Propagation and Long-Term Storage of Rhaponticum carthamoides Under In Vitro Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Disinfection and In Vitro Culture Initiation
2.3. In Vitro Micropropagation
2.4. Deposition of Aseptic In Vitro Cultures
2.5. In Vitro Rooting
2.6. Ex Vitro Rooting
2.7. Quantitative Determination of 20-Hydroxyecdysone in Plant Extracts
2.8. Evaluation of Genetic Stability of R. Carthamoides Plants
2.9. Statistical Analyses
3. Results
3.1. Collection and Characterization of R. carthamoides Samples
3.2. In Vitro Germination of Rhaponticum Carthamoides Seeds
3.3. In Vitro Micropropagation of Rhaponticum Carthamoides Explants
3.4. Optimization of In Vitro Storage Conditions for Rhaponticum Carthamoides
3.5. In Vitro Rooting and Acclimatization of Rhaponticum Carthamoides Regenerants
3.6. Quantitative Analysis of 20-Hydroxyecdysone in Rhaponticum Carthamoides Plants
3.7. Analysis of Genetic Stability in R. carthamoides Plants by ISSR Marker Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ANOVA | Analysis of variance |
| BAP | 6-benzylaminopurine |
| CCC | Chlorocholine chloride |
| HPLC | High-performance liquid chromatography |
| HPR | Hypocotyl with primary rootlet |
| IBA | Indole-3-butyric acid |
| iPBS | inter Primer Binding Site |
| ISSR | Inter simple sequence repeats |
| Kin | Kinetin |
| MS | Murashige and Skoog |
| mT | Meta-Topolin |
| PEG | Polyethyleneglycol |
| PGR | Plant growth regulator |
| POD | Peroxidase |
| PR-1 | Plants regenerated after long-term storage and rooted ex vitro |
| PR-2 | Regenerated plants cultivated in a nursery |
| RAPD | Random Amplified Polymorphic DNA |
| SA | Shoot apex with cotyledons |
| SOD | Superoxide dismutase |
| SSR | Simple sequence repeats |
| WP | Wild plant |
| 20-HE | 20-hydroxyecdysone |
References
- Indu, I.; Mehta, B.K.; Shashikumara, P.; Gupta, G.; Dikshit, N.; Chand, S.; Yadav, P.K.; Ahmed, S.; Singhal, R.K. Forage crops: A repository of functional trait diversity for current and future climate adaptation. Crop Pasture Sci. 2023, 74, 961–977. [Google Scholar] [CrossRef]
- Chen, C.; Chang, H.; Pang, X.; Liu, Q.; Xue, L.; Yin, C. Genetic diversity analysis and conservation strategy recommendations for ex situ conservation of Cupressus chengiana. BMC Plant Biol. 2025, 25, 552. [Google Scholar] [CrossRef]
- Lopes Paulo, M.; Machado Leitea, D.; Gabriel Do Carmob, D.; Montenegro Valls, J.F.; Borghetti, F.; Ebling Brondanic, G. A germination and micropropagation protocol for an endangered grass, Gymnopogon doellii, for ex situ conservation. Seed Sci. Technol. 2024, 52, 41–55. [Google Scholar] [CrossRef]
- Todorova, V.; Savova, M.S.; Ivanova, S.; Ivanov, K.; Georgiev, M.I. Anti-adipogenic activity of Rhaponticum carthamoides and its secondary metabolites. Nutrients 2023, 15, 3061. [Google Scholar] [CrossRef]
- Timofeev, N.P. Experience of Rhaponticum carthamoides (Willd.) Iliin cultivation as a natural source of ecdysterone under the conditions of the Arkhangelsk region. Agric. Biol. 2023, 58, 114–141. [Google Scholar] [CrossRef]
- Kubentaev, S.A.; Danilova, A.N. Evaluation of ecological and biological characteristics of Rhaponticum carthamoides (Willd.) Iljin and its resource indicators on the Ridge of Ivanovo (Eastern Kazakhstan). Biology 2017, 37, 31–46. [Google Scholar] [CrossRef]
- Zhmud, E.V. Rhaponticum carthamoides (Asteraceae) in the Altai Republic: Assessment of the State of the Plant Affected by Human Activities. J. Sib. Fed. Biol. 2022, 15, 92–106. [Google Scholar] [CrossRef]
- Baitulin, I.O. (Ed.) The Red Book of Kazakhstan (Plants); Art Print XXI: Astana, Kazakhstan, 2014; 452p. (In Russian) [Google Scholar]
- Bikov, B.K. (Ed.) Rare and Endangered Species of Animals and Plants. Part 2. Plants. In Red Data Book of Kazakh SSR; Nauka: Alma-Ata, Kazakhstan, 1981; 260p. (In Russian) [Google Scholar]
- Tarkowská, D.; Strnad, M. Plant ecdysteroids: Plant sterols with intriguing distributions, biological effects and relations to plant hormones. Planta 2016, 244, 545–555. [Google Scholar] [CrossRef]
- Parr, M.K.; Botrè, F.; Naß, A.; Hengevoss, J.; Diel, P.; Wolber, G. Ecdysteroids: A novel class of anabolic agents? Biol. Sport 2015, 32, 169–173. [Google Scholar] [CrossRef]
- Głazowska, J.; Kamiński, M.M.; Kamiński, M. Chromatographic separation, determination and identification of ecdysteroids: Focus on Maral root (Rhaponticum carthamoides, Leuzea carthamoides). J. Sep. Sci. 2018, 41, 4304–4314. [Google Scholar] [CrossRef]
- Kosović, E.; Lino, K.; Kuchař, M. HPLC-MS Methodology for R. carthamoides extract quality evaluation: A simultaneous determination of eight bioactive compounds. Diversity 2022, 14, 880. [Google Scholar] [CrossRef]
- Ulrikh, E.V.; Smolovskaya, O.V.; Pak, N.G. Study of antibacterial and antioxidant properties of medical plant extracts. BIO Web Conf. 2022, 47, 06001. [Google Scholar] [CrossRef]
- Yang, Y.; Asyakina, L.; Babich, O.; Dyshluk, L.; Sukhikh, S.; Popov, A.; Kostyushina, N. Physicochemical properties and biological activity of extracts of dried biomass of callus and suspension cells and in vitro root cultures. Food Process Tech. Technol. 2020, 50, 480–492. [Google Scholar] [CrossRef]
- Myrzagaliyeva, A.; Irsaliyev, S.; Tustubayeva, S.; Samarkhanov, T.; Orazov, A.; Alemseitova, Z. Natural Resources of Rhaponticum carthamoides in the Tarbagatai State National Nature Park. Diversity 2024, 16, 676. [Google Scholar] [CrossRef]
- Zhdanova, I.N. The effect of vitamin-herbal flour from R. Carthamoides on the blood parameters of young cattle. Agrar. Nauka 2022, N2, 28–31. [Google Scholar] [CrossRef]
- Mamyrova, S.; Kupriyanov, A.; Ishmuratova, M.; Ivashchenko, A.; Myrzagaliyeva, A.; Orazov, A.; Kubentayev, S. The current state of populations of Rhaponticum altaicum (Asteraceae) in the northern and central Kazakhstan. Diversity 2025, 17, 206. [Google Scholar] [CrossRef]
- Maysak, G.P.; Matolinets, D.A. Seed productivity of Maral root in the Perm Territory. Kormoproizvodstvo 2021, 2, 32–35. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; Henriques, A.B.; Sato, A. In vitro conservation of Mandevilla moricandiana (Apocynaceae): Short-term storage and encapsulation–dehydration of nodal segments. In Vitro Cell. Dev. Biol.-Plant 2014, 50, 326–336. [Google Scholar] [CrossRef]
- Jalli, R.; Aravind, J.; Pandey, A. Conservation and management of endemic and threatened plant species in India: An overview. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 461–486. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Cavanagh, A.P.; Macnaghten, P. Agricultural biotechnology: Potential, challenges, and debate. Plants People Planet 2025, early view. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Prosekov, A.Y.; Kozlova, O.V.; Vesnina, A.D. Biotechnology of Cultivation of Rhaponticum Carthamoides (willd.) Suspension Cells: A Prospective Source of antitumor Substances. Russ. Agric. Sci. 2022, 48, 197–202. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, V.; Quraishi, A. In vitro conservation through slow-growth storage. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 397–416. [Google Scholar] [CrossRef]
- Nhut, D.T.; Van Le, B.; Fukai, S.; Tanaka, M.; Van, K.T.T. Effects of activated charcoal, explant size, explant position and sucrose concentration on plant and shoot regeneration of Lilium longiflorum via young stem culture. Plant Growth Regul. 2001, 33, 59–65. [Google Scholar] [CrossRef]
- Rademacher, W. Biochemical effects of plant growth retardants. In Plant Biochemical Regulators; CRC Press: Boca Raton, FL, USA, 2020; pp. 169–200. [Google Scholar]
- Ma, Z.B.; Dong, X.R.; Fang, M.Y.; Wang, Q.; Yan, P.; Wang, Q.Y.; Lu, L.; Dong, Z.Q. Effects of basic application of chlorocholine chloride combined with nitrogen fertilizer on nitrogen use of summer maize in North China Plain. Ying Yong Sheng Tai Xue Bao 2021, 32, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Interactions of gibberellins with phytohormones and their role in stress responses. Horticulturae 2022, 8, 241. [Google Scholar] [CrossRef]
- Hua, W.R.; Yong, L.; Sheng, W.; Hua, N.X. Liu Peng Effect of plant growth retardants on the growth and development of potted rose. Acta Bot. Boreali-Occidentalia Sin. 2012, 32, 767–773. [Google Scholar]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Angelini, L.G. Effect of two plant growth retardants on steviol glycosides content and antioxidant capacity in Stevia (Stevia rebaudiana Bertoni). Acta Physiol. Plant 2014, 36, 1211–1219. [Google Scholar] [CrossRef]
- Ju, S.; Xu, D.; Zhang, C.; Lu, J.; Jiang, X.; Ji, L. Induction of tolerance by chlorocholine chloride in Sequoia sempervirens seedlings under natural cooling and drought. J. For. Sci. 2020, 66, 236–243. [Google Scholar] [CrossRef]
- Andrade, D.S.S.; Souza, E.H.; Costa, E.M.R.; Souza, F.V.D. In vitro conservation of three endemic Bromeliaceae species and reintroduction into their natural habitat. J. Nat. Conserv. 2025, 84, 126839. [Google Scholar] [CrossRef]
- Lu, X.; Sun, P.; Liu, R.; Wang, C.; Tong, L.; Tahir, M.M.; Ma, X.; Bao, J.; Zhang, D.; Wang, M.; et al. In vitro slow-growth conservation, acclimatization, and genetic stability of virus-free apple plants. Hortic. Adv. 2024, 2, 30. [Google Scholar] [CrossRef]
- Pan, X.J.; Zhang, W.E.; Li, X. In Vitro Conservation of Native Chinese Wild Grape (Vitis Heyneana Roem. & Schult) by Slow Growth Culture. Vitis 2015, 53, 207. [Google Scholar] [CrossRef]
- Alleweldt, G.; Harstlangenbucher, M. The effect of growth-inhibitors on long-term storage of in vitro cultures of grapevine. Vitis 1987, 26, 57–64. [Google Scholar]
- Mitrofanova, I.V.; Ivanova, N.N.; Brailko, V.A. Clematis plants conservation under in vitro GeneBank conditions. Acta Hortic. 2020, 1298, 167–174. [Google Scholar] [CrossRef]
- Du, Y.; Li, W.; Zhang, M.; He, H.; Jia, G. The establishment of a slow-growth conservation system in vitro for two wild lily species. Afr. J. Biotechnol. 2012, 11, 1981–1990. [Google Scholar] [CrossRef]
- Mitrofanova, I.V.; Brailko, V.A.; Lesnikova-Sedoshenko, N.P.; Ivanova, N.N.; Mitrofanova, O.V. Structure of Vegetative Organs in Essential Oil Rose Under Standard Culture Conditions and Long-Term Conservation In Vitro; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2021. [Google Scholar] [CrossRef]
- Dey, A.; Kundu, S.; Bandyopadhyay, A.; Bhattacharjee, A. Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. Comptes Rendus Biol. 2013, 336, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tikendra, L.; Potshangbam, A.M.; Dey, A.; Devi, T.R.; Sahoo, M.R.; Nongdam, P. RAPD, ISSR, and scot markers based genetic stability assessment of micropropagated Dendrobium fimbriatum Lindl. var. oculatum Hk. f.- an important endangered orchid. Physiol. Mol. Biol. Plants 2021, 27, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B. Plant regeneration from hypocotyls of black carrot via direct somatic embryogenesis and determination of its genetic stability by RAPD and ipbs methods. Indian J. Genet. Plant Breed. 2018, 78, 373–377. [Google Scholar] [CrossRef]
- Kalendar, R.; Schulman, A.H. Transposon-based tagging: IRAP, REMAP, and iPBS. Molecular plant taxonomy: Methods and protocols. Methods Mol. Biol. 2014, 1115, 233–255. [Google Scholar] [CrossRef]
- Tikendra, L.; Choudhary, R.; Sanayaima Devi, R.; Dey, A.; Potshangbam, A.M.; Nongdam, P. Micropropagation of bamboos and clonal fidelity assessment using molecular markers. In Biotechnological Advances in Bamboo; Ahmad, Z., Ding, Y., Shahzad, A., Eds.; Springer: Singapore, 2021; pp. 145–185. [Google Scholar] [CrossRef]
- Kalendar, R.; Muterko, A.; Boronnikova, S. Retrotransposable Elements: DNA Fingerprinting and the Assessment of Genetic Diversity. In Molecular Plant Taxonomy; Besse, P., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2222. [Google Scholar] [CrossRef]
- Etminan, A.; Pour-Aboughadareh, A.; Mohammadi, R.; Ahmadi-Rad, A.; Noori, A.; Mahdavian, Z.; Moradi, Z. Applicability of start codon targeted (SCoT) and inter-simple sequence repeat (ISSR) markers for genetic diversity analysis in durum wheat genotypes. Biotechnol. Biotechnol. Equip. 2016, 30, 1075–1081. [Google Scholar] [CrossRef]
- Bornet, B.; Branchard, M. Nonanchored inter simple sequence repeat (ISSR) markers: Reproducible and specific tools for genome fingerprinting. Plant Mol. Biol. Report. 2001, 19, 209–215. [Google Scholar] [CrossRef]
- Raizer, O.; Tagimanova, D.; Nagmetova, G.; Khapilina, O. Introduction to in vitro culture of Rhaponticum carthamoides. Eurasian J. Appl. Biotechnol. 2024, 2, 61–69. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Zhou, J.; Wang, X.; Guo, L. Isolation and purification of three ecdysteroids from the stems of Diploclisia glaucescens by high-speed countercurrent chromatography and their anti-inflammatory activities in vitro. Molecules 2017, 22, 1310. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Nazari, M.; Kordrostami, M.; Ghasemi-Soloklui, A.A. Conservation of medicinal plants by tissue culture techniques. In Medicinal Plants: Biodiversity, Biotechnology and Conservation Sustainable; Jha, S., Halder, M., Eds.; Springer: Singapore, 2023; Volume 33, pp. 801–818. [Google Scholar] [CrossRef]
- Sarasan, V.; Cripps, R.; Ramsay, M.M.; Atherton, C.; McMichen, M.; Prendergast, G.; Rowntree, J.K. Conservation in vitro of threatened plants—Progress in the past decade. In Vitro Cell. Dev. Biol.-Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Chauhan, R.; Keshavkant, S.; Jadhav, S.K.; Quraishi, A. In vitro slow-growth storage of Chlorophytum borivilianum Sant. et Fernand: A critically endangered herb. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 315–321. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. In vitro minimum growth for conservation of Drosophyllum lusitanicum. Biol. Plant 2007, 51, 795–798. [Google Scholar] [CrossRef]
- Trejgell, A.; Kamińska, M.; Tretyn, A. In vitro slow growth storage of Senecio macrophyllus shoots. Acta Physiol. Plant 2015, 37, 234. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Senula, A.; Leunufna, S.; Grübe, M. Slow growth storage and cryopreservation—Tools to facilitate germplasm maintenance of vegetatively propagated crops in living plant collections. Int. J. Refrig. 2006, 29, 411–417. [Google Scholar] [CrossRef]
- Arora, K.; Rai, M.K.; Sharma, A.K. Tissue culture mediated biotechnological interventions in medicinal trees: Recent progress. Plant Cell Tiss. Organ. Cult. 2022, 150, 267–287. [Google Scholar] [CrossRef]
- Benelli, C.; Tarraf, W.; İzgü, T.; Anichini, M.; Faraloni, C.; Salvatici, M.C.; Jouini, N.; Germanà, M.A.; Danti, R.; Lambardi, M. Long-term conservation for the safeguard of Abies nebrodensis: An endemic and endangered species of Sicily. Plants 2024, 13, 1682. [Google Scholar] [CrossRef]
- Krishnan, P.N.; Decruse, S.W.; Radha, R.K. Conservation of medicinal plants of Western Ghats, India and its sustainable utilization through in vitro technology. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 110–122. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and conservation of plant biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Wildi, E.; Schaffner, W.; Berger Büter, K. In vitro propagation of Petasites hybridus (Asteraceae) from leaf and petiole explants and from inflorescence buds. Plant Cell Rep. 1998, 18, 336–340. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Barone, G.; Di Gristina, E.; Sottile, F.; Domina, G. Micropropagation of endemic endangered taxa of the Italian flora: Adenostyles alpina subsp. macrocephala (Asteraceae), as a case study. Plants 2023, 12, 1530. [Google Scholar] [CrossRef]
- Skała, E.; Grąbkowska, R.; Sitarek, P.; Kuźma, Ł.; Błauż, A.; Wysokińska, H. Rhaponticum carthamoides regeneration through direct and indirect organogenesis, molecular profiles and secondary metabolite production. Plant Cell Tiss. Organ. Cult. 2015, 123, 83–98. [Google Scholar] [CrossRef]
- Skała, E.; Kicel, A.; Olszewska, M.A.; Kiss, A.K.; Wysokińska, H. Establishment of hairy root cultures of Rhaponticum carthamoides (Willd.) Iljin for the production of biomass and caffeic acid derivatives. BioMed Res. Int. 2015, 2015, 181098. [Google Scholar] [CrossRef]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef]
- Davidović Gidas, J.; Zeljković, S.; Đekić, N.; Đurić, G. LED lights and plant growth regulators enhance the in vitro mass propagation of rosemary (Rosmarinus officinalis L.). Eur. J. Hortic. Sci. 2025, 90, 0008. [Google Scholar] [CrossRef]
- Skała, E.; Olszewska, M.A.; Tabaka, P.; Kicel, A. Light-emitting diodes and liquid system affect the caffeoylquinic acid derivative and flavonoid production and shoot growth of Rhaponticum carthamoides (Willd.) Iljin. Molecules 2024, 29, 2145. [Google Scholar] [CrossRef] [PubMed]
- Krakhmaleva, I.L.; Molkanova, O.I.; Orlova, N.D.; Koroleva, O.V.; Mitrofanova, I.V. In vitro morpho-anatomical and regeneration features of cultivars of Actinidia kolomikta (Maxim.) Maxim. Horticulturae 2024, 10, 1335. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Husain, F.M.; Khanam, M.N.; Javed, S.B. Effect of meta-Topolin on morphological, physiochemical, and molecular dynamics during in vitro regeneration of Salix tetrasperma Roxb. BMC Plant Biol. 2025, 25, 121. [Google Scholar] [CrossRef] [PubMed]
- San José, M.C.; Cernadas, M.J.; Janeiro, L.V. Optimization of micropropagation protocols in some woody plants using meta-topolin. In Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Ahmad, N., Strnad, M., Eds.; Springer: Singapore, 2021; pp. 221–240. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tiss. Organ. Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Ptak, A.; Szewczyk, A.; Simlat, M.; Błażejczak, A.; Warchoł, M. Meta-Topolin-induced mass shoot multiplication and biosynthesis of valuable secondary metabolites in Stevia rebaudiana Bertoni bioreactor culture. Sci. Rep. 2023, 13, 15520. [Google Scholar] [CrossRef]
- Domingues, J.; Eira, A.; Ramalho, I.; Barrocas, I.; Gonçalves, J.C. In vitro Propagation and Conservation of Lavandula stoechas subsp. luisieri and Pterospartum tridentatum, Two Important Medicinal and aromatic Species from Portugal. Plants 2024, 13, 2124. [Google Scholar] [CrossRef]
- Akhtar, Z.; Alireza, B.; Reza, O.; Elham, D. Study on callus induction and plant regeneration of Leuzea carthamoides via tissue culture system. J. Med. Plants Res. 2014, 8, 260–268. [Google Scholar] [CrossRef]
- Duškova, J.; Dušek, J. Leuzea carthamoides DC. in vitro. Herba Pol. 1995, 41, 165–169. [Google Scholar]
- Engelmann, F. Present development and use of in vitro culture techniques for the conservation of plant genetic resources. Acta Hortic. 1997, 447, 471–476. [Google Scholar] [CrossRef]
- Hussain, I.; Saleem, M.H.; Mumtaz, S.; Rasheed, R.; Ashraf, M.A.; Maqsood, F.; Rehman, M.; Yasmin, H.; Ahmed, S.; Ishtiaq, M.; et al. Choline chloride mediates chromium tolerance in spinach (Spinacia oleracea L.) by restricting its uptake in relation to morpho-physio-biochemical attributes. J. Plant Growth Regul. 2022, 41, 1594–1614. [Google Scholar] [CrossRef]
- Koroleva, O.V.; Molkanova, O.I.; Mishanova, E.V. Biotechnological methods of reproduction and preservation of species and cultivars of the genus Syringa L. Acta Hortic. 2022, 1339, 87–92. [Google Scholar] [CrossRef]
- Mitrofanova, I.V.; Brailko, V.A.; Ivanova, N.N.; Mitrofanova, O.V. Some special features of the conservation of valuable, essential oil rose cultivars: In vitro deposition and cryopreservation. Acta Hortic. 2019, 1234, 195–202. [Google Scholar] [CrossRef]
- Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs combined with CHO sources and CCC priming PLB regeneration of Phalaenopsis. Horticulturae 2019, 5, 34. [Google Scholar] [CrossRef]
- Poniewozik, M.; Parzymies, M.; Szot, P. Effect of activated charcoal and ascorbic acid on in vitro morphogenesis and o-dihydroxyphenols content in Paphiopedilum insigne. Hortic. Sci. 2022, 49, 48–51. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Wawrzyniak, M.K.; Kalemba, E.M.; Ley-López, J.M.; Lira, J.M.S.; Chmielarz, P. In vitro rooting of Quercus robur, activated charcoal vs. exogenous auxin: A morphophysiological approach. Plant Cell Tiss. Organ. Cult. 2024, 156, 24. [Google Scholar] [CrossRef]
- Timofeev, N.P. Accumulation and variability of ecdysteroid content in medicinal raw materials of Leuzea carthamoides. Agric. Biol. 2009, 44, 106–117. (In Russian) [Google Scholar]
- Timofeev, N.P. Productivity of aboveground phytomass and ecdysterone content in the agropopulation of Leuzea carthamoides for 27 years of ontogenesis. In New and Unconventional Plants and Prospects for Their Use; Federal Scientific Center of Vegetable Growing: Vniissok, Russia, 2017; Number S13; pp. 167–170. (In Russian) [Google Scholar]
- Thiem, B.; Kikowska, M.; Maliński, M.P.; Kruszka, D.; Napierała, M.; Florek, E. Ecdysteroids: Production in plant in vitro cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytoecdysteroids: Distribution, structural diversity, biosynthesis, activity, and crosstalk with phytohormones. Int. J. Mol. Sci. 2022, 23, 8664. [Google Scholar] [CrossRef]
- Ravishankar, G.A.; Mehta, A.R. Control of Ecdysterone Biogenesis in Tissue Cultures of Trianthema portulacastrum. J. Nat. Prod. 1979, 42, 152–158. [Google Scholar] [CrossRef] [PubMed]


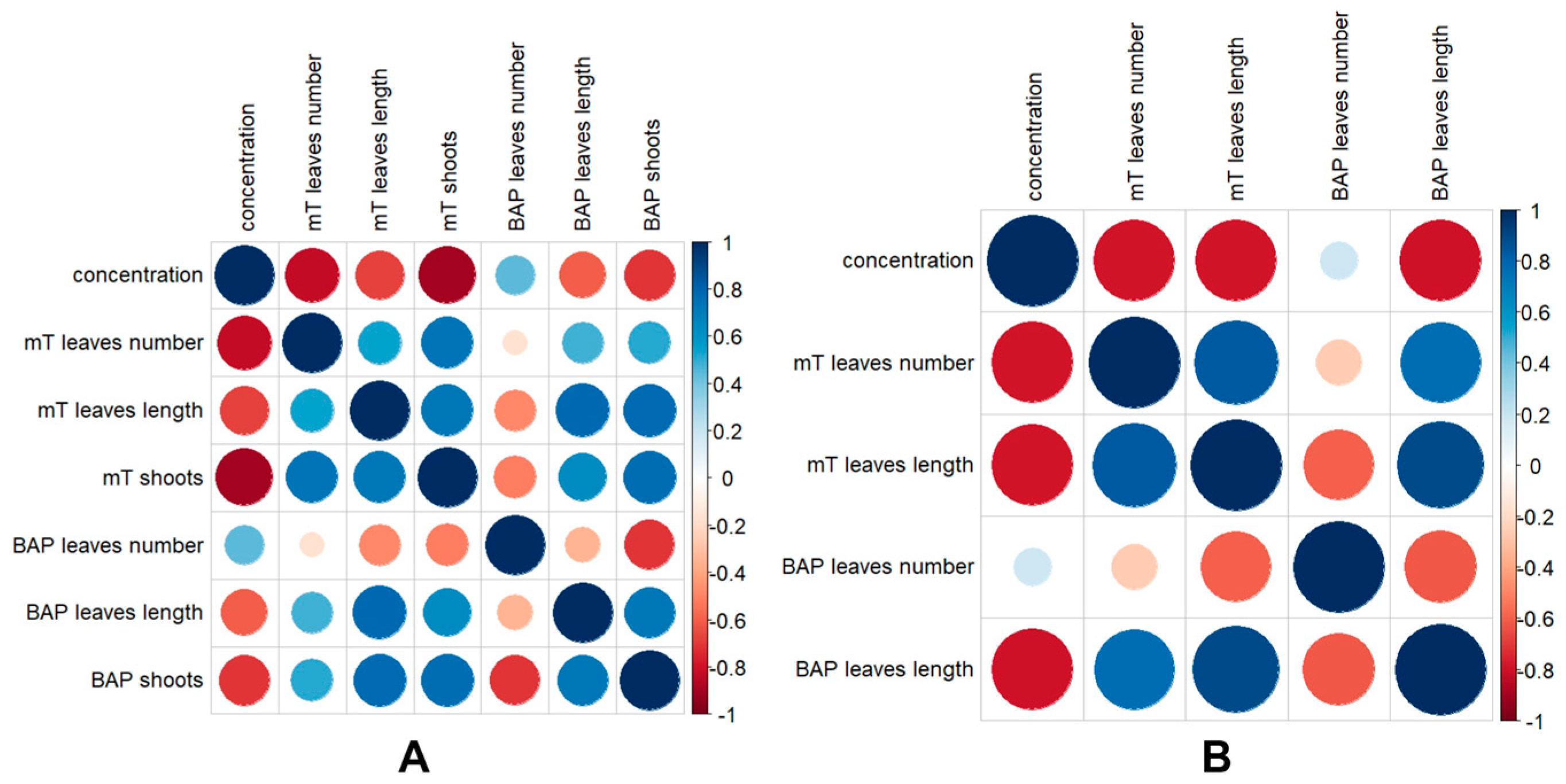
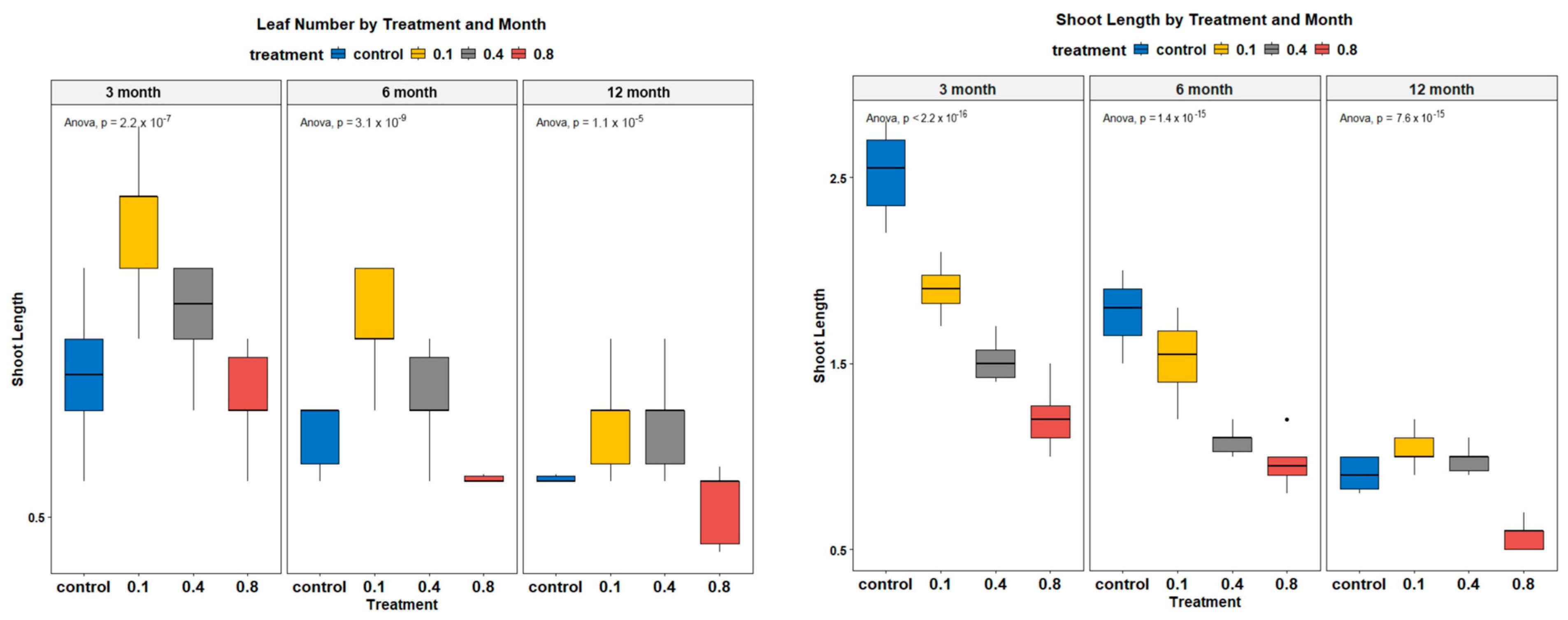

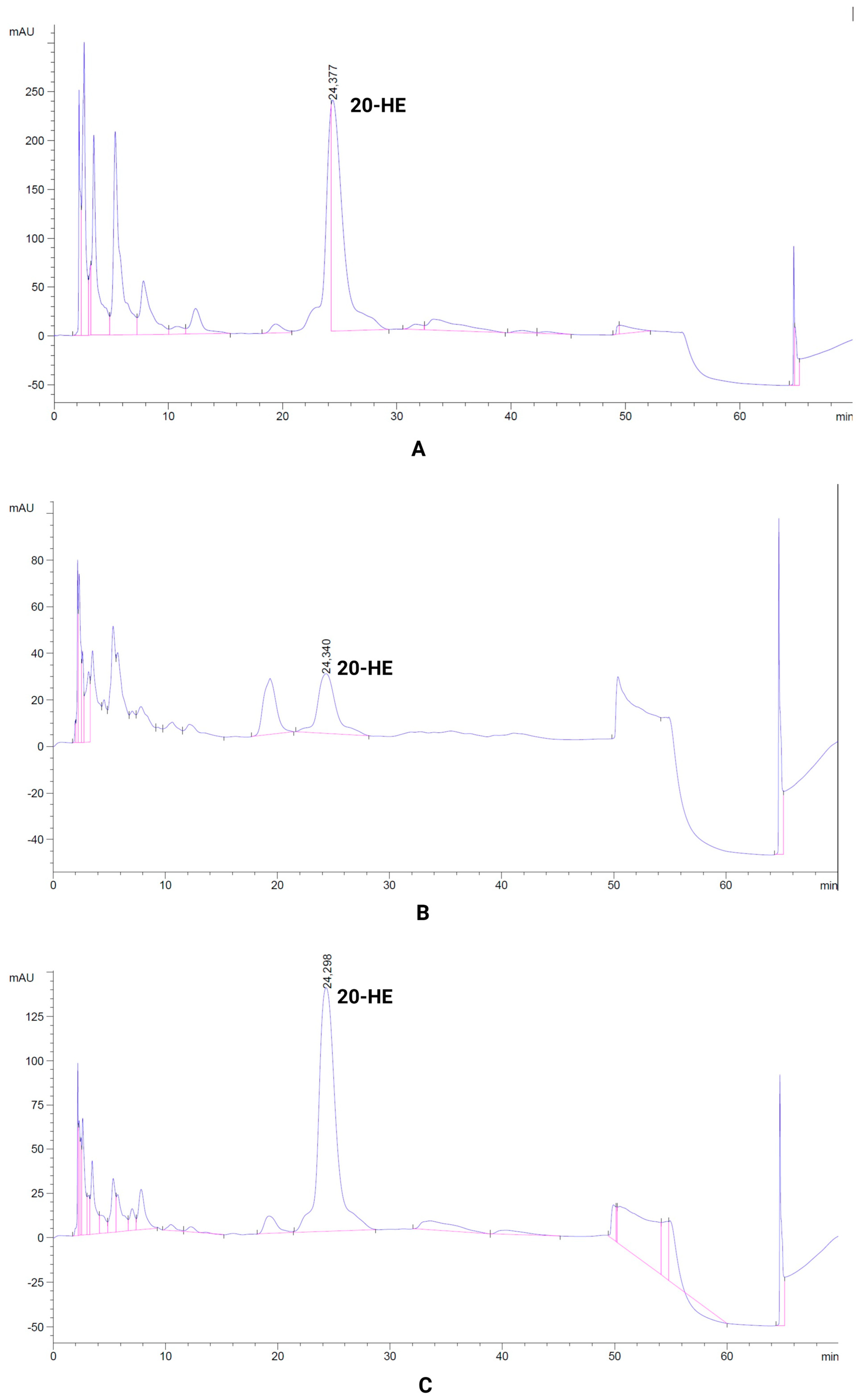
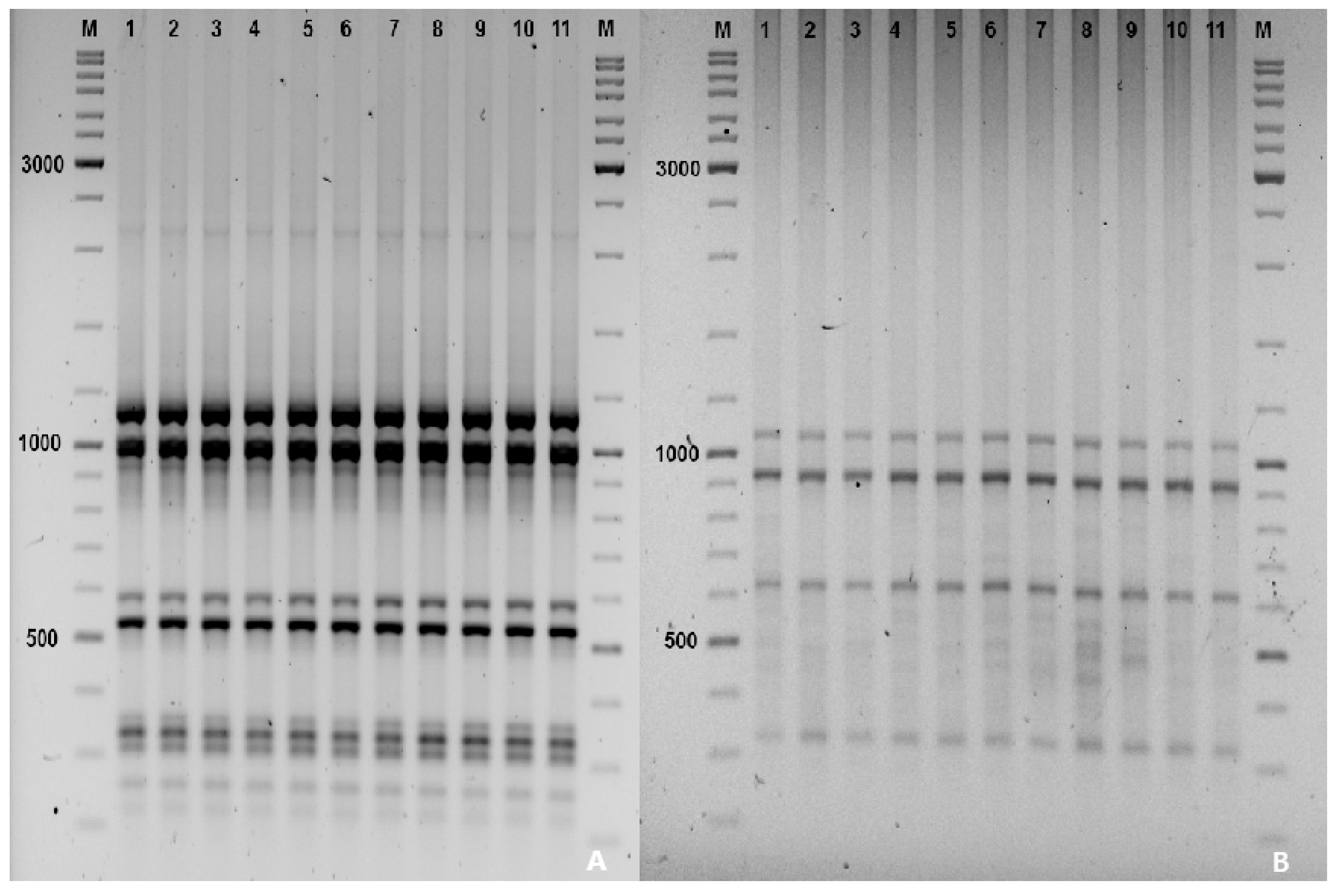
| Growth Regulator (Cytokinin) | Concentration (mg L−1) | Average Number of Leaves/Explant | Average Length of Leaves (cm) | Average Number of Shoots/Explant |
|---|---|---|---|---|
| SA | ||||
| mT | 0.5 | 33 ± 2.1 ab | 10.4 ± 2.0 a | 7.5 ± 1.5 a |
| 1.0 | 31 ± 2.4 d | 6.1 ± 1.5 c | 6.5 ± 1.2 c | |
| 2.0 | 31 ± 3.7 bd | 4.8 ± 1.0 d | 4.5 ± 0.9 e | |
| 5.0 | 22 ± 2.6 e | 3.8 ± 1.5 b | 1.5 ± 0.2 d | |
| BAP | 0.5 | 14 ± 1.3 f | 5.6 ± 0.9 cd | 3.0 ± 0.4 b |
| 1.0 | 11 ± 1.0 c | 3.7 ± 0.5 b | 2.5 ± 0.3 b | |
| 2.0 | 34 ± 3.1 a | 3.6 ± 0.6 b | 1.5 ± 0.5 d | |
| 5.0 | 23 ± 2.0 e | 3.1 ± 0.4 b | 1.5 ± 0.2 d | |
| Significance level | 0.000 *** | 0.000 *** | 0.000 *** | |
| HPR | ||||
| mT | 0.5 | 16 ± 1.6 a | 4.5 ± 0.6 a | 1 a |
| 1.0 | 9 ± 1.4 c | 3.5 ± 0.5 c | 1 a | |
| 2.0 | 8 ± 1.3 e | 1.2 ± 0.2 f | 1 a | |
| 5.0 | 5 ± 0.9 g | 1.1 ± 0.1 ef | 1 a | |
| BAP | 0.5 | 6 ± 1.2 f | 2.9 ± 0.5 b | 1 a |
| 1.0 | 4 ± 0.7 b | 2.6 ± 0.4 b | 1 a | |
| 2.0 | 13 ± 1.2 d | 0.9 ± 0.1 e | 1 a | |
| 5.0 | 7 ± 1.0 ef | 0.7 ± 0.1 d | 1 a | |
| Significance level | 0.000 *** | 0.000 *** | n.s. | |
| Concentration IBA, mg L−1 | Average Number of Roots, pcs | Average Root Length, cm |
|---|---|---|
| (control) | 1.5 ± 0.5 | 4.5 ± 1.5 |
| 1.0 | 1.7 ± 0.8 | 5.6 ± 1.9 |
| 2.0 | 9.7 ± 0.7 | 19.7 ± 0.6 |
| 5.0 | 9.5 ± 0.5 | 18.3 ± 0.5 |
| Sample | Retention Time of Main Peak (min) | Main Peak Area (mAU·s) | Share of Main Peak (%) | Content of 20-HE (mg/mL) |
|---|---|---|---|---|
| WP (leaf rosette phase) | 24.377 | 16,690.2 | 31.54 | 7.88 |
| PR-1 (after deposition, ex vitro rooting) | 24.34 | 8235.4 | 14.96 | 3.88 |
| PR-2 (after deposition, nursery, leaf rosette phase) | 24.298 | 19,574.7 | 42.25 | 9.24 |
| Sequence | Tm (°C) | Amplicon Size (bp) | Number of Amplicons |
|---|---|---|---|
| AGCAGCAGCAGCAGCAGCC | 67 | 350–1300 | 80 |
| CTCCTCCTCCTCCTCCTCG | 61 | 550–2500 | 60 |
| GAGAGAGAGAGAGAGAGAGAC | 54 | 300–1200 | 40 |
| CTCTCTCTCTCTCTCTCTCTG | 54 | 250–1800 | 20 |
| ACACACACACACACACACACC | 60 | 450–1500 | 90 |
| ACACACACACACACACACACG | 60 | 250–1500 | 30 |
| ACACACACACACACACACACT | 58 | 300–1800 | 50 |
| AGAGAGAGAGAGAGAGAGAGC | 56 | 400–1500 | 110 |
| TGTGTGTGTGTGTGTGTGTGC | 60 | 200–1200 | 60 |
| CACCACCACCACCACCACCACT | 67 | 150–1000 | 60 |
| TGTGTGTGTGTGTGTGTGTGA | 58 | 350–1600 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiser, O.; Tagimanova, D.; Turzhanova, A.; Magzumova, S.; Nagmetova, G.; Akhmetkarimova, Z.; Premina, N.; Filippova, N.; Khapilina, O. Propagation and Long-Term Storage of Rhaponticum carthamoides Under In Vitro Conditions. Horticulturae 2025, 11, 952. https://doi.org/10.3390/horticulturae11080952
Raiser O, Tagimanova D, Turzhanova A, Magzumova S, Nagmetova G, Akhmetkarimova Z, Premina N, Filippova N, Khapilina O. Propagation and Long-Term Storage of Rhaponticum carthamoides Under In Vitro Conditions. Horticulturae. 2025; 11(8):952. https://doi.org/10.3390/horticulturae11080952
Chicago/Turabian StyleRaiser, Olesya, Damelya Tagimanova, Ainur Turzhanova, Saule Magzumova, Gulden Nagmetova, Zhanar Akhmetkarimova, Nataliya Premina, Nadezhda Filippova, and Oxana Khapilina. 2025. "Propagation and Long-Term Storage of Rhaponticum carthamoides Under In Vitro Conditions" Horticulturae 11, no. 8: 952. https://doi.org/10.3390/horticulturae11080952
APA StyleRaiser, O., Tagimanova, D., Turzhanova, A., Magzumova, S., Nagmetova, G., Akhmetkarimova, Z., Premina, N., Filippova, N., & Khapilina, O. (2025). Propagation and Long-Term Storage of Rhaponticum carthamoides Under In Vitro Conditions. Horticulturae, 11(8), 952. https://doi.org/10.3390/horticulturae11080952







