Expression of the Protein Phosphatase Gene SlPP2C28 Confers Enhanced Tolerance to Bacterial Wilt in Tobacco
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation of Pathogenic Inoculum
2.2. Plant Materials and Inoculation Methods
2.3. Preliminary Analysis of the Response of SlPP2C28 to R. solanacearum in Tomato
2.4. Virus-Induced Gene Silencing in Tomato
2.5. Construction of Plant Overexpression Vector
2.6. Acquisition of Tobacco Transgenic Lines
2.7. Expression Analysis of SlPP2C28, Defense Enzyme-Related Genes, and Pathogenesis-Related Genes Following Inoculation with R. solanacearum
2.8. Determination of Defense-Related Enzyme Activity in N. benthamiana Leaves
2.9. Determination of the Tobacco Plant Disease Index
2.10. Detection of the Number of R. solanacearum Infections in N. benthamiana Stems
2.11. Tissue Staining Analysis of Leaves
2.12. Anatomical Observation of the N. benthamiana Stems
2.13. Statistical Analysis
3. Results
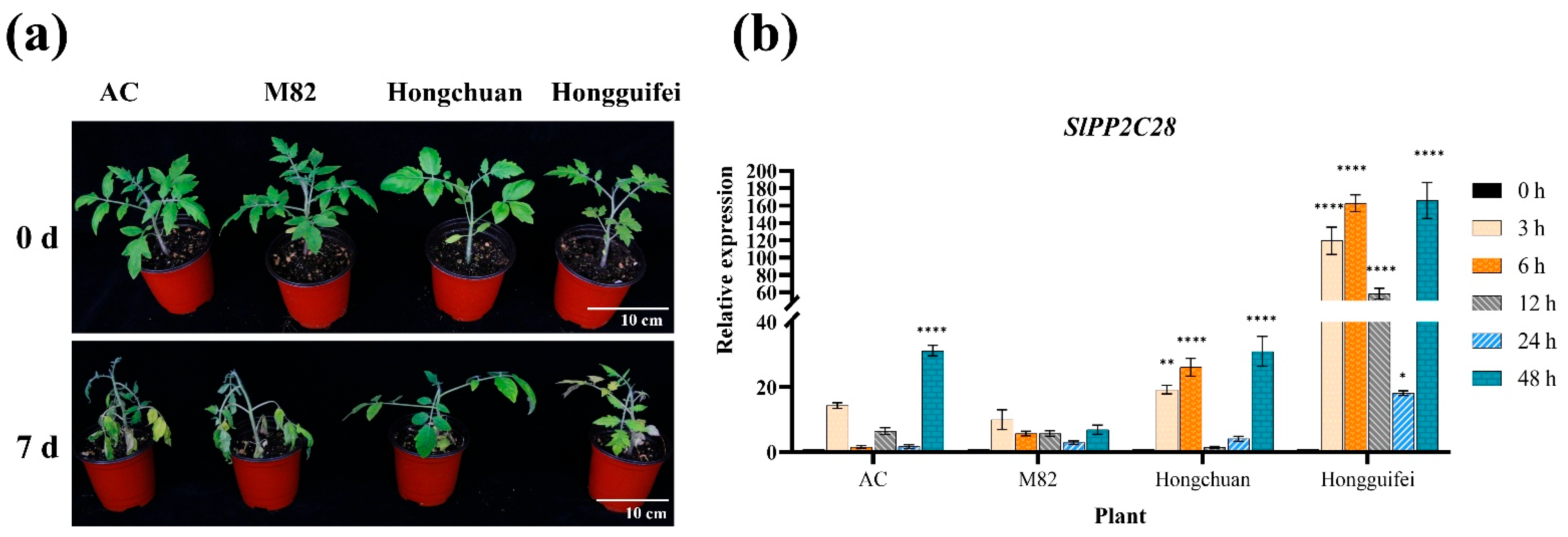
3.1. SlPP2C28 Was Highly Expressed in R. solanacearum-Resistant Tomato Plants
3.2. SlPP2C28-Silencing Reduced the Resistance of Tomato to Bacterial Wilt
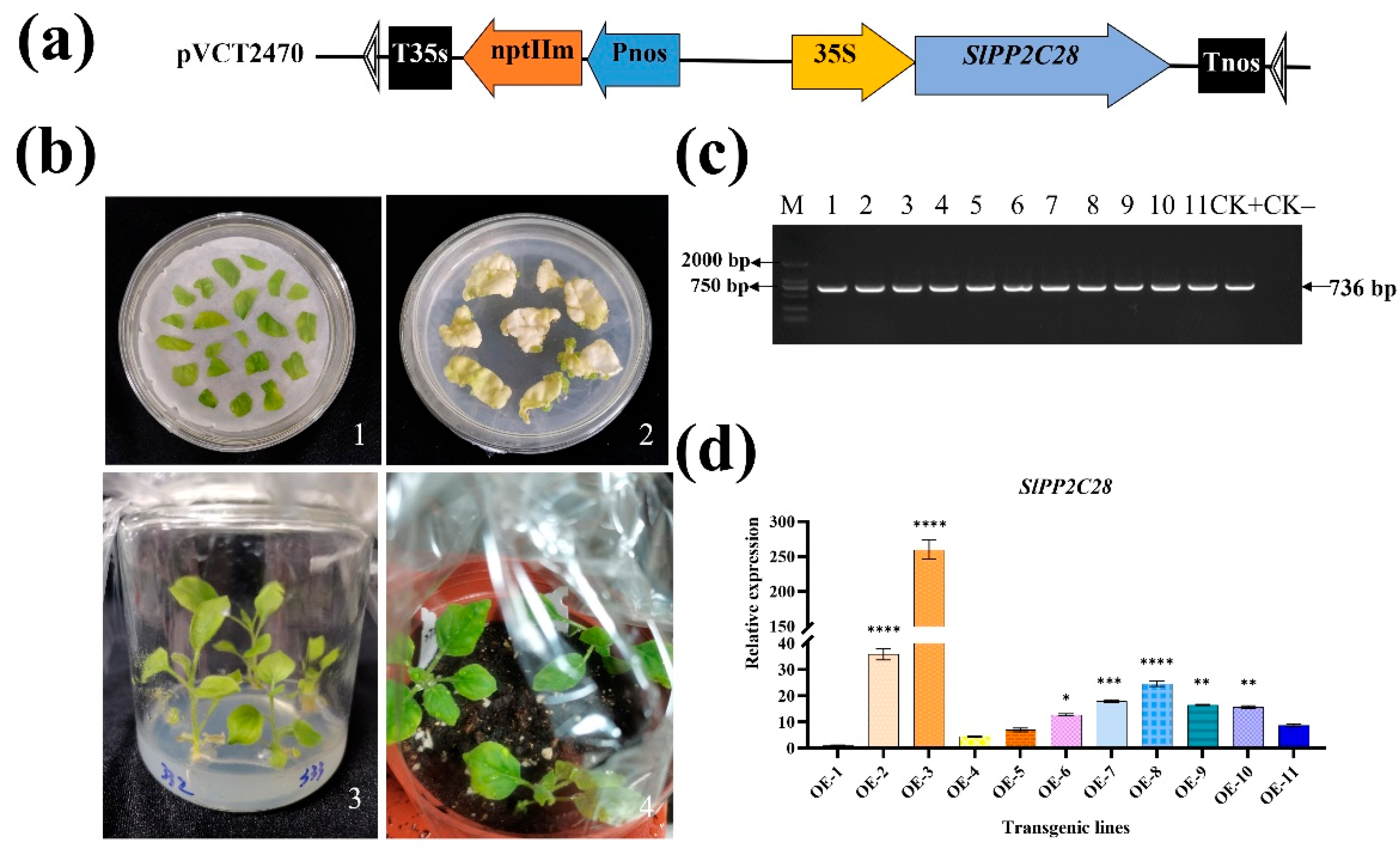
3.3. SlPP2C28-Overexpression Tobacco Lines Were Obtained
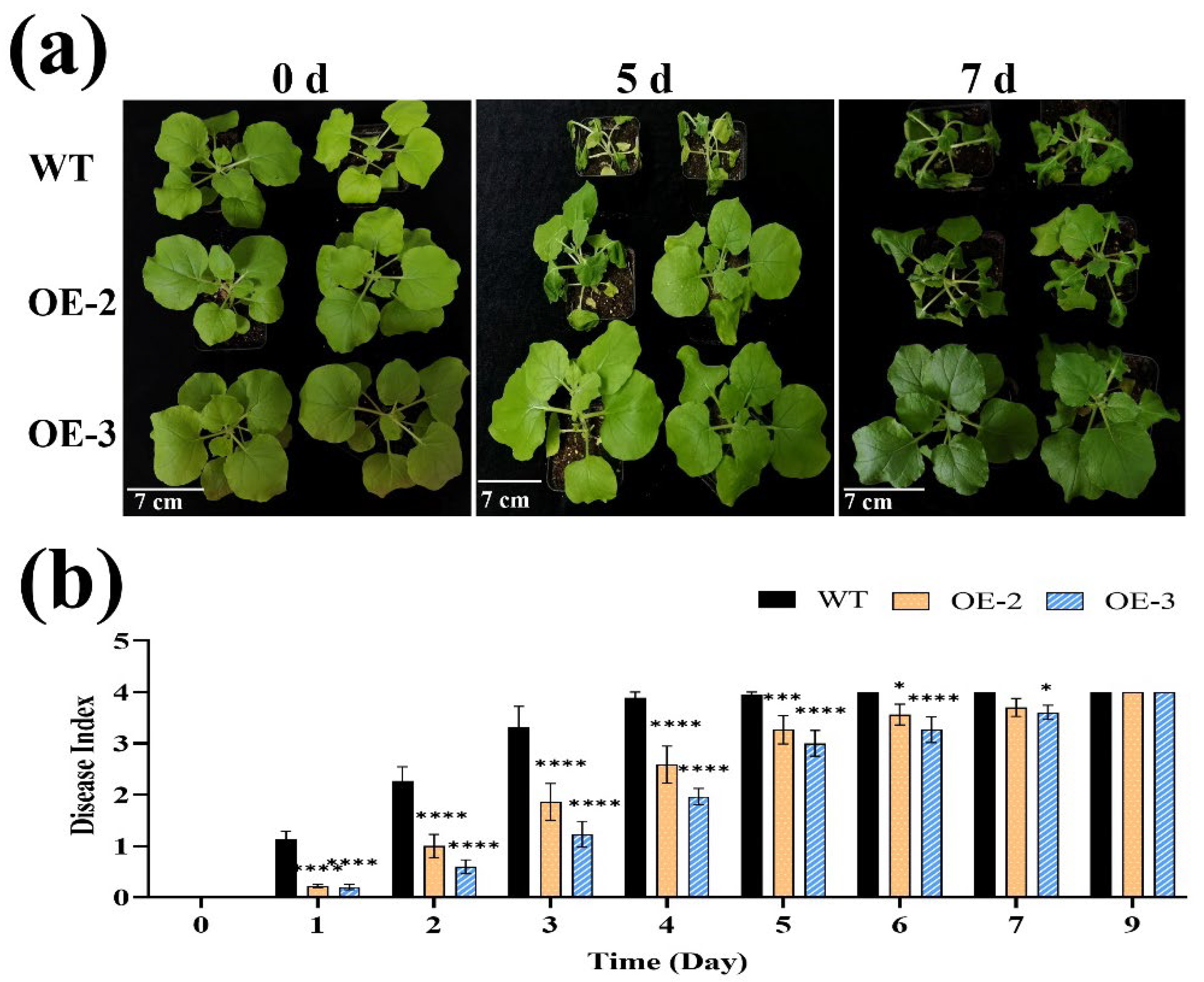
3.4. SlPP2C28-Overexpressing Improved Transgenic Tobacco Resistance to R. solanacearum Inoculation
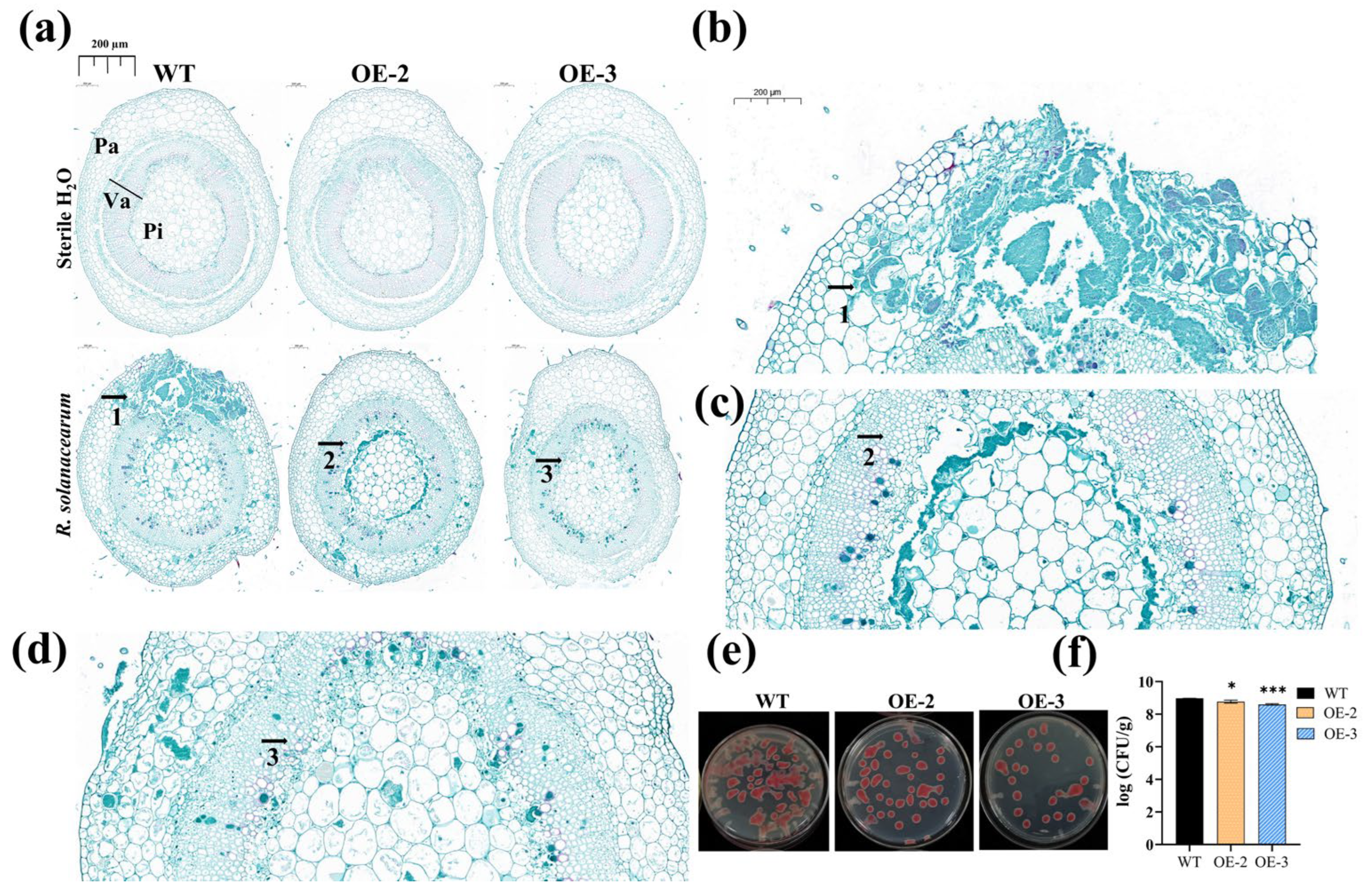
3.5. Overexpression of SlPP2C28 Enhanced Transgenic Stem Tolerance to R. solanacearum Infection
3.6. Overexpression of SlPP2C28 Enhanced the Disease Tolerance of Transgenic Leaves by ROS Scavenging
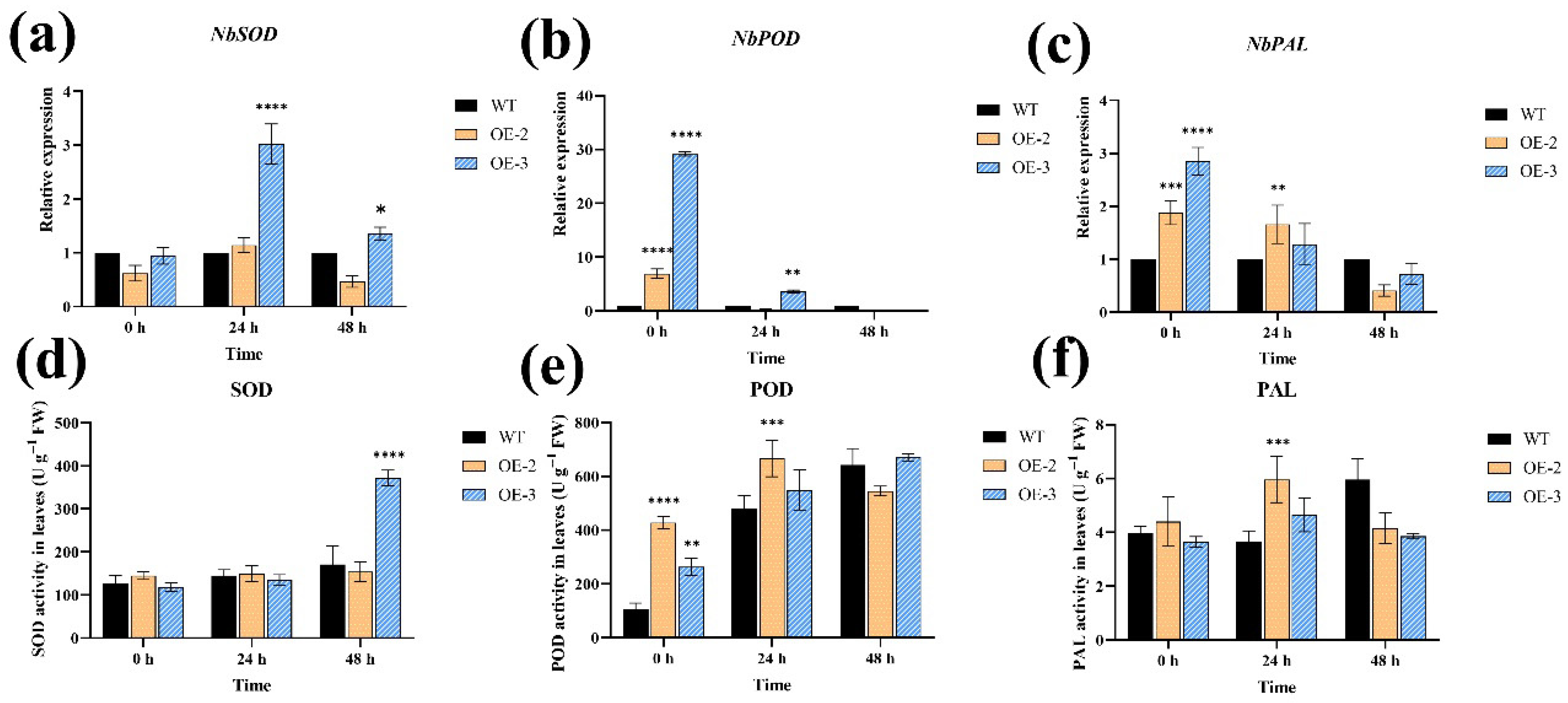
3.7. Overexpression of SlPP2C28 Increased the Expression of Defense Enzyme-Related Genes and Enhanced the Activity of Defense-Related Enzymes in Transgenic Tobacco
3.8. Overexpression of SlPP2C28 Increased Expression of Pathogenesis-Related Genes in Transgenic Tobacco
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salanoubat, M.; Genin, S.; Artiguenave, F.; Gouzy, J.; Mangenot, S.; Arlat, M.; Billault, A.; Brottier, P.; Camus, J.C.; Cattolico, L.; et al. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 2002, 415, 497–502. [Google Scholar] [CrossRef]
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Lowe-Power, T.M.; Khokhani, D.; Allen, C. How Ralstonia solanacearum exploits and thrives in the flowing plant xylem environment. Trends Microbiol. 2018, 26, 929–942. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial production of reactive oxygen species. Biochemistry 2013, 78, 1490–1511. [Google Scholar] [CrossRef]
- Galluzzi, L.; Morselli, E.; Kepp, O.; Vitale, I.; Rigoni, A.; Vacchelli, E.; Michaud, M.; Zischka, H.; Castedo, M.; Kroemer, G. Mitochondrial gateways to cancer. Mol. Asp. Med. 2010, 31, 1–20. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- De Gara, L.; de Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol. Biochem. 2003, 41, 863–870. [Google Scholar] [CrossRef]
- Allen, R.D. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995, 107, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Sandauo, L.M.; Del Río, L.A. Manganese superoxide dismutase and higher plant chloroplasts: A reappraisal of a controverted cellular localization. J. Plant Physiol. 1986, 125, 427–439. [Google Scholar] [CrossRef]
- Hernaīndez, J.; Ferrer, M.; Jimeīnez, A.; Ros-Barceloī, A.; Sevilla, F. Antioxidant systems and O2·−/H2O2 production in the apoplast of Pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef]
- Tognolli, M.; Penel, C.; Greppin, H.; Simon, P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 2002, 288, 129–138. [Google Scholar] [CrossRef]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhao, Y.; Han, G.; Zhu, S. Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 2015, 566, 95–108. [Google Scholar] [CrossRef]
- Mariutto, M.; Duby, F.; Adam, A.; Bureau, C.; Fauconnier, M.-L.; Ongena, M.; Thonart, P.; Dommes, J. The elicitation of a systemic resistance by Pseudomonas putida BTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol. 2011, 11, 29. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef] [PubMed]
- Fritig, B.; Heitz, T.; Legrand, M. Antimicrobial proteins in induced plant defense. Curr. Opin. Immunol. 1998, 10, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. J. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- van Loon, L.C.; Pierpoint, W.S.; Boller, T.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Kerk, D.; Templeton, G.; Moorhead, G.B. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef]
- Singh, A.; Giri, J.; Kapoor, S.; Tyagi, A.K.; Pandey, G.K. Protein phosphatase complement in rice: Genome-wide identification and transcriptional analysis under abiotic stress conditions and reproductive development. BMC Genomics 2010, 11, 435. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, A.; Srivastava, A.K.; Tran, L.-S.P.; Pandey, G.K. Plant protein phosphatases 2C: From genomic diversity to functional multiplicity and importance in stress management. Crit. Rev. Biotechnol. 2016, 36, 1023–1035. [Google Scholar] [CrossRef]
- Hu, X.; Song, F.; Zheng, Z. Molecular characterization and expression analysis of a rice protein phosphatase 2C gene, OsBIPP2C1, and overexpression in transgenic tobacco conferred enhanced disease resistance and abiotic tolerance. Physiol. Plant. 2006, 127, 225–236. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Li, G.; Yang, Y.; Zheng, Z.; Song, F. Ectopic expression of a rice protein phosphatase 2C gene OsBIPP2C2 in tobacco improves disease resistance. Plant Cell Rep. 2009, 28, 985–995. [Google Scholar] [CrossRef]
- Akimoto-Tomiyama, C.; Tanabe, S.; Kajiwara, H.; Minami, E.; Ochiai, H. Loss of chloroplast-localized protein phosphatase 2Cs in Arabidopsis thaliana leads to enhancement of plant immunity and resistance to Xanthomonas campestris pv. campestris infection. Mol. Plant Pathol. 2018, 19, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Sobol, G.; Chakraborty, J.; Martin, G.B.; Sessa, G. The emerging role of PP2C phosphatases in tomato immunity. Mol. Plant-Microbe Interact. 2022, 35, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Shui, D.; Su, S.; Xiong, Z.; Zai, W. Gene enrichment and co-expression analysis shed light on transcriptional responses to Ralstonia solanacearum in tomato. BMC Genomics 2023, 24, 159. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Ni, L.; Xia, X.; Chen, S.; Zhang, Y.; Lang, M.; Li, M.; Liu, B.; Pan, Y.; Li, J.; et al. Genome-wide analysis of the protein phosphatase 2C genes in Tomato. Genes 2022, 13, 604. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, L.; Chen, S.; Qin, Y.; Ding, X.; Li, J.; Pan, Y.; Zhang, X. Pterostilbene production of tomato transformed with resveratrol synthase and resveratrol O-methyltransferase genes. Plant Sci. 2022, 322, 111343. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Liu, Y.; Zhang, L.; Ding, W. Overexpression of NtPR-Q up-regulates multiple defense-related genes in Nicotiana tabacum and enhances plant resistance to Ralstonia solanacearum. Front. Plant Sci. 2017, 8, 1963. [Google Scholar] [CrossRef]
- Chen, N.; Shao, Q.; Lu, Q.; Li, X.; Gao, Y. Transcriptome analysis reveals differential transcription in tomato (Solanum lycopersicum) following inoculation with Ralstonia solanacearum. Sci. Rep. 2022, 12, 22137. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Tang, Y.; Chen, J.; Ding, W. Overexpression of NtWRKY50 Increases Resistance to Ralstonia solanacearum and Alters Salicylic Acid and Jasmonic Acid Production in Tobacco. Front. Plant Sci. 2017, 8, 1710. [Google Scholar] [CrossRef]
- Miller, A.-F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef]
- Rosli, H.G.; Zheng, Y.; Pombo, M.A.; Zhong, S.; Bombarely, A.; Fei, Z.; Collmer, A.; Martin, G.B. Transcriptomics-based screen for genes induced by flagellin and repressed by pathogen effectors identifies a cell wall-associated kinase involved in plant immunity. Genome Biol. 2013, 14, R139. [Google Scholar] [CrossRef]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1386. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, M.; Liu, B.; Qin, Y.; Li, J.; Pan, Y.; Zhang, X. Effects of Phytochelatin-like Gene on the Resistance and Enrichment of Cd2+ in Tobacco. Int. J. Mol. Sci. 2022, 23, 16167. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Wu, K.; Ding, Y.; Tang, M.; Zhang, X.; Pan, Y.; Wu, L.; Su, C.; Hong, Z.; et al. The DnaJ1 heat shock protein interacts with the flavanone 3-hydroxylase-like protein F3HL to synergistically enhance drought tolerance by scavenging reactive oxygen species in tomato. Plant J. 2025, 121, e70097. [Google Scholar] [CrossRef] [PubMed]
- Montalbini, P.; Buonaurio, R. Effect of tobacco mosaic virus infection of levels of soluble superoxide dismutase (SOD) in nicotiana tabacum and nicotiana glutinosa leaves. Plant Sci. 1986, 47, 135–143. [Google Scholar] [CrossRef]
- Buonaurio, R.; Della Torre, G.; Montalbini, P. Soluble superoxide dismutase (SOD) in susceptible and resistant host-parasite complexes of Phaseolus vulgaris and Uromyces phaseoli. Physiol. Mol. Plant Pathol. 1987, 31, 173–184. [Google Scholar] [CrossRef]
- Lu, W.; Duanmu, H.; Qiao, Y.; Jin, X.; Yu, Y.; Yu, L.; Chen, C. Genome-wide identification and characterization of the soybean SOD family during alkaline stress. PeerJ 2020, 8, e8457. [Google Scholar] [CrossRef]
- Welinder, K.G. Superfamily of plant, fungal and bacterial peroxidases. Curr. Opin. Struct. Biol. 1992, 2, 388–393. [Google Scholar] [CrossRef]
- Hiraga, S.; Yamamoto, K.; Ito, H.; Sasaki, K.; Matsui, H.; Honma, M.; Nagamura, Y.; Sasaki, T.; Ohashi, Y. Diverse expression profiles of 21 rice peroxidase genes. FEBS Lett. 2000, 471, 245–250. [Google Scholar] [CrossRef]
- González, A.M.; Marcel, T.C.; Kohutova, Z.; Stam, P.; van der Linden, C.G.; Niks, R.E.; Grebe, M. Peroxidase profiling reveals genetic linkage between peroxidase gene clusters and basal host and non-host resistance to rusts and mildew in barley. PLoS ONE 2010, 5, e10495. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hewezi, T.; Baum, T.J. Arabidopsis peroxidase AtPRX53 influences cell elongation and susceptibility to Heterodera schachtii. Plant Signal. Behav. 2011, 6, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Wei-Feng, X.; Wei-Ming, S.; Ueda, A.; Takabe, T. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana carrying a peroxisomal ascorbate peroxidase gene from barley. Pedosphere 2008, 18, 486–495. [Google Scholar] [CrossRef]
- Coego, A.; Ramirez, V.; Ellul, P.; Mayda, E.; Vera, P. The H2O2-regulated Ep5C gene encodes a peroxidase required for bacterial speck susceptibility in tomato. Plant J. 2005, 42, 283–293. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, Y.J.; Lee, S.C.; Hong, J.K.; Hwang, B.K. Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol. 2007, 145, 890–904. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Sun, M.; Sun, F.; Deng, S.; Dong, H. Riboflavin-induced priming for pathogen defense in Arabidopsis thaliana. J. Integr. Plant Biol. 2009, 51, 167–174. [Google Scholar] [CrossRef]
- Pant, S.; Huang, Y. Genome-wide studies of PAL genes in sorghum and their responses to aphid infestation. Sci. Rep. 2022, 12, 22537. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.A.; Zanetti, M.E.; Daleo, G.R. Calcium-dependent Protein Kinases are Involved in Potato Signal Transduction in Response to Elicitors from the Oomycete Phytophthora infestans. J. Phytopathol. 2008, 156, 53–61. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, B.K. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Schweighofer, A.; Hirt, H.; Meskiene, I. Plant PP2C phosphatases: Emerging functions in stress signaling. Trends Plant Sci. 2004, 9, 236–243. [Google Scholar] [CrossRef]
- Widjaja, I.; Lassowskat, I.; Bethke, G.; Eschen-Lippold, L.; Long, H.-H.; Naumann, K.; Dangl, J.L.; Scheel, D.; Lee, J. A protein phosphatase 2C, responsive to the bacterial effector AvrRpm1 but not to the AvrB effector, regulates defense responses in Arabidopsis. Plant J. 2010, 61, 249–258. [Google Scholar] [CrossRef]
- van Loon, L.; Rep, M.; Pieterse, C. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Alexander, D.; Goodman, R.M.; Gut-Rella, M.; Glascock, C.; Weymann, K.; Friedrich, L.; Maddox, D.; Ahl-Goy, P.; Luntz, T.; Ward, E. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc. Natl. Acad. Sci. USA 1993, 90, 7327–7331. [Google Scholar] [CrossRef]
- Hugot, K.; Aimé, S.; Conrod, S.; Poupet, A.; Galiana, E. Developmental regulated mechanisms affect the ability of a fungal pathogen to infect and colonize tobacco leaves. Plant J. 1999, 20, 163–170. [Google Scholar] [CrossRef]
- Liu, F.; Wei, F.; Wang, L.; Liu, H.; Zhu, X.; Liang, Y. Riboflavin activates defense responses in tobacco and induces resistance against Phytophthora parasitica and Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2010, 74, 330–336. [Google Scholar] [CrossRef]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Fritig, B.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans). Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef]
- Suhaimi, A.H.; Rajendram, A.; Khaidizar, F.D.; Mir, P.; Pulido-Lucas, E.; Quirce, S.; Pedrosa, M.; Rodriguez-Perez, R.; Al-Idrus, A. Occurrences of allergenicity to banana pathogenesis-related-10 (PR10) protein variants. Food Funct. 2024, 15, 11715–11725. [Google Scholar] [CrossRef]
- Chen, Z.; Brown, R.L.; Damann, K.E.; Cleveland, T.E. PR10 expression in maize and its effect on host resistance against Aspergillus flavus infection and aflatoxin production. Mol. Plant Pathol. 2010, 11, 69–81. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Qin, Y.; Wang, M.; Qiu, J.; Tang, D.; Chen, L.; Wu, L.; Li, J.; Pan, Y.; Zhang, X. Expression of the Protein Phosphatase Gene SlPP2C28 Confers Enhanced Tolerance to Bacterial Wilt in Tobacco. Horticulturae 2025, 11, 937. https://doi.org/10.3390/horticulturae11080937
Ni L, Qin Y, Wang M, Qiu J, Tang D, Chen L, Wu L, Li J, Pan Y, Zhang X. Expression of the Protein Phosphatase Gene SlPP2C28 Confers Enhanced Tolerance to Bacterial Wilt in Tobacco. Horticulturae. 2025; 11(8):937. https://doi.org/10.3390/horticulturae11080937
Chicago/Turabian StyleNi, Lei, Yafei Qin, Mei Wang, Jianfang Qiu, Daodao Tang, Liantian Chen, Lang Wu, Jinhua Li, Yu Pan, and Xingguo Zhang. 2025. "Expression of the Protein Phosphatase Gene SlPP2C28 Confers Enhanced Tolerance to Bacterial Wilt in Tobacco" Horticulturae 11, no. 8: 937. https://doi.org/10.3390/horticulturae11080937
APA StyleNi, L., Qin, Y., Wang, M., Qiu, J., Tang, D., Chen, L., Wu, L., Li, J., Pan, Y., & Zhang, X. (2025). Expression of the Protein Phosphatase Gene SlPP2C28 Confers Enhanced Tolerance to Bacterial Wilt in Tobacco. Horticulturae, 11(8), 937. https://doi.org/10.3390/horticulturae11080937






