li, J.X. Comprehensive evaluation of aroma and taste properties of different parts from the wampee fruit. Food Chem. 2023, 19, 100–835. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.X.; Wang, Y.J.; Wu, B.H.; Fang, J.B.; Li, S.H. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- He, J.J.; Zhou, Y.Z.; Li, Y.L.; Zhou, H.J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chem. 2022, 373, 131–587. [Google Scholar]
- Wen, Y.Q.; Zhong, G.Y.; Gao, Y.; Lan, Y.B.; Duan, C.Q.; Pan, Q.H. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biol. 2015, 15, 240. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Martinez, M.C.; Santiago, J.L.; Simal-Gandara, J. Floral, spicy and herbaceous active odorants in Gran Negro grapes from shoulders and tips into the cluster, and comparison with Brancellao and Mouratón varieties. Food Chem. 2012, 135, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wu, Y.S.; Chen, Y.J.; Zheng, Q.Z.; Ma, C.; Xu, W.P.; Zhang, C.X.; Wang, S.P. Aroma characteristics of three Kyoho grapevine series. Shanghai Jiao Tong Univ. (Agric. Sci.) 2018, 36, 51–59, 66. [Google Scholar]
- Wu, Y.S.; Duan, S.Y.; Zhao, L.P.; Gao, Z.; Luo, M.; Song, S.R.; Xu, W.P.; Zhang, C.X.; Ma, C.; Wang, S.P. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31–116. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Cai, J. Study on Aroma Quality Improvement for Cabernet Sauvignon Wines Based on Pre-Fermentation Technology from North Slope of Tianshan Mountains. Doctoral Thesis, China Agricultural University, Beijing, China, 2014. [Google Scholar]
- Jogaiah, S.; Oulkar, D.P.; Banerjee, K.; Sharma, J.; Patil, A.G.; Maske, S.R.; Somkuwar, R.G. Biochemically induced variations during some phenological stages in Thompson Seedless grapevines grafted on different rootstocks. S. Afr. J. Enol. Vitic. 2013, 34, 36–45. [Google Scholar] [CrossRef]
- Qiu, L.L.; Jiang, B.; Fang, J.; Shen, Y.K.; Fang, Z.X.; RM, S.K.; Yi, K.K.; Shen, C.J.; Yan, D.L.; Zheng, B.S. Analysis of transcriptome in hickory (Carya cathayensis), and uncover the dynamics in the hormonal signaling pathway during graft process. BMC Genom. 2016, 17, 935. [Google Scholar] [CrossRef] [PubMed]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; Von-Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- He, L.; Xu, X.Q.; Wang, Y.; Chen, W.K.; Sun, R.Z.; Cheng, G.; Liu, B.; Chen, W.; Duan, C.Q.; Wang, J.; et al. Modulation of volatile compound metabolome and transcriptome in grape berries exposed to sunlight under dry-hot climate. BMC Plant Biol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Burdack-Freitag, A.; Schieberle, P. Changes in the key odorants of Italian Hazelnuts (Coryllus avellana L. var. Tonda Romana) induced by roasting. Agric. Food Chem. 2010, 58, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.G.; Chen, H.T. The Technology of Food Flavor, 3rd ed.; Chemical Industry Press: Beijing, China, 2016. [Google Scholar]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans, Punter and Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Du, X.F.; Finn, C.E.; Qian, M.C. Volatile composition and odour-activity value of thornless ‘Black Diamond’ and ‘Marion’ blackberries. Food Chem. 2010, 119, 1127–1134. [Google Scholar] [CrossRef]
- Spillman1, P.J.; Sefton, M.A.; Gawel, R. The contribution of volatile compounds derived during oak barrel maturation to the aroma of a Chardonnay and Cabernet Sauvignon wine. Aust. J. Grape Wine Res. 2004, 103, 227–235. [Google Scholar] [CrossRef]
- Buhr, K.; Köhnhofer, B.; Heilig, A.; Hinrichs, J.; Schieberle, P. Behaviour of selected flavour compounds in dairy matrices: Stability and release. Food Chem. 2010, 408, 165–168. [Google Scholar]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Lu, M.; An, H.M. Comparative analysis of chemical components of traditional Chinese medicine and antioxidants between Rosa roxburghii and R. sterilis fruits. Fruit Sci. 2023, 40, 111–125. [Google Scholar]
- Negri, A.S.; Prinsi, B.; Rossoni, M.; Failla, O.; Scienza, A.; Cocucci, M.; Espen, L. Proteome changes in the skin of the grape cultivar Barbera among different stages of ripening. BMC Genom. 2008, 9, 378. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, A.; Chai, L.; Zhou, W.; Deng, X. Transcriptome analysis of a spontaneous mutant in sweet orange citrus sinensis osbeck during fruit development. Exp. Bot. 2009, 60, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, Y.; Wu, D.; Qin, C.; Yang, C.; Jiang, Y.; Hou, Z. Enhancement of antioxidant capacity and preservation of quality in postharvest apricots through led light quality treatment. Hortic. Environ. Biotechnol. 2025, 66, 253–268. [Google Scholar] [CrossRef]
- Luo, C.D.; Feng, C.M.; Li, J.Q.; Li, X.R.; Wei, Y.; Yang, L.Y.; Liu, X.J.; Tan, H.; Ren, E.F.; Luo, X.J. Analysis of differential aroma volatiles of tainong no.1 mango of different ripeness by non-targeted metabolomics based on gas chromatography-mass spectrometry. Sci. Agric. Sin. 2025, 58, 564–581. [Google Scholar]
- Terzoudis, K.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Dynamic labelling reveals central carbon metabolism responses to stepwise decreasing hypoxia and reoxygenation during postharvest in pear fruit. Postharvest Biol. Technol. 2022, 186, 111–816. [Google Scholar] [CrossRef]
- Wang, S.F.; Deng, Y.P.; Zhang, K.M.; Jiang, Z.R.; Chen, Z.Y.; Miao, Y.; Huang, K.L.; Hu, C.; Wang, Z. Volatile fatty acids production and sulfur metabolism pathways altered in a two-phase anaerobic sulfate-reducing system due to high organic and sulfate loading rates. Water Process Eng. 2025, 70, 106948. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, K.J.; Wang, N.; Xiao, X.J.; Leng, Y.J.; Fan, J.; Du, Y.; Wang, S.H. Sulfur dioxide-free wine with polyphenols promotes lipid metabolism via the Nrf2 pathway and gut microbiota modulation. Food Chem. X 2024, 21, 101079. [Google Scholar] [CrossRef]
- Ji, X.A.; Yu, X.W.; Xu, Y.; Wu, Q.; Madadi, M.; Mousavi Khaneghah, A. Initial acidity regulates microbial sulfur metabolism in the spontaneous fermentation of sesame flavor-type baijiu. Int. J. Food Microbiol. 2025, 435, 111182. [Google Scholar] [CrossRef]
- Pinsorn, P.; Sanachai, K.; Rungrotmongkol, T.; Hoefgen, R.; Watanabe, M.; Brueckner, F.; Nakabayashi, R.; Mori, T.; Oikawa, A.; Sasaki, R.; et al. Sulfur metabolism in durian pulps: Factors contributing to the production of volatile sulfur compounds during fruit ripening. Postharvest Biol. Technol. 2023, 206, 112533. [Google Scholar] [CrossRef]
- Song, X.S.; Shang, Z.W.; Yin, Z.P.; Ren, J.; Sun, M.C.; Ma, X.L. Mechanism of xanthophyll-cycle-mediated photoprotection in Cerasus humilis seedlings under water stress and subsequent recovery. Photosynthetica 2011, 49, 523–530. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, Y.; Wang, J.C.; Zhuo, Y.L.; Sun, Y.H.; Wang, J.G.; Zhang, G.G. Four new compounds from the fruits of Chinese dwarf cherry (prunus humilis bunge). Nat. Prod. Res. 2024, 38, 1207–1215. [Google Scholar] [CrossRef]
- Li, L.Y.; Chen, C.Y.; Wang, W.L.; Jin, J.H.; Ma, M.S.; Jin, J.Z. Evaluation of volatile compounds from Chinese dwarf cherry (cerasus humilis (bge.) sok.) germplasms by headspace solid-phase microextraction and gas chromatography–mass spectrometry. Food Chem. 2017, 217, 389–397. [Google Scholar]
- Zhang, L.; Han, X.; Zhang, S.; Du, J.; Zhang, J.; Gao, Y.G.; Wang, P.; Mu, X. A systematic profiling of the volatile compounds in 53 cerasus humilis genotypes using headspace solid-phase microextraction and gas chromatography-mass spectrometry. Horticulturae 2023, 9, 806. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Z.X.; Jiang, H.Y.; Wang, C.Z.; Chen, L.L.; Li, L.G. Effect of Different Rootstocks on Volatile Components in Fruits of ‘Fuji’ Apple; Shandong Academy of Agricultural Sciences: Jinan, China, 2018. [Google Scholar]
- Zhang, H.; Li, X.L.; Li, Y.; Chen, Z.W.; Le, Y.Z.; Zhai, J.H. The influence of rootstock on the fruit quality of Chinese kiwifruit “Jianxiang”. Fruit Trees S. 2024, 53, 161–168. [Google Scholar]
- Wang, N.; Zhang, X.; Guo, Q.; Yan, G.; Wang, J.; Wu, C.; Zhou, Y.; Zhou, J.; Zhang, K.; Li, T.; et al. Effects of different rootstocks on fruit quality and non-volatile flavor-related compounds of sweet cherry ‘summit’. Food Chem. 2025, 463 Pt 4, 141512. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.K.; Gao, X.T.; He, L.; Yang, X.H.; He, F.; Duan, C.Q.; Wang, J. Rootstock-mediated effects on Cabernet Sauvignon performance: Vine growth, berry ripening, flavonoids, and aromatic profiles. Int. J. Mol. Sci. 2019, 20, 401. [Google Scholar] [CrossRef]
- Niu, S.K.; Yang, L.L.; Sun, C.W. Grape aromatic substance factors affecting research progress. China Foreign Grape Wine 2017, 4, 100–103. [Google Scholar]
- El-Sharkawy, I.; Manríquez, D.; Flores, F.B.; Regad, F.; Bouzayen, M.; Latché, A.; Pech, J.C. Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. identification of the crucial role of a threonine residue for enzyme. Plant Mol. Biol. 2005, 59, 345–362. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Luan, F.; Mosandl, A.; Munch, A.; Wust, M. Metabolism of geraniol in grape berry mesocarp of Vitis vinifera L. cv. Scheurebe: Demonstration of stereoselective reduction, E/Z-isomerization, oxidation and glycosylation. Phytochemistry 2005, 66, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.T.; Bohlmann, J. The molecular basis for wine grape quality—A volatile subject. Science 2006, 311, 804–805. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling rootstock × scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef]
- Harada, T. Grafting and RNA transport via phloem tissue in horticultural plants. Sci. Hortic. 2010, 125, 545–550. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; Shen, Q.; Yuan, H.; Cui, F.; Yan, D.; Ding, W.; Wang, X.; Zheng, B. Hormone Fluctuation and Gene Expression During Early Stages of the Hickory Grafting Process. Plants 2025, 14, 2229. [Google Scholar] [CrossRef]
- Miroslav, B.; Jana, R.; Miroslav, P. Comparative analysis of genetic diversity in Prunus L. as revealed by RAPD and SSR markers. Sci. Hortic. 2006, 108, 253–259. [Google Scholar] [CrossRef]
- Li, X.Y.; Song, L.Q.; Li, M.Y.; Wang, H.J.; Liu, J.Z.; Wu, J.K.; Zhang, L.B. Advances in Biosynthesis and Metabolism Regulation of Fruit Volatile Compounds. J. Agric. Biotechnol. 2023, 31, 629–642. [Google Scholar]
- Pan, F.; Zhao, X.; Liu, F.; Luo, Z.; Chen, S.; Liu, Z.; Zhao, Z.; Liu, M.; Wang, L. Triterpenoids in jujube: A review of composition, content diversity, pharmacological effects, synthetic pathway, and variation during domestication. Plants 2023, 12, 1501. [Google Scholar] [CrossRef]
- Hao, H.; Xue, Z.; Li, Y.; Ma, H.; Wen, Q.; Lin, L.; Zhu, H. Genome-wide identification and characterization of lipoxygenases gene family in luffa aegyptiaca revealed downregulation of lox genes under heat stress. Sci. Rep. 2025, 15, 17696. [Google Scholar] [CrossRef]
- Huang, M.L.; Li, K.; Cheng, Y.; Li, M.Y.; Liu, L.; Mur, L.A.J.; Luo, J.; Fang, C.Y. PeWRKY20 represses PeMDH1 to modulate malic acid metabolism and flavor formation in postharvest passion fruit. Postharvest Biol. Technol. 2024, 218, 113164. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.C.; Wang, H.J.; Ablimit, A.; Sun, Q.; Dong, H.J. Identification of characteristic volatiles and metabolomic pathways during the fermentation of red grapefruit by Monascus purpureus using HS-SPME-GC–MS and metabolomics. Food Chem. 2025, 464 Pt 3, 141786. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Yoshinari, Y. Carbohydrate and protein metabolism. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Liu, R.H.; Jia, J.W.; Wang, C.W.; Wu, Q.P.; Du, L.; Li, W.Q.; Yang, W.W.; Ma, J.J.; Zhang, D.; Xing, L.B.; et al. Transcriptomic and primary metabolic profiles reveal the mechanism of development and maturation of fuji apple grafted onto different dwarfed intermediate rootstocks. Sci. Hortic. 2025, 343, 114060. [Google Scholar] [CrossRef]
- Wei, J.; Guo, R.F.; Zhou, Y.; Shi, B.S.; Liu, Y.G.; Li, J.Q.; Zhou, X.H. Phenylacetaldehyde reductase gene (SpPAR) regulates the biosynthesis of Phenylethanol and Benzylalcohol in Syringa pubescens. Sci. Hortic. 2025, 343, 114075. [Google Scholar] [CrossRef]
- Jahan, T.; Huda, M.N.; Zhang, K.X.; He, Y.Q.; Lai, D.L.; Dhami, N.; Quinet, M.; Ali, M.A.; Kreft, I.; Woo, S.H.; et al. Plant secondary metabolites against biotic stresses for sustainable crop protection. Biotechnol. Adv. 2025, 79, 108520. [Google Scholar] [CrossRef] [PubMed]


| Number | Combinations | Code Name |
|---|---|---|
| 1 | Cerasus humilis Nongda 4/Amygdalus communis | ZG/BT |
| 2 | Cerasus humilis Nongda 4/Cerasus tomentosa | ZG/MYT |
| 3 | Cerasus humilis Nongda 4/Armeniaca sibirica | ZG/SX |
| 4 | Cerasus humilis Nongda 4 | ZG |
| OPLS-DA Model | Component | R2X (cum) | R2Y (cum) |
|---|---|---|---|
| ZG vs. ZG/BT | 1 + 1 + 0 | 0.81 | 1 |
| ZG vs. ZG/MYT | 1 + 1 + 0 | 0.761 | 0.998 |
| ZG vs. ZG/SX | 1 + 1 + 0 | 0.8 | 1 |
| Taste Component | Thresholds (μg·kg−1) | Odor Description | OAVs | Aroma Series | |||
|---|---|---|---|---|---|---|---|
| ZG/BT | ZG/MYT | ZG/SX | ZG | ||||
| Butanoic acid, butyl ester | 10 | Banana, strawberry, apple, grape | 1.13 | 2.19 | 0.94 | 0.84 | 1 |
| Hexanoic acid, ethyl ester | 0.01 | Pineapple | 71.01 | 127.25 | 80.39 | 18.00 | 1 |
| Acetic acid, hexyl ester | 2 | Pears, berries | 62.06 | 77.06 | 48.61 | 34.53 | 1 |
| Octanoic acid, ethyl ester | 0.58 | Sweet, pineapple | 20.36 | 46.31 | 23.23 | 3.70 | 1 |
| Octanal | 0.7 | Fruit | 4.35 | 11.31 | 4.38 | 4.71 | 1 |
| 2-Vinylethyl acetate | 1 | Pears, apples | 10.37 | 17.44 | 7.47 | 4.95 | 1 |
| Hexanal | 4.5 | Green leaves | 3.56 | 5.48 | 2.46 | 1.70 | 2 |
| 3-Hexenal | 0.25 | Green flavor | 10.51 | 7.67 | 3.76 | 15.82 | 2 |
| 2-Hexenal, (E)- | 17 | Grassy green | 0.95 | 1.34 | 0.63 | 0.67 | 2 |
| 3-Hexen-1-ol, acetate, (Z)- | 1 | Grassy green | 6.64 | 8.50 | 6.05 | 2.43 | 2 |
| 2,4-Hexadienal, (E,E)- | 0.05 | Grassy green | 8.79 | 11.32 | 6.47 | 4.04 | 2 |
| Linalool | 0.22 | Rose, fresh floral–woody | 80.95 | 162.21 | 69.46 | 60.24 | 3 |
| trans-Calamenene | 0.005 | Piney, woody | 0.91 | 0.00 | 0.79 | 1.27 | 4 |
| 2-Nonenal, (E)- | 0.08 | Cucumber, green leaves | 2.54 | 3.88 | 1.74 | 3.22 | 5 |
| Estragole | 10 | Fennel, sweet | 1.03 | 1.15 | 0.59 | 0.30 | 5 |
| 1-Octen-3-one | 0.001 | Mushroom-like | 37.93 | 79.43 | 38.49 | 32.05 | 6 |
| trans-3-Nonen-2-one | 0.25 | Mushroom-like | 0.93 | 2.07 | 1.09 | 0.68 | 6 |
| 1-Heptanol, 4-methyl- | 1 | Fatty, cheese-like | 7.26 | 8.72 | 4.54 | 3.65 | 9 |
| 1-Butanol, 3-methyl-, acetate | 2 | Sweet, banana | 9.34 | 8.18 | 5.95 | 1.89 | 1,5 |
| 4-Hexen-1-ol, acetate | 1 | Grassy green, fruity | 15.41 | 40.62 | 26.55 | 22.65 | 1,2 |
| Decanal | 0.1 | Sweet, citrus, dairy | 0.91 | 2.15 | 1.08 | 1.27 | 5,8,9 |
| Nonanal | 1 | Aldehyde, citrus note, dairy | 16.78 | 30.05 | 13.32 | 9.32 | 7,8,9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, G.; Xie, J.; Zhang, C.; Liu, Y.; Guo, X.; Jia, Q.; Zhang, C.; Xu, M. GC-MS Non-Target Metabolomics-Based Analysis of the Volatile Aroma in Cerasus humilis After Grafting with Different Rootstocks. Horticulturae 2025, 11, 972. https://doi.org/10.3390/horticulturae11080972
Qiao G, Xie J, Zhang C, Liu Y, Guo X, Jia Q, Zhang C, Xu M. GC-MS Non-Target Metabolomics-Based Analysis of the Volatile Aroma in Cerasus humilis After Grafting with Different Rootstocks. Horticulturae. 2025; 11(8):972. https://doi.org/10.3390/horticulturae11080972
Chicago/Turabian StyleQiao, Gaixia, Jun Xie, Chun’e Zhang, Yujuan Liu, Xiaojing Guo, Qiaoxia Jia, Caixia Zhang, and Meilong Xu. 2025. "GC-MS Non-Target Metabolomics-Based Analysis of the Volatile Aroma in Cerasus humilis After Grafting with Different Rootstocks" Horticulturae 11, no. 8: 972. https://doi.org/10.3390/horticulturae11080972
APA StyleQiao, G., Xie, J., Zhang, C., Liu, Y., Guo, X., Jia, Q., Zhang, C., & Xu, M. (2025). GC-MS Non-Target Metabolomics-Based Analysis of the Volatile Aroma in Cerasus humilis After Grafting with Different Rootstocks. Horticulturae, 11(8), 972. https://doi.org/10.3390/horticulturae11080972





