Acrostalagmus luteoalbus as the Novel Causing Agent of Root Rot on Strawberry and In Vitro Screening of Effective Fungicides
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation of the Fungi
2.2. Pathogenicity Tests
2.3. Identification of Pathogenic Fungus
2.4. In Vitro Screening of Effective Fungicides
2.5. Statistical Analysis
3. Results
3.1. Isolation and Pathogenicity of Strains Causing Strawberry Root Rot
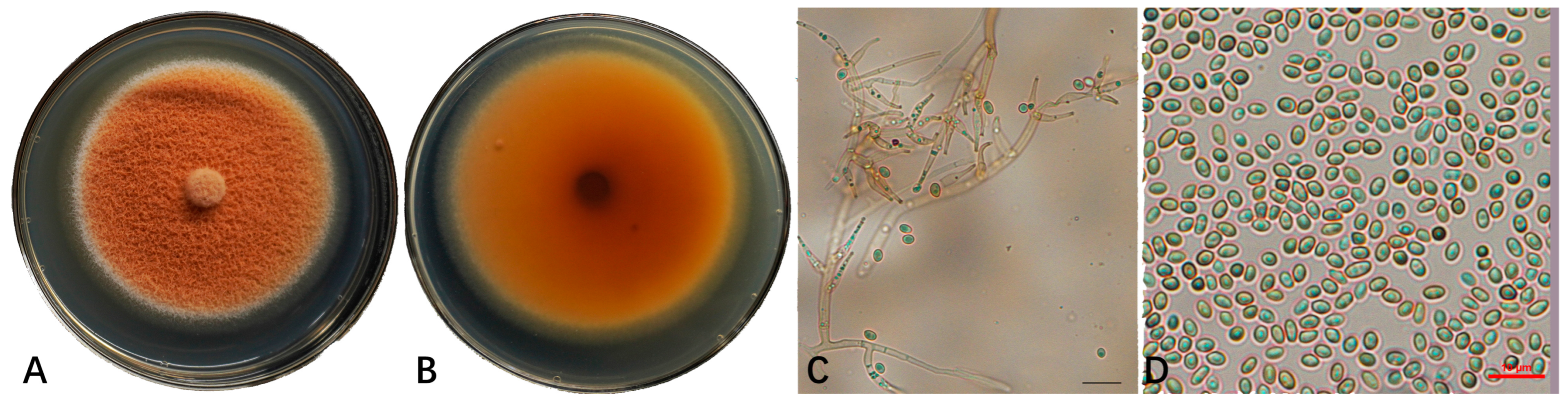
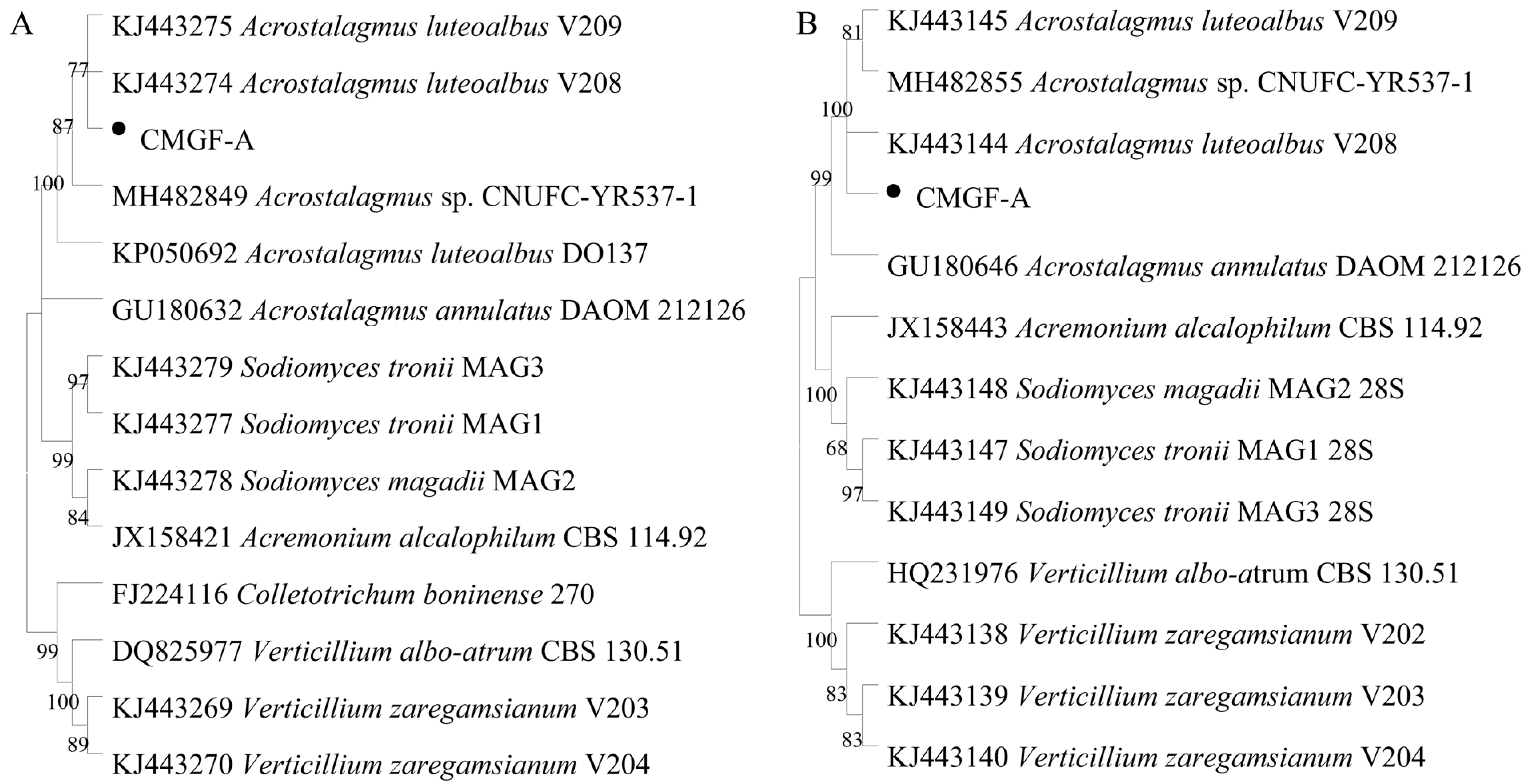
3.2. Identification of the Pathogenic Fungi
3.3. Regression Equation of Test Fungicides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A. luteoalbus | Acrostalagmus luteoalbus |
| PDA | Potato Dextrose Agar Medium |
| EC | Emulsifiable Concentrate |
| SC | Suspension Concentrate |
| WP | Wettable Powder |
| AS | Aqueous Solution |
| Co., Ltd. | Company Limited |
| ITS | Internal Transcribed Spacer |
| LSU | The Large Subunit of 28S |
| EC50 | Half Maximal Effective Concentration |
| PCR | Polymerase Chain Reaction |
| DNA | Deoxyribonucleic Acid |
References
- Husaini, A.; Neri, D.; Garrido, C.; González-Rodríguez, V.E.; Carbu, M.; Husaini, A.M.; Cantoral, J.M. Fungal Diseases of Strawberry and their Diagnosis. In Strawberry: Growth, Development and Diseases; CAB International: Wallingford, UK, 2021. [Google Scholar]
- Zhang, X.Y.; Huo, H.L.; Zhao, Z.Y.; Dong, B.Z.; Wang, W. First Report of a Root rot Caused by Binucleate Rhizoctonia AG-A on Strawberries in Inner Mongolia, China. Plant Dis. 2016, 100, 1011. [Google Scholar] [CrossRef]
- Gilardi, G.; Bergeretti, F.; Gullino, M.L.; Garibaldi, A. First Report of Neopestalotiopsis clavispora Causing Root and Crown Rot on Strawberry in Italy. Plant Dis. 2019, 103, 2959. [Google Scholar] [CrossRef]
- Ishiguro, Y.; Otsubo, K.; Watanabe, H.; Suzuki, M.; Nakayama, K.; Fukuda, T.; Fujinaga, M.; Suga, H.; Kageyama, K. Root and crown rot of strawberry caused by Pythium helicoides and its distribution in strawberry production areas of Japan. J. Gen. Plant Pathol. 2014, 80, 423–429. [Google Scholar] [CrossRef]
- Borrero, C.; Avilés-García, I.; López, N.; Avilés, M. First Report of Root Rot on Strawberry Caused by Binucleate Rhizoctonia AG-K in Spain. Plant Dis. 2018, 103, 376. [Google Scholar] [CrossRef]
- Baiswar, P.; Ngachan, S.V. First Report of Root and Collar Rot of Strawberry (Fragaria × ananassa) Caused by Ceratobasidium sp. AG-B(o) (Binucleate Rhizoctonia) in India. Plant Dis. 2017, 102, 1035. [Google Scholar] [CrossRef]
- Pastrana, A.M.; Capote, N.; De los Santos, B.; Romero, F.; Basallote-Ureba, M.J. First report of Fusarium solani causing crown and root rot on strawberry crops in southwestern Spain. Plant Dis. 2014, 98, 161. [Google Scholar] [CrossRef]
- Sun, X.; Xin, Z.; Xin, X.; Wu, B. First Report of Strawberry Root Rot Caused by Fusarium falciforme in China. Plant Dis. 2024, 108, 2233. [Google Scholar] [CrossRef]
- Payán-Arzapalo, M.A.; López-Cuén, P.I.; Vega-Gutiérrez, T.A.; Molina-Cárdenas, L.; López-Orona, C.A.; Valenzuela-Tirado, G.A.; Tirado-Ramírez, M.A. First report of Fusarium falciforme causing root rot and wilt on strawberry in Sinaloa, Mexico. Plant Dis. 2024, 108, 2223. [Google Scholar] [CrossRef]
- Shrestha, U.; Dee, M.E.; Littrell, J.; Rice, J.H.; Ouma, W.; Ownley, B.H.; Butler, D.M. First report of root rot of strawberry caused by Fusarium cugenangense, a member of the F. oxysporum species complex, in Tennessee, USA. Plant Dis. 2024, 108, 2238. [Google Scholar] [CrossRef]
- Chamorro, M.; Aguado, A.; De los Santos, B. First report of root and crown rot caused by Pestalotiopsis clavispora (Neopestalotiopsis clavispora) on strawberry in Spain. Plant Dis. 2016, 100, 1495. [Google Scholar] [CrossRef]
- Obregón, V.G.; Meneguzzi, N.G.; Ibañez, J.M.; Lattar, T.E.; Kirschbaum, D.S. First report of Neopestalotiopsis clavispora causing root and crown rot on strawberry plants in Argentina. Plant Dis. 2018, 102, 1856. [Google Scholar] [CrossRef]
- Park, K.; Han, I.; Lee, S.M.; Choi, S.L.; Kim, M.C.; Lee, H. Crown and root rot of strawberry caused by Neopestalotiopsis clavispora in Korea. Korean J. Mycol. 2019, 47, 427–435. [Google Scholar]
- Sun, Q.; Harishchandra, D.; Jia, J.; Zuo, Q.; Zhang, G.; Wang, Q.; Yan, J.; Zhang, W.; Li, X. Role of Neopestalotiopsis rosae in causing root rot of strawberry in Beijing, China. Crop Prot. 2021, 147, 105710. [Google Scholar] [CrossRef]
- Duzan, H.M.; Abied, M.A. First report of Macrophomina phaseolina causing crown and root rot of strawberry in Tripoli, Libya. J. Appl. Plant Prot. 2020, 9, 83–84. [Google Scholar] [CrossRef]
- Qamar, M.I.; Ghanzafar, M.U.; Abbas, M.F.; Zainab, R.; Andleeb, S.; Shafiq, M.; Mushtaq, S.J. First report of Macrophomina phaseolina causing crown and root rot on strawberry in Sargodha, Pakistan. Plant Dis. 2019, 103, 1420. [Google Scholar] [CrossRef]
- Zhang, G.; Peng, W.J.; Xu, Z.Q.; Xu, H.; Feng, X.H. First report of Phytopythium helicoides causing crown and root rot on strawberry in China. ACTA Phytopathol. Sin. 2023, 53, 990–993. [Google Scholar]
- Habibi, A.; Ghaderi, F. First record of Dactylonectria macrodidyma causing black root rot on strawberry. Mycol. Iran. 2020, 7, 241–246. [Google Scholar]
- Weber, R.W.; Entrop, A.-P. Dactylonectria torresensis as the main component of the black root rot complex of strawberries and raspberries in Northern Germany. Erwerbs-Obstbau 2017, 59, 157–169. [Google Scholar] [CrossRef]
- Wilhelm, S.; Nelson, P.E.; Thomas, H.E.; Johnson, H. Pathology of strawberry root rot caused by Ceratobasidium species. Phytopathology 1972, 62, 700–705. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, S.L.; Zhang, X.G.; Ma, X.Y.; Zhong, S.; Zhang, G.Z. Dactylonectria species associated with black root rot of strawberry in China. Australas. Plant Pathol. 2021, 50, 501–511. [Google Scholar] [CrossRef]
- Vigliecca, M.; González, P.; Machín, A.; Vicente, E.; Silvera-Pérez, E. First report of root and crown rot caused by Dactylonectria novozelandica on strawberry in Uruguay. Agrociencia Urug. 2022, 26, e962. [Google Scholar] [CrossRef]
- Amatayakul, T. The synthesis of fibrinolysin by fungi. Ohio J. Sci. 1955, 55, 343–353. [Google Scholar]
- Rojas, N.L.; Voget, C.E.; Hours, R.A.; Cavalitto, S.F. Purification and characterization of a novel alkaline α-L-rhamnosidase produced by Acrostalagmus luteo albus. J. Ind. Microbiol. Biotechnol. 2011, 38, 1515–1522. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.Q.; Wang, Z.M.; Zheng, L.; Yu, Y.Y.; Dai, J.J.; Shi, D.Y. Bioactivity-guided screening of antimicrobial secondary metabolites from Antarctic cultivable fungus Acrostalagmus luteoalbus CH-6 combined with molecular networking. Mar. Drugs 2022, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Chudinova, E.M.; Vedmedenko, D.V.; Platonov, V.A.; Elansky, A.S.; Belosokhov, A.F.; Elansky, S.N. First report of potato tuber disease caused by Acrostalagmus luteoalbus. J. Plant Pathol. 2022, 104, 1203. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Tang, C.Y. First Report of Acrostalagmus luteo-albus Causing Red Rust of Needle Mushroom (Flammulina velutipes) in China. Plant Dis. 2015, 99, 158. [Google Scholar] [CrossRef] [PubMed]
- Acosta-González, U.; Leyva-Mir, S.G.; Silva-Rojas, H.V.; Rebollar-Alviter, A. Preventive and curative effects of treatments to manage strawberry root and crown rot caused by Neopestalotiopsis rosae. Plant Dis. 2024, 108, 1278–1288. [Google Scholar] [CrossRef]
- da Costa, C.A.; Reis, A.; Mizubuti, E.S.; Lourenco, V., Jr. Sensitivity of Septoria lycopersici Speg. isolates to fungicides in Brazil. Crop Prot. 2025, 190, 107059. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phillips, A.J.; Jayawardena, R.S.; Promputtha, I.; Hyde, K.D. Importance of molecular data to identify fungal plant pathogens and guidelines for pathogenicity testing based on Koch’s Postulates. Pathogens 2021, 10, 1096. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hee Lee, S.; Jeong Jeon, S.; Burm Lee, H. First records of rare ascomycete fungi, Acrostalagmus luteoalbus, Bartalinia robillardoides, and Collariella carteri from freshwater samples in Korea. Mycobiology 2019, 47, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; He, C.; Zhang, P.; Zhao, L.; Liu, W.; Jin, N.; Guo, Y. Isolation, identification, and in vitro fungicide screening of the pathogen associated with pear dry blight. Pathogens 2025, 14, 432. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W.; Schroers, H.-J. The type species of Verticillium is not congeneric with the plant-pathogenic species placed in Verticillium and it is not the anamorph of ‘Nectria’ inventa. Mycol. Res. 2004, 108, 576–582. [Google Scholar] [CrossRef]
- Grum-Grzhimaylo, A.A.; Georgieva, M.L.; Bondarenko, S.A.; Debets, A.J.; Bilanenko, E.N. On the diversity of fungi from soda soils. Fungal Divers. 2016, 76, 27–74. [Google Scholar] [CrossRef]
- Bondarenko, S.; Georgieva, M.; Bilanenko, E. Alkalitolerant micromycetes in acidic and neutral soils of the temperate zone. Microbiology 2016, 85, 737–744. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Sinitsyna, N.A.; Georgieva, M.L.; Bilanenko, E.N.; Tereshina, B.M. Dynamics of the cytosol soluble carbohydrates and membrane lipids in response to ambient pH in alkaliphilic and alkalitolerant fungi. Microbiology 2018, 87, 21–32. [Google Scholar] [CrossRef]
- Rojas, N.L.; Cavalitto, S.F.; Cabello, M.; Hours, R.A.; Voget, C.E. Alkaline polysaccharidases produced in solid state cultures by alkalophilic fungi isolated from Argentina. J. Pure. Appl. Microbiol. 2008, 2, 1–10. [Google Scholar]
- Kavitha, P.G.; Sudha, A.; Devi, P.A.; Kumaran, K. A comparative study on Forest soil microbial diversity and biomass in Nilgiri biosphere of southern India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3701–3715. [Google Scholar] [CrossRef]
- Monoson, H.L.; Conway, T.D.; Nelson, R.E. Four endoparasitic nematode destroying fungi isolated from Sand Ridge State Forest soil. Mycopathologia 1975, 57, 59–62. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Thomas, T. Diversity, host-specificity and stability of sponge-associated fungal communities of co-occurring sponges. PeerJ 2018, 6, e4965. [Google Scholar] [CrossRef]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive overview on the chemistry and biological activities of selected alkaloid producing marine-derived fungi as a valuable reservoir of drug entities. Biomedicines 2021, 9, 485. [Google Scholar] [CrossRef]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Zhang, W.M.; Tian, X.P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorganic Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus Acrostalagmus luteoalbus HDN13-530. RSC Advances 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Z.; Wang, Y.; Feng, J.; He, J.; Wu, H.; Ma, Z. Indoor screening of biofungicides for strawberry root rot and verification of field control efficacy. Chin. J. Pestic. Sci. 2022, 24, 1456–1465. [Google Scholar]
- Iqbal, M.; Jamshaid, M.; Zahid, M.A.; Andreasson, E.; Vetukuri, R.R.; Stenberg, J.A. Biological control of strawberry crown rot, root rot and grey mould by the beneficial fungus Aureobasidium pullulans. BioControl 2021, 66, 535–545. [Google Scholar] [CrossRef]

| Fungicides Tested | Concentration Tested (mg/L) | Regression Equation | Correlation Coefficient (R2) | EC50 Value (mg/L) |
|---|---|---|---|---|
| prochloraz EC | 0.01, 0.1, 0.5, 1, 10 | y = 0.1983x + 0.8814 | 0.9994 | 0.0119 |
| tebuconazole SC | 0.5, 1, 5, 25, 50 | y = 0.3622x + 0.2796 | 0.9797 | 4.0617 |
| thiophanate-methyl WP | 0.5, 1, 5, 25, 50 | y = 0.5752x + 0.1218 | 0.9666 | 4.5446 |
| difenoconazole·azoxystrobin WP | 0.5, 1, 5, 25, 50 | y = 0.3092x + 0.2773 | 0.9294 | 5.2517 |
| pyraclostrobin SC | 0.5, 1, 5, 25, 50 | y = 0.235x + 0.1321 | 0.9983 | 36.7901 |
| oxadixyl·mancozeb WP | 0.5, 1, 5, 25, 50 | y = 0.2448x + 0.0786 | 0.9047 | 52.6635 |
| propiconazole·difenoconazole EC | 0.5, 1, 5, 25, 50 | y = 0.2211x + 0.0477 | 0.9757 | 111.1162 |
| triadimefon WP | 0.5, 1, 5, 25, 50 | y = 0.1667x + 0.1189 | 0.8238 | 193.1738 |
| metalaxyl·hymexazol AS | 0.5, 1, 5, 25, 50 | y = 0.0747x + 0.0928 | 0.9705 | 2.8064 × 105 |
| matrine EC | 0.5, 1, 5, 25, 100 | y = 0.0302x + 0.0845 | 0.9600 | 5.8272 × 1013 |
| matrine·osthol AS | 0.5, 1, 5, 25, 100 | y = 0.0227x + 0.0111 | 0.9221 | 3.1719 × 1021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Fu, C.; Zhang, H.; Li, Z.; Sun, P. Acrostalagmus luteoalbus as the Novel Causing Agent of Root Rot on Strawberry and In Vitro Screening of Effective Fungicides. Horticulturae 2025, 11, 940. https://doi.org/10.3390/horticulturae11080940
Zhang L, Fu C, Zhang H, Li Z, Sun P. Acrostalagmus luteoalbus as the Novel Causing Agent of Root Rot on Strawberry and In Vitro Screening of Effective Fungicides. Horticulturae. 2025; 11(8):940. https://doi.org/10.3390/horticulturae11080940
Chicago/Turabian StyleZhang, Lei, Chongyi Fu, Hongling Zhang, Zhengnan Li, and Pingping Sun. 2025. "Acrostalagmus luteoalbus as the Novel Causing Agent of Root Rot on Strawberry and In Vitro Screening of Effective Fungicides" Horticulturae 11, no. 8: 940. https://doi.org/10.3390/horticulturae11080940
APA StyleZhang, L., Fu, C., Zhang, H., Li, Z., & Sun, P. (2025). Acrostalagmus luteoalbus as the Novel Causing Agent of Root Rot on Strawberry and In Vitro Screening of Effective Fungicides. Horticulturae, 11(8), 940. https://doi.org/10.3390/horticulturae11080940





