Amelioration of Olive Tree Indices Related to Salinity Stress via Exogenous Administration of Amino Acid Content: Real Agronomic Effectiveness or Mechanistic Restoration Only?
Abstract
1. Introduction
2. Materials and Methods
3. Results
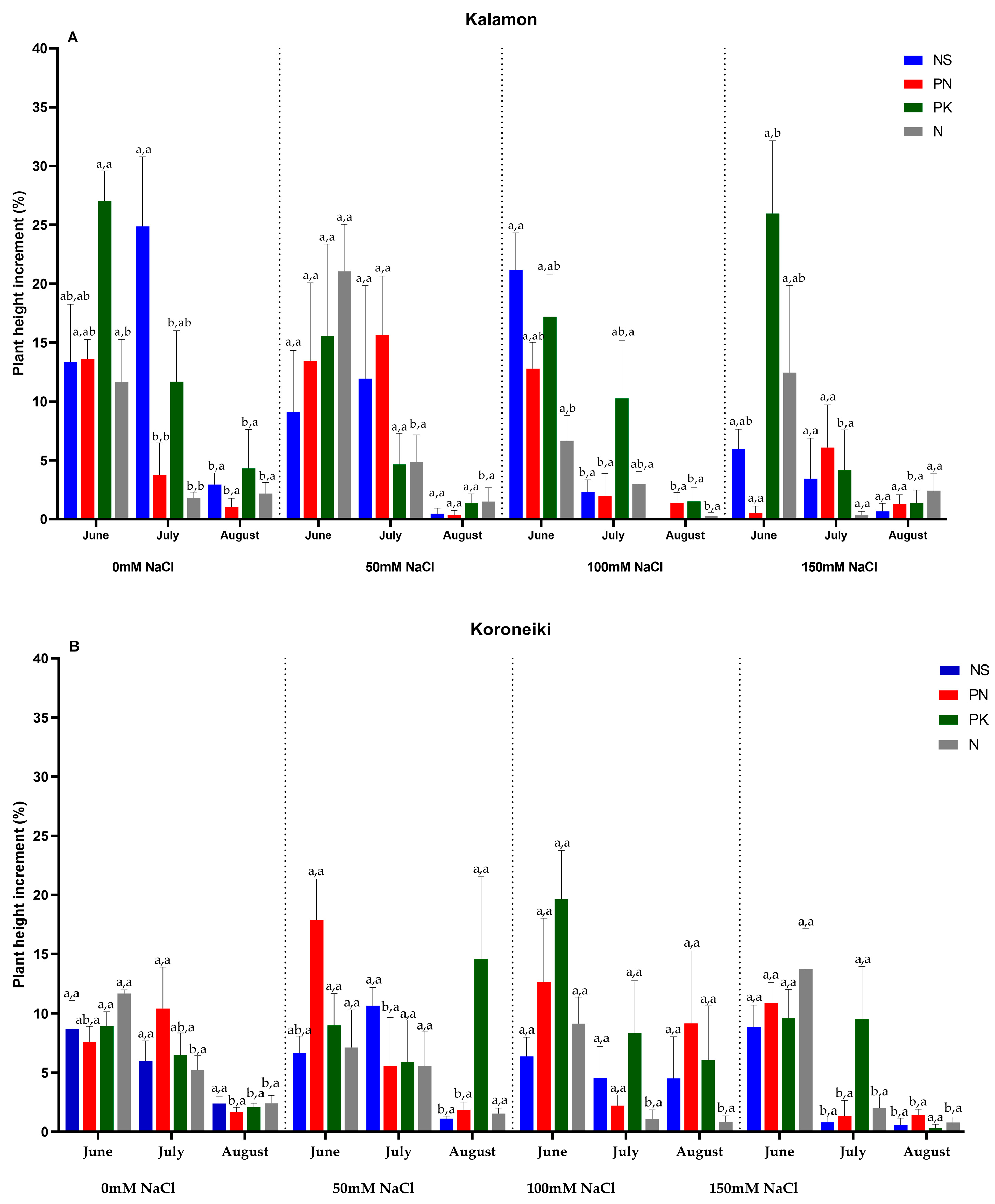
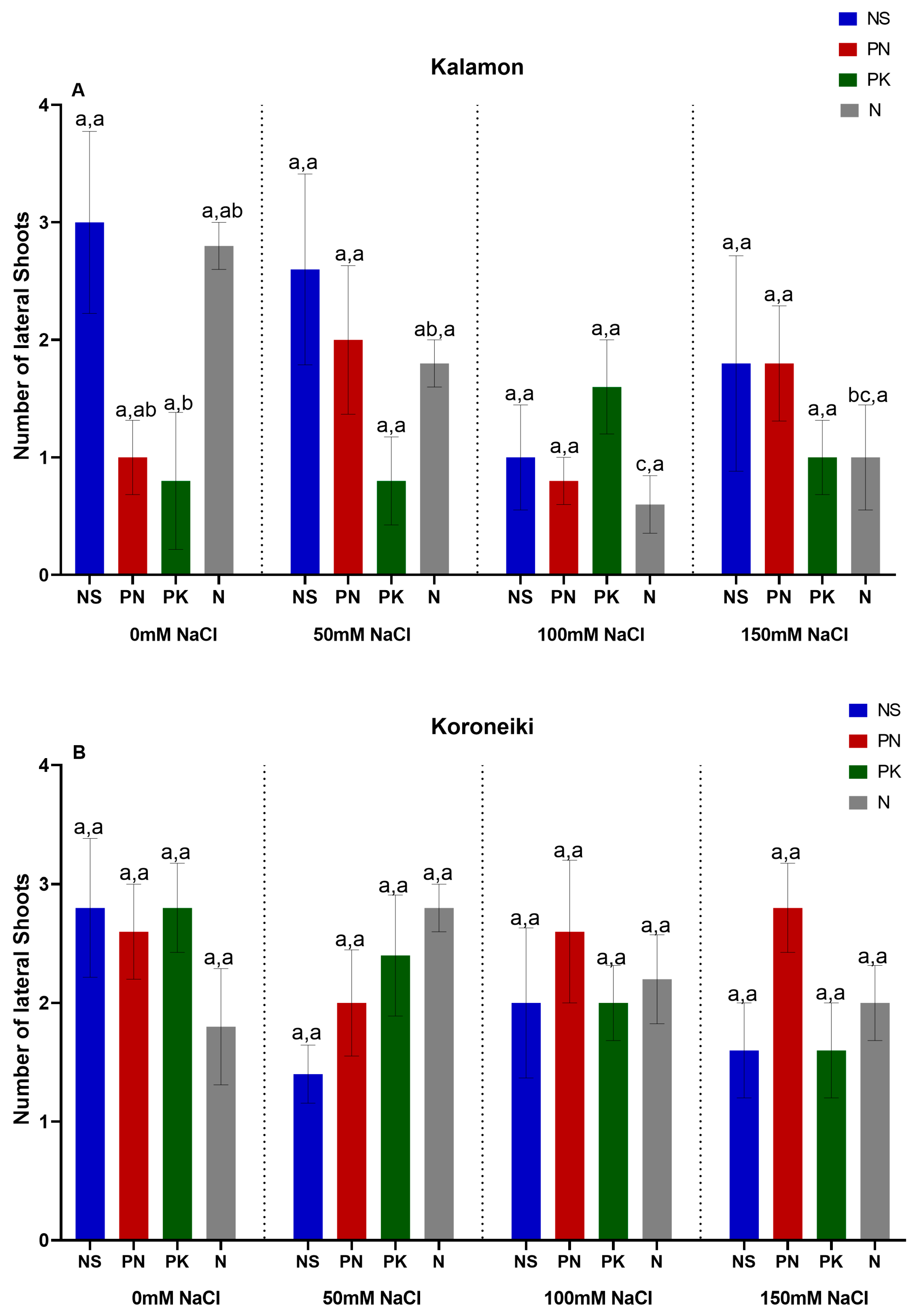
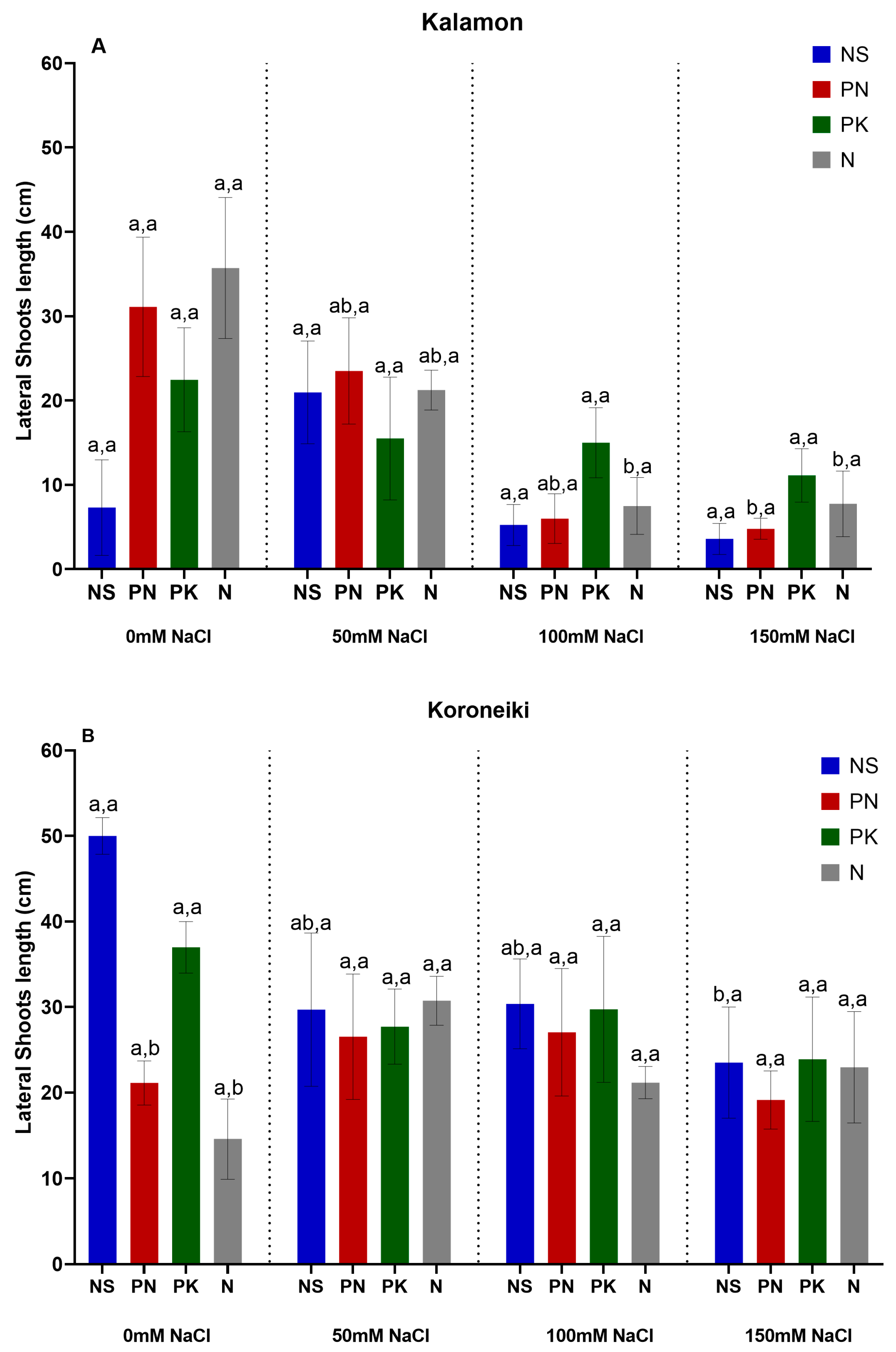
3.1. Olive Growth Rate, Lateral Number, and Length
3.2. Photosynthetic Rates
3.3. Leaf Transpiration
3.4. Stomatal Conductance
3.5. Water Use Efficiency
3.6. Leaf Quantum Yield
3.7. Cin/Cout Ratio
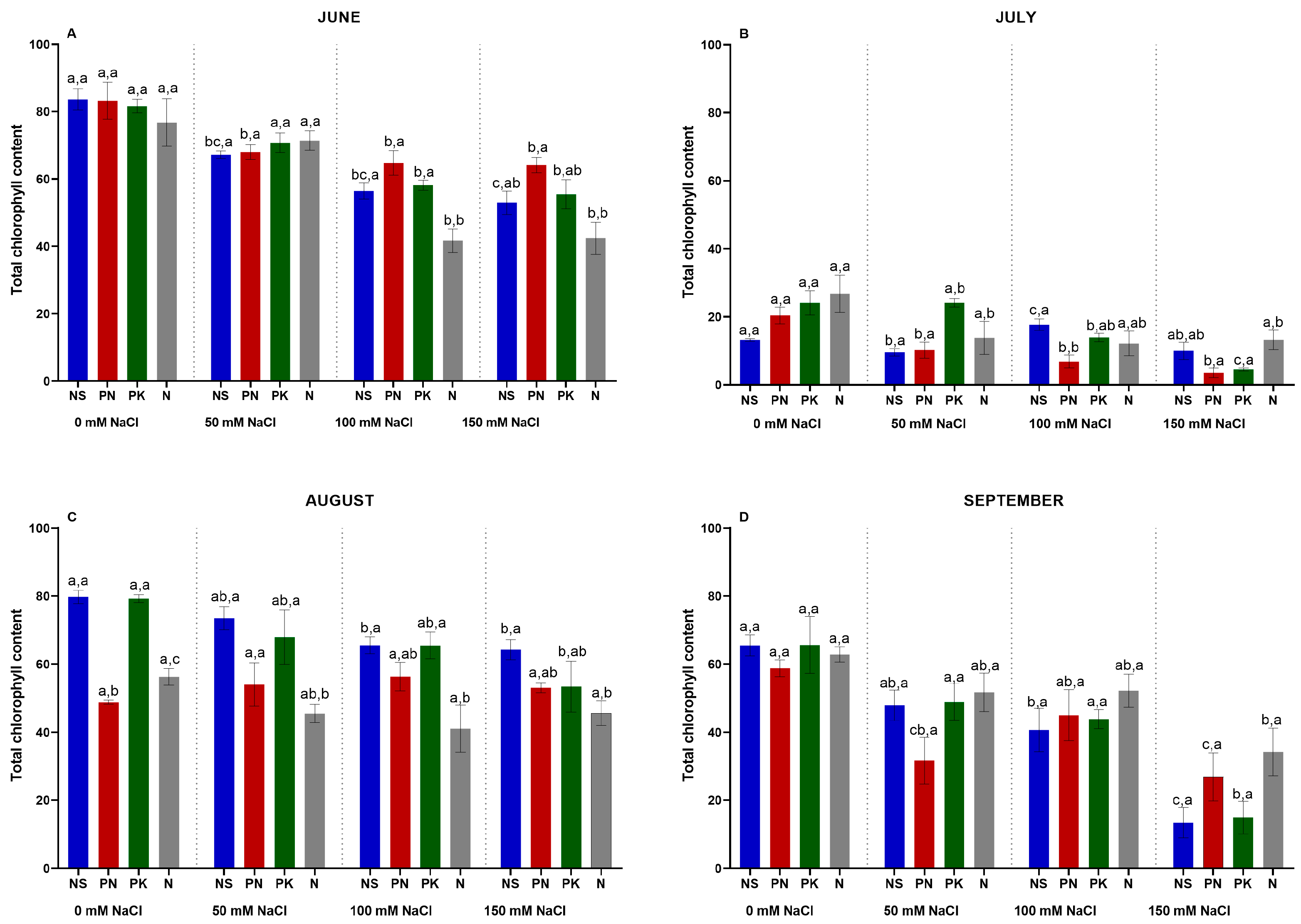
3.8. Total Chlorophyll Content
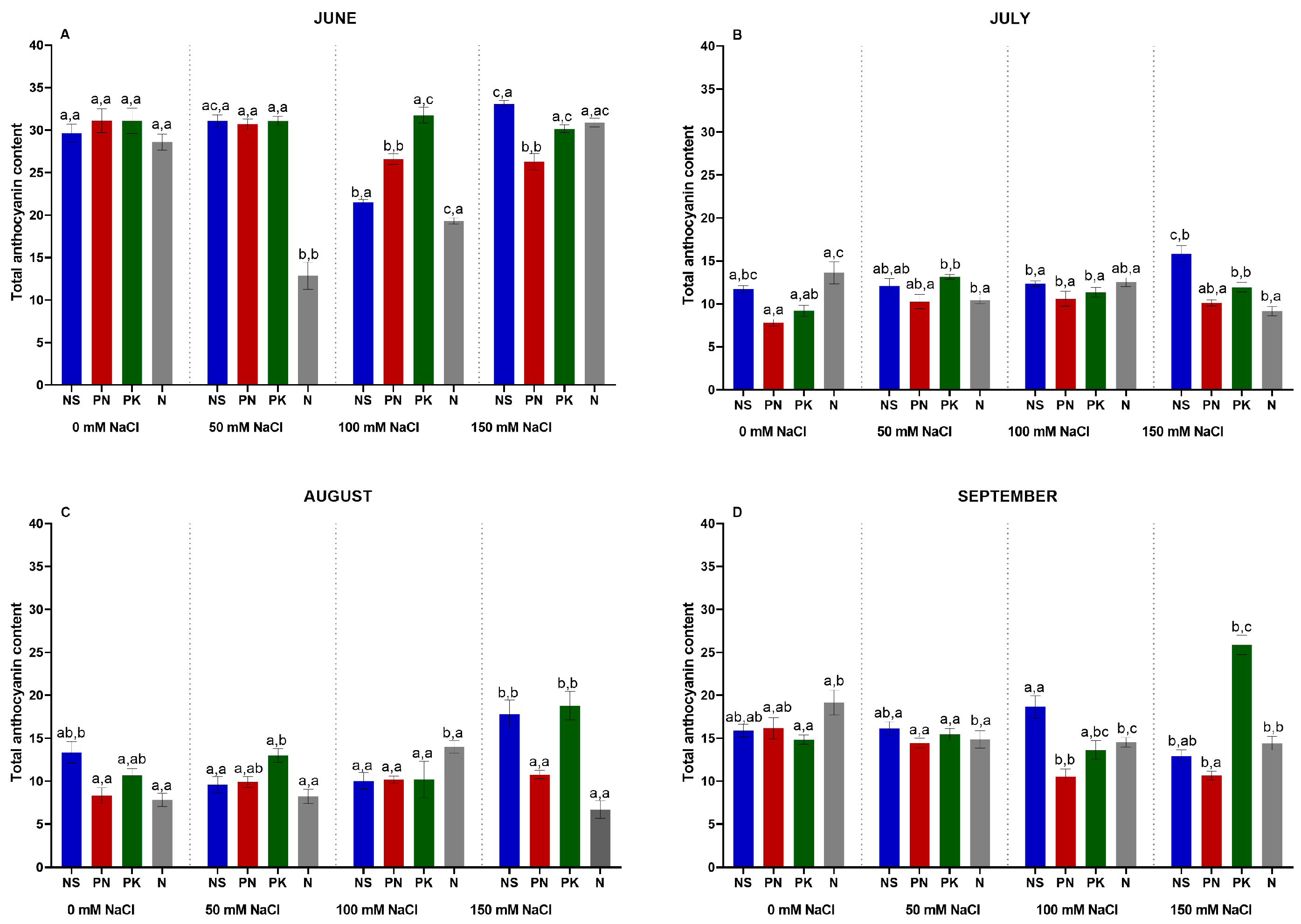
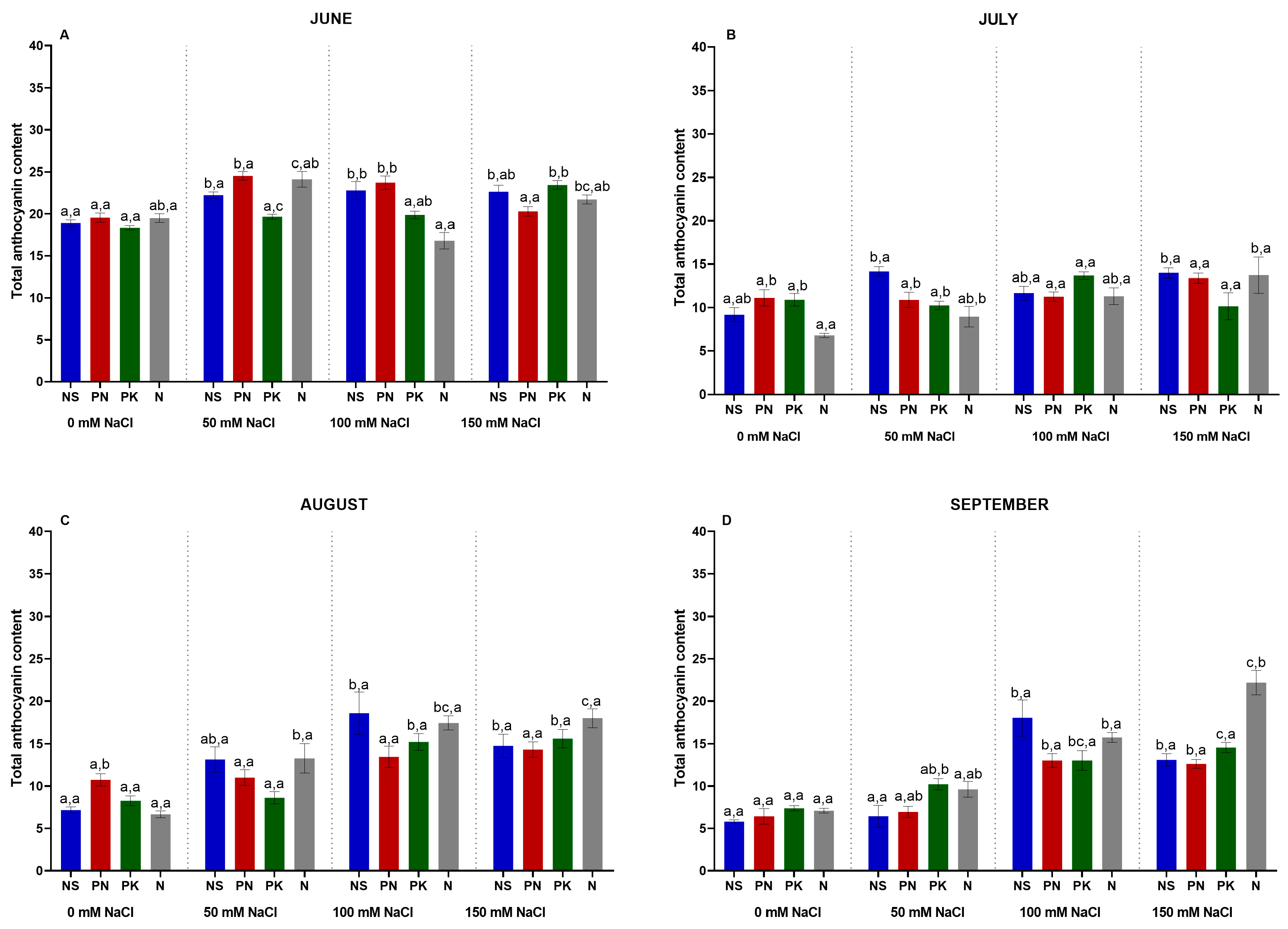
3.9. Leaf Anthocyanin Content
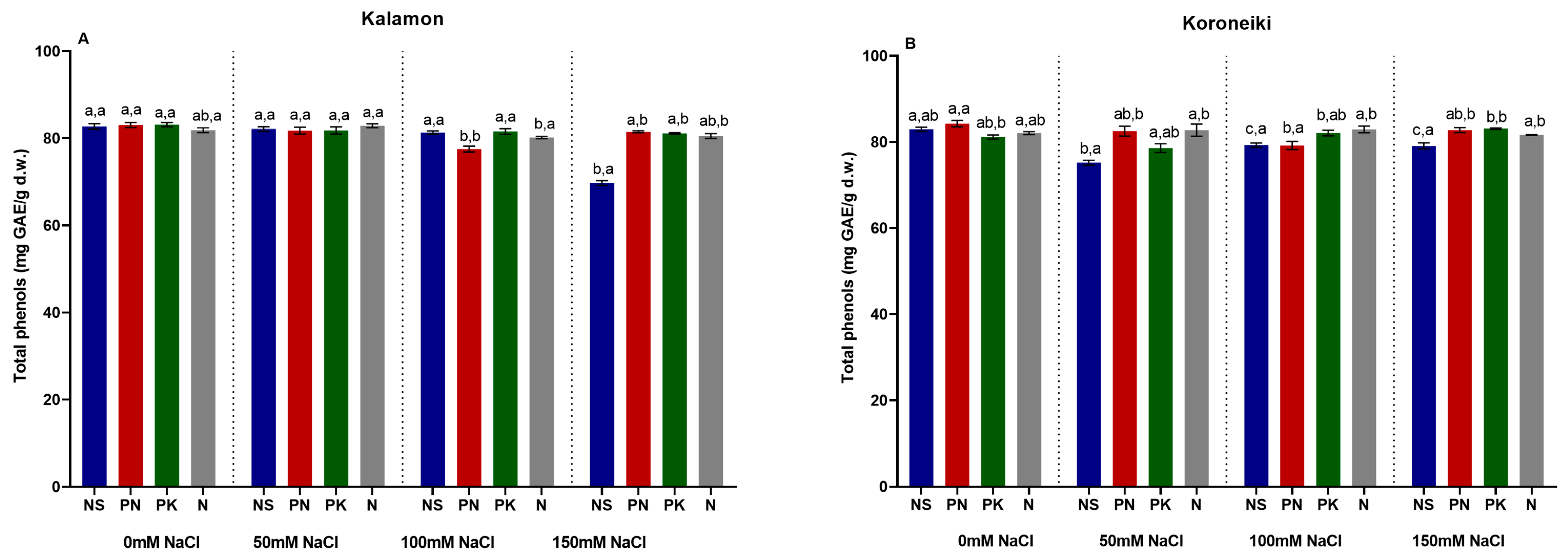
3.10. Total Phenolics
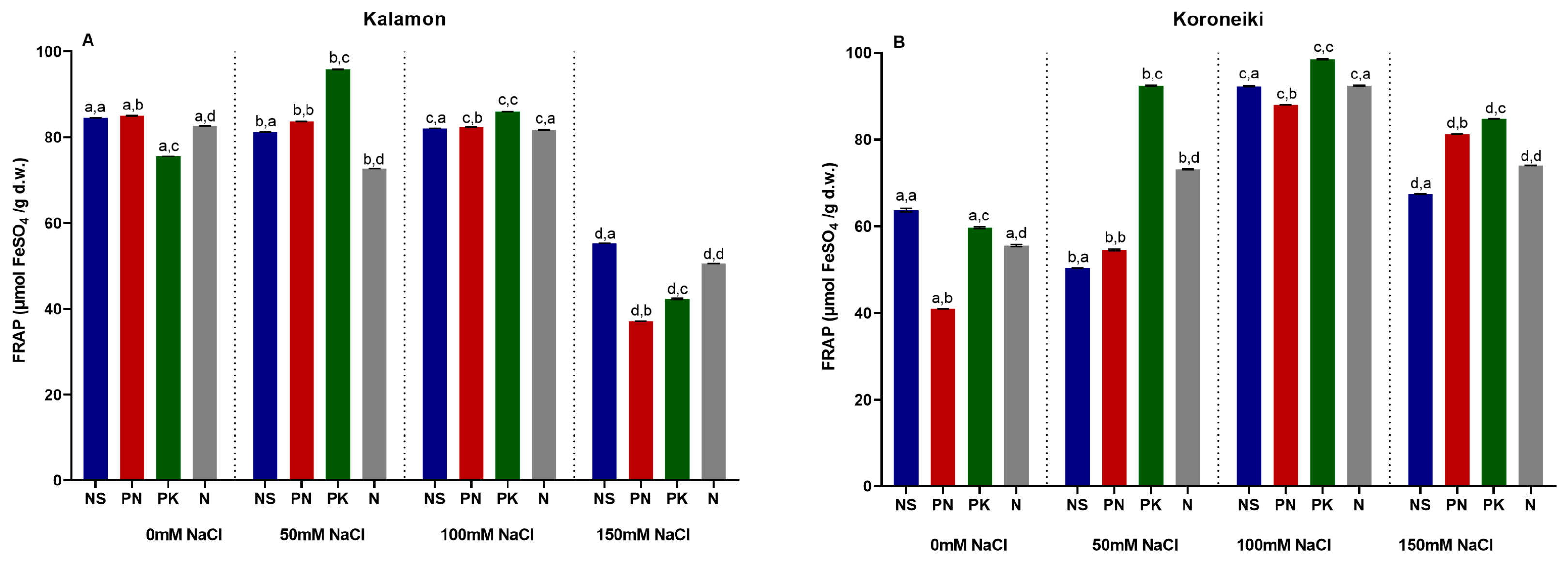
3.11. Antioxidant Activity (FRAP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loumou, A.; Giourga, C. Olive Groves: “The Life and Identity of the Mediterranean”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Włodarczyk, Z. The Seven Plant Species—A Basis of Nutrition of Ancient Israel. Biomed. J. Sci. Tech. Res. 2020, 25, 19369–19373. [Google Scholar] [CrossRef]
- Barazani, O.; Dag, A.; Dunseth, Z. The History of Olive Cultivation in the Southern Levant. Front. Plant Sci. 2023, 14, 1131557. [Google Scholar] [CrossRef]
- Avramidou, E.V.; Boutsios, S.; Korakaki, E.; Malliarou, E.; Solomou, A.; Petrakis, P.V.; Koubouris, G. Olive, a Monumental Tree; Multidimensional Perspective from Origin to Sustainability. In Economically Important Trees: Origin, Evolution, Genetic Diversity and Ecology; Uthup, T.K., Karumamkandathil, R., Eds.; Sustainable Development and Biodiversity; Springer Nature: Singapore, 2024; Volume 37, pp. 51–80. ISBN 978-981-97-5939-2. [Google Scholar]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive Oil Consumption and Human Health: A Narrative Review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Schicchi, R.; Speciale, C.; Amato, F.; Bazan, G.; Di Noto, G.; Marino, P.; Ricciardo, P.; Geraci, A. The Monumental Olive Trees as Biocultural Heritage of Mediterranean Landscapes: The Case Study of Sicily. Sustainability 2021, 13, 6767. [Google Scholar] [CrossRef]
- Villalobos, F.J.; López-Bernal, Á.; García-Tejera, O.; Testi, L. Is Olive Crop Modelling Ready to Assess the Impacts of Global Change? Front. Plant Sci. 2023, 14, 1249793. [Google Scholar] [CrossRef]
- Kalorizou, H.; Makanikas, S.; Giannoulis, P.; Papachatzis, A. Foliar Application of Urea as Alternative Nitrogen Nutritional Delivery Scheme for Konservolia and Kalamon Commercial Olive Orchards. Agric. Food 2023, 11, 233–243. [Google Scholar] [CrossRef]
- Eid, M.M.; Ali, S.E.; Mostafa, D.M.M.; Elsorady, M.E. Roasted Olive Stones Powder: Promising Alternative of Non-Caffeinated Coffee. Int. J. Fam. Stud. Food Sci. Nutr. Health 2021, 2, 110–123. [Google Scholar] [CrossRef]
- López Gómez, M.; Cultrone, G. The Use of Expanded Polystyrene and Olive Stones in the Manufacture of Lightweight Bricks: Evaluation of Their Properties and Durability. Materials 2023, 16, 1330. [Google Scholar] [CrossRef] [PubMed]
- Kafkaletou, M.; Ouzounidou, G.; Tsantili, E. Fruit Ripening, Antioxidants and Oil Composition in Koroneiki Olives (Olea europea L.) at Different Maturity Indices. Agronomy 2021, 11, 122. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Miho, H.; Díez, C.M.; Mena-Bravo, A.; Sánchez De Medina, V.; Moral, J.; Melliou, E.; Magiatis, P.; Rallo, L.; Barranco, D.; Priego-Capote, F. Cultivar Influence on Variability in Olive Oil Phenolic Profiles Determined through an Extensive Germplasm Survey. Food Chem. 2018, 266, 192–199. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G. Genotypic Variation of Total Phenol and Oleuropein Concentration and Antioxidant Activity of 11 Greek Olive Cultivars (Olea europaea L.). HortScience 2012, 47, 339–342. [Google Scholar] [CrossRef]
- Tsantili, E. Quality Attributes and Their Relations in Fresh Black Ripe ‘Kalamon’ Olives (Olea europaea L.) for Table Use—Phenolic Compounds and Total Antioxidant Capacity. Int. J. Food Sci. Technol. 2014, 49, 657–665. [Google Scholar] [CrossRef]
- Calderon Pincay, J.M.; Pincay Cantos, M.F. Analysis of Soil Salinization as an Environmental Issue in Latin America. J. Ecol. Eng. 2024, 25, 146–152. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global Predictions of Primary Soil Salinization under Changing Climate in the 21st Century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium Chloride (NaCl)-Induced Physiological Alteration and Oxidative Stress Generation in Pisum Sativum (L.): A Toxicity Assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef]
- Boland, A.M.; Jerie, P.; Maas, E. Long-Term Effects of Salinity on Fruit Trees. Acta Hortic. 1997, 449, 599–606. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef]
- Sofo, A.; Palese, A.M.; Casacchia, T.; Dichio, B.; Xiloyannis, C. Sustainable Fruit Production in Mediterranean Orchards Subjected to Drought Stress. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 105–129. ISBN 978-1-4614-0633-4. [Google Scholar]
- Chartzoulakis, K.S. Salinity and Olive: Growth, Salt Tolerance, Photosynthesis and Yield. Agric. Water Manag. 2005, 78, 108–121. [Google Scholar] [CrossRef]
- Roussos, P.A.; Dimou, A.; Assimakopoulou, A.; Gasparatos, D.; Kostelenos, G.; Bouchaghier, P.; Argyrokastritis, I. Spatial Distribution of Nutrients and Morpho-Physiological Indicators of Salinity Tolerance among Five Olive Cultivars—The Use of Relative Nutrient Concentration as an Efficient Tolerance Index. J. Plant Nutr. 2019, 42, 2269–2286. [Google Scholar] [CrossRef]
- El Yamani, M.; Cordovilla, M.D.P. Tolerance Mechanisms of Olive Tree (Olea europaea) under Saline Conditions. Plants 2024, 13, 2094. [Google Scholar] [CrossRef]
- Skodra, C.; Michailidis, M.; Moysiadis, T.; Stamatakis, G.; Ganopoulou, M.; Adamakis, I.-D.S.; Angelis, L.; Ganopoulos, I.; Tanou, G.; Samiotaki, M.; et al. Disclosing the Molecular Basis of Salinity Priming in Olive Trees Using Proteogenomic Model Discovery. Plant Physiol. 2023, 191, 1913–1933. [Google Scholar] [CrossRef]
- Amir, R.; Hacham, Y. Methionine Metabolism in Plants. In Agronomy Monographs; Jez, J., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2008; pp. 251–279. ISBN 978-0-89118-186-6. [Google Scholar]
- Ravanel, S.; Gakière, B.; Job, D.; Douce, R. The Specific Features of Methionine Biosynthesis and Metabolism in Plants. Proc. Natl. Acad. Sci. USA 1998, 95, 7805–7812. [Google Scholar] [CrossRef]
- Gomez-Jimenez, M.C.; Paredes, M.A.; Gallardo, M.; Fernandez-Garcia, N.; Olmos, E.; Sanchez-Calle, I.M. Tissue-Specific Expression of Olive S-Adenosyl Methionine Decarboxylase and Spermidine Synthase Genes and Polyamine Metabolism during Flower Opening and Early Fruit Development. Planta 2010, 232, 629–647. [Google Scholar] [CrossRef]
- Dancs, G.; Kondrák, M.; Bánfalvi, Z. The Effects of Enhanced Methionine Synthesis on Amino Acid and Anthocyanin Content of Potato Tubers. BMC Plant Biol. 2008, 8, 65. [Google Scholar] [CrossRef]
- Ortega, M.L.S.; Orellana-Palacios, J.C.; Garcia, S.R.; Rabanal-Ruiz, Y.; Moreno, A.; Hadidi, M. Olive Leaf Protein: Extraction Optimization, in Vitro Digestibility, Structural and Techno-Functional Properties. Int. J. Biol. Macromol. 2024, 256, 128273. [Google Scholar] [CrossRef]
- Procida, G.; Cichelli, A.; Lagazio, C.; Conte, L.S. Relationships between Volatile Compounds and Sensory Characteristics in Virgin Olive Oil by Analytical and Chemometric Approaches. J. Sci. Food Agric. 2016, 96, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Vichi, S.; Cortés-Francisco, N.; Romero, A.; Caixach, J. Determination of Volatile Thiols in Virgin Olive Oil by Derivatisation and LC–HRMS, and Relation with Sensory Attributes. Food Chem. 2014, 149, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Bianco, L.; Alagna, F.; Baldoni, L.; Finnie, C.; Svensson, B.; Perrotta, G. Proteome Regulation during Olea europaea Fruit Development. PLoS ONE 2013, 8, e53563. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; De Feudis, M.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli, D.; Proietti, P. The Selenium Supplementation Influences Olive Tree Production and Oil Stability Against Oxidation and Can Alleviate the Water Deficiency Effects. Front. Plant Sci. 2018, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, A.M.; Regni, L.; Di Michele, A.; Gentile, A.; Del Buono, D.; Proietti, P.; Palmerini, C.A. Effects of Selenium-Methionine against Heat Stress in Ca2+-Cytosolic and Germination of Olive Pollen Performance. Agriculture 2022, 12, 826. [Google Scholar] [CrossRef]
- Bastam, N.; Baninasab, B.; Mobli, M.; Goli, S.A.H. Effects of Foliar Applications of Zinc in the Forms of Free Mineral or Amino Acid Complexed on Qualitative Characteristics of Olive Oil. J. Am. Oil Chem. Soc. 2021, 98, 173–184. [Google Scholar] [CrossRef]
- Salama, A. Effect of Chemical Loosening Agents on Harvesting Efficiency and Fruit Quality of Olive Trees. Alex. Sci. Exch. J. 2023, 44, 25–35. [Google Scholar] [CrossRef]
- Faris, A.S.; Abdulqader, S.M. Response of Olive Trees (Olea europaea L.) cv. Zaity to Bio Health and Foliar Spray of Tecamin Max and Boron. Kufa J. Agri. Sci. 2024, 16, 113–130. [Google Scholar] [CrossRef]
- Massenti, R.; Ciaccio, V.; Lo Bianco, R. Foliar Applications with SUNRED® Biostimulant Advance and Uniform Fruit Ripening in Orange and Olive. Int. J. Plant Anim. Environ. Sci. 2015, 5, 227–232. [Google Scholar]
- Shahid, S.; Kausar, A.; Zahra, N.; Hafeez, M.B.; Raza, A.; Ashraf, M.Y. Methionine-Induced Regulation of Secondary Metabolites and Antioxidants in Maize (Zea mays L.) Subjected to Salinity Stress. Gesunde Pflanzen 2023, 75, 1143–1155. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, Y.U.; Heo, I.K.; Kim, H.A.; Seo, C.I.; Kim, J.E.; Son, S.K.; Lee, S.M.; Jhon, S.H.; Lee, H.J.; et al. Microorganism Producing O-Acetyl-Homoserine and the Method of Producing o-Acetyl-Homoserine Using the Microorganism. U.S. Patent No US20110053252A1, 17 December 2013. [Google Scholar]
- Kim, H.; Saremi, B.; Park, S.; Jung, M.; Yun, Y.; Son, J.; Lee, J.; Kim, J.-W.; Won, W. Comparative Life Cycle Assessment for the Sustainable Production of Fermentation-Based L-Methionine. J. Clean. Prod. 2024, 462, 142700. [Google Scholar] [CrossRef]
- Kim, J.-W. Plant-Scale Circular Economy Using Biological Reuse of Electrolyte Residues in the Amino Acid Industry. Bioengineering 2025, 12, 24. [Google Scholar] [CrossRef]
- Kirby, A. Exploratory Bibliometrics: Using VOSviewer as a Preliminary Research Tool. Publications 2023, 11, 10. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Fernández-Prior, A.; Luisa Castejón, M.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Extraction of Polyphenols Associated with Pectin from Olive Waste (Alperujo) with Choline Chloride. Food Chem. 2023, 419, 136073. [Google Scholar] [CrossRef]
- Fanali, C.; Della Posta, S.; Dugo, L.; Gentili, A.; Mondello, L.; De Gara, L. Choline-Chloride and Betaine-Based Deep Eutectic Solvents for Green Extraction of Nutraceutical Compounds from Spent Coffee Ground. J. Pharm. Biomed. Anal. 2020, 189, 113421. [Google Scholar] [CrossRef]
- Wahdan, M.T. Effects of Cobalt Sulfate and Choline Chloride on Fruiting and Fruit Quality of Mango cv. Succary Abiad. Life Sci. J. 2011, 8, 337–343. [Google Scholar] [CrossRef]
- Çetinbaş, M.; Koyuncu, F.; Butar, S. Application of GA3, AVG and Choline Chloride to Peach Trees: Effects on Nutritional Status of Fruit. J. Plant Nutr. 2016, 39, 1783–1795. [Google Scholar] [CrossRef]
- Teragishi, A.; Kanbara, Y.; Ono, H. Effects of Foliar Application of Choline Chloride on the Quality of Winter-Cropped Fig cv. Masui-Dauphine Grown in Hydroponics. Engei Gakkai Zasshi 2000, 69, 390–395. [Google Scholar] [CrossRef]
- Li, T.; He, M.; Zeng, J.; Chen, Z.; Hongxia, Q.; Duan, X.; Jiang, Y. Choline Chloride Alleviates the Pericarp Browning of Harvested Litchi Fruit by Inhibiting Energy Deficiency Mediated Programmed Cell Death. Postharvest Biol. Technol. 2020, 167, 111224. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, G.; Li, J.; Wang, C. Choline Chloride Treatments Promote Ornamental Quality of Lilium Oriental Hybrids ‘Sorbonne’. Acta Hortic. 2016, 1129, 123–126. [Google Scholar] [CrossRef]
- Ikeda, N.; Kamimura, M.; Uesugi, K.; Kobayashi, T.; Che, F.-S. Choline Chloride and N -Allylglycine Promote Plant Growth by Increasing the Efficiency of Photosynthesis. Biosci. Biotechnol. Biochem. 2024, 89, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bligny, R.; Foray, M.F.; Roby, C.; Douce, R. Transport and Phosphorylation of Choline in Higher Plant Cells. J. Biol. Chem. 1989, 264, 4888–4895. [Google Scholar] [CrossRef]
- Akpinar, A.; Cansev, A. Choline Supplementation Reduces Cadmium Uptake and Alleviates Cadmium Toxicity in Solanum lycopersicum Seedlings. BMC Plant Biol. 2024, 24, 977. [Google Scholar] [CrossRef]
- Hussain, I.; Saleem, M.H.; Mumtaz, S.; Rasheed, R.; Ashraf, M.A.; Maqsood, F.; Rehman, M.; Yasmin, H.; Ahmed, S.; Ishtiaq, M.; et al. Choline Chloride Mediates Chromium Tolerance in Spinach (Spinacia oleracea L.) by Restricting Its Uptake in Relation to Morpho-Physio-Biochemical Attributes. J. Plant Growth Regul. 2022, 41, 1594–1614. [Google Scholar] [CrossRef]
- Zhao, H.; Tan, J.; Qi, C. Photosynthesis of Rehmannia glutinosa Subjected to Drought Stress is Enhanced by Choline Chloride through Alleviating Lipid Peroxidation and Increasing Proline Accumulation. Plant Growth Regul. 2007, 51, 255–262. [Google Scholar] [CrossRef]
- Hu, K.; Xu, S.; Gao, Y.; He, Y.; Wang, X. Choline Chloride and Rhamnolipid Combined with Organic Manures Improve Salinity Tolerance, Yield, and Quality of Tomato. J. Plant Growth Regul. 2023, 42, 4118–4130. [Google Scholar] [CrossRef]
- Salama, K.H.A.; Mansour, M.M.F. Choline Priming-Induced Plasma Membrane Lipid Alterations Contributed to Improved Wheat Salt Tolerance. Acta Physiol. Plant. 2015, 37, 170. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Zhang, X.; Yang, Q.; Huang, B. Up-regulation of Lipid Metabolism and Glycine Betaine Synthesis Are Associated with Choline-induced Salt Tolerance in Halophytic Seashore Paspalum. Plant Cell Environ. 2020, 43, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Rathinasabapathi, B.; Chamberlin, B.; Gage, D.A. Comparative Physiological Evidence That β-Alanine Betaine and Choline- O -Sulfate Act as Compatible Osmolytes in Halophytic Limonium Species. Plant Physiol. 1991, 97, 1199–1205. [Google Scholar] [CrossRef]
- Jones, R.G.W.; Rippin, A.J.; Storey, R. Metabolism of Choline in the Rhizosphere and its Possible Influence on Plant Growth. Pestic. Sci. 1973, 4, 375–383. [Google Scholar] [CrossRef]
- Pinheiro, G.H.R.; Marques, R.F.; Araújo, P.P.S.; Martins, D.; Marchi, S.R. Hormesis Effect of 2,4-D Choline Salt on Soybean Biometric Variables. Chil. J. Agric. Res. 2021, 81, 536–545. [Google Scholar] [CrossRef]
- Blunden, G. Betaines in the Plant Kingdom and Their Use in Ameliorating Stress Conditions in Plants. Acta Hortic. 2003, 597, 23–29. [Google Scholar] [CrossRef]
- Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules 2023, 28, 4824. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, N.H.; Hamada, A.; Yamada, N.; Rai, V.; Hibino, T.; Takabe, T. Regulation of Betaine Synthesis by Precursor Supply and Choline Monooxygenase Expression in Amaranthus tricolor. J. Exp. Bot. 2007, 58, 4203–4212. [Google Scholar] [CrossRef]
- Hanson, A.D.; Scott, N.A. Betaine Synthesis from Radioactive Precursors in Attached, Water-Stressed Barley Leaves. Plant Physiol. 1980, 66, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Tsutsumi, K.; Rai, V.; Theerawitaya, C.; Cha-um, S.; Yamada-Kato, N.; Sakakibara, S.; Tanaka, Y.; Takabe, T. Isolation and Functional Characterization of 3-Phosphoglycerate Dehydrogenase Involved in Salt Responses in Sugar Beet. Protoplasma 2017, 254, 2305–2313. [Google Scholar] [CrossRef]
- Wang, W.; Liu, A.; Chen, X.; Zheng, X.; Fu, W.; Wang, G.; Ji, J.; Jin, C.; Guan, C. The Potential Role of Betaine in Enhancement of Microbial-Assisted Phytoremediation of Benzophenone-3 Contaminated Soil. Chemosphere 2022, 307, 135783. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; Yavaş, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Dai, T.; Ban, S.; Han, L.; Li, L.; Zhang, Y.; Zhang, Y.; Zhu, W. Effects of Exogenous Glycine Betaine on Growth and Development of Tomato Seedlings under Cold Stress. Front. Plant Sci. 2024, 15, 1332583. [Google Scholar] [CrossRef]
- Jarin, A.; Ghosh, U.K.; Hossain, M.S.; Mahmud, A.; Khan, M.A.R. Glycine Betaine in Plant Responses and Tolerance to Abiotic Stresses. Discov. Agric. 2024, 2, 127. [Google Scholar] [CrossRef]
- Kong, Q.; Zheng, S.; Li, W.; Liang, H.; Zhou, L.; Yang, H.; Jiang, X.; Feng, S.; Chen, T.; Ding, C. Performance of Camellia Oleifera Seedlings Under Alkali Stress Improved by Spraying with Types of Exogenous Biostimulants. Agriculture 2025, 15, 274. [Google Scholar] [CrossRef]
- Satake, F.; Okada, K.; Yoshida, Y.; Sakata, W.; Motoki, S. Glycine Betaine Material Affects the Growth and Yield of Asparagus in the Long-Term Harvest Production System in a Semi-Forcing Culture. Acta Hortic. 2024, 1404, 1319–1324. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of Glycine Betaine in the Thermotolerance of Plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The Role of Glycine Betaine in the Protection of Plants from Stress: Clues from Transgenic Plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Metwally, R.A. The Potential Utilization of Mycorrhizal Fungi and Glycine Betaine to Boost the Fenugreek (Trigonella foenum-graecum L.) Tolerance to Chromium Toxicity. J. Soil Sci. Plant Nutr. 2025, 25, 259–278. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Zheng, X.; Zhuo, C.; Hou, Q.; Yao, L.; Wang, X.; Wang, J.; Ni, W. Foliar Application of Glycinebetaine and Zn Fertilizer Improves Both the Apparent and Functional Qualities of Albino Tea [Camellia Sinensis (L.) O. Kuntze]. Food Funct. 2021, 12, 9476–9485. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Ebrahim, N.E.S.; Mohamed, G.Z. Effect of Water Stress and Foliar Application of Chitosan and Glycine Betaine on Lettuce. Sci. Rep. 2023, 13, 17274. [Google Scholar] [CrossRef] [PubMed]
- Makonya, G.M.; Bryla, D.R.; Hardigan, M.A.; Hoashi-Erhardt, W.; DeVetter, L.W. Biostimulants with Glycine Betaine or Kelp Extract Alleviate Heat Stress in Red Raspberry (Rubus idaeus). Sci. Rep. 2025, 15, 2251. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous Application of Glycinebetaine and Potassium for Improving Water Relations and Grain Yield of Wheat under Drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The Synthesis and Role of β-Alanine in Plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Balestrieri, M.L.; Ferrari, G.; Cautela, D.; Castaldo, D. Occurrence of Pipecolic Acid and Pipecolic Acid Betaine (Homostachydrine) in Citrus Genus Plants. J. Agric. Food Chem. 2012, 60, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Trinchant, J.-C.; Boscari, A.; Spennato, G.; Van De Sype, G.; Le Rudulier, D. Proline Betaine Accumulation and Metabolism in Alfalfa Plants under Sodium Chloride Stress. Exploring Its Compartmentalization in Nodules. Plant Physiol. 2004, 135, 1583–1594. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B. Role of Proline in Cell Wall Synthesis and Plant Development and its Implications in Plant Ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of Proline and GABA in Sexual Reproduction of Angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A. Role of Proline and Pyrroline-5-Carboxylate Metabolism in Plant Defense against Invading Pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Disruptive Effects of Exogenous Proline on Chloroplast and Mitochondrial Ultrastructure in Arabidopsis Leaves. S. Afr. J. Bot. 2002, 68, 393–396. [Google Scholar] [CrossRef]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline Metabolism and Transport in Plant Development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef]
- Chartzoulakis, K.S. The Potential of Saline and Residual Water Use in Olive Growing. Acta Hortic. 2014, 1057, 257–273. [Google Scholar] [CrossRef]
- Perica, S.; Goreta, S.; Selak, G.V. Growth, Biomass Allocation and Leaf Ion Concentration of Seven Olive (Olea europaea L.) Cultivars under Increased Salinity. Sci. Hortic. 2008, 117, 123–129. [Google Scholar] [CrossRef]
- Regni, L.; Del Pino, A.M.; Mousavi, S.; Palmerini, C.A.; Baldoni, L.; Mariotti, R.; Mairech, H.; Gardi, T.; D’Amato, R.; Proietti, P. Behavior of Four Olive Cultivars During Salt Stress. Front. Plant Sci. 2019, 10, 867. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Substituting Far-Red for Traditionally Defined Photosynthetic Photons Results in Equal Canopy Quantum Yield for CO2 Fixation and Increased Photon Capture During Long-Term Studies: Implications for Re-Defining PAR. Front. Plant Sci. 2020, 11, 581156. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statics in Biological Research; A Series of Books in Biology; Freeman: San Francisco, CA, USA, 1969; ISBN 978-0-7167-0663-2. [Google Scholar]
- Chartzoulakis, K.S. The Use of Saline Water for Irrigation of Olives: Effects on Growth, Physiology, Yield and Oil Quality. Acta Hortic. 2011, 888, 97–108. [Google Scholar] [CrossRef]
- Zhao, W.; Xiao, J.; Wang, S.; Gai, X.; Chen, G. Bone Biochar and Humic Acid Improved Soil Quality and Promoted Olea Europaea Growth in Coastal Saline Soil by Enhancing the Stoichiometric Homeostasis of Nutrient Elements. Biochar 2025, 7, 70. [Google Scholar] [CrossRef]
- Ziskin, R.; Dag, A.; Yermiyahu, U.; Levy, G.J. Different Amendments for Combating Soil Sodicity in an Olive Orchard. Agric. Water Manag. 2024, 299, 108837. [Google Scholar] [CrossRef]
- Rud, R.; Cohen, Y.; Alchanatis, V.; Beiersdorf, I.; Klose, R.; Presnov, E.; Levi, A.; Brikman, R.; Agam, N.; Dag, A.; et al. Characterization of Salinity-Induced Effects in Olive Trees Based on Thermal Imagery. In Precision Agriculture’15; Stafford, J.V., Ed.; Brill|Wageningen Academic: Wageningen, The Netherlands, 2015; pp. 511–518. ISBN 978-90-8686-267-2. [Google Scholar]
- Gharsallah, M.E.; Aichi, H.; Stambouli, T.; Ben Rabah, Z.; Ben Hassine, H. Assessment and Mapping of Soil Salinity Using Electromagnetic Induction and Landsat 8 OLI Remote Sensing Data in an Irrigated Olive Orchard under Semi-Arid Conditions. Soil Water Res. 2022, 17, 15–28. [Google Scholar] [CrossRef]
- Liopa-Tsakalidi, A.; Kalorizou, H.; Gana, V.; Thomopoulos, V.; Giannaros, A. Integrating Sensor Technology in Organic Table Olive Cultivation: A Case Study. Smart Agric. Technol. 2025, 11, 101037. [Google Scholar] [CrossRef]
- Mirlas, V.; Anker, Y.; Aizenkod, A.; Goldshleger, N. Irrigation Quality and Management Determine Salinization in Israeli Olive Orchards. Geosci. Model Dev. 2022, 15, 129–143. [Google Scholar] [CrossRef]
- Isidoro, D.; Aragüés, R. Modeling Survival of Young Olive Trees (Olea europaea L. cv. Arbequina) in Saline and Waterlogging Field Conditions. Agron. J. 2006, 98, 795–799. [Google Scholar] [CrossRef]
- Martinez, G.; Martínez-García, J.M.; Giráldez, J.V.; Laguna, A.M.; Ramos, T. Exploratory Modeling of Saline Irrigation of Olive Trees Using Artificially Built Contrasting Soil Barriers 2025. In Proceedings of the EGU General Assembly, Vienna, Austria, 14–19 April 2024. [Google Scholar] [CrossRef]
- D’ Andria, R.; Lavini, A. Irrigation. In Production Techniques in Olive Growing; Tombesi, A., Tombesi, S., d’Andria, R., Lavini, A., Saavedra, M.M.S., Jardak, T., Fernández-Escobar, R., Eds.; International Olive Council: Madrid, Spain, 2007; pp. 169–210. [Google Scholar]
- Rallo, P.; Rapoport, H. Early Growth and Development of the Olive Fruit Mesocarp. J. Hortic. Sci. Biotechn. 2001, 76, 408–412. [Google Scholar] [CrossRef]
- Loupassaki, M.H.; Chartzoulakis, K.S.; Digalaki, N.B.; Androulakis, I.I. The Concentration of Mineral Elements in the Leaves, Stems and Roots of Six Olive Cultivars Under Saline and Normal Irrigation Regimes. Acta Hortic. 2002, 586, 411–414. [Google Scholar] [CrossRef]
- Golz, J.F.; Hudson, A. Signalling in Plant Lateral Organ Development. Plant Cell 2002, 14, S277–S288. [Google Scholar] [CrossRef] [PubMed]
- Kalorizou, H.; Giannoulis, P.; Leontopoulos, S.; Angelakis, C.; Sorovigka, M. Coastal Almond-Leaved Pear (Pyrus spinosa) Seedlings’ Responses to Saline Stress Alleviated by Formulated L-Methionine and Bacterial Exogenous Soil Application. Horticulturae 2024, 10, 849. [Google Scholar] [CrossRef]
- Lin, Y.C.; Araguirang, G.E.; Ngo, A.H.; Lin, K.T.; Angkawijaya, A.E.; Nakamura, Y. The Four Arabidopsis Choline/Ethanolamine Kinase Isozymes Play Distinct Roles in Metabolism and Development. Plant Physiol. 2020, 183, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Kraner, M.E.; Link, K.; Melzer, M.; Ekici, A.B.; Uebe, S.; Tarazona, P.; Feussner, I.; Hofmann, J.; Sonnewald, U. Choline Transporter-like1 (CHER 1) Is Crucial for Plasmodesmata Maturation in Arabidopsis Thaliana. Plant J. 2017, 89, 394–406. [Google Scholar] [CrossRef]
- Rabeler, C.; Chen, M.; Kaplinsky, N. BUMPY STEM Is an Arabidopsis Choline/Ethanolamine Kinase Required for Normal Development and Chilling Responses. Front. Plant Sci. 2022, 13, 851960. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Chloride in Soil: From Nutrient to Soil Pollutant. Environ. Exp. Bot. 2019, 157, 299–309. [Google Scholar] [CrossRef]
- Martin, P.K.; Koebner, R.M.D. Sodium and Chloride Ions Contribute Synergistically to Salt Toxicity in Wheat. Biol. Plant 1995, 37, 265–271. [Google Scholar] [CrossRef]
- Yang, S.; Lee, H. Salinity-Triggered Responses in Plant Apical Meristems for Developmental Plasticity. Int. J. Mol. Sci. 2023, 24, 6647. [Google Scholar] [CrossRef]
- Long, L.; Gu, L.; Wang, S.; Cai, H.; Wu, J.; Wang, J.; Yang, M. Progress in the Understanding of WRKY Transcription Factors in Woody Plants. Int. J. Biol. Macromol. 2023, 242, 124379. [Google Scholar] [CrossRef]
- Rong, S.; Wu, Z.; Cheng, Z.; Zhang, S.; Liu, H.; Huang, Q. Genome-Wide Identification, Evolutionary Patterns, and Expression Analysis of bZIP Gene Family in Olive (Olea europaea L.). Genes 2020, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Escaray, F.; Felipo-Benavent, A.; Vera, P. Linking Plant Metabolism and Immunity through Methionine Biosynthesis. Molecular Plant 2022, 15, 6–8. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic Pathways of Hormones in Plants. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Denaxa, N.-K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative Effects of Exogenous Glycine Betaine, Kaolin Clay Particles and Ambiol on Photosynthesis, Leaf Sclerophylly Indexes and Heat Load of Olive cv. Chondrolia Chalkidikis under Drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Che, F.-S.; Sato, F.; Hyeon, S.-B.; Isogai, A.; Yamada, Y.; Suzuki, A. Stimulation of Photosynthesis and Growth of Photoautotrophically Cultured Plant Cells by Choline and Its Analogs. Plant Cell Rep. 1993, 12, 691–697. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous Proline Effects on Photosynthetic Performance and Antioxidant Defense System of Young Olive Tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Zouari, M.; Elloumi, N.; Labrousse, P.; Ben Rouina, B.; Ben Abdallah, F.; Ben Ahmed, C. Olive Trees Response to Lead Stress: Exogenous Proline Provided Better Tolerance than Glycine Betaine. S. Afr. J. Bot. 2018, 118, 158–165. [Google Scholar] [CrossRef]
- Riaz, S.; Hussain, I.; Ibrahim, M.; Rasheed, R.; Ashraf, M.A. Choline Chloride Mediates Salinity Tolerance in Cluster Bean (Cyamopsis tetragonoloba L.) by Improving Growth, Oxidative Defense, and Secondary Metabolism. Dose-Response 2021, 19, 15593258211055026. [Google Scholar] [CrossRef]
- Roussos, P.A.; Assimakopoulou, A.; Nikoloudi, A.; Salmas, I.; Nifakos, K.; Kalogeropoulos, P.; Kostelenos, G. Intra- and Inter-Cultivar Impacts of Salinity Stress on Leaf Photosynthetic Performance, Carbohydrates and Nutrient Content of Nine Indigenous Greek Olive Cultivars. Acta Physiol. Plant 2017, 39, 136. [Google Scholar] [CrossRef]
- Boussadia, O.; Zgallai, H.; Mzid, N.; Zaabar, R.; Braham, M.; Doupis, G.; Koubouris, G. Physiological Responses of Two Olive Cultivars to Salt Stress. Plants 2023, 12, 1926. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Espejo, A.; Hafidi, B.; Fernandez, J.E.; Palomo, M.J.; Sinoquet, H. Transpiration and Photosynthesis of the Olive Tree: A Model Approach. Acta Hortic. 2002, 586, 457–460. [Google Scholar] [CrossRef]
- Azimi, M.; Khoshzaman, T.; Taheri, M.; Dadras, A. Evaluation of Salinity Tolerance of Three Olive (Olea europaea L.) Cultivars. J. Cent. Eur. Agric. 2021, 22, 571–581. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Wang, C.; Han, K.; Hu, L.; Niu, T.; Yang, Y.; Chang, Y.; Xie, J. Exogenous Proline Enhances Systemic Defense against Salt Stress in Celery by Regulating Photosystem, Phenolic Compounds, and Antioxidant System. Plants 2023, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- ÖdemiŞ, B.; Büyüktaş, D.; Çalişkan, M.E. Effects of Saline Irrigation Water and Proline Applications on Yield, Vegetative and Physiological Characteristics of Potato Crop (Solanum tuberosum L.). Derim 2019, 36, 54–63. [Google Scholar] [CrossRef]
- Tabssum, F.; Zaman, Q.U.; Chen, Y.; Riaz, U.; Ashraf, W.; Aslam, A.; Ehsan, N.; Nawaz, R.; Aziz, H.; Shah, S.U.S. Exogenous Application of Proline Improved Salt Tolerance in Rice through Modulation of Antioxidant Activities. Pak. J. Agric. Res. 2019, 32, 140. [Google Scholar] [CrossRef]
- Huo, J.; Yu, M.; Feng, N.; Zheng, D.; Zhang, R.; Xue, Y.; Khan, A.; Zhou, H.; Mei, W.; Du, X.; et al. Integrated Transcriptome and Metabolome Analysis of Salinity Tolerance in Response to Foliar Application of Choline Chloride in Rice (Oryza sativa L.). Front. Plant Sci. 2024, 15, 1440663. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Li, S.; Chen, J.; Amin, A.S.; Wang, G.; Xiaojun, S.; Zain, M.; Gao, Y. Linking Exogenous Foliar Application of Glycine Betaine and Stomatal Characteristics with Salinity Stress Tolerance in Cotton (Gossypium hirsutum L.) Seedlings. BMC Plant Biol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Kchaou, H.; Larbi, A.; Chaieb, M.; Sagardoy, R.; Msallem, M.; Morales, F. Genotypic Differentiation in the Stomatal Response to Salinity and Contrasting Photosynthetic and Photoprotection Responses in Five Olive (Olea europaea L.) Cultivars. Sci. Hortic. 2013, 160, 129–138. [Google Scholar] [CrossRef]
- Despotaki, E.; Linos, A.; Hagidimitriou, M. Studying the Genetic Variation Among Clones of ‘Kalamon’ and ‘Koroneiki’ Using Molecular Techniques. Acta Hortic. 2011, 924, 335–339. [Google Scholar] [CrossRef]
- Galicia-Campos, E.; García-Villaraco, A.; Montero-Palmero, M.B.; Gutiérrez-Mañero, F.J.; Ramos-Solano, B. Bacillus G7 Improves Adaptation to Salt Stress in Olea europaea L. Plantlets, Enhancing Water Use Efficiency and Preventing Oxidative Stress. Sci. Rep. 2023, 13, 22507. [Google Scholar] [CrossRef]
- Ahmali, A.; Mandi, L.; Loutfi, K.; El Ghadraoui, A.; El Mansour, T.E.; El Kerroumi, A.; Hejjaj, A.; Del Bubba, M.; Ouazzani, N. Agro-Physiological Responses of Koroneiki Olive Trees (Olea europaea L.) Irrigated by Crude and Treated Mixture of Olive Mill and Urban Wastewaters. Sci. Hortic. 2020, 263, 109101. [Google Scholar] [CrossRef]
- Pervaiz, A.; Iqbal, A.; Khalid, A.; Manzoor, A.; Noreen, S.; Ayaz, A.; Zafar, Z.U.; Athar, H.-R.; Ashraf, M. Proline Induced Modulation in Physiological Responses in Wheat Plants. J. Agric. Environ. Sci. 2019, 8, 112–119. [Google Scholar] [CrossRef]
- Messedi, D.; Farhani, F.; Hamed, K.B.; Trabelsi, N.; Ksouri, R.; Athar, H.-U.-R.; Abdelly, C. Highlighting the Mechanisms by Which Proline Can Confer Tolerance to Salt Stress in Cakile maritima. Pak. J. Bot. 2016, 48, 417–427. [Google Scholar]
- Nakhaie, A.; Habibi, G.; Vaziri, A. Exogenous Proline Enhances Salt Tolerance in Acclimated Aloe vera by Modulating Photosystem II Efficiency and Antioxidant Defense. S. Afr. J. Bot. 2022, 147, 1171–1180. [Google Scholar] [CrossRef]
- Wani, A.S.; Faraz, A.; Faizan, M.; Ahmad, A.; Hayat, S.; Tahir, I. Foliar Spray of Proline Enhanced the Photosynthetic Efficiency and Antioxidant System in Brassica juncea. Not. Bot. Horti Agrobot. 2017, 45, 112–119. [Google Scholar] [CrossRef]
- Estaji, A.; Kalaji, H.M.; Karimi, H.R.; Roosta, H.R.; Moosavi-Nezhad, S.M. How Glycine Betaine Induces Tolerance of Cucumber Plants to Salinity Stress? Photosynthetica 2019, 57, 753–761. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Q.; Zhang, Y.; Zhang, M.; Li, Z. Glycine Betaine Increases Salt Tolerance in Maize (Zea mays L.) by Regulating Na+ Homeostasis. Front. Plant Sci. 2022, 13, 978304. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Loupassaki, M.; Bertaki, M.; Androulakis, I. Effects of NaCl Salinity on Growth, Ion Content and CO2 Assimilation Rate of Six Olive Cultivars. Sci. Hortic. 2002, 96, 235–247. [Google Scholar] [CrossRef]
- Melgar, J.C.; Syvertsen, J.P.; García-Sánchez, F. Can Elevated CO2 Improve Salt Tolerance in Olive Trees? J. Plant Physiol. 2008, 165, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Hajilou, J.; Tabatabaei, S.J. Photosynthetic and Growth Responses of Olive to Proline and Salicylic Acid under Salinity Condition. Not. Bot. Horti. Agrobo. 2016, 44, 579–585. [Google Scholar] [CrossRef]
- Poury, N.; Seifi, E.; Alizadeh, M. Effects of Salinity and Proline On Growth and Physiological Characteristics of Three Olive Cultivars. Gesunde Pflanzen 2023, 75, 1169–1180. [Google Scholar] [CrossRef]
- DemiRal, M.A.; Uygun, D.A.; Uygun, M.; Kasirğa, E.; Karagözler, A.A. Biochemical Response of Olea europaea cv. Gemlik to Short-Term Salt Stress. Turk. J. Biol. 2011, 35, 433–442. [Google Scholar] [CrossRef]
- Minh, L.T.; Khang, D.T.; Thu Ha, P.T.; Tuyen, P.T.; Minh, T.N.; Quan, N.V.; Xuan, T.D. Effects of Salinity Stress on Growth and Phenolics of Rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Pungin, A.; Lartseva, L.; Loskutnikova, V.; Shakhov, V.; Popova, E.; Skrypnik, L.; Krol, O. Effect of Salinity Stress on Phenolic Compounds and Antioxidant Activity in Halophytes Spergularia marina (L.) Griseb. and Glaux maritima L. Cultured In Vitro. Plants 2023, 12, 1905. [Google Scholar] [CrossRef]
- Zrig, A.; Tounekti, T.; Vadel, A.M.; Ben Mohamed, H.; Valero, D.; Serrano, M.; Chtara, C.; Khemira, H. Possible Involvement of Polyphenols and Polyamines in Salt Tolerance of Almond Rootstocks. Plant Physiol. Biochem. 2011, 49, 1313–1322. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic Content Changes in Plants Under Salt Stress. In Ecophysiology and Responses of Plants Under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 283–314. ISBN 978-1-4614-4746-7. [Google Scholar]
- Kim, N.S.; Kim, J.K.; Sathasivam, R.; Park, H.W.; Nguyen, B.V.; Kim, M.C.; Cuong, D.M.; Chung, Y.S.; Park, S.U. Impact of Betaine Under Salinity on Accumulation of Phenolic Compounds in Safflower (Carthamus tinctorius L.) Sprouts. Nat. Prod. Commun. 2021, 16, 1934578X211015090. [Google Scholar] [CrossRef]
- Saddique, M.; Kausar, A.; Iqra, I.; Akhter, N.; Mujahid, N.; Parveen, A.; Zaman, Q.; Hussain, S. Amino Acids Application Alleviated Salinity Stress in Spinach (Spinacia oleracea L.) by Improving Oxidative Defense, Osmolyte Accumulation, and Nutrient Balance. Turk. J. Agric. For. 2022, 46, 875–887. [Google Scholar] [CrossRef]
- Ahmadzai, A.S.; Hu, C.; Zhang, C.; Li, Y. Mechanisms of Anthocyanin-Mediated Salt Stress Alleviation and Cellular Homeostasis in Plants. Plant Growth Regul. 2025, 105, 655–673. [Google Scholar] [CrossRef]
- Wang, D.-R.; Yang, K.; Wang, X.; Lin, X.-L.; Rui, L.; Liu, H.-F.; Liu, D.-D.; You, C.-X. Overexpression of MdZAT5, an C2H2-Type Zinc Finger Protein, Regulates Anthocyanin Accumulation and Salt Stress Response in Apple Calli and Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1897. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-Induced Changes in Phenolic Compounds in Leaves and Roots of Four Olive Cultivars (Olea europaea L.) and Their Relationship to Antioxidant Activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Dinkeloo, K.; Boyd, S.; Pilot, G. Update on Amino Acid Transporter Functions and on Possible Amino Acid Sensing Mechanisms in Plants. Semin. Cell Dev. Biol. 2018, 74, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Svennerstam, H.; Jämtgård, S. Timing is Everything—Obtaining Accurate Measures of Plant Uptake of Amino Acids. New Phytol. 2022, 234, 311–318. [Google Scholar] [CrossRef]
- Manolikaki, I.; Digalaki, N.; Psarras, G.; Tzerakis, C.; Sergentani, C.; Papamanolioudaki, A.; Tul, S.; Koubouris, G. Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties. Int. J. Plant Biol. 2022, 13, 95–105. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants During Variable Environmental Conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Das Bhowmik, S.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L.T.M. Rapid Accumulation of Proline Enhances Salinity Tolerance in Australian Wild Rice Oryza Australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef]
- Gupta, N.; Thind, S.K.; Bains, N.S. Glycine Betaine Application Modifies Biochemical Attributes of Osmotic Adjustment in Drought Stressed Wheat. Plant Growth Regul. 2014, 72, 221–228. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential Distribution of Amino Acids in Plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Reeve, J.R.; Smith, J.L.; Carpenter-Boggs, L.; Reganold, J.P. Soil-Based Cycling and Differential Uptake of Amino Acids by Three Species of Strawberry (Fragaria spp.) Plants. Soil Biol. Biochem. 2008, 40, 2547–2552. [Google Scholar] [CrossRef]
- Sekula, B.; Ruszkowski, M.; Dauter, Z. S-Adenosylmethionine Synthases in Plants: Structural Characterization of Type I and II Isoenzymes from Arabidopsis thaliana and Medicago truncatula. Int. J. Biol. Macromol. 2020, 151, 554–565. [Google Scholar] [CrossRef]
- Van De Poel, B.; Bulens, I.; Oppermann, Y.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Sauter, M.; Geeraerd, A.H. S-adenosyl-L-methionine Usage during Climacteric Ripening of Tomato in Relation to Ethylene and Polyamine Biosynthesis and Transmethylation Capacity. Physiol. Plant. 2013, 148, 176–188. [Google Scholar] [CrossRef]
- Espartero, J.; Pintor-Toro, J.A.; Pardo, J.M. Differential Accumulation of S-Adenosylmethionine Synthetase Transcripts in Response to Salt Stress. Plant Mol. Biol. 1994, 25, 217–227. [Google Scholar] [CrossRef]
- Lu, Y.; Bu, Q.; Chuan, M.; Cui, X.; Zhao, Y.; Zhou, D. Metabolic Regulation of the Plant Epigenome. Plant J. 2023, 114, 1001–1013. [Google Scholar] [CrossRef]
- Camilloni, G. The Evolutionary Reasons of Epigenetics. DNA 2025, 5, 6. [Google Scholar] [CrossRef]
- Roy, S.; Soni, P. Unraveling the Epigenetic Landscape for Salt Tolerance in Plants. Int. J. Plant Biol. 2022, 13, 443–462. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Epigenetic Regulation during Salinity and Drought Stress in Plants: Histone Modifications and DNA Methylation. Plant Gene 2017, 11, 199–204. [Google Scholar] [CrossRef]
- Singroha, G.; Kumar, S.; Gupta, O.P.; Singh, G.P.; Sharma, P. Uncovering the Epigenetic Marks Involved in Mediating Salt Stress Tolerance in Plants. Front. Genet. 2022, 13, 811732. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Wei, J.; Pan, Y.; Su, C.; Zhang, X. SpPKE1, a Multiple Stress-Responsive Gene Confers Salt Tolerance in Tomato and Tobacco. Int. J. Mol. Sci. 2019, 20, 2478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, D.; Zhang, Y.; Li, G.; Sun, D.; Zhou, B.; Li, J. Insights into the Epigenetic Basis of Plant Salt Tolerance. Int. J. Mol. Sci. 2024, 25, 11698. [Google Scholar] [CrossRef]
- Lai, S.-J.; Lai, M.-C.; Lee, R.-J.; Chen, Y.-H.; Yen, H.E. Transgenic Arabidopsis Expressing Osmolyte Glycine Betaine Synthesizing Enzymes from Halophilic Methanogen Promote Tolerance to Drought and Salt Stress. Plant Mol. Biol. 2014, 85, 429–441. [Google Scholar] [CrossRef][Green Version]
- Singhal, N.K.; Sternbach, S.; Fleming, S.; Alkhayer, K.; Shelestak, J.; Popescu, D.; Weaver, A.; Clements, R.; Wasek, B.; Bottiglieri, T.; et al. Betaine Restores Epigenetic Control and Supports Neuronal Mitochondria in the Cuprizone Mouse Model of Multiple Sclerosis. Epigenetics 2020, 15, 871–886. [Google Scholar] [CrossRef]
- Steiger, H.; Casey, K.F.; Burdo, J.; Marcil, V.; Harvison, M.; Meyerfreund, J.; Breton, É.; Nemoda, Z.; Thaler, L.; St-Hilaire, A.; et al. Elevated Plasma B12 and Betaine Levels in Women with Anorexia Nervosa: Possible Role in Illness Pathophysiology and Epigenetic Regulation. J. Psychiatry Neurosci. 2025, 50, E85–E91. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Regni, L.; Bocchini, M.; Mariotti, R.; Cultrera, N.G.M.; Mancuso, S.; Googlani, J.; Chakerolhosseini, M.R.; Guerrero, C.; Albertini, E.; et al. Physiological, Epigenetic and Genetic Regulation in Some Olive Cultivars under Salt Stress. Sci. Rep. 2019, 9, 1093. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Papasotiropoulos, V.; Letsiou, S.; Gerakari, M.; Abraham, E.; Bebeli, P.J. Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change. Int. J. Mol. Sci. 2025, 26, 3160. [Google Scholar] [CrossRef]
- Sena, S.; Prakash, A.; Van Staden, J.; Kumar, V. Epigenetic Control of Plant Regeneration: Unraveling the Role of Histone Methylation. Curr. Plant Biol. 2024, 40, 100408. [Google Scholar] [CrossRef]
- Haimon, M.L.J.; Estrada-Cortés, E.; Amaral, T.F.; Martin, H.; Jeensuk, S.; Block, J.; Heredia, D.; Venturini, M.; Rojas, C.S.; Gonella-Diaza, A.M.; et al. Provision of Choline Chloride to the Bovine Preimplantation Embryo Alters Postnatal Body Size and DNA Methylation. Biol. Reprod. 2024, 111, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, S.; Esmaeilpour, K.; Shabani, M.; Sepehri, G.; Rajizadeh, M.A.; Maneshian, M.; Joushi, S.; Sheibani, V. Choline Chloride Modulates Learning, Memory, and Synaptic Plasticity Impairments in Maternally Separated Adolescent Male Rats. Int. J. Dev. Neurosci. 2022, 82, 19–38. [Google Scholar] [CrossRef]
- McNeil, S.D.; Nuccio, M.L.; Ziemak, M.J.; Hanson, A.D. Enhanced Synthesis of Choline and Glycine Betaine in Transgenic Tobacco Plants That Overexpress Phosphoethanolamine N -Methyltransferase. Proc. Nat. Acad. Sci. USA 2001, 98, 10001–10005. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, M.L.; McNeil, S.D.; Ziemak, M.J.; Hanson, A.D.; Jain, R.K.; Selvaraj, G. Choline Import into Chloroplasts Limits Glycine Betaine Synthesis in Tobacco: Analysis of Plants Engineered with a Chloroplastic or a Cytosolic Pathway. Metab. Eng. 2000, 2, 300–311. [Google Scholar] [CrossRef]
- Tekaya, M.; Dahmen, S.; Ben Mansour, M.; Ferhout, H.; Chehab, H.; Hammami, M.; Attia, F.; Mechri, B. Foliar Application of Fertilizers and Biostimulant Has a Strong Impact on the Olive (Olea europaea) Rhizosphere Microbial Community Profile and the Abundance of Arbuscular Mycorrhizal Fungi. Rhizosphere 2021, 19, 100402. [Google Scholar] [CrossRef]
- Kakagianni, M.; Tsiknia, M.; Feka, M.; Vasileiadis, S.; Leontidou, K.; Kavroulakis, N.; Karamanoli, K.; Karpouzas, D.G.; Ehaliotis, C.; Papadopoulou, K.K. Above- and below-Ground Microbiome in the Annual Developmental Cycle of Two Olive Tree Varieties. FEMS Microbes 2023, 4, xtad001. [Google Scholar] [CrossRef]
- Azeem, M.; Sultana, R.; Ahmed, N.; Abbasi, M.W.; Hasan, K.A.; Dong, R.; Alamri, S.; Alfagham, A.T. Salinity Stress Resilience in Sorghum Bicolor through Pseudomonas- Mediated Modulation of Growth, Antioxidant System, and Eco-Physiological Adaptations. ACS Omega 2025, 10, 940–954. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, H.-M.; Yuan, J.-L.; Xu, L.; Sun, J.-Q. A Comprehensive Genomic Analysis Provides Insights on the High Environmental Adaptability of Acinetobacter Strains. Front. Microbiol. 2023, 14, 1177951. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.P.; Akram, M.S.; Rafiq, K.; Basheer, S.; Iqbal, N. Staphylococcus Sciuri SAT-17 Facilitated in Vitro Regenerated Sugarcane Plantlets Cultivation in Saline Soil by Harmonizing Oxidative Signaling, Photosynthetic Efficiency and Nutrients Uptake Patterns. J. Soil Sci. Plant Nutr. 2023, 23, 163–176. [Google Scholar] [CrossRef]
- Banaei-Asl, F.; Farajzadeh, D.; Bandehagh, A.; Komatsu, S. Comprehensive Proteomic Analysis of Canola Leaf Inoculated with a Plant Growth-Promoting Bacterium, Pseudomonas Fluorescens, under Salt Stress. BBA—Proteins Proteom. 2016, 1864, 1222–1236. [Google Scholar] [CrossRef]
- Nakada, Y.; Nishijyo, T.; Itoh, Y. Divergent Structure and Regulatory Mechanism of Proline Catabolic Systems: Characterization of the putAP Proline Catabolic Operon of Pseudomonas Aeruginosa PAO1 and Its Regulation by PruR, an AraC/XylS Family Protein. J. Bacteriol. 2002, 184, 5633–5640. [Google Scholar] [CrossRef][Green Version]
- Rogan, C.J.; Pang, Y.-Y.; Mathews, S.D.; Turner, S.E.; Weisberg, A.J.; Lehmann, S.; Rentsch, D.; Anderson, J.C. Transporter-Mediated Depletion of Extracellular Proline Directly Contributes to Plant Pattern-Triggered Immunity against a Bacterial Pathogen. Nat. Commun. 2024, 15, 7048. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, H.W.; Abee, T.; Kleefsman, A.W.; Melgers, B.; Kortstee, G.J.; Konings, W.N.; Zehnder, A.J. Energetics of Alanine, Lysine, and Proline Transport in Cytoplasmic Membranes of the Polyphosphate-Accumulating Acinetobacter johnsonii Strain 210A. J. Bacteriol. 1994, 176, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Deutch, C.E. L-Proline Nutrition and Catabolism in Staphylococcus saprophyticus. Antonie Leeuwenhoek 2011, 99, 781–793. [Google Scholar] [CrossRef]
- Blasco, R.; Martínez-Luque, M.; Madrid, M.; Castillo, F.; Moreno-Vivián, C. Rhodococcus sp. RB1 Grows in the Presence of High Nitrate and Nitrite Concentrations and Assimilates Nitrate in Moderately Saline Environments. Arch. Microbiol. 2001, 175, 435–440. [Google Scholar] [CrossRef]
- Bae, J.H.; Anderson, S.H.; Miller, K.J. Identification of a High-Affinity Glycine Betaine Transport System in Staphylococcus aureus. Appl. Environ. Microbiol. 1993, 59, 2734–2736. [Google Scholar] [CrossRef]
- Wargo, M.J. Homeostasis and Catabolism of Choline and Glycine Betaine: Lessons from Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2013, 79, 2112–2120. [Google Scholar] [CrossRef]
- Galinski, E.A.; Trüper, H.G. Microbial Behaviour in Salt-Stressed Ecosystems. FEMS Microbiol. Rev. 1994, 15, 95–108. [Google Scholar] [CrossRef]
- Zou, H.; Chen, N.; Shi, M.; Xian, M.; Song, Y.; Liu, J. The Metabolism and Biotechnological Application of Betaine in Microorganism. Appl. Microbiol. Biotechnol. 2016, 100, 3865–3876. [Google Scholar] [CrossRef]
- Scholz, A.; Stahl, J.; De Berardinis, V.; Müller, V.; Averhoff, B. Osmotic Stress Response in Acinetobacter baylyi: Identification of a Glycine–Betaine Biosynthesis Pathway and Regulation of Osmoadaptive Choline Uptake and Glycine–Betaine Synthesis through a Choline-responsive BetI Repressor. Environ. Microbiol. Rep. 2016, 8, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Salvano, M.A.; Lisa, T.A.; Domenech, C.E. Choline Transport in Pseudomonas aeruginosa. Mol. Cell Biochem. 1989, 85, 81–89. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Citrus Plants Exude Proline and Phytohormones under Abiotic Stress Conditions. Plant Cell Rep. 2017, 36, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; De Vries, F.T. Plant Root Exudation under Drought: Implications for Ecosystem Functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, Z.; Sun, Y.; Wang, E.; Tian, C.; Sun, Y.; Liu, J.; Sun, C.; Tian, L. Current Studies of the Effects of Drought Stress on Root Exudates and Rhizosphere Microbiomes of Crop Plant Species. Int. J. Mol. Sci. 2022, 23, 2374. [Google Scholar] [CrossRef] [PubMed]

| Saline Soil Stress (NaCl) | Treat.(2) | Pn (μmol m−2.s−1) | E (mmol m−2.s−1) | gs (mmol m−2.s−1) | WUE (μmol CO2/mmol H2O) | QY | Cin/Cout |
|---|---|---|---|---|---|---|---|
| June | |||||||
| 0 mM | NS | 11.44 ± 0.03ab,a,a | 1.73 ± 0.02a,a,a | 0.146 ± 0.003a,a,a | 6.60 ± 0.07a,a,a | 0.70 ± 0.02a,a,a | 0.58 ± 0.01a,a,a |
| PN | 9.87 ± 0.04a,a,a | 1.16 ± 0.02a,b,a | 0.090 ± 0.000a,b,a | 8.49 ± 0.18a,b,a | 0.88 ± 0.03a,b,a | 0.48 ± 0.01a,a,a | |
| PK | 11.43 ± 0.12a,a,a | 1.64 ± 0.01a,a,a | 0.143 ± 0.003a,a,a | 6.95 ± 0.10a,ab,a | 0.78 ± 0.03a,ab,a | 0.57 ± 0.01a,a,a | |
| N | 6.88 ± 0.77a,b,ab | 1.02 ± 0.17ab,b,ab | 0.073 ± 0.018ac,b,ab | 6.87 ± 0.67ab,a,a | 0.45 ± 0.05a,c,a | 0.55 ± 0.05a,a,a | |
| 50 mM | NS | 6.54 ± 0.48a,a,b | 0.99 ± 0.06a,a,b | 0.056 ±0.006a,a,b | 6.58 ± 0.31a,a,a | 0.31 ± 0.01a,a,b | 0.50 ± 0.03a,a,a |
| PN | 6.78 ± 0.15a,a,b | 0.89 ± 0.00ab,a,b | 0.063 ± 0.003ab,a,b | 7.71 ± 0.06a,b,a | 0.40 ± 0.01a,bc,b | 0.47 ± 0.00a,a,a | |
| PK | 6.47 ± 0.06a,a,b | 0.92 ± 0.03a,a,b | 0.060 ± 0.000a,a,b | 7.02 ± 0.18a,c,a | 0.38 ± 0.01a,c,b | 0.51 ± 0.01bc,a,b | |
| N | 9.50 ± 0.09a,b,b | 1.58 ± 0.01a,b,a | 0.110 ± 0.000a,b,a | 6.00 ± 0.05a,a,a | 0.48 ± 0.00a,d,a | 0.56 ± 0.01a,a,a | |
| 100 mM | NS | 4.14 ± 0.13a,a,c | 0.77 ± 0.04a,a,bc | 0.046 ± 0.003a,a,bc | 5.37 ± 0.12a,a,b | 0.24 ± 0.02bc,a,c | 0.59 ± 0.00a,a,a |
| PN | 5.98 ± 0.30a,a,b | 0.83 ± 0.03a,a,b | 0.046 ± 0.003a,a,c | 7.44 ± 0.55a,a,a | 0.35 ± 0.03a,a,b | 0.46 ± 0.05a,a,a | |
| PK | 6.07 ± 0.15a,a,b | 0.87 ± 0.05a,a,b | 0.050 ± 0.005a,a,b | 6.99 ± 0.30a,a,a | 0.36 ± 0.01a,a,bc | 0.48 ± 0.02ab,a,b | |
| N | 5.98 ± 1.14a,a,a | 1.09 ± 0.20a,a,ab | 0.070 ± 0.015a,a,ab | 5.61 ± 0.75a,a,a | 0.44 ± 0.14a,a,a | 0.59 ± 0.05a,a,a | |
| 150 mM | NS | 3.11 ± 0.13ab,a,c | 0.59 ± 0.06a,a,c | 0.030 ± 0.005ab,a,c | 5.30 ± 0.37a,a,b | 0.18 ± 0.01c,a,d | 0.58 ± 0.03a,a,a |
| PN | 3.95 ± 0.10a,a,c | 0.54 ± 0.02a,a,c | 0.030 ± 0.000ab,a,d | 7.29 ± 0.27a,b,a | 0.23 ± 0.01a,bc,c | 0.44 ± 0.04a,b,a | |
| PK | 5.82 ± 0.28a,b,b | 0.91 ± 0.06a,b,b | 0.053 ± 0.006a,b,b | 6.39 ± 0.12a,ab,a | 0.30 ± 0.01a,cd,c | 0.51 ± 0.01ab,ab,b | |
| N | 5.27 ± 0.22a,b,a | 0.78 ± 0.03a,ab,b | 0.043 ± 0.003a,ab,b | 6.76 ± 0.03a,b,a | 0.32 ± 0.03a,d,a | 0.48 ± 0.01ab,ab,a | |
| July | |||||||
| 0 mM | NS | 14.74 ± 1.72a,a,a | 1.55 ± 0.11a,a,a | 0.153 ± 0.017a,a,a | 9.44 ± 0.43b,a,a | 0.78 ± 0.11a,a,a | 0.48 ± 0.02a,a,a |
| PN | 12.85 ± 0.07b,a,a | 1.53 ± 0.03b,a,a | 0.163 ± 0.006b,a,a | 8.37 ± 0.14a,a,a | 0.70 ± 0.03b,a,a | 0.56 ± 0.01a,a,a | |
| PK | 11.37 ± 0.67a,a,a | 1.39 ± 0.03a,a,a | 0.146 ± 0.003a,a,a | 8.19 ± 0.68a,a,a | 0.65 ± 0.052,a,a | 0.58 ± 0.033,a,a | |
| N | 10.64 ± 0.05b,a,a | 1.36 ± 0.09a,a,a | 0.123 ± 0.008a,a,a | 7.86 ± 0.57b,a,a | 0.54 ± 0.00a,a,a | 0.55 ± 0.023,a,a | |
| 50 mM | NS | 6.19 ± 0.54a,a,b | 0.94 ± 0.00a,a,b | 0.066 ± 0.003a,a,b | 6.54 ± 0.56a,a,b | 0.35 ± 0.04a,a,b | 0.57 ± 0.03a,a,a |
| PN | 9.79 ± 0.57b,b,b | 1.34 ± 0.24a,a,a | 0.116 ± 0.023a,a,a | 7.73 ± 1.21a,a,a | 0.61 ± 0.01b,b,a | 0.55 ± 0.05a,a,a | |
| PK | 6.78 ± 0.54a,b,b | 1.16 ± 0.08a,a,a | 0.090 ± 0.010b,a,b | 5.83 ± 0.02b,a,bc | 0.33 ± 0.02a,a,b | 0.63 ± 0.00a,a,a | |
| N | 5.99 ± 0.03b,b,b | 0.97 ± 0.02b,a,b | 0.070± 0.000b,a,b | 6.14 ± 0.16a,a,b | 0.30 ± 0.01b,a,b | 0.59 ± 0.01a,a,a | |
| 100 mM | NS | 4.46 ± 0.13a,a,b | 0.62 ± 0.06ab,a,b | 0.040 ± 0.000a,a,b | 7.26 ± 0.53b,a,b | 0.34 ± 0.04c,a,b | 0.57 ± 0.01a,a,a |
| PN | 6.95 ± 0.26a,c,c | 1.22 ± 0.07b,b,ab | 0.070 ± 0.005a,b,b | 5.68 ± 0.18a,bc,a | 0.37 ± 0.01a,a,b | 0.53 ± 0.02a,ab,a | |
| PK | 6.17 ± 0.29a,bc,b | 1.17 ± 0.05b,b,a | 0.076 ± 0.003b,b,b | 5.25 ± 0.18a,c,c | 0.40 ± 0.05a,a,b | 0.61 ± 0.01a,a,a | |
| N | 5.56 ± 0.28a,ab,bc | 0.85 ± 0.05ab,a,b | 0.046 ± 0.003ab,a,c | 6.51 ± 0.10a,ab,ab | 0.31 ± 0.01a,a,b | 0.48 ± 0.01a,b,b | |
| 150 mM | NS | 3.48 ± 0.49a,a,b | 0.66 ± 0.08a,a,b | 0.033 ± 0.003a,a,b | 5.27 ± 0.14a,a,b | 0.17 ± 0.02bc,a,b | 0.56 ± 0.01a,a,a |
| PN | 3.89 ± 0.42ab,a,d | 0.67 ± 0.13a,a,b | 0.036 ± 0.006a,a,b | 5.99 ± 0.51ac,ab,a | 0.21 ± 0.03ab,ab,b | 0.57 ± 0.03a,a,a | |
| PK | 5.24 ± 0.38a,a,b | 0.73 ± 0.03ab,a,b | 0.036 ± 0.003ab,a,c | 7.17 ± 0.29a,b,ab | 0.28 ± 0.02a,b,b | 0.39 ± 0.02b,b,b | |
| N | 4.94 ± 0.24ac,a,c | 0.89 ± 0.03a,a,b | 0.046 ± 0.003a,a,c | 5.51 ± 0.16a,a,b | 0.27 ± 0.01b,b,b | 0.54 ± 0.01bc,a,ab | |
| August | |||||||
| 0 mM | NS | 7.83 ± 1.04b,a,a | 1.47 ± 0.26a,a,a | 0.070 ± 0.015b,a,a | 5.43 ± 0.38a,ab,a | 0.47 ± 0.06b,a,a | 0.48 ± 0.04a,a,ab |
| PN | 6.81 ± 0.15c,ab,a | 1.21 ± 0.07a,a,a | 0.060 ± 0.005c,a,a | 5.66 ± 0.23b,a,a | 0.49 ± 0.03c,a,a | 0.50 ± 0.02a,ab,a | |
| PK | 3.63 ± 1.10b,b,a | 0.86 ± 0.17b,a,a | 0.040 ± 0.010b,a,a | 4.05 ± 0.41b,b,a | 0.28 ± 0.09b,a,a | 0.63 ± 0.03a,b,a | |
| N | 6.29 ± 0.86ac,ab,a | 1.16 ± 0.12ab,a,a | 0.060 ± 0.010c,a,a | 5.37 ± 0.34a,ab,a | 0.53 ± 0.04a,a,a | 0.55 ± 0.02a,ab,a | |
| 50 mM | NS | 3.35 ± 0.55b,ab,b | 0.71 ± 0.05b,ab,b | 0.026 ± 0.003b,a,b | 4.62 ± 0.40a,a,a | 0.19 ± 0.03c,a,b | 0.54 ± 0.05a,a,ab |
| PN | 2.86 ± 0.25c,b,b | 0.63 ± 0.12bc,b,b | 0.020 ± 0.005b,a,b | 4.79 ± 0.77a,a,a | 0.17 ± 0.01c,a,b | 0.52 ± 0.08a,a,a | |
| PK | 5.86 ± 0.54a,a,a | 0.98 ± 0.03a,a,a | 0.043 ± 0.003ac,b,a | 5.54 ±0.28b,a,a | 0.35 ± 0.02a,b,a | 0.47 ± 0.02b,a,a | |
| N | 2.98 ± 0.77c,b,b | 0.56 ± 0.03c,b,b | 0.023 ± 0.003c,a,b | 5.28 ± 1.23a,a,a | 0.17 ± 0.05c,a,b | 0.51 ± 0.09a,a,a | |
| 100 mM | NS | 2.35 ± 0.25b,a,b | 0.40 ± 0.02bc,a,b | 0.023 ± 0.003b,a,b | 5.76 ± 0.31ab,a,a | 0.13 ± 0.01ab,a,b | 0.63 ± 0.02a,a,a |
| PN | 2.11 ± 0.77b,a,b | 0.35 ± 0.06c,a,b | 0.020 ± 0.005c,a,b | 6.79 ± 2.89a,a,a | 0.13 ± 0.05b,a,b | 0.60 ± 0.16a,a,a | |
| PK | 3.30 ± 0.79b,a,a | 0.47 ± 0.04c,a,b | 0.030± 0.000c,a,a | 6.84 ± 1.08a,a,a | 0.20 ± 0.04b,a,a | 0.59 ± 0.07ab,a,a | |
| N | 2.68 ± 0.53a,a,b | 0.28 ± 0.03b,a,b | 0.016 ± 0.003b,a,b | 9.36 ± 0.95b,a,b | 0.15 ± 0.03a,a,b | 0.44 ± 0.05a,a,a | |
| 150 mM | NS | 2.15 ± 0.22bc,a,b | 0.21 ± 0.02b,a,b | 0.010 ± 0.000c,a,b | 10.00 ± 0.64b,a,b | 0.14 ± 0.02ab,a,b | 0.43 ± 0.03a,a,b |
| PN | 3.15 ± 0.21b,b,b | 0.32 ± 0.01a,b,b | 0.020 ± 0.000ab,ab,b | 9.65 ± 0.35b,a,a | 0.16 ± 0.01bc,a,b | 0.26 ± 0.01b,b,a | |
| PK | 3.04 ± 0.08b,b,a | 0.49 ± 0.04bc,c,b | 0.033 ± 0.003bc,cd,a | 6.22 ± 0.38a,b,a | 0.16 ± 0.01b,a,a | 0.62 ± 0.03a,c,a | |
| N | 2.75 ± 0.15c,ab,b | 0.40 ± 0.01b,bc,b | 0.023 ± 0.003b,bd,b | 6.79 ± 0.52a,b,a | 0.15 ± 0.01c,a,b | 0.59 ± 0.03c,c,a | |
| September | |||||||
| 0 mM | NS | 2.84 ± 0.50c,a,a | 0.68 ± 0.14b,a,a | 0.033 ± 0.008b,a,a | 4.47 ± 1.37a,a,a | 0.14 ± 0.02c,a,a | 0.62 ± 0.11a,a,a |
| PN | 4.33 ± 0.17d,a,a | 0.57 ± 0.08c,a,a | 0.033 ± 0.003d,a,a | 7.78 ± 0.92a,a,a | 0.22 ± 0.01d,a,a | 0.44 ± 0.05a,a,a | |
| PK | 2.89 ± 0.34b,a,a | 0.39 ± 0.04c,a,a | 0.016 ± 0.003b,a,a | 7.35 ± 0.31a,a,a | 0.15 ± 0.02b,a,a | 0.43 ± 0.03b,a,a | |
| N | 4.12 ± 0.65c,a,a | 0.62 ± 0.10b,a,a | 0.033 ± 0.008c,a,a | 6.68 ± 0.43ab,a,a | 0.21 ± 0.04b,a,a | 0.50 ± 0.02a,a,a | |
| 50 mM | NS | 1.26 ± 0.10c,a,a | 0.21 ± 0.01c,a,b | 0.010 ± 0.000b,a,b | 5.97 ± 0.70a,a,a | 0.07 ± 0.01d,a,a | 0.50 ± 0.05a,a,a |
| PN | 2.67 ± 0.28c,a,a | 0.43 ± 0.04c,a,a | 0.016 ± 0.003b,a,a | 6.17 ± 0.04a,a,ab | 0.13 ± 0.01c,a,a | 0.47 ± 0.01a,a,a | |
| PK | 2.46 ± 0.56b,a,a | 0.46 ± 0.10b,a,a | 0.020 ± 0.005c,a,a | 5.25 ± 0.18b,a,a | 0.12 ± 0.03b,a,a | 0.55 ± 0.01c,a,a | |
| N | 2.10 ± 0.73c,a,a | 0.34 ± 0.08c,a,a | 0.013 ± 0.003c,a,a | 6.53 ± 1.22a,a,a | 0.11 ± 0.03c,a,a | 0.52 ± 0.05a,a,a | |
| 100 mM | NS | 2.42 ± 0.62b,a,a | 0.37 ± 0.06c,a,b | 0.013 ± 0.003b,a,ab | 6.27 ± 0.50ab,a,a | 0.12 ± 0.030,a,a | 0.44 ± 0.03b,a,a |
| PN | 2.41 ± 0.86b,a,a | 0.37 ± 0.12c,a,a | 0.016 ± 0.006c,a,a | 6.31 ± 0.28a,a,ab | 0.11 ± 0.04b,a,a | 0.41 ± 0.02a,a,a | |
| PK | 2.24 ± 0.32b,a,a | 0.32 ± 0.02c,a,a | 0.010± 0.000d,a,a | 6.81 ± 0.63a,a,a | 0.11 ± 0.02b,a,a | 0.39 ± 0.05b,a,a | |
| N | 2.73 ± 0.99a,a,a | 0.49 ± 0.20b,a,a | 0.020 ± 0.010b,a,a | 5.74 ± 0.38a,a,a | 0.13 ± 0.04a,a,a | 0.46 ± 0.04a,a,a | |
| 150 mM | NS | 1.49 ± 0.08c,a,a | 0.37 ± 0.06b,a,b | 0.013 ± 0.003bc,a,ab | 4.30 ± 0.95a,a,a | 0.07 ± 0.00a,a,a | 0.60 ± 0.08a,a,a |
| PN | 2.22 ± 0.57b,a,a | 0.40 ± 0.08a,a,a | 0.016 ± 0.003b,a,a | 5.37 ± 0.37c,a,b | 0.11 ± 0.02c,ab,a | 0.49 ± 0.03a,a,a | |
| PK | 2.76 ± 0.07b,a,a | 0.38 ± 0.06c,a,a | 0.016 ± 0.003c,a,a | 6.86 ± 0.85a,a,a | 0.15 ± 0.02b,ab,a | 0.38 ± 0.06b,a,a | |
| N | 3.23 ± 0.75c,a,a | 0.50 ± 0.10b,a,a | 0.020 ± 0.005b,a,a | 6.41 ± 0.15a,a,a | 0.17 ± 0.03bc,b,a | 0.42 ± 0.01a,a,a | |
| Saline Stress (NaCl) | Treat.(2) | Pn (μmol m−2.s−1) | E (mmol m−2.s−1) | gs (mmol m−2.s−1) | WUE (μmol CO2/mmol H2O) | QY | Cin/Cout |
|---|---|---|---|---|---|---|---|
| June | |||||||
| 0 mM | NS | 10.03 ± 0.18a,ab,a | 1.51 ± 0.14a,a,a | 0.103 ± 0.012a,a,a | 6.72 ± 0.57a,a,a | 0.91 ± 0.03a,a,a | 0.49 ± 0.05a,a,a |
| PN | 11.31 ± 0.06a,bc,a | 1.64 ± 0.08a,a,a | 0.106 ± 0.008a,a,a | 6.94 ± 0.39a,a,a | 0.89 ± 0.03a,a,a | 0.47 ± 0.03a,a,a | |
| PK | 9.08 ± 0.54a,a,a | 1.31 ± 0.09a,a,a | 0.066 ± 0.003a,b,a | 6.93 ± 0.11ab,a,ab | 0.45 ± 0.01a,b,a | 0.38 ± 0.00a,a,a | |
| N | 11.74 ± 0.18a,c,a | 1.57 ± 0.07a,a,a | 0.106 ± 0.008a,a,a | 7.50 ± 0.26a,a,a | 0.59 ± 0.01a,c,a | 0.44 ± 0.02a,a,a | |
| 50 mM | NS | 8.29 ± 0.09a,a,b | 1.09 ± 0.02a,a,ab | 0.063 ± 0.003a,a,b | 7.61 ± 0.06a,a,a | 0.45 ± 0.00a,a,bc | 0.43 ± 0.01ab,a,a |
| PN | 9.91 ± 0.05a,b,a | 1.28 ± 0.04a,a,b | 0.083 ± 0.006a,a,ab | 7.73 ± 0.33ab,a,ab | 0.54 ± 0.00a,a,b | 0.43 ± 0.03a,a,a | |
| PK | 8.01 ± 0.25a,a,a | 1.14 ± 0.05a,a,ab | 0.066 ± 0.008a,a,a | 7.03 ± 0.24ab,a,ab | 0.51 ± 0.01a,a,ab | 0.45 ± 0.03a,a,a | |
| N | 9.19 ± 0.65a,ab,b | 1.25 ± 0.13a,a,ab | 0.080 ± 0.011a,a,ab | 7.39 ± 0.32a,a,a | 0.48 ± 0.04a,a,a | 0.44 ± 0.03a,a,a | |
| 100 mM | NS | 7.42 ± 0.44a,ab,b | 0.96 ± 0.06a,a,b | 0.060 ± 0.005a,a,b | 7.40 ± 0.17a,a,a | 0.42 ± 0.03a,a,c | 0.44 ± 0.02a,a,a |
| PN | 7.62 ± 0.68a,ab,b | 1.14 ± 0.07a,a,b | 0.070 ± 0.005a,a,bc | 6.66 ± 0.17a,a,a | 0.40 ± 0.03a,a,c | 0.43 ± 0.01ab,a,a | |
| PK | 8.83 ± 0.37a,a,a | 1.17 ± 0.04a,a,ab | 0.070 ± 0.005a,a,a | 7.54 ± 0.14a,a,a | 0.45 ± 0.03a,a,a | 0.41 ± 0.01a,a,a | |
| N | 6.16 ± 0.29a,b,c | 0.91 ± 0.05a,a,bc | 0.050 ± 0.005a,a,b | 6.78 ± 0.42ab,a,a | 0.32 ± 0.006a,b,b | 0.46 ± 0.03a,a,a | |
| 150 mM | NS | 7.14 ± 0.30a,ab,b | 1.01 ± 0.10a,a,b | 0.066 ± 0.008a,a,b | 7.13 ± 0.55a,a,a | 0.52 ± 0.02a,a,b | 0.50 ± 0.04a,a,a |
| PN | 7.10 ± 0.49a,ab,b | 0.80 ± 0.06a,a,c | 0.053 ± 0.003a,a,c | 8.88 ± 0.09a,b,b | 0.560 ± 0.02a,a,b | 0.44 ± 0.00a,ab,a | |
| PK | 6.22 ± 0.28a,b,b | 0.93 ± 0.01a,a,b | 0.053 ± 0.003a,a,a | 6.66 ± 0.21b,a,b | 0.57 ± 0.04a,a,b | 0.55 ± 0.01b,a,b | |
| N | 8.18 ± 0.23a,a,b | 0.80 ± 0.04a,a,c | 0.053 ± 0.003a,a,b | 10.27 ± 0.35a,b,b | 0.58 ± 0.04a,a,a | 0.35 ± 0.03a,b,a | |
| July | |||||||
| 0 mM | NS | 7.24 ± 0.04b,a,a | 1.20 ± 0.09ab,a,a | 0.076 ± 0.008ab,a,a | 6.08 ± 0.48a,a,a | 0.49 ± 0.02b,ab,a | 0.57 ± 0.03a,a,b |
| PN | 8.51 ±0.30b,b,a | 1.42 ± 0.07ab,a,a | 0.100 ± 0.005a,a,a | 5.98 ± 0.22ab,a,a | 0.57 ± 0.03b,b,a | 0.58 ± 0.02b,a,bc | |
| PK | 8.58 ± 0.07a,b,a | 1.43 ± 0.04ab,a,a | 0.096 ± 0.008b,a,a | 6.02 ± 0.23bc,a,a | 0.56 ± 0.02b,b,a | 0.56 ± 0.03b,a,ab | |
| N | 6.78 ± 0.43b,ac,a | 1.17 ± 0.09b,a,a | 0.083 ± 0.008ab,a,a | 5.79 ± 0.08b,a,a | 0.44 ± 0.02b,a,a | 0.60 ± 0.01bc,a,a | |
| 50 mM | NS | 6.16 ± 0.01b,ac,b | 0.84 ± 0.04b,a,b | 0.053 ± 0.003a,a,ab | 7.32 ± 0.40a,ab,ab | 0.34 ± 0.01b,a,b | 0.52 ± 0.03c,ab,ab |
| PN | 8.49 ± 0.40b,b,a | 1.03 ± 0.04b,a,b | 0.076 ± 0.003a,a,ab | 8.24 ± 0.13ab,b,b | 0.45 ± 0.02b,b,ab | 0.48 ± 0.01a,a,ab | |
| PK | 5.99 ± 0.56b,c,b | 0.88 ± 0.10b,a,b | 0.056 ± 0.008a,a,b | 6.82 ± 0.18ab,a,a | 0.33 ± 0.02b,a,b | 0.55 ± 0.01a,ab,ab | |
| N | 6.53 ± 0.45b,a,a | 0.97 ± 0.05a,a,a | 0.070 ± 0.005a,a,a | 6.73 ± 0.22ab,a,ab | 0.37 ± 0.03a,ab,a | 0.57 ± 0.02b,b,ab | |
| 100 mM | NS | 4.65 ± 0.26b,a,c | 0.57 ± 0.02b,a,c | 0.033 ± 0.003bc,a,b | 8.11 ± 0.08a,a,b | 0.24 ± 0.01b,a,c | 0.44 ± 0.01a,a,a |
| PN | 7.07 ± 0.71ab,b,a | 0.92 ± 0.05ab,b,b | 0.050 ± 0.005b,a,bc | 7.61 ± 0.46a,a,ab | 0.38 ± 0.05a,b,bc | 0.403± 0.04a,a,a | |
| PK | 5.12 ± 0.16b,a,bc | 0.72 ± 0.08b,ab,b | 0.043 ± 0.008ab,a,b | 7.25 ± 0.60a,a,a | 0.28 ± 0.01b,ab,b | 0.49 ± 0.04a,a,a | |
| N | 4.58 ± 0.39b,a,b | 0.58 ± 0.10b,a,b | 0.033 ± 0.003ab,a,b | 8.15 ± 0.73a,a,b | 0.25 ± 0.02b,a,b | 0.48 ± 0.03ab,a,b | |
| 150 mM | NS | 3.41 ± 0.06b,a,d | 0.49 ± 0.02b,a,c | 0.036± 0.003b,a,b | 6.96 ± 0.47ab,a,ab | 0.240± 0.00b,a,c | 0.60 ± 0.03a,a,b |
| PN | 3.96 ± 0.19b,ab,b | 0.56 ± 0.07ab,a,c | 0.046 ± 0.008ab,a,c | 7.26 ± 0.82ab,a,ab | 0.29 ± 0.02b,ab,c | 0.62 ± 0.04b,a,c | |
| PK | 3.95 ± 0.12b,ab,c | 0.57 ± 0.00b,a,b | 0.046 ± 0.003a,a,b | 6.90 ± 0.21ab,a,a | 0.34 ± 0.03b,b,b | 0.63 ± 0.01a,a,b | |
| N | 4.65 ± 0.19b,b,b | 0.54 ± 0.02b,a,b | 0.040 ± 0.000b,a,b | 8.54 ± 0.53a,a,b | 0.24 ± 0.01b,a,b | 0.53 ± 0.03b,a,ab | |
| August | |||||||
| 0 mM | NS | 4.99 ± 0.21c,a,a | 0.99 ± 0.08b,a,a | 0.053 ± 0.006b,a,a | 5.11 ± 0.54a,a,a | 0.38 ± 0.01b,a,a | 0.60 ± 0.05a,a,a |
| PN | 6.40 ± 0.15c,b,a | 1.20 ± 0.08bc,a,a | 0.066 ± 0.006b,a,a | 5.33 ± 0.21b,a,a | 0.42 ± 0.02c,a,ab | 0.57 ± 0.02ab,a,a | |
| PK | 7.96 ± 0.47ab,b,a | 1.61 ± 0.06b,b,a | 0.090 ± 0.005b,b,a | 4.94 ± 0.28c,a,a | 0.47 ± 0.03ab,a,a | 0.59 ± 0.02b,a,b | |
| N | 6.78 ± 0.28b,b,a | 1.31 ± 0.04ab,b,a | 0.063 ± 0.003bc,a,a | 5.18 ± 0.24b,a,a | 0.40 ± 0.02b,a,a | 0.53 ± 0.02b,a,a | |
| 50 mM | NS | 3.47 ± 0.18c,ab,b | 0.53 ± 0.03c,a,b | 0.020 ± 0.000b,a,b | 6.57 ± 0.14ab,a,a | 0.20 ± 0.01c,a,b | 0.37 ± 0.01a,a,b |
| PN | 5.20 ± 0.31c,b,a | 0.50 ± 0.05c,a,b | 0.033 ± 0.003b,a,b | 10.80 ± 1.75b,a,b | 0.31 ± 0.03c,b,bc | 0.40 ± 0.08a,ab,a | |
| PK | 3.37 ± 0.49c,ab,b | 0.34 ± 0.05c,a,b | 0.020 ± 0.005b,a,b | 10.05 ± 1.17a,a,b | 0.19 ± 0.02c,a,b | 0.41 ± 0.02a,ab,a | |
| N | 3.31 ± 0.18c,a,b | 0.44 ± 0.02b,a,b | 0.030 ± 0.000b,a,b | 7.43 ± 0.33a,a,b | 0.19 ± 0.01b,a,b | 0.57 ± 0.02b,b,a | |
| 100 mM | NS | 2.91 ± 0.26c,a,cb | 0.40 ± 0.07bc,a,bc | 0.026 ± 0.003cd,a,b | 7.64 ± 1.06a,a,a | 0.18 ± 0.02b,a,b | 0.58 ± 0.04b,a,a |
| PN | 5.13 ± 0.12ab,b,a | 0.73 ± 0.04bc,b,b | 0.046 ± 0.003b,a,ab | 7.03 ± 0.37a,a,ab | 0.50 ± 0.01a,b,a | 0.55 ± 0.03b,a,a | |
| PK | 2.98 ± 0.39c,a,b | 0.50 ± 0.00bc,a,b | 0.036 ± 0.006bc,a,b | 5.91 ± 0.69a,a,a | 0.24 ± 0.06bc,a,ab | 0.63 ± 0.05b,a,b | |
| N | 3.17 ± 0.31c,a,b | 0.52 ± 0.03bc,a,b | 0.026 ± 0.006bc,a,bc | 6.05 ± 0.25b,a,ab | 0.27 ± 0.02ab,a,b | 0.57 ± 0.01b,a,a | |
| 150 mM | NS | 2.08 ± 0.08c,a,c | 0.33 ± 0.04b,a,c | 0.013 ± 0.003b,a,b | 6.43 ± 0.98ab,a,a | 0.19 ± 0.02b,a,b | 0.53 ± 0.06a,a,a |
| PN | 2.55 ± 0.90bc,a,b | 0.48 ± 0.14ab,a,b | 0.023 ± 0.008b,a,b | 5.21 ± 0.56b,a,b | 0.22 ± 0.07bc,a,c | 0.57 ± 0.06ab,a,a | |
| PK | 2.13 ± 0.71b,a,b | 0.26 ± 0.07c,a,b | 0.013 ± 0.003b,a,b | 7.97 ± 0.45a,a,a | 0.20 ± 0.08bc,a,b | 0.40 ± 0.02c,a,a | |
| N | 2.40 ± 0.25c,a,b | 0.37 ± 0.01c,a,b | 0.010 ± 0.000c,a,c | 6.41 ± 0.39b,a,ab | 0.22 ± 0.03b,a,b | 0.43 ± 0.04ab,a,b | |
| September | |||||||
| 0 mM | NS | 5.50 ± 0.27c,a,a | 0.92 ± 0.04b,a,a | 0.070 ± 0.005ab,a,a | 5.99 ± 0.22a,a,a | 0.37 ± 0.06b,a,a | 0.64 ± 0.01a,a,b |
| PN | 4.89 ± 0.33d,a,a | 0.86 ± 0.07c,a,a | 0.060 ± 0.005b,a,a | 5.67 ± 0.25ab,a,a | 0.35 ± 0.02c,a,a | 0.63 ± 0.01b,a,a | |
| PK | 5.863 ± 0.3b,a,a | 0.76 ± 0.04c,a,a | 0.050 ± 0.005a,a,a | 7.69 ± 0.61a,b,a | 0.45 ± 0.03a,a,a | 0.50 ± 0.04b,b,a | |
| N | 4.57 ± 0.15c,a,a | 0.78 ± 0.00c,a,a | 0.050 ±0.000c,a,a | 5.85 ± 0.20b,a,ab | 0.39 ± 0.01b,a,a | 0.61 ± 0.01c,c,a | |
| 50 mM | NS | 2.18 ± 0.04d,ab,b | 0.34 ± 0.01d,a,b | 0.010 ± 0.000b,a,b | 6.30 ± 0.14b,a,a | 0.14 ± 0.01d,a,b | 0.47 ± 0.01bc,a,a |
| PN | 1.76 ± 0.03d,b,b | 0.30 ± 0.02c,a,b | 0.010 ± 0.000c,a,b | 5.88 ± 0.51a,a,a | 0.10 ± 0.00d,a,b | 0.48 ± 0.04a,a,b | |
| PK | 3.05 ± 0.37c,a,b | 0.53 ± 0.06c,b,ab | 0.023 ± 0.003b,bc,b | 5.87 ± 1.10b,a,a | 0.19 ± 0.02c,b,b | 0.49 ± 0.08a,a,a | |
| N | 2.26 ± 0.12c,ab,b | 0.39 ± 0.03b,ab,b | 0.016 ± 0.003b,ac,b | 5.75 ± 0.29b,a,ab | 0.15 ± 0.01b,ab,b | 0.50 ± 0.01ab,a,b | |
| 100 mM | NS | 1.95 ± 0.29c,a,b | 0.30 ± 0.02c,a,b | 0.010 ± 0.000d,a,b | 6.37 ± 0.42a,bc,a | 0.13 ± 0.03b,a,b | 0.44 ± 0.03a,a,a |
| PN | 1.62 ± 0.04c,a,b | 0.34 ± 0.01c,a,b | 0.010 ± 0.000c,a,b | 4.74 ± 0.18b,a,a | 0.10 ± 0.00b,a,b | 0.55 ± 0.01b,b,ab | |
| PK | 1.80 ± 0.04c,a,b | 0.31 ± 0.01c,a,b | 0.010 ± 0.000c,a,b | 5.76 ± 0.19a,ab,a | 0.10 ± 0.00c,a,b | 0.47 ± 0.01a,a,a | |
| N | 1.85 ± 0.08c,a,b | 0.26 ± 0.00c,a,c | 0.010 ± 0.000c,a,b | 7.01 ± 0.15ab,c,b | 0.11 ± 0.00c,a,c | 0.34 ± 0.01c,c,c | |
| 150 mM | NS | 1.50 ± 0.12c,a,b | 0.40 ± 0.09b,a,b | 0.020 ± 0.005b,a,b | 3.99 ± 0.61b,a,b | 0.09 ± 0.01c,a,b | 0.65 ± 0.07a,a,b |
| PN | 1.23 ± 0.17c,a,b | 0.21 ± 0.03b,a,b | 0.010 ± 0.000b,a,b | 5.82 ± 0.09b,b,a | 0.08 ± 0.02c,a,b | 0.52 ± 0.02ab,a,b | |
| PK | 2.00 ± 0.51b,a,b | 0.38 ± 0.09bc,a,b | 0.016 ± 0.006b,a,b | 5.15 ± 0.04c,ab,a | 0.13 ± 0.02c,a,b | 0.60 ± 0.00ab,a,a | |
| N | 1.24 ± 0.11d,a,c | 0.24 ± 0.02c,a,c | 0.010 ± 0.000c,a,b | 5.17 ± 0.42b,ab,a | 0.09 ± 0.01c,a,c | 0.63 ± 0.01b,a,a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalorizou, H.; Giannoulis, P.; Leontopoulos, S.; Koubouris, G.; Chavalina, S.; Sorovigka, M. Amelioration of Olive Tree Indices Related to Salinity Stress via Exogenous Administration of Amino Acid Content: Real Agronomic Effectiveness or Mechanistic Restoration Only? Horticulturae 2025, 11, 890. https://doi.org/10.3390/horticulturae11080890
Kalorizou H, Giannoulis P, Leontopoulos S, Koubouris G, Chavalina S, Sorovigka M. Amelioration of Olive Tree Indices Related to Salinity Stress via Exogenous Administration of Amino Acid Content: Real Agronomic Effectiveness or Mechanistic Restoration Only? Horticulturae. 2025; 11(8):890. https://doi.org/10.3390/horticulturae11080890
Chicago/Turabian StyleKalorizou, Helen, Paschalis Giannoulis, Stefanos Leontopoulos, Georgios Koubouris, Spyridoula Chavalina, and Maria Sorovigka. 2025. "Amelioration of Olive Tree Indices Related to Salinity Stress via Exogenous Administration of Amino Acid Content: Real Agronomic Effectiveness or Mechanistic Restoration Only?" Horticulturae 11, no. 8: 890. https://doi.org/10.3390/horticulturae11080890
APA StyleKalorizou, H., Giannoulis, P., Leontopoulos, S., Koubouris, G., Chavalina, S., & Sorovigka, M. (2025). Amelioration of Olive Tree Indices Related to Salinity Stress via Exogenous Administration of Amino Acid Content: Real Agronomic Effectiveness or Mechanistic Restoration Only? Horticulturae, 11(8), 890. https://doi.org/10.3390/horticulturae11080890










