Salinity Tolerance of Novel and Established Olive (Olea europaea L.) Cultivars for Super-High-Density Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Experimental Design
2.2. Irrigation Management
2.3. Plant Material Analysis
2.4. Physiological Parameters
2.4.1. Chlorophyll Fluorescence
2.4.2. Stomatal Conductance
2.4.3. Soil Plant Analysis Development (SPAD)
2.5. Statistical Analysis
3. Results
3.1. Growth Parameters
3.1.1. Fresh and Dry Weight
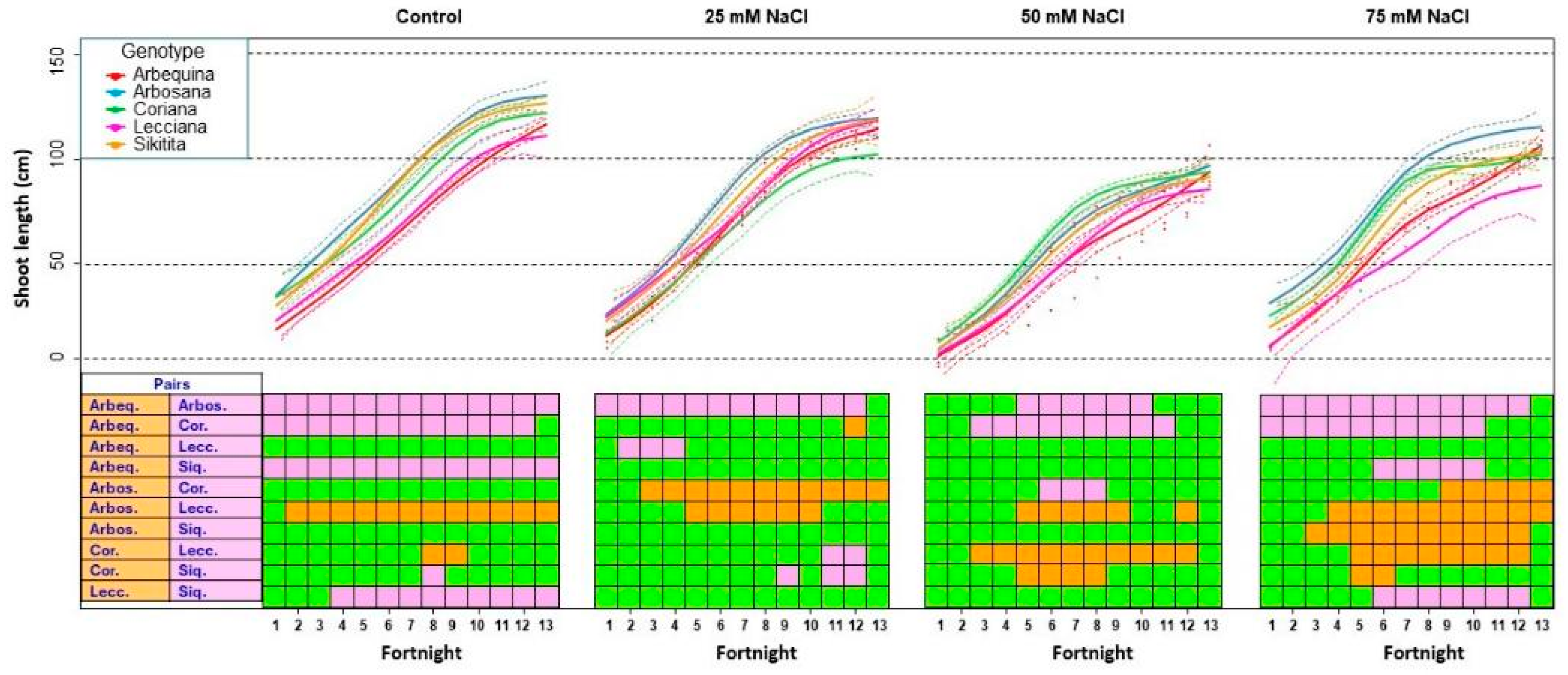
3.1.2. Shoot Length
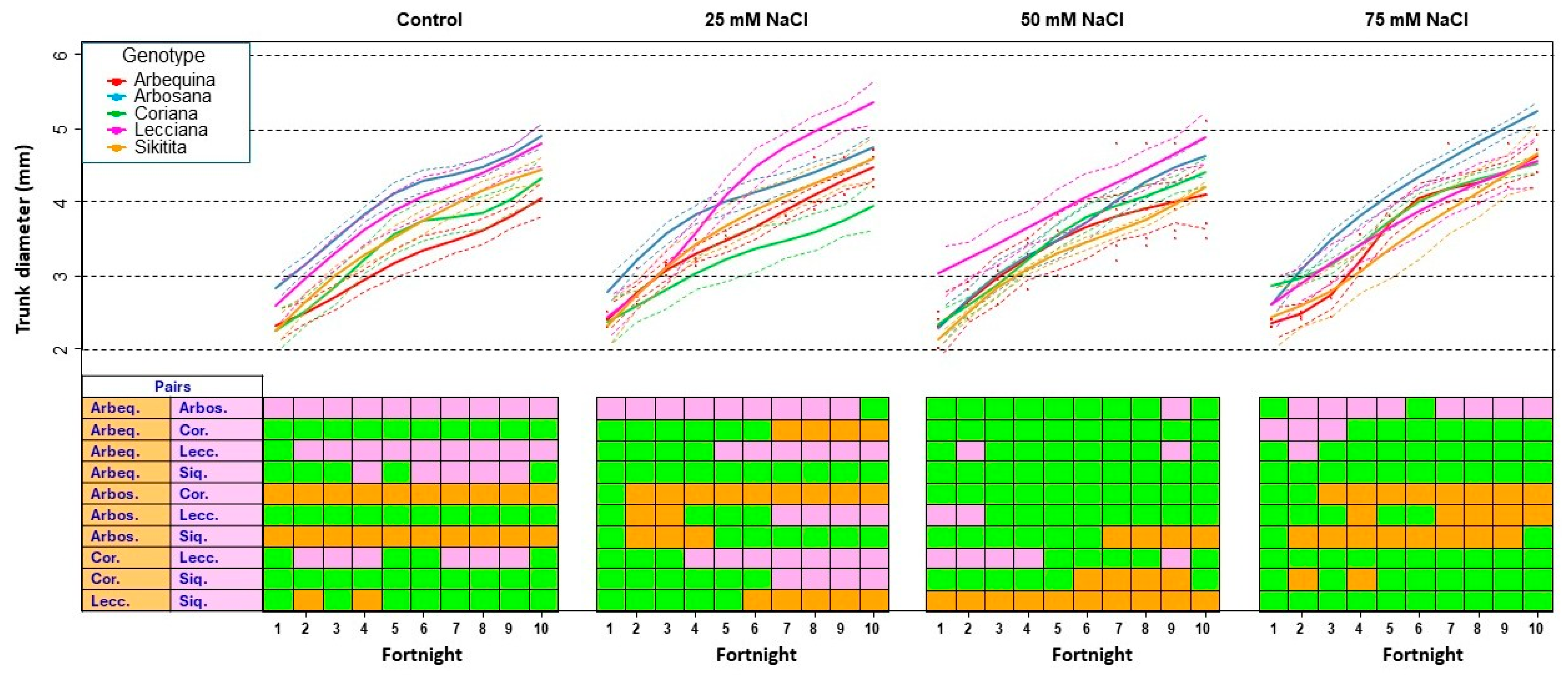
3.1.3. Trunk Diameter
3.2. Physiological Responses Under Salt Treatments
3.2.1. Fluorimetry
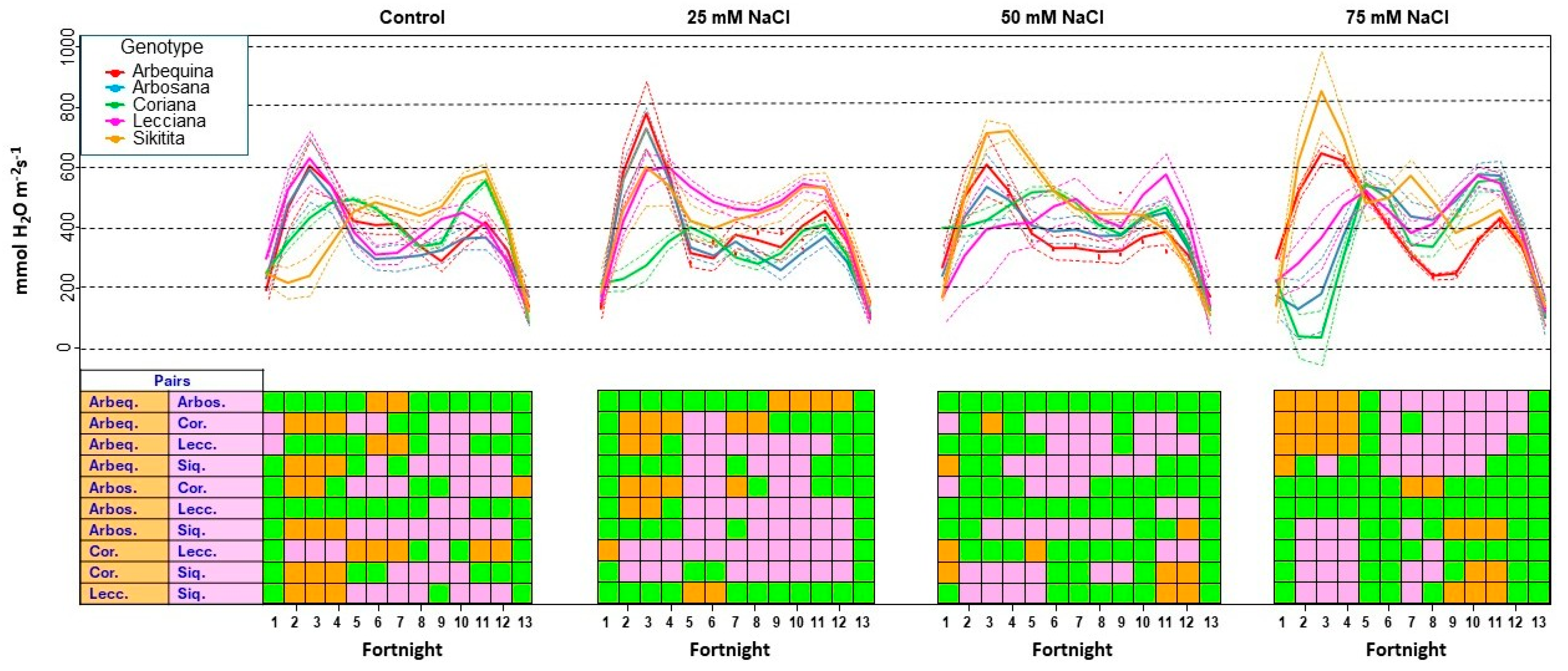
3.2.2. Gas Exchange
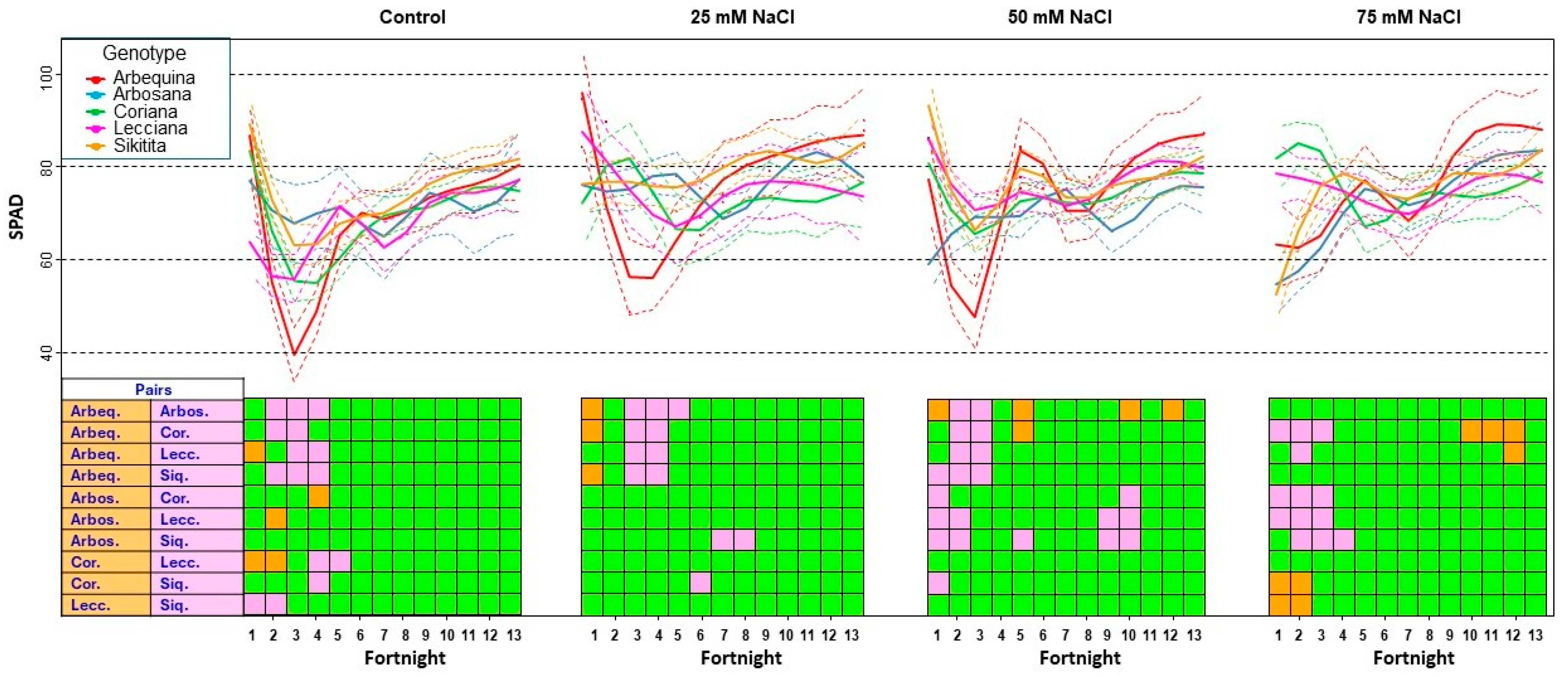
3.2.3. Chlorophyll Content Analysis by Soil–Plant Analysis Development (SPAD)
3.3. Changes in Mineral Content
3.3.1. Calcium Content and Distribution
3.3.2. Potassium Accumulation Patterns
3.3.3. Chloride Accumulation and Distribution
3.3.4. Sodium Content and Distribution
3.3.5. Potassium/Sodium Ratio Analysis
3.3.6. Calcium/Sodium Ratio Analysis
4. Discussion
4.1. Differential Growth and Resource Allocation Under Salinity
4.2. Physiological Adjustments: Photosynthesis and Stomatal Regulation
4.3. Ion Homeostasis as the Core Mechanism of Salt Tolerance
4.3.1. Role of Calcium in Mitigating Sodium Toxicity
4.3.2. Potassium Accumulation Patterns
4.3.3. Sodium and Chloride Exclusion as a Primary Defense
4.3.4. Ionic Ratios as Key Indicators of Tolerance
4.4. Genotype Performance Comparison
4.5. Genotype-Specific Applications
4.6. Study Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | atomic absorption spectrometry |
| ACA | autoinhibited Ca2+-ATPase |
| AIC | Akaike information criterion |
| AKT | Arabidopsis K+ transporter |
| ANOVA | analysis of variance |
| APX | ascorbate peroxidase |
| BIC | Bayesian information criterion |
| CAT | catalase |
| CAX | cation/H+ exchanger |
| CI | confidence interval |
| CV | coefficient of variation |
| DIo/CSo | energy dissipated per cross-section |
| DIo/RC | energy dissipated per reaction center |
| DREB | dehydration-responsive element-binding |
| EC | electrical conductivity |
| ETo/RC | electron transport rate per reaction center |
| F0 | initial fluorescence |
| Fm | maximum fluorescence |
| Fv | variable fluorescence |
| Fv/Fm | maximum quantum yield of PSII |
| Fv/F0 | variable to initial fluorescence ratio |
| F0/Fm | initial to maximum fluorescence ratio |
| GR | glutathione reductase |
| HAK | high-affinity K+ transporter |
| HKT | high-affinity K+ transporter |
| KT | K+ transporter |
| KUP | K+ uptake permease |
| MYB | myeloblastosis |
| N | turnover number |
| NHX | Na+/H+ exchanger |
| OJIP | chlorophyll fluorescence transient phases |
| P5CS | Δ1-pyrroline-5-carboxylate synthetase |
| PI(abs) | performance index on absorption basis |
| ProDH | proline dehydrogenase |
| PSII | photosystem II |
| pXRF | portable X-ray fluorescence |
| QA | primary quinone electron acceptor |
| REo/RC | electron transport rate beyond QA per reaction center |
| ROS | reactive oxygen species |
| SAR | sodium absorption ratio |
| SD | standard deviation |
| SE | standard error |
| SHD | super-high-density |
| Sm | normalized total complementary area |
| SOD | superoxide dismutase |
| SOS | salt overly sensitive |
| SPAD | soil-plant analysis development |
| SW | Shapiro-Wilk test |
| Vj | relative variable fluorescence at J-step |
| WRKY | WRKY transcription factor family |
| φ(Po) | maximum quantum yield of primary photochemistry |
| ψ(Eo) | efficiency of electron movement into the electron transport chain |
References
- Vilar, J.; Hernández, M.L.; Pereira, J.A.; Hoyo, R. International Olive Growing: Worldwide Analysis and Summary; Fundación Caja Rural Jaén: Jaén, Spain, 2018; p. 153. [Google Scholar]
- Camposeo, S.; Vivaldi, G.A.; Russo, G.; Melucci, F.M. Intensification in olive growing reduces global warming potential under both integrated and organic farming. Sustainability 2022, 14, 6389. [Google Scholar] [CrossRef]
- Sobreiro, J.; Patanita, M.I.; Patanita, M.; Tomaz, A. Sustainability of high-density olive orchards: Hints for irrigation management and agroecological approaches. Water 2023, 15, 2486. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Proietti, P.; Regni, L.; Caruso, T. Planting systems for modern olive growing: Strengths and weaknesses. Agriculture 2021, 11, 494. [Google Scholar] [CrossRef]
- DeAndreis, P. One-Third of Global Olive Oil Production Comes from Intensive Farming. Available online: https://www.oliveoiltimes.com/production/one-third-of-global-olive-oil-production-comes-from-intensive-farming/112809 (accessed on 8 August 2025).
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-scale soil salinization dynamics from global to pore scale: A review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Shokri, N.; Hassani, A.; Sahimi, M. Soil salinization: A rising threat to ecosystems and global food security. Eos 2024, 105. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2021, 38, 39–67. [Google Scholar] [CrossRef]
- FAO. Global Status of Salt-Affected Soils; FAO: Rome, Italy, 2024; p. 240. [Google Scholar] [CrossRef]
- Chartzoulakis, K.S. Salinity and olive: Growth, salt tolerance, photosynthesis and yield. Agric. Water Manag. 2005, 78, 108–121. [Google Scholar] [CrossRef]
- Jung, E.; Park, N.; Park, J. Composite modeling for evaluation of groundwater and soil salinization on the multiple reclaimed land due to sea-level rise. Transp. Porous Media 2020, 136, 271–293. [Google Scholar] [CrossRef]
- Carr, M.K.V. The water relations and irrigation requirements of olive (Olea europaea L.): A review. Exp. Agric. 2013, 49, 597–639. [Google Scholar] [CrossRef]
- Hoque, M.N.; Imran, S.; Hannan, A.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Sarker, P.; Irin, I.J.; Brestic, M.; Rhaman, M.S. Organic amendments for mitigation of salinity stress in plants: A review. Life 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Khademi, F.; Ghasemnezhad, M.; Salehi, M.M.; Seyedi, A. Evaluation of salinity tolerance in three Olea europaea L. cultivars. Russ. J. Plant Physiol. 2023, 70, 85. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. In Agricultural Salinity Assessment and Management, 2nd ed.; Wallender, W.W., Tanji, K.K., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 405–459. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance—Current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Kchaou, H.; Larbi, A.; Gargouri, K.; Chaieb, M.; Morales, F.; Msallem, M. Assessment of tolerance to NaCl salinity of five olive cultivars, based on growth characteristics and Na+ and Cl− exclusion mechanisms. Sci. Hortic. 2010, 124, 306–315. [Google Scholar] [CrossRef]
- Soda, N.; Ephrath, J.E.; Dag, A.; Beiersdorf, I.; Presnov, E.; Yermiyahu, U.; Ben-Gal, A. Root growth dynamics of olive (Olea europaea L.) affected by irrigation induced salinity. Plant Soil 2016, 411, 305–318. [Google Scholar] [CrossRef]
- Tabatabaei, S.J. Effects of salinity and N on the growth, photosynthesis and N status of olive (Olea europaea L.) trees. Sci. Hortic. 2006, 108, 432–438. [Google Scholar] [CrossRef]
- Tabatabaei, S. Salinity stress and olive: An overview. Plant Stress 2007, 1, 105–112. [Google Scholar]
- Bracci, T.; Minnocci, A.; Sebastiani, L. In vitro olive (Olea europaea L.) cvs Frantoio and Moraiolo microshoot tolerance to NaCl. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2008, 142, 563–571. [Google Scholar] [CrossRef]
- Karimi, S.; Rahemi, M.; Zeinanloo, A.A. Ion compartmentalization determines salinity tolerance in olive cultivars. Erwerbs-Obstbau 2023, 65, 2527–2536. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Loupassaki, M.; Bertaki, M.; Androulakis, I. Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. Sci. Hortic. 2002, 96, 235–247. [Google Scholar] [CrossRef]
- Claros, M.G.; Bullones, A.; Castro, A.J.; Lima-Cabello, E.; Viruel, M.Á.; Suárez, M.F.; Romero-Aranda, R.; Fernández-Pozo, N.; Veredas, F.J.; Belver, A.; et al. Multi-omic advances in olive tree (Olea europaea subsp. europaea L.) under salinity: Stepping towards ‘smart oliviculture’. Biology 2025, 14, 287. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of four olive cultivars during salt stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- El Yamani, M.; Cordovilla, M.d.P. Tolerance mechanisms of olive tree (Olea europaea) under saline conditions. Plants 2024, 13, 2094. [Google Scholar] [CrossRef]
- Boussadia, O.; Zgallai, H.; Mzid, N.; Zaabar, R.; Braham, M.; Doupis, G.; Koubouris, G. Physiological responses of two olive cultivars to salt stress. Plants 2023, 12, 1926. [Google Scholar] [CrossRef]
- Mousavi, S.; Regni, L.; Bocchini, M.; Mariotti, R.; Cultrera, N.G.M.; Mancuso, S.; Googlani, J.; Chakerolhosseini, M.R.; Guerrero, C.; Albertini, E.; et al. Physiological, epigenetic and genetic regulation in some olive cultivars under salt stress. Sci. Rep. 2019, 9, 1093. [Google Scholar] [CrossRef]
- Ayaz, M.; Varol, N.; Yolcu, S.; Pelvan, A.; Kaya, Ü.; Aydoğdu, E.; Bor, M.; Özdemir, F.; Türkan, İ. Three (Turkish) olive cultivars display contrasting salt stress-coping mechanisms under high salinity. Trees 2021, 35, 1283–1298. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; College of Agriculture, University of California; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; p. 31. [Google Scholar]
- Perica, S.; Goreta, S.; Selak, G.V. Growth, biomass allocation and leaf ion concentration of seven olive (Olea europaea L.) cultivars under increased salinity. Sci. Hortic. 2008, 117, 123–129. [Google Scholar] [CrossRef]
- Sapkota, Y.; McDonald, L.M.; Griggs, T.C.; Basden, T.J.; Drake, B.L. Portable x-ray fluorescence spectroscopy for rapid and cost-effective determination of elemental composition of ground forage. Front. Plant Sci. 2019, 10, 317. [Google Scholar] [CrossRef]
- Antonangelo, J.; Zhang, H. Soil and plant nutrient analysis with a portable XRF probe using a single calibration. Agronomy 2021, 11, 2118. [Google Scholar] [CrossRef]
- Towett, E.K.; Shepherd, K.D.; Lee Drake, B. Plant elemental composition and portable X-ray fluorescence (pXRF) spectroscopy: Quantification under different analytical parameters. X-Ray Spectrom. 2016, 45, 117–124. [Google Scholar] [CrossRef]
- McGladdery, C.; Weindorf, D.C.; Chakraborty, S.; Li, B.; Paulette, L.; Podar, D.; Pearson, D.; Kusi, N.Y.O.; Duda, B. Elemental assessment of vegetation via portable X-ray fluorescence (pXRF) spectrometry. J. Environ. Manag. 2018, 210, 210–225. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, N.; Singh, V.K. Application of X-ray fluorescence spectrometry in plant science: Solutions, threats, and opportunities. X-Ray Spectrom. 2021, 51, 304–327. [Google Scholar] [CrossRef]
- Jones, J.B.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. In Soil testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 2018; Volume 3, pp. 389–427. [Google Scholar]
- Kalra, Y. Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 1997; p. 320. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lilliefors, H.W. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust tests for the equality of variances. J. Am. Stat. Assoc. 1974, 69, 364. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wilcox, R.R. Introduction to Robust Estimation and Hypothesis Testing, 5th ed.; Academic Press: London, UK, 2021. [Google Scholar]
- Welch, B.L. On the comparison of several mean values: An alternative approach. Biometrika 1951, 38, 330–336. [Google Scholar] [CrossRef]
- Mond, C.E.D.; Lenth, R.V. A robust confidence interval for location. Technometrics 1987, 29, 211. [Google Scholar] [CrossRef]
- Wilcox, R.R.; Tian, T.S. Measuring effect size: A robust heteroscedastic approach for two or more groups. J. Appl. Stat. 2011, 38, 1359–1368. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies. With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd ed.; Springer: New York, NY, USA, 2015; p. 582. [Google Scholar] [CrossRef]
- Friedman, J.H. Multivariate adaptive regression splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 2006, 8, 551–561. [Google Scholar] [CrossRef]
- Krzanowski, W.J. An Introduction to Statistical Modelling; Wiley: London, UK, 2010; p. 272. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika 2008, 95, 759–771. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Naeini, M.R. Salinity effect on concentration, uptake, and relative translocation of mineral nutrients in four olive cultivars. J. Plant Nutr. 2008, 31, 1243–1256. [Google Scholar] [CrossRef]
- Azimi, M.; Khoshzaman, T.; Taheri, M.; Dadras, A. Evaluation of salinity tolerance of three olive (Olea europaea L.) cultivars. J. Cent. Eur. Agric. 2021, 22, 571–581. [Google Scholar] [CrossRef]
- Rahemi, M.; Karimi, S.; Sedaghat, S.; Rostami, A.A. Physiological responses of olive cultivars to salinity stress. Adv. Hortic. Sci. 2017, 31, 53–60. [Google Scholar] [CrossRef]
- Tadić, J.; Dumičić, G.; Veršić Bratinčević, M.; Vitko, S.; Radić Brkanac, S. Physiological and biochemical response of wild olive (Olea europaea subsp. europaea var. sylvestris) to salinity. Front. Plant Sci. 2021, 12, 712005. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Boukhris, M. Changes in water relations, photosynthetic activity and proline accumulation in one-year-old olive trees (Olea europaea L. cv. Chemlali) in response to NaCl salinity. Acta Physiol. Plant. 2008, 30, 553–560. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Trupiano, D.; Polzella, A.; De Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Ben Youssef, N.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Tan, J.; Ben-Gal, A.; Shtein, I.; Bustan, A.; Dag, A.; Erel, R. Root structural plasticity enhances salt tolerance in mature olives. Environ. Exp. Bot. 2020, 179, 104224. [Google Scholar] [CrossRef]
- Melgar, J.C.; Mohamed, Y.; Serrano, N.; García-Galavís, P.A.; Navarro, C.; Parra, M.A.; Benlloch, M.; Fernández-Escobar, R. Long term responses of olive trees to salinity. Agric. Water Manag. 2009, 96, 1105–1113. [Google Scholar] [CrossRef]
- Bsoul, E.Y.; Shahrestani, S.A.; Shdiefat, S.M. Growth, nutrient acquisition, and physiological responses of three olive cultivars to induced salt stress. J. Plant Nutr. 2017, 40, 1955–1968. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Role of vacuolar membrane transport systems in plant salinity tolerance. J. Plant Growth Regul. 2022, 42, 1364–1401. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Kchaou, H.; Larbi, A.; Chaieb, M.; Sagardoy, R.; Msallem, M.; Morales, F. Genotypic differentiation in the stomatal response to salinity and contrasting photosynthetic and photoprotection responses in five olive (Olea europaea L.) cultivars. Sci. Hortic. 2013, 160, 129–138. [Google Scholar] [CrossRef]
- Hagagg, L.F.; Abd El-Migeed, M.M.M.; Shahin, M.F.M.; Mustafa, N.S.; El-Hady, E.S. Response of some olive cultivars to different salinity levels under shade house conditions. Middle East J. Appl. Sci. 2022, 12, 212–219. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Anjum, M.A. Physiological and molecular basis of salinity tolerance in fruit crops. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 445–464. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Tester, M. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Sodini, M.; Astolfi, S.; Francini, A.; Sebastiani, L.; Qian, S. Multiple linear regression and linear mixed models identify novel traits of salinity tolerance in Olea europaea L. Tree Physiol. 2022, 42, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.; Feificova, D.; Rasmussen, N.F.; Bauer-Gottwein, P. Plant uptake of NaCl in relation to enzyme kinetics and toxic effects. Environ. Exp. Bot. 2008, 64, 1–7. [Google Scholar] [CrossRef]
- Gerós, H.; Bazakos, C.; Manioudaki, M.E.; Sarropoulou, E.; Spano, T.; Kalaitzis, P. 454 Pyrosequencing of olive (Olea europaea L.) transcriptome in response to salinity. PLoS ONE 2015, 10, e0143000. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.; Xie, D.-Y.; Sebastiani, L. Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- León, L.; de la Rosa, R.; Velasco, L.; Belaj, A. Using wild olives in breeding programs: Implications on oil quality composition. Front. Plant Sci. 2018, 9, 232. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rius-García, X.; Videgain-Marco, M.; Casanova-Gascón, J.; Acuña-Rello, L.; Martín-Ramos, P. Salinity Tolerance of Novel and Established Olive (Olea europaea L.) Cultivars for Super-High-Density Systems. Horticulturae 2025, 11, 957. https://doi.org/10.3390/horticulturae11080957
Rius-García X, Videgain-Marco M, Casanova-Gascón J, Acuña-Rello L, Martín-Ramos P. Salinity Tolerance of Novel and Established Olive (Olea europaea L.) Cultivars for Super-High-Density Systems. Horticulturae. 2025; 11(8):957. https://doi.org/10.3390/horticulturae11080957
Chicago/Turabian StyleRius-García, Xavier, María Videgain-Marco, José Casanova-Gascón, Luis Acuña-Rello, and Pablo Martín-Ramos. 2025. "Salinity Tolerance of Novel and Established Olive (Olea europaea L.) Cultivars for Super-High-Density Systems" Horticulturae 11, no. 8: 957. https://doi.org/10.3390/horticulturae11080957
APA StyleRius-García, X., Videgain-Marco, M., Casanova-Gascón, J., Acuña-Rello, L., & Martín-Ramos, P. (2025). Salinity Tolerance of Novel and Established Olive (Olea europaea L.) Cultivars for Super-High-Density Systems. Horticulturae, 11(8), 957. https://doi.org/10.3390/horticulturae11080957










