1. Introduction
Controlled environment agriculture (CEA) has transformed crop production by allowing the precise control of growth conditions to improve yield and quality attributes, including nutritional value, leaf color, and sensory characteristics [
1,
2]. In CEA production systems, factors such as temperature, carbon dioxide levels, relative humidity, and light can be finely tuned to meet the specific needs of various crops [
3]. Recent advances in light-emitting diode (LED) technology present a more energy-efficient alternative to traditional horticultural lighting methods like high-pressure sodium and fluorescent lamps [
3,
4,
5]. LEDs offer superior energy efficiency, longer operational life, reduced heat emission, and precise control over the light spectrum, enabling customized lighting to enhance both the yield and quality of CEA-grown crops throughout their growth stages [
3,
4,
6,
7]. Investigating how different light spectra impact these aspects is crucial for refining and optimizing cultivation techniques.
Basil (
Ocimum basilicum L.) is a widely cultivated herb known for its culinary and medicinal properties. It is known for its aromatic leaves, which play a fundamental role in a variety of culinary applications, as well as its profile of bioactive compounds, including essential oils, phenolic compounds, and flavonoids [
8]. These compounds not only contribute to basil’s distinct aroma and flavor, but also provide potential health benefits, such as antioxidant and anti-inflammatory properties [
9,
10,
11]. Recent studies have highlighted the importance of these bioactive compounds, particularly linalool, eugenol, and methyl chavicol, which are major contributors to basil’s sensory attributes and therapeutic potential [
12,
13,
14,
15]. Due to basil’s commercial significance and strong market demand, CEA systems provide an ideal solution for optimizing the production of this high-value crop. CEA systems offer precise control over environmental conditions, including light, temperature, and humidity, which are crucial for ensuring consistent high-quality yields [
3,
16]. Among these factors, the light spectrum plays a particularly significant role in regulating plant growth, secondary metabolite production, and volatile compound synthesis, which in turn affect basil’s sensory qualities and market value [
17,
18].
The photosynthetic portion of the light spectrum contains wavebands of blue (BL; 400–499 nm), green (GR; 500–599 nm), red (R; 600–699 nm), and far-red (FR; 700–799 nm), and influences plant growth by affecting various physiological and developmental processes. Different wavelengths are absorbed by specific photoreceptors, which are plant proteins that detect light and modulate plant responses through signal transduction pathways [
19]. Phytochromes, which absorb R and FR light, and cryptochromes, which absorb BL light, both regulate photomorphogenesis [
20,
21,
22]. BL light enhances chlorophyll
a content, boosting photosynthetic efficiency, but generally reduces stem and leaf extension, leading to a more compact growth habit compared to R light [
23,
24]. BL light also promotes stomatal opening and increases the production of secondary metabolites like flavonoids, improving plant defense mechanisms [
25,
26]. GR light, while traditionally considered less effective than R or BL light, penetrates more deeply into plant canopies, enhancing photosynthesis in the lower leaves and thus improving overall biomass [
27,
28]. GR light also balances photomorphogenic responses, such as stem elongation and leaf expansion, resulting in a more uniform plant structure [
28]. R light is essential for photosynthesis, stimulating stem elongation and leaf expansion, and significantly increasing biomass [
29,
30]. FR light enhances these effects when combined with R light, triggering shade-avoidance responses and increasing stem elongation as plants attempt to capture more light [
31,
32,
33]. Additionally, FR light, when combined with R light, enhances photosynthesis through the Emerson enhancement effect, which involves simultaneous excitation of photosystem I (PSI) by FR and photosystem II (PSII) by R light [
34,
35,
36]. This synergy increases electron transport rates, carbon fixation, and overall photosynthetic output. While current research highlights the role of the light spectrum on plant growth, morphology, and photosynthesis, more research is needed to determine the specific effects of supplemental light wavebands on basil growth attributes.
The light spectrum significantly influences the synthesis of aromatic compounds in basil, which are key contributors to its flavor, aroma, and medicinal properties [
26]. BL light, in particular, enhances the production of essential oils and other secondary metabolites that give basil its characteristic scent. Linalool and eugenol, two major compounds responsible for basil’s aromatic profile, are significantly increased under BL light, improving basil’s medicinal value due to the antioxidant and anti-inflammatory properties associated with these compounds [
26]. Studies have shown that BL light boosts the synthesis of phenolic compounds and flavonoids, which contribute to aroma and flavor and enhance the plant’s defense mechanisms [
37,
38,
39]. While the effects of BL light on volatile compounds are well-documented, the role of other wavebands, such as GR and FR light, in shaping basil’s aromatic profile remains less explored. GR light, which penetrates deeper into the plant canopy, may promote the synthesis of volatile compounds in lower leaves, contributing to a more uniform distribution of aroma throughout the plant [
27,
28]. FR light, when combined with R light, triggers shade-avoidance responses and alters the allocation of resources, potentially enhancing the production of volatile oils like methyl chavicol and linalool in response to light stress [
33,
36]. These synergistic effects between different light wavelengths can influence the concentration of aromatic compounds, though more research is needed to fully understand the interaction between light quality and essential oil synthesis in basil.
The relationship between light spectrum and aromatic quality is especially important for consumer preferences for basil, as compounds like linalool and eugenol are key determinants of basil’s aromatic and flavor profile. However, there is still a gap in understanding how specific light treatments affect volatiles related to aroma in commercially grown basil, particularly in terms of how different light spectra influence consumer-perceived quality of different cultivars of basil [
17]. Furthermore, based on our knowledge, there are no comprehensive basil studies that investigate the effects of the light spectrum on growth and volatile compounds, paired with a sensory analysis by trained panelists on both green and purple leaf basil. Therefore, research that investigates volatile compound profiles along with sensory attributes, like aroma, is needed to further optimize basil production for consumer preferences. In addition to improving aromatic qualities, basil production yields must also be maintained to ensure profitability. In line with this, the objectives of this study were to evaluate green and purple leaf basil coloration, growth, morphology, and photosynthetic rates under different supplemental light treatments and to determine the effects of supplemental narrowband light on volatile compounds related to aroma. It was hypothesized that FR light would increase basil growth, stem elongation, leaf size, and photosynthetic rates, but decrease sensory attributes and volatile compounds. Conversely, it was expected that BL light would enhance sensory attributes, increase volatile compound concentrations, and reduce growth and photosynthetic rates.
2. Materials and Methods
2.1. Plant Growth Conditions
Experiments were conducted at the USDA-ARS Beltsville Agriculture Research Center, MD, USA, using walk-in temperature-controlled Environmental Growth Chambers (Model GR-48; Chagrin Falls, OH, USA). Prospera
® Compact DMR (PL4) F1 Pelleted Genovese basil seeds and Genovese Purple Basil Amethyst Improved seeds (Johnny Seeds; Winslow, ME, USA) were selected due to their commercial relevance and different pigment profiles. Seed were sown into hydrated 200-cell Rockwool sheets (Grodan; Roermond, The Netherlands). The seeds were germinated in darkness for 24 h at 28 °C and 70% relative humidity. Following germination, the basil plants were exposed to a total photon flux density (TPFD; 315–800 nm) of 200 µmol∙m
−2∙s
−1 of white light (5000 K) with a 20 h photoperiod. On day 7, humidity domes were removed, and the basil seedlings were transplanted into deep water culture (DWC) hydroponic systems and grown under their respective light treatments (
Table 1).
Five DWC systems were set up, each utilizing a 56 cm × 114 cm Active Aqua flood bath (Shoemakersville, PA, USA) with recirculating nutrient solutions. In each bath, a 2.5 cm polystyrene foam raft (Uline; Pleasant Prairie, WI, USA) was fitted with 82 holes spaced 7.6 cm apart, using 2.5 cm net cups (Lapond). The nutrient solution was composed of 15-5-20 N-P-K fertilizer (150 ppm N; Allentown, PA, USA) at a concentration of 0.66 g/L, and supplemented with Pennington Epsom salt (9.8% magnesium, 12.9% combined sulfur; Madison, Georgia) at 0.26 g/L. Nutrients were replenished one week after transplanting, and the pH and electrical conductivity (EC) of the DWC reservoirs were monitored using a Groline HI9814 waterproof portable pH/EC/TDS meter (Smithfield, RI, USA), with the pH maintained at 5.8–6.2 and EC at 1.5 dS∙m−1.
Mylar-covered panels (Ontario, CA, USA) were used to separate the different light treatments, preventing light pollution and enhancing light uniformity within each treatment. Additionally, HOBO 4-channel analog data loggers (Bourne, MA, USA) monitored chamber temperature and relative humidity. The temperature was maintained at 28 ± 0.86 °C with a relative humidity of 45 ± 3.1%.
2.2. Light Treatments
Three Alina touch lights (RAYN; Middleton, WI, USA) were suspended above each DWC, spaced 25 cm apart and positioned 50 cm above the growing surface. Seedlings were initially exposed to a white light spectrum at a TPFD of 200 µmol∙m
−2∙s
−1 with a photoperiod of 20 h from day 1 through day 7. From day 7 until harvest, the light spectrum was modified by applying 160 µmol∙m
−2∙s
−1 of white light (LW; 5000 K), supplemented with an additional 80 µmol∙m
−2∙s
−1 of either the same white light (HW; 5000 K), BL (peak = 451 nm), GR (peak = 525 nm), or FR (peak = 735 nm) light, resulting in a TPFD of 240 µmol∙m
−2∙s
−1 (
Table 1) delivered for a 20 h photoperiod. Light treatments were measured and averaged across nine representative locations at the plant canopy using a LI-COR-180 portable spectroradiometer (LI-COR; Tucson, AZ, USA). The objective of the light treatments was to test the effects of specific supplemental wavebands in addition to white light, and to compare white light with other supplemental treatments at the same total intensity.
2.3. Growth, Morphology, and Chlorophyll Fluorescence Measurements
On day 24, the plants were harvested to conduct growth, morphology, and chlorophyll fluorescence measurements. Five plants from each cultivar under each light treatment were randomly selected for chlorophyll fluorescence and stomatal conductance measurements using an LI-600 porometer/fluorometer (LI-600; LI-COR, Lincoln, NE, USA). Dark-adapted fluorescence and stomatal conductance measurements were taken before the light period began, following 4 h of darkness, and light-adapted measurements were collected after 1 h of light exposure. For each set of measurements, the top two fully expanded leaves on each plant (n = 10) were measured. The same five plants and the same two leaves were used for both dark- and light-adapted measurements. Leaf tissue was clamped into the device with a 0.75 cm2 aperture and a flow rate of 150 μmol∙s−1. Measurements were automatically recorded once the relative humidity and flow rate had stabilized.
Ten plants from each cultivar and treatment were randomly selected for growth and morphology assessments. After harvesting the plants, the number of leaves greater than 1 cm was counted, and stem length (cm) was measured. Plant shoots and leaves were then placed into pre-weighed bags, and fresh mass (g) was measured using a Thomas Scientific scale. Finally, the plants were placed in paper bags and dried at 80 °C for 10 days before dry mass (g) of each plant was measured and recorded.
2.4. GC/MS Sample Preparation and Analysis
Volatile compound analysis was performed using headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC–MS), following the protocol outlined by Bai et al. [
40]. Immediately after harvest, 0.5 g of basil tissue was homogenized with 2 mL of sodium chloride solution using a mortar and pestle. The homogenate was then transferred to a 20 mL vial, and 2.5 µL of the internal standard solution (10 mM 4-methyl-2-pentanone in water) was added. The vials were sealed with Teflon/silicone septa and stored at −80 °C for up to one week until analysis. Prior to analysis, the samples were thawed under running tap water and placed in the autosampler (Model MPS2, Gerstel Inc., Linthicum, MD, USA), which was equipped with a cooled tray holder controlled by a Peltier thermostat (Laird Tech, Göteborg, Sweden; CTC Analytics, Zwingen, Switzerland). The samples were maintained at 4 °C in the cooled tray for up to 16 h before analysis.
For extraction, the samples were incubated at 40 °C for 30 min, and a 2 cm triple-phase SPME fiber (50/30 μm DVB/Carboxen/PDMS, Supelco, Bellefonte, PA, USA) was exposed to the headspace for 30 min. After exposure, the fiber was inserted into the injector of an Agilent 7890 GC, equipped with a DB-5 column (60 m × 0.25 mm i.d., 1.00 μm film thickness, J&W Scientific, Folsom, CA, USA), and coupled to a 5975 MS detector (Agilent Technologies, Palo Alto, CA, USA). The volatiles were desorbed at 250 °C for 15 min. The column temperature was initially set to 40 °C, ramped to 230 °C at a rate of 4 °C min−1, and then increased to 260 °C at 100 °C min−1. The ionization voltage was set to 70 eV, and ions were detected from m/z 33 to 250.
Each day, a Kovats mixture of C-5 to C-20 n-alkanes was analyzed to calculate retention indices (RIs) and ensure inter-day reproducibility. Volatile compounds were identified by comparison to the NIST 23 database
http://chemdata.nist.gov (accessed on 10 April 2024), and identities were confirmed using authentic standards under the same chromatographic conditions. Additionally, compound identities were verified using a column of opposite polarity (DB-Wax capillary column; 60 m × 0.25 mm i.d., 0.5 μm film thickness; J&W Scientific, Folsom, CA, USA) as described by Molyneux and Schieberle [
41].
The data were analyzed and peak areas were quantified using MassHunter Quantitative Analysis software (Version 12.1; Agilent, Santa Clara, CA, USA). Five biological replicates were measured along with three technical replicates. Their averages are reported.
2.5. Sensory Training and Evaluation
Whole basil plants were harvested at 9:00 am from the growth chamber and transported to the sensory laboratory approximately one hour before evaluation. Thirty minutes prior to the sensory evaluation, basil leaves were randomly selected and inspected to ensure they were free from damage and deterioration. The leaves were clipped from the plants, placed into 3 oz souffle cups, and sealed with lids labeled with random three-digit blinding codes. Each panelist was provided with 3–4 leaves of similar size per session. The leaves were served to participants individually at room temperature.
The sensory panel consisted of five panelists (three female, two male) recruited from the USDA-Beltsville campus, all aged 18–65, with no known vision, taste, or smell disorders. These participants had extensive experience evaluating agricultural products, each with over 40 h of previous sensory panel experience. The panelists were trained for eight hours over four sessions using a generic Descriptive Analysis approach. A preliminary lexicon was developed by published sensory lexicons for fresh basil [
42,
43,
44]. During the training, panelists were exposed to various fresh and processed basil products to develop a sensory lexicon. The lexicon was finalized through consensus and conducted practice evaluations to ensure consistency and eliminate redundancies.
Aroma and texture evaluations were conducted in a quiet room with minimal distractions. Samples were evaluated in triplicate across three sessions, with each sample presented once per session in a randomized order. Panelists were instructed to rate the aroma and texture attributes on a 0–100 intensity scale using Compusense Cloud software (Version 24.0.4) [
45]. For aroma evaluation, participants were instructed to tear a leaf perpendicular to the stem, and a second leaf was provided for texture assessment. Between samples, coffee beans were smelled by panelists to reset their sense of smell. A mandatory 60 s break was enforced between samples, during which participants were instructed to wipe their hands to remove any oil residue.
2.6. Data Analysis
R statistical analysis software (Version 4.3.1) [
46] was used for statistical analysis and the creation of figures. Analysis of variance and Tukey’s HSD test (α = 0.05) were conducted using R packages ‘dplyr’ [
47] and ‘agricolae’ [
48]. For sensory variables, a principal component analysis (PCA) was applied to the data produced (quantitative descriptive analysis) by the trained panelists to group attributes based on patterns of correlation.
4. Discussion
This study examined the effects of FR, BL, and GR light on growth, morphology, photosynthetic efficiency, volatile profiles, and sensory attributes in two basil cultivars, Prospera (green) and Amethyst (purple). The results showed that the light spectrum strongly influenced physiological and morphological responses, with clear cultivar differences. These findings have important implications for optimizing basil production in controlled environments by tailoring light treatments to enhance traits like size, aroma, and appearance.
Growth and morphology were assessed to determine how supplemental narrowband light treatments influenced basil architecture, which is critical for optimizing productivity and space efficiency in controlled environments. Traits such as stem elongation, leaf number, and biomass accumulation reflect underlying resource allocation patterns, and are driven by photosynthetic efficiency and photoreceptor signaling. For example, FR light significantly increased stem elongation and biomass in both Prospera and Amethyst. These findings are consistent with the previous work of Legendre and van Iersel [
32], showing a significant increase in dry mass, leaf length, and leaf width of lettuce under increasing FR light. Additionally, in another study, FR light increased leaf area and thus light capture and plant growth of lettuce and basil [
49]. Furthermore, adding additional white light to the spectrum (HW) increased fresh and dry mass in both cultivars compared to LW, though it only slightly increased stem height of Amethyst basil. These findings suggest that while the higher TPFD provided by HW light promotes biomass accumulation, shade-avoidance responses remain most pronounced under FR light. These results are consistent with the role of phytochrome-mediated signaling in the shade-avoidance syndrome, a plant’s adaptive response to low-red to far-red light ratios encountered in dense canopies [
31,
50]. When plants are exposed to increased FR light, the balance between the active form of phytochrome (Pfr) shifts toward the inactive form of phytochrome (Pr) [
21]. This shift inactivates phyB, which would normally inhibit elongation growth, and allows phytochrome interacting factors (PIFs) to become more active [
51]. The activation of PIFs leads to the upregulation of genes responsible for cell elongation and division, particularly in the stem and petioles, thus promoting stem elongation and leaf expansion [
51]. This elongation response is crucial for optimizing light capture by allowing plants to grow taller and extend their leaves to reach more favorable light conditions [
50]. However, increased stem elongation often results in leggy plants, with elongated internodes, which can reduce plant compactness and structural stability, traits that may be undesirable in horticultural markets that prioritize compact growth forms. The connection between growth and light-driven physiological responses is further supported by the increased leaf number under FR light, which expands the light-capturing surface area. Prospera exhibited a 38.2% increase in leaf number under FR light compared to LW, and Amethyst showed a 98.0% increase. These results demonstrate that while FR light enhances stem elongation and leaf development, its effects on compactness may limit its suitability for specific markets.
In contrast to FR, BL light inhibited stem elongation and promoted a more compact growth habit in both basil cultivars. Specifically, stem height was reduced in Amethyst basil under BL light compared to FR light but was similar to LW and other supplemental light treatments. These results are consistent with other studies where additional or a higher fraction of BL light did not affect stem length of fresh mass of basil [
49,
52]. This response is largely controlled by cryptochrome signaling, which plays a key role in counteracting the elongation effects driven by PIFs under FR light [
53]. Cryptochromes are sensitive to BL light wavelengths and, when activated, inhibit PIF activity, thereby suppressing the shade-avoidance response and promoting more compact growth than plants grown under FR light [
20,
53]. By inhibiting PIF activity, cryptochromes effectively prevent the excessive elongation of stems and promote the development of shorter internodes and broader leaves. Additionally, BL light promotes lateral growth by encouraging leaf expansion rather than vertical elongation [
23,
54]. This compact growth form is especially desirable in CEA, where space efficiency and uniformity are critical, such as in vertical farming systems or high-density plantings [
23]. The ability of BL light to suppress vertical growth while enhancing lateral leaf expansion makes it a valuable tool for growers aiming to produce basil plants that are both visually appealing and compact.
These findings illustrate the contrasting roles of FR and BL light in regulating basil growth and morphology through distinct photoreceptor pathways. FR light, via phytochrome signaling and the activation of PIFs, drives stem elongation and biomass accumulation, but at the expense of plant compactness, often resulting in leggy plants. On the other hand, BL light suppresses PIF activity through cryptochrome signaling, leading to compact growth with shorter stems and broader leaves, making it ideal for growers aiming for compact, uniform plants. This interplay between morphology and photosynthetic capacity highlights the importance of balancing light spectrum composition to optimize plant architecture and maximize yield based on production system needs.
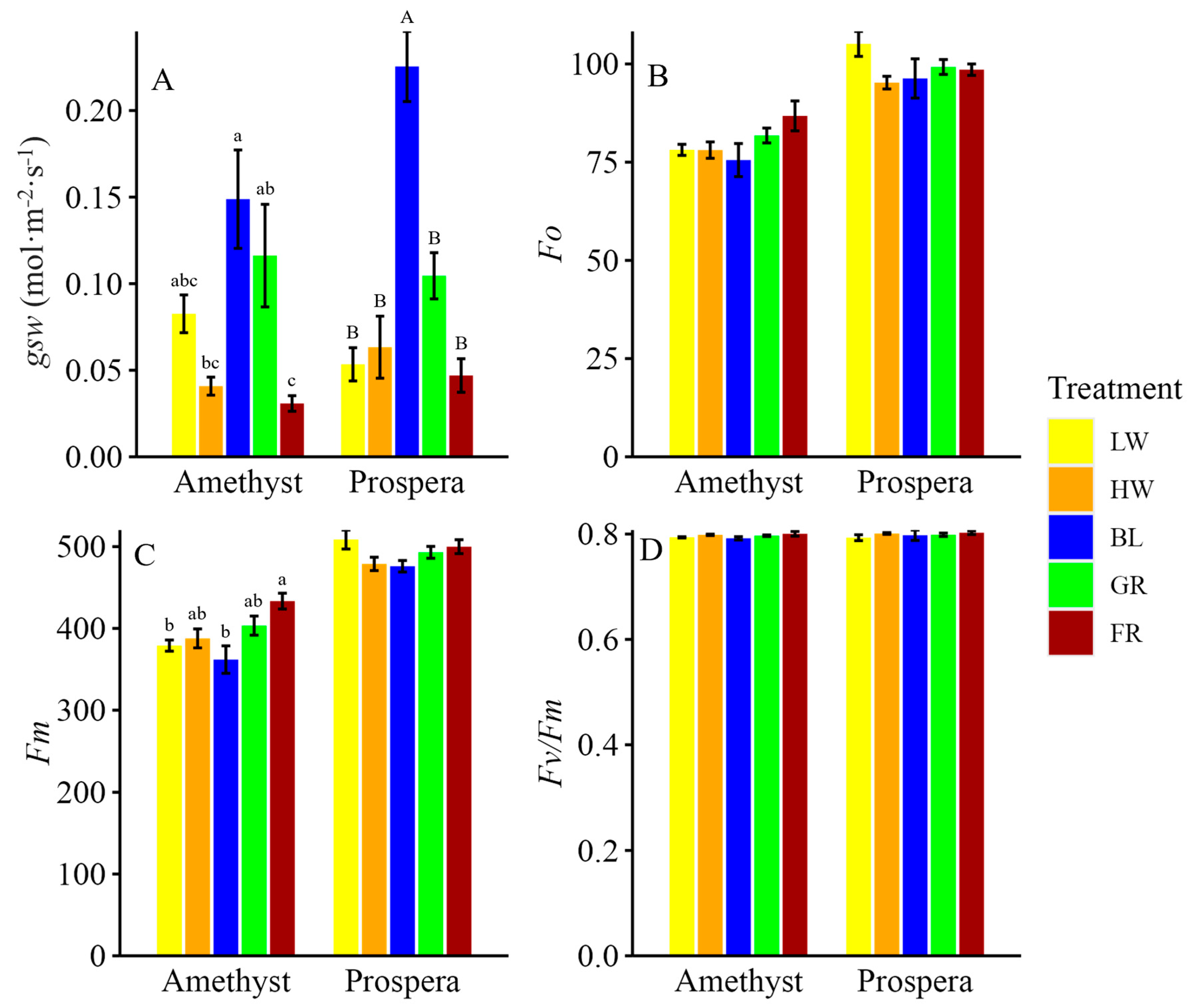
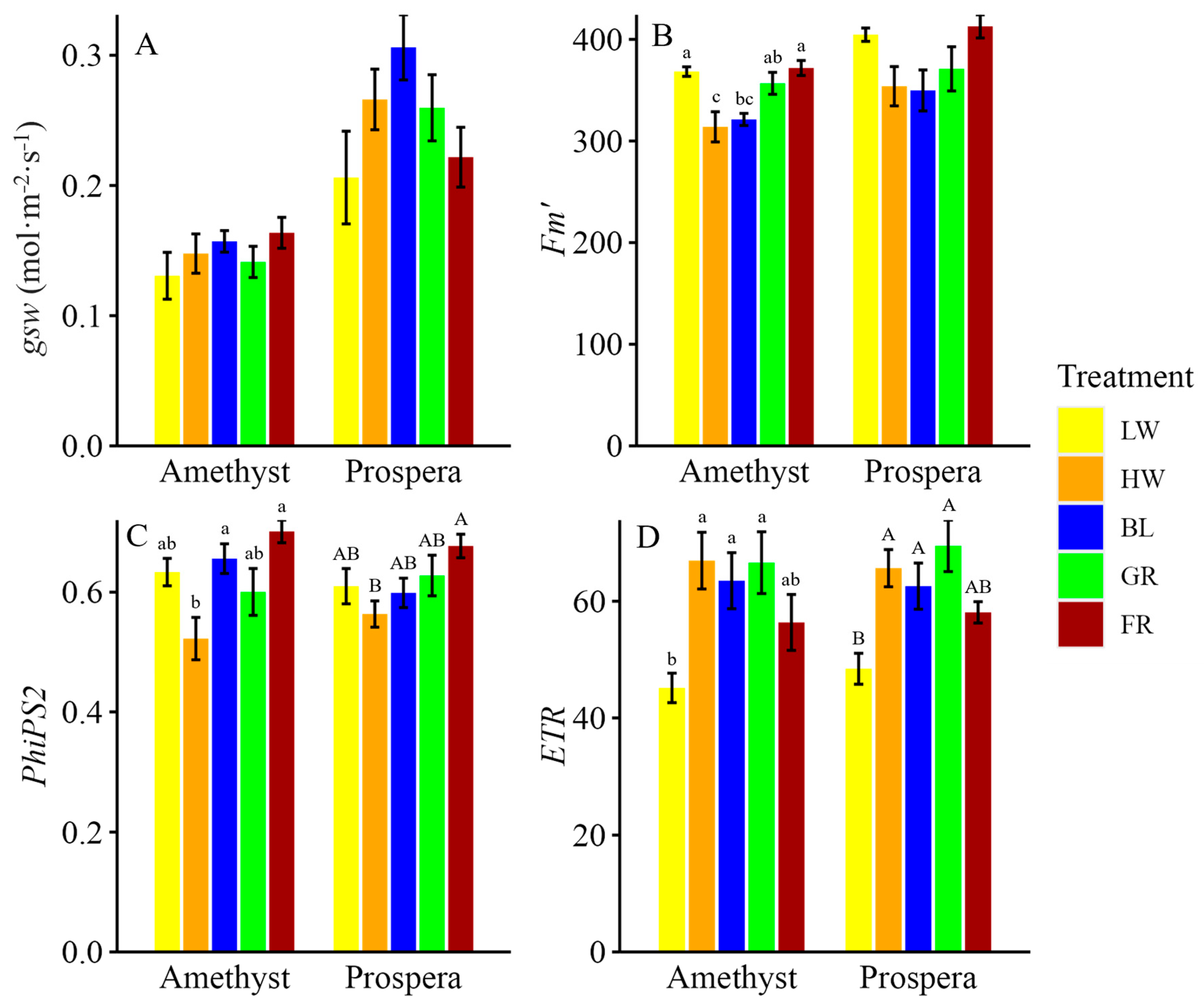
The measurements of
gsw,
phiPS2, and
ETR were included to elucidate how different light wavelengths influence the photosynthetic machinery and subsequently drive plant growth. For example, this study revealed that FR light reduced stomatal conductance by approximately 80% in both Prospera and Amethyst basil compared to BL light, highlighting a significant impact on gas exchange dynamics. This reduction, however, did not compromise photosynthetic efficiency; rather, an increase of up to 34% in
phiPS2 was observed under FR light compared to HW light, demonstrating the distinct role of FR light in regulating photosynthetic processes. This aligns with earlier findings that while FR light enhances PSI activity [
36], it efficiently drives PSII like BL or R light [
22]. The impact of FR light on PSII efficiency arises from its role in driving the shade-avoidance syndrome and promoting elongation growth rather than optimizing photosynthetic electron transport [
33]. FR light predominantly excites PSI, which functions in the later stages of the electron transport chain, leading to the production of ATP and NADPH [
55,
56]. However, without sufficient excitation of PSII, which is responsible for splitting water and generating the electrons needed for downstream photosynthetic reactions, the overall photosynthetic efficiency remains suboptimal [
22]. When combined with R light, FR light enhances photosynthesis through the Emerson enhancement effect by simultaneously exciting PSI and PSII, particularly in dense CEA systems where light distribution is critical [
22,
34]. These findings highlight the potential of integrating FR light with R or white light to maximize photosynthetic efficiency and plant growth in controlled environments.
Additionally, BL light significantly increased
gsw compared to LW or HW light, allowing greater CO
2 diffusion into the leaves and supporting higher photosynthetic rates. In particular, BL light enhanced
ETR by 15.8% in Prospera basil compared to LW light, demonstrating its role in driving photochemical efficiency through PSII. Furthermore, BL light directly enhanced photosynthetic efficiency by improving
ETR and
phiPS2, particularly in Amethyst basil compared to LW or HW light. BL light is well-known for its role in optimizing PSII function, because chlorophyll absorbs shorter wavelengths more effectively, driving higher photochemical efficiency [
23]. This increased light absorption by PSII leads to enhanced excitation of electrons, improving the efficiency of photochemical reactions and supporting higher rates of carbon fixation.
FR light and BL light have distinct effects on photosynthesis and stomatal conductance, with FR light enhancing growth through shade-avoidance responses and increased PSII efficiency, while BL light optimizes stomatal function and photosynthetic electron transport, and enhances photoprotective mechanisms. Additionally, when combined with R-containing light, FR light can significantly boost photosynthetic efficiency through the Emerson enhancement effect, demonstrating its value in improving photosynthetic capacity when properly integrated with other light spectra. For instance, tailoring light regimes with more FR light during early growth to drive elongation and biomass, followed by BL light later to enhance photoprotection and nutritional quality, can help growers balance growth, photosynthetic efficiency, and crop quality in CEA systems.
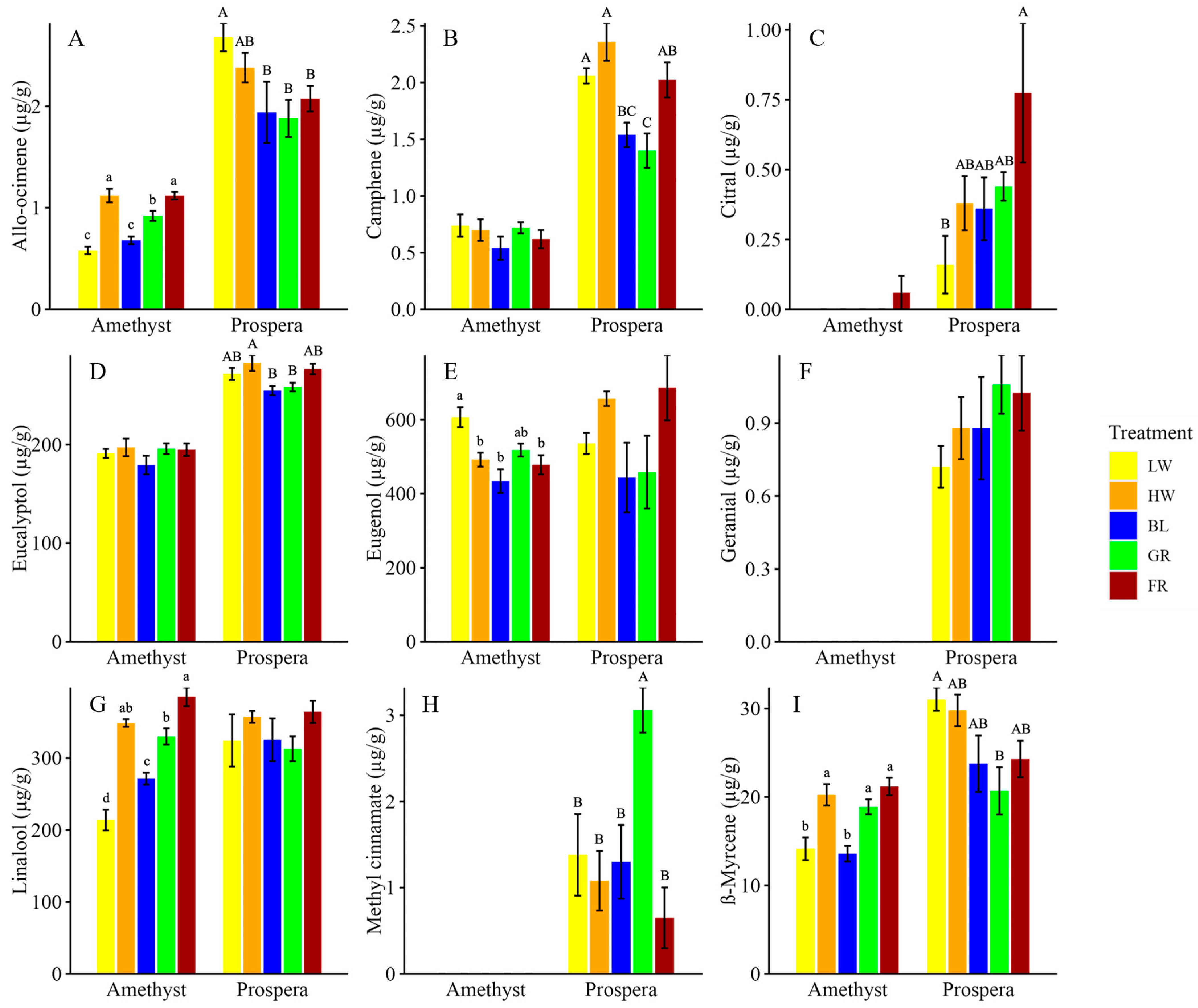
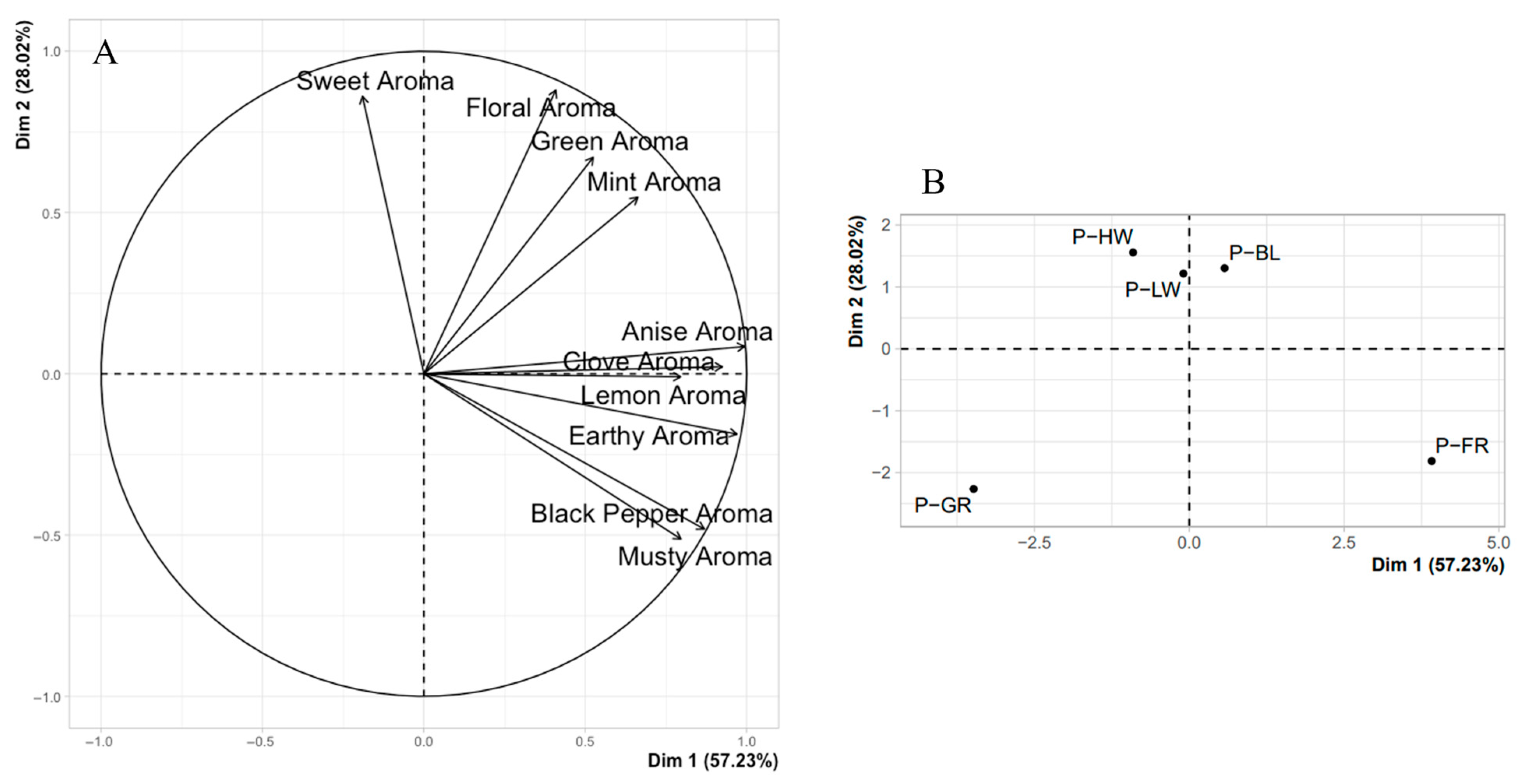
The effects of light spectra extended beyond growth and photosynthesis, significantly impacting the volatile compound profiles and sensory attributes of basil, reflecting both cultivar-specific and light-specific responses. The analysis of volatile compounds and sensory attributes was designed to connect the biochemical responses of basil to light treatments with their implications for consumer-perceived quality and marketability. Key aroma-related compounds in basil, such as citral, linalool, and allo-ocimene, are synthesized through the terpenoid and phenylpropanoid pathways and were influenced by the light spectrum [
26]. The production of these volatile compounds is often regulated by light-induced changes in gene expression associated with these pathways, which can be modulated by different photoreceptors and signaling pathways that respond to specific wavelengths of light [
17,
57,
58]. These results reveal connections between the metabolic pathways activated under specific wavelengths and their sensory outcomes.
Phytochromes respond to changes in the red to far-red light ratio, triggering a cascade of gene expression changes that upregulate enzymes involved in terpenoid biosynthesis, enhancing the production of volatile compounds like citral [
31,
59]. It was found that FR light significantly increased the concentration of specific volatile compounds, particularly citral in Prospera basil, where concentrations increased by over 371.0% compared to low-intensity white (LW) light. Similarly, Carvalho et al. [
17] found that FR light can stimulate the production of floral and citrus aroma compounds, such as citral, in basil and other herbs. Additionally, allo-ocimene, a floral-sweet monoterpene, increased by 89.0% in Amethyst basil under FR light, further supporting the role of FR light in enhancing monoterpene production, as noted in other studies investigating light quality and volatile biosynthesis in basil [
17]. Sensory evaluations further supported these biochemical findings. For instance, basil grown under FR light was rated higher in floral and citrus aromas, linking volatile compound profiles and perceived sensory quality. This suggests that FR light plays a crucial role in enhancing the aromatic complexity of basil, particularly in sun-adapted species, where FR light may trigger a stress-related increase in volatile compounds as a defense mechanism [
50]. In contrast, BL light reduced the concentrations of key volatiles such as eucalyptol and eugenol compared to FR. BL light typically redirects the plant’s metabolic focus towards the production of phenolic compounds and flavonoids, which are closely associated with plant defense mechanisms and antioxidant properties [
16,
60]. This shift occurs through the activation of cryptochrome signaling, which upregulates flavonoid biosynthesis pathways, reducing the pool of precursors available for volatile terpenoid synthesis [
18,
61]. Similarly, Hogewoning et al. [
23] found that BL light enhances photosynthetic efficiency and secondary metabolite production. These results support the idea that BL light can lead to a trade-off between aromatic intensity and nutritional quality, as the focus shifts toward increasing antioxidant activity, which is important for human health.
The results indicate that the effects of light on basil volatile compound synthesis and concentration directly influence the perceived aroma of both basil cultivars. The sensory findings are consistent with those reported in other studies examining basil’s volatile profiles under different light spectra. This relationship between light spectra and volatile compound production highlights the role of metabolic shifts in determining sensory outcomes and marketability. For instance, FR light enhanced the production of citral and linalool, two key aroma compounds. The sensory findings align with previous studies; for example, Carvalho et al. [
17] found that basil exposed to combinations of narrowband light exhibited changes in the concentrations of volatiles such as monoterpenoids and sesquiterpenoids, with FR light enhancing both. These results are consistent with those of that study, as an increase in citral and linalool production under FR light was found to correlate with a stronger citrus aroma, especially in Amethyst basil. Furthermore, eugenol concentrations in Amethyst basil decreased under BL light compared to LW, highlighting the trade-off between aroma intensity and the potential for increased antioxidant production under BL light. These differences in volatile production between FR and BL light highlight how light quality can shift metabolic pathways, particularly those involved in monoterpene biosynthesis, influencing both flavor and aroma. Moreover, studies by Walters et al. [
15] indicate that consumer preferences often favor basil with higher concentrations of volatiles such as eugenol and linalool, both of which are enhanced under FR light treatments. This suggests that growers targeting consumer demand for aromatic complexity can utilize FR light to increase the production of desirable volatiles like citral, eugenol, and linalool. This study reported similar intensity ratings during sensory evaluation, with basil samples grown under FR light receiving higher scores for aroma intensity, due to the enhanced production of key volatiles like eugenol, linalool, and citral. This suggests that optimizing light spectra in CEA systems can enhance flavor profiles while meeting consumer demand for more aromatic complexity.
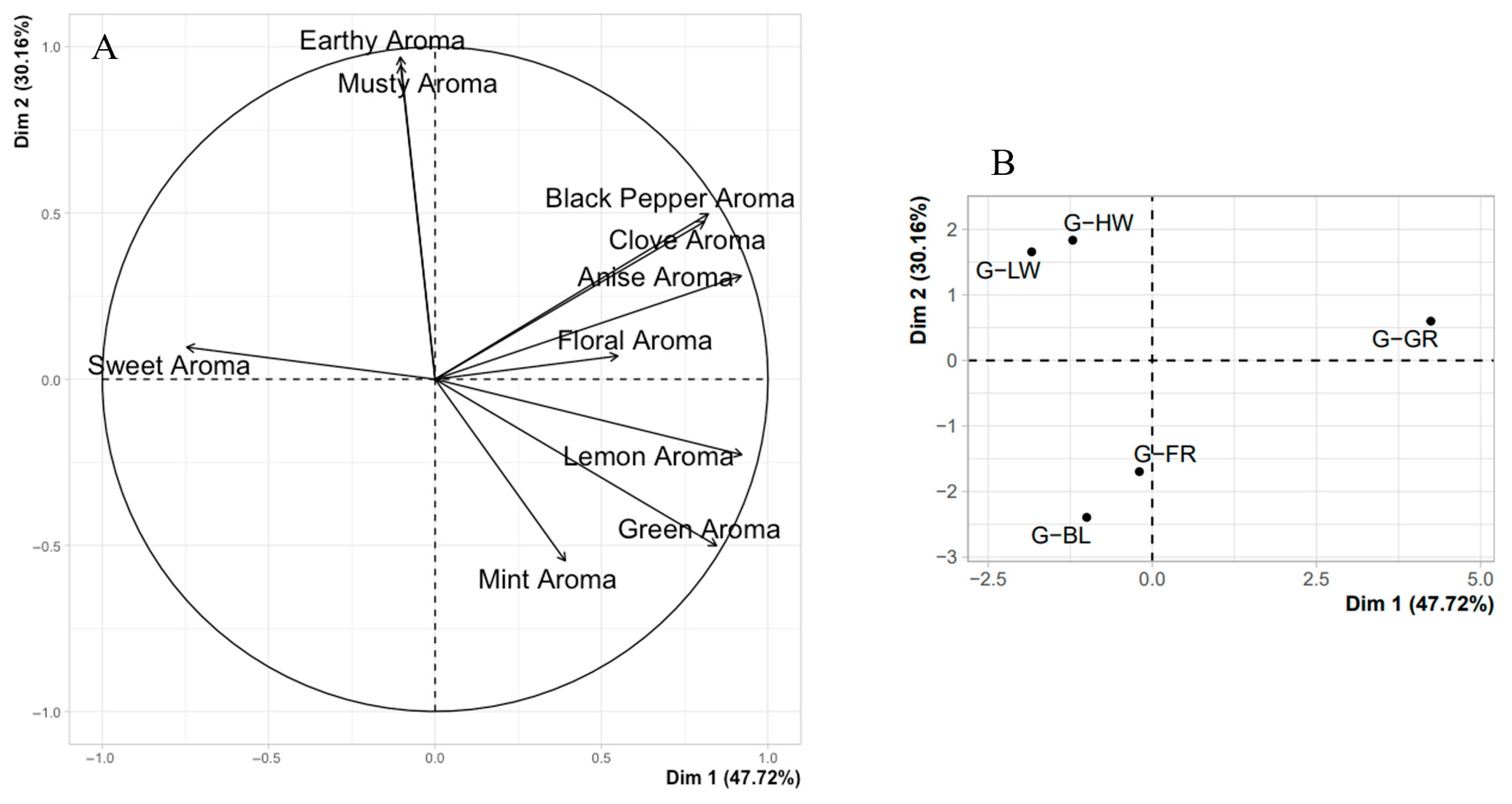
The relationship between the light spectrum and aromatic compound production has direct implications for sensory science and consumer preferences. Sensory panels and consumer testing are crucial in evaluating the organoleptic properties of basil, such as flavor and aroma which, as seen in our study and others, are heavily influenced by the concentrations of volatile compounds [
17,
62]. These findings further demonstrate that specific light treatments directly link volatile compound synthesis to perceived sensory quality. For example, FR light significantly enhanced monoterpene production, creating an intense aromatic profile favored in sensory evaluations. Compounds like citral and linalool are key determinants of the floral and citrus aromas that consumers typically associate with high-quality basil [
44]. In the current study, FR light, by enhancing monoterpene production, created a more intense aromatic profile, appealing to consumers seeking aromatic complexity. Conversely, the reduction in volatiles such as eugenol and linalool under BL light compared to FR light led to a milder aroma, which could be less desirable in markets prioritizing strong, pungent basil flavors. This trade-off between aroma intensity and nutritional quality reflects the need for growers to balance production goals with market demands, depending on whether the focus is on sensory enhancement or nutritional properties. Furthermore, the trade-off between aroma intensity and nutritional quality suggests that different consumer demographics may have varying preferences depending on whether they prioritize flavor or potential health benefits. For instance, health-conscious consumers may prefer basil grown under BL light for its potential antioxidant benefits, while culinary markets may favor basil grown under FR light for its enhanced sensory attributes. Consumer panels play a vital role in determining how different segments of the market respond to light-induced changes in basil’s sensory attributes. Furthermore, understanding consumer preferences is key to the success of specific basil cultivars under controlled light conditions. Sensory testing, combined with chemical analysis of volatile compounds, allows growers to fine-tune light environments to produce basil with the most marketable flavor and aromatic profiles, enhancing both yield and market appeal.
The ability to manipulate basil’s volatile profiles through light spectra has important practical implications for basil production in controlled environments. FR light could be used to enhance citrusy and floral aromas, making basil more appealing to consumers who prefer these sensory attributes. This targeted approach can help growers cater to niche markets, such as gourmet culinary herbs or premium aromatics for specialty foods and beverages. This is particularly useful for the culinary and aromatic herb markets, where flavor and aroma are key drivers of consumer preference. Additionally, the elongation growth promoted by FR light complements its effects on aroma by increasing overall plant biomass and leaf surface area, which may further amplify volatile compound production. The combination of increased yield and enhanced aroma profiles makes FR light particularly advantageous for markets prioritizing both yield and sensory quality, although FR can promote extensive extension growth. In contrast, BL light, while reducing the intensity of some aroma compounds, enhances leaf coloration and nutritional content, making it valuable for growers targeting health-conscious consumers or those seeking visually appealing basil with high antioxidant content.
This study also suggests that growers should consider cultivar-specific responses when designing light regimes for basil production. For instance, Amethyst basil responded more strongly to FR light in terms of allo-ocimene production, while Prospera basil showed greater increases in citral. The distinct responses highlight how each cultivar’s metabolic pathways are differentially regulated by specific light wavelengths, underscoring the importance of cultivar-specific optimization. Additionally, Prospera basil had more volatile compounds detected, indicating it might have a more complex chemical makeup. This highlights the importance of tailoring light treatments to the specific needs of each cultivar to optimize both growth and sensory quality.