Effects of 1-Methylcyclopropene Fumigant on Texture and Nutritional Quality of ‘Yanshu 25’ Sweet Potato During Shelf-Life and Long-Term Storage at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Design
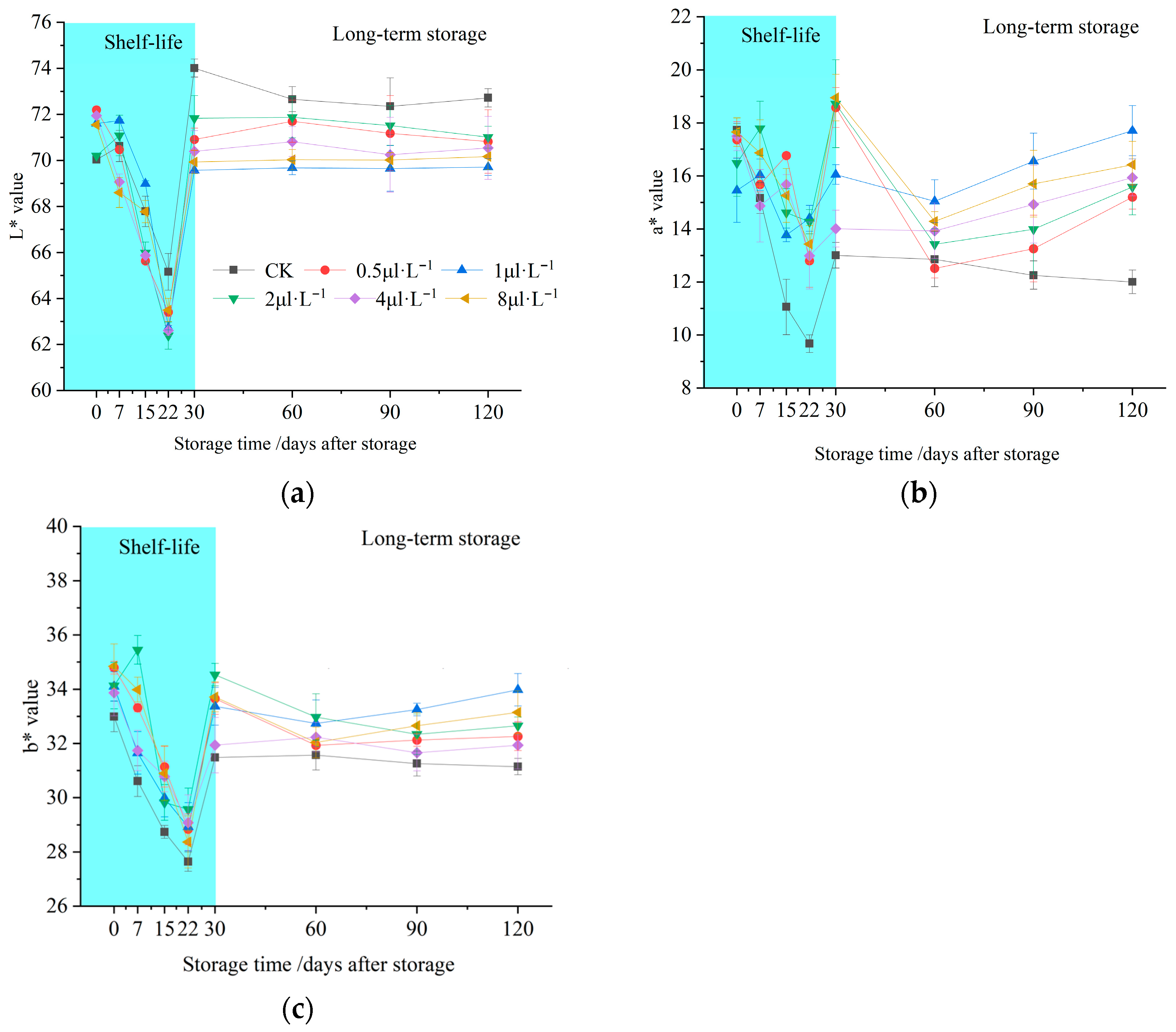
2.2. Flesh Colour
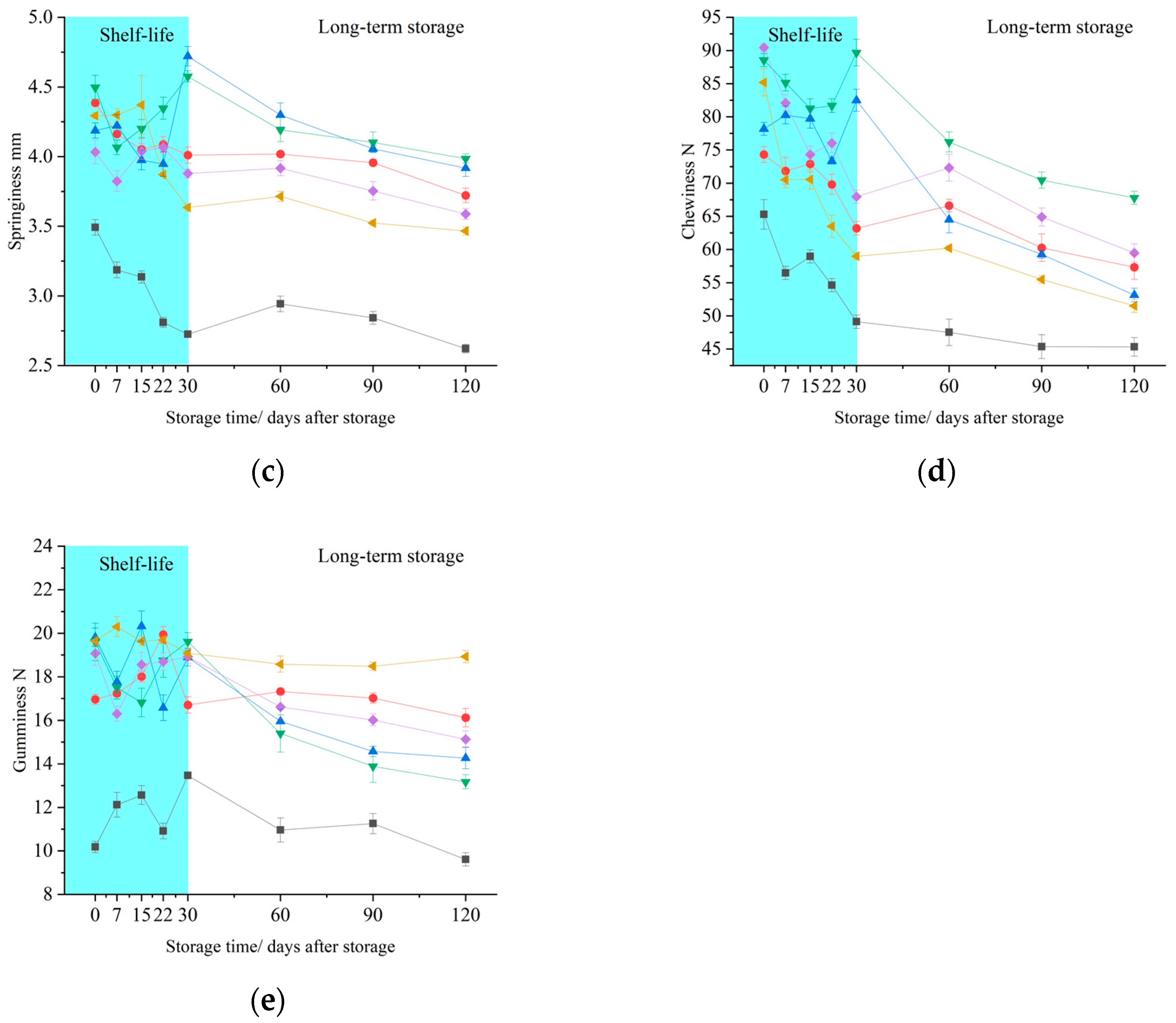
2.3. Texture Properties
2.4. Amylose Content
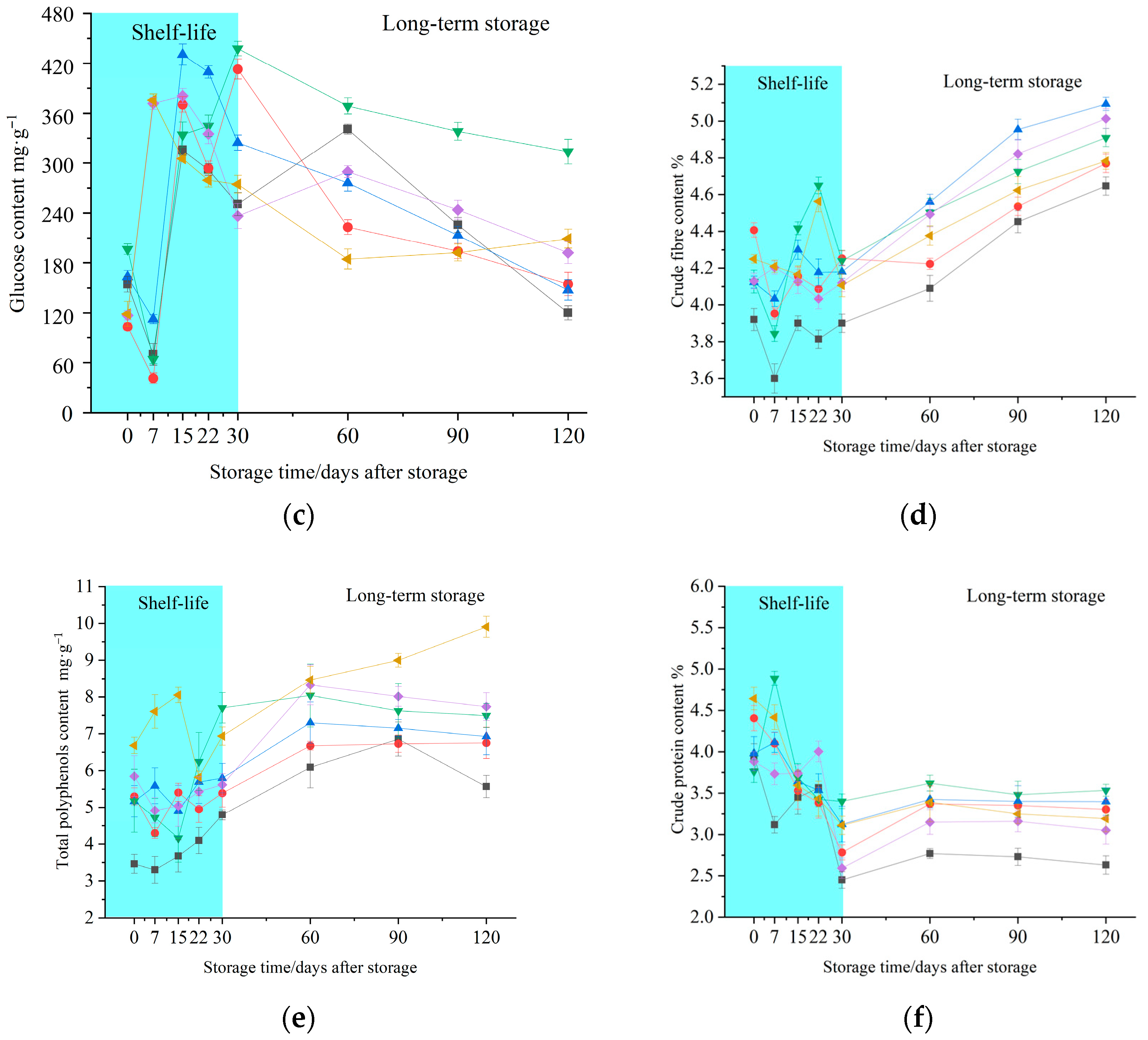
2.5. Glucose Content
2.6. Dry Matter Content
2.7. Total Polyphenols Content
2.8. Crude Protein Content
2.9. Crude Fibre Content
2.10. Statistical Analysis
3. Results
3.1. Effects of 1-MCP on Flesh Colour
3.2. Effects of 1-MCP on Flesh Texture Properties
3.3. Effects of 1-MCP on Chemical and Nutritional Properties
3.4. PCA
0.056 × X9 + 0.319 × X10 + 0.287 × X11 + 0.397 × X12 + 0.366 × X13 + 0.358 × X14
0.415 × X9 − 0.331 × X10 + 0.229 × X11 − 0.046 × X12 − 0.050 × X13 − 0.240 × X14
0.293 × X9 + 0.081 × X10 + 0.184 × X11 + 0.117 × X12 + 0.235 × X13 − 0.067 × X14
0.329 × X7 + 0.164 × X8 + 0.392 × X9 − 0.130 × X10 − 0.059 × X11 + 0.131 × X12 + 0.007 × X13 + 0.128 × X14
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS | Days after storage |
| 1-MCP | Methylcyclopropene |
References
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Pang, L.J.; Guo, K.R.; Lu, X.H.; Lu, G.Q.; Cheng, J.Y.; Wang, L.H.; Li, M.S.; Rui, Y.K. Comparison of Heavy Metals and Trace Elements Contents in Two Sweet Potato Varieties for Different Eating Ways. Fresenius Environ. Bull. 2020, 29, 1493–1494. [Google Scholar]
- Wu, J.; Zhang, J.; Ni, W.; Xu, X.; George, M.S.; Lu, G. Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage. Foods 2023, 12, 4352. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.D.; Costa, P.R.D.; Brito, F.; Assis, J.; Marcelino, A.; Santos, V.N.; Silva, L.J.V.; Silveira, F.P.D.; Junior, A.P.B.; Simoes, A.D. Quality of packaged refrigerated biofortified sweet potato cultivars. Rev. Bras. Eng. Agric. E Ambient. 2024, 28, e275946. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Mujumdar, A.S.; Luo, Z. Intelligent Quality Control of Starch-Rich Root and Tuber Products in the Cold Chain Logistics: Research Progress and Challenges. Food Rev. Int. 2025, 1–26. [Google Scholar] [CrossRef]
- Kou, J.; Zang, X.; Li, M.; Li, W.; Zhang, H.; Chen, Y.; Zhu, G. Effects of Ethylene and 1-Methylcyclopropene on the Quality of Sweet Potato Roots during Storage: A Review. Horticulturae 2023, 9, 667. [Google Scholar] [CrossRef]
- de Araújo, N.O.; Santos, M.N.d.S.; de Araujo, F.F.; Véras, M.L.M.; Tello, J.P.d.J.; Arruda, R.d.S.; Fugate, K.K.; Finger, F.L. Balance between oxidative stress and the antioxidant system is associated with the level of cold tolerance in sweet potato roots. Postharvest Biol. Technol. 2021, 172, 111359. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, W.; Yuan, J.; Zhang, J.; Zhu, L.; Xu, X.; Pan, S. Cyclic variable temperature conditioning-maintained storage quality of sweet potatoes by enhancing antioxidant activity. Food Biosci. 2024, 61, 104689. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.-D.; Laborda, P.; Wang, H.-L.; Wang, R.; Chen, X.; Liu, F.-Q.; Yang, D.-J.; Wang, S.-Y.; Shi, X.-C.; et al. Mode of action and efficacy of quinolinic acid for the control of Ceratocystis fimbriata on sweet potato. Pest. Manag. Sci. 2021, 77, 4564–4571. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Chromatographic Methods for Detection and Quantification of Carbendazim in Food. J. Agric. Food Chem. 2020, 68, 11880–11894. [Google Scholar] [CrossRef]
- Lima, P.C.C.; Santos, M.N.D.; Guimaraes, M.E.D.; de Araújo, N.O.; Krause, M.R.; Finger, F.L. Ethylene and its inhibitors affect the quality of processed sweet potatoes. Food Sci. Technol. 2021, 41, 825–832. [Google Scholar] [CrossRef]
- De Paepe, A.; Van Der Straeten, D. Ethylene Biosynthesis and Signaling: An Overview. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 72, pp. 399–430. [Google Scholar]
- Chen, S.-P.; Kuo, Y.-W.; Lin, J.-S. Review: Defense responses in sweetpotato (Ipomoea batatas L.) against biotic stress. Plant Sci. 2023, 337, 111893. [Google Scholar] [CrossRef]
- Pang, L.; Lu, G.; Cheng, J.; Lu, X.; Ma, D.; Li, Q.; Li, Z.; Zheng, J.; Zhang, C.; Pan, S. Physiological and biochemical characteristics of sweet potato (Ipomoea batatas (L.) Lam) roots treated by a high voltage alternating electric field during cold storage. Postharvest Biol. Technol. 2021, 180, 111619. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Wasternack, C.; Xie, D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 2014, 21, 112–119. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, N.O.; Véras, M.L.M.; Santos, M.N.d.S.; de Araújo, F.F.; Tello, J.P.d.J.; Finger, F.L. Sucrose degradation pathways in cold-induced sweetening and its impact on the non-enzymatic darkening in sweet potato root. Food Chem. 2020, 312, 125904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Wang, X.K.; Zhang, K.S.; Wang, H.; Xu, X.Z.; Zhang, Y.; Wang, C.H.; Yuan, Y.B.; Yang, S.L.; Cheng, C.X. Optimizing storage conditions for ‘Harlikar’ apples: The role of 1-methyl cyclopropene and harvest stage. Food Chem. 2025, 473, 143016. [Google Scholar] [CrossRef]
- Zhou, H.J.; Ye, Z.W.; Wang, L.F.; Zhang, S.Y.; Yuan, Z.Y.; Su, M.S.; Zhang, X.N.; Du, J.H.; Li, X.W.; Zhang, M.H.; et al. 1-MCP regulates taste development in cold-stored peach fruit through modulation of sugar, organic acid, and polyphenolic metabolism. Postharvest Biol. Technol. 2025, 225, 113518. [Google Scholar] [CrossRef]
- Tedeschi, P.; Marzocchi, S.; Marchetti, N.; Barba, F.J.; Maietti, A. Influence of Post-Harvest 1-Methylcyclopropene (1-MCP) Treatment and Refrigeration on Chemical Composition, Phenolic Profile and Antioxidant Modifications during Storage of Abate Fétel Pears. Antioxidants 2023, 12, 1955. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Álvarez, F.; Arena, M.; Auteri, D.; Batista Leite, S.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; et al. Peer review of the pesticide risk assessment of the active substance 1-methylcyclopropene. EFSA J. 2024, 22, e8977. [Google Scholar] [CrossRef]
- Lv, J.; Bai, L.; Han, X.; Xu, D.; Ding, S.; Li, C.; Ge, Y.; Li, J. Effects of 1-MCP treatment on sprouting and preservation of ginger rhizomes during storage at room temperature. Food Chem. 2021, 349, 129004. [Google Scholar] [CrossRef]
- Cao, J.X.; Liu, P.; Wang, X.J.; Wang, Q.G.; Shi, J.Y. Combination of wound healing with 1-methylcyclopropene and wound detection by iodine solution to maintain the quality of sweet potato during long-term storage. Int. J. Agric. Biol. Eng. 2021, 14, 241–246. [Google Scholar] [CrossRef]
- Cheema, M.U.A.; Rees, D.; Colgan, R.J.; Taylor, M.; Westby, A. The effects of ethylene, 1-MCP and AVG on sprouting in sweetpotato roots. Postharvest Biol. Technol. 2013, 85, 89–93. [Google Scholar] [CrossRef]
- Amoah, R.; Terry, L.A. Biochemical and Physiological Changes in Stored Sweet Potatoes as Mediated by 1-Methylcyclopropene (1-MCP). In Proceedings of the 7th International Postharvest Symposium, Kuala Lumpur, Malaysia, 25–29 June 2012; pp. 345–351. [Google Scholar]
- Nie, X.; Zhao, Y.; Li, Y.; Lv, H. 1-Methylcyclopropylene and heat treatment alleviate chilling injury in purple sweet potato by regulating ROS homeostasis. Sci. Hortic. 2024, 324, 112606. [Google Scholar] [CrossRef]
- Amoah, R.S.; Terry, L.A. 1-Methylcyclopropene (1-MCP) effects on natural disease resistance in stored sweet potato. J. Sci. Food Agric. 2018, 98, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, X.; Zhang, H.; Lv, Z.; Xia, J.; Cheng, P.; George, M.S.; Chen, Y.; Pang, L.; Lu, G. Effects of Foliar Application of Uniconazole on the Storage Quality of Tuberous Roots in Sweetpotato. Agronomy 2022, 12, 2983. [Google Scholar] [CrossRef]
- Dong, W.; Li, L.; Cao, R.; Xu, S.; Cheng, L.; Yu, M.; Lv, Z.; Lu, G. Changes in cell wall components and polysaccharide-degrading enzymes in relation to differences in texture during sweetpotato storage root growth. J. Plant Physiol. 2020, 254, 153282. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Chen, K.; Zhang, H.; Zhou, S.; Lv, Z.; Chen, Y.; Cui, P.; Cui, Z.; Lu, G. Comprehensive Evaluation of Raw Eating Quality in 81 Sweet Potato (Ipomoea batatas (L.) Lam) Varieties. Foods 2023, 12, 261. [Google Scholar] [CrossRef]
- Li, Y. Determination of Fructose, Glucose and Sucrose in Tea by High Performance Liquid Chromatography-Differential Refraction Detector. Guangdong Chem. Ind. 2016, 43, 187–188. [Google Scholar]
- Shi, J.; Fang, D.; Sui, Y.; Xiong, T.; Chen, X.; Fan, C.; Zhou, D.; Cai, F.; Mei, X. Polyphenol content, antioxidant capacity, and composition in different varieties of sweet potato (Ipomoea batatas L.) leaves during growth stages. Sci. Hortic. 2025, 342, 113925. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 984.13 Protein (Crude) in Animal Feed and Pet Food: Copper Catalyst Kjeldahl Method. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; AOAC Publications: New York, NY, USA, 2023; p. C4-31. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 978.10 Fiber (Crude) in Animal Feed and Pet Food. In Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; AOAC Publications: New York, NY, USA, 2023; p. C4-46. [Google Scholar] [CrossRef]
- Sakaigaichi, T.; Suematsu, K.; Kawata, Y.; Kobayashi, A.; Kamada, E.; Kai, Y. Comparison of root dry matter content and root dry matter weight in sweet potato genotypes cultivated by transplanting and direct planting. Plant Prod. Sci. 2023, 26, 440–447. [Google Scholar] [CrossRef]
- Lyu, R.; Ahmed, S.; Fan, W.; Yang, J.; Wu, X.; Zhou, W.; Zhang, P.; Yuan, L.; Wang, H. Engineering Properties of Sweet Potato Starch for Industrial Applications by Biotechnological Techniques including Genome Editing. Int. J. Mol. Sci. 2021, 22, 9533. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; Al-Maqtari, Q.A.; He, H.-J.; Othman, N. Evaluation of amylose content: Structural and functional properties, analytical techniques, and future prospects. Food Chem. X 2024, 24, 101830. [Google Scholar] [CrossRef]
- Zaccari, F.; Cabrera, M.C.; Saadoun, A. Glucose Content and In Vitro Bioaccessibility in Sweet Potato and Winter Squash Varieties during Storage. Foods 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Rana, Z.H.; Islam, S.N. Comparison of the Proximate Composition, Total Carotenoids and Total Polyphenol Content of Nine Orange-Fleshed Sweet Potato Varieties Grown in Bangladesh. Foods 2016, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Pankomera, P.; Heyes, J.A.; Lewthwaite, S.L.; Roskruge, N. Effects of ethylene and 1-methylcyclopropene on sweetpotato storage root quality. Acta Hortic. 2016, 1118, 163–170. [Google Scholar] [CrossRef]
- Amoah, R.S.; Landahl, S.; Terry, L.A. The timing of exogenous ethylene supplementation differentially affects stored sweetpotato roots. Postharvest Biol. Technol. 2016, 120, 92–102. [Google Scholar] [CrossRef]
- Gunes, N.T.; Dumanoglu, H.; Poyrazoglu, E.S. Use of 1-MCP for Keeping Postharvest Quality of ‘Ekmek’ Quince Fruit. In Proceedings of the 28th International Horticultural Congress on Science and Horticulture for People (IHC)/International Symposium on Postharvest Technology in the Global Market, Lisbon, Portugal, 22–27 August 2010; pp. 297–302. [Google Scholar]
- de Melo, R.B.; Figueiredo, R.W.; Maia, G.A.; Alves, R.E.; Silva, E.O. Storability of Orange Flesh Melons Treated with 1-Mcp. Acta Hortic. 2008, 768, 317–322. [Google Scholar] [CrossRef]
- Bakoglu, N.; Gunes, N.T. The effect of 1-MCP concentration on skin and flesh color polyphenol oxidase activity during cold storage and shelf life periods in ‘Ankara’ pear (Pyrus communis L.). In Proceedings of the 30th International Horticultural Congress (IHC)—Bridging the World Through Horticulture / International Symposium on Strategies and Technologies to Maintain Quality and Reduce Postharvest Losses, Turkish Soc Hort Sci, Istanbul, Turkey, 12–16 August 2018; pp. 221–227. [Google Scholar]
- Zhang, X.; Mi, L.; Yu, Q.; Chen, F.; Qi, Y.; Deng, Y.; Liu, W.; Shi, Y. Effect of 1-Methylcyclopropene on the Quality of Sweet Potato Storage Root during Storage. Food Sci. 2016, 37, 250. [Google Scholar] [CrossRef]
- Lu, X.; Nock, J.F.; Ma, Y.; Liu, X.; Watkins, C.B. Effects of repeated 1-methylcyclopropene (1-MCP) treatments on ripening and superficial scald of ‘Cortland’ and ‘Delicious’ apples. Postharvest Biol. Technol. 2013, 78, 48–54. [Google Scholar] [CrossRef]
- Wei, J.; Wu, S.; Lyu, Z.; Cui, P.; Xu, X.; Pang, L.; Lu, G.; Ji, S. Effects of Different 1-MCP Treatments on Fresh-keeping and Antioxidant Capacity of Sweet Potato During Storage. J. Nucl. Agric. Sci. 2022, 36, 1596–1606. [Google Scholar] [CrossRef]



| Components | Initial Eigenvalue | Extracting Square SUM and Loading | ||||
|---|---|---|---|---|---|---|
| Total | Variance/% | Accumulate/% | Total | Variance/% | Accumulate/% | |
| PC1 | 5.177 | 36.978 | 36.978 | 5.177 | 36.978 | 36.978 |
| PC2 | 2.874 | 20.527 | 57.505 | 2.874 | 20.527 | 57.505 |
| PC3 | 1.716 | 12.259 | 69.764 | 1.716 | 12.259 | 69.764 |
| PC4 | 1.080 | 7.715 | 77.479 | 1.080 | 7.715 | 77.479 |
| PC5 | 0.796 | 5.683 | 83.163 | |||
| PC6 | 0.598 | 4.268 | 87.431 | |||
| PC7 | 0.448 | 3.202 | 90.632 | |||
| PC8 | 0.430 | 3.070 | 93.702 | |||
| PC9 | 0.338 | 2.411 | 96.114 | |||
| PC10 | 0.225 | 1.609 | 97.723 | |||
| PC11 | 0.119 | 0.847 | 98.570 | |||
| PC12 | 0.104 | 0.744 | 99.314 | |||
| PC13 | 0.058 | 0.412 | 99.726 | |||
| PC14 | 0.038 | 0.274 | 100.000 | |||
| Indexs | PC1 (Y1) | PC2 (Y2) | PC3 (Y3) | PC4 (Y4) |
|---|---|---|---|---|
| Dry matter content (X1) | 0.310 | 0.328 | 0.485 | −0.631 |
| L* value (X2) | −0.040 | 0.617 | −0.636 | −0.090 |
| a* value (X3) | 0.635 | 0.419 | −0.343 | −0.036 |
| b* value (X4) | 0.533 | 0.613 | −0.542 | 0.050 |
| Amylose content (X5) | −0.578 | −0.334 | 0.065 | 0.467 |
| Glucose content (X6) | 0.731 | −0.138 | −0.073 | 0.286 |
| Crude fibre content (X7) | −0.019 | 0.666 | 0.482 | 0.342 |
| Crude protein content (X8) | 0.617 | −0.344 | −0.284 | 0.170 |
| Total Polyphenols content (X9) | 0.128 | 0.704 | 0.384 | 0.407 |
| Firmness (X10) | 0.726 | −0.561 | 0.106 | −0.135 |
| Cohesiveness (X11) | 0.653 | 0.388 | 0.241 | −0.061 |
| Springiness (X12) | 0.903 | −0.078 | 0.153 | 0.136 |
| Gumminess (X13) | 0.833 | −0.085 | 0.308 | 0.007 |
| Chewiness (X14) | 0.815 | −0.407 | −0.088 | 0.133 |
| Indexs | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| X1 | 0.136 | 0.193 | 0.370 | −0.607 |
| X2 | −0.018 | 0.364 | −0.486 | −0.087 |
| X3 | 0.279 | 0.247 | −0.262 | −0.035 |
| X4 | 0.234 | 0.362 | −0.414 | 0.048 |
| X5 | −0.254 | −0.197 | 0.050 | 0.449 |
| X6 | 0.321 | −0.081 | −0.056 | 0.275 |
| X7 | −0.008 | 0.393 | 0.368 | 0.329 |
| X8 | 0.271 | −0.203 | −0.217 | 0.164 |
| X9 | 0.056 | 0.415 | 0.293 | 0.392 |
| X10 | 0.319 | −0.331 | 0.081 | −0.130 |
| X11 | 0.287 | 0.229 | 0.184 | −0.059 |
| X12 | 0.397 | −0.046 | 0.117 | 0.131 |
| X13 | 0.366 | −0.050 | 0.235 | 0.007 |
| X14 | 0.358 | −0.240 | −0.067 | 0.128 |
| Scene | Recommended Concentration | Application Scenarios |
|---|---|---|
| Shelves (in the market or supermarket ≤15 DAS, 20–30 °C) | High concentration (8–10 μL·L−1) | Mid-to-high-end fresh produce market and Convenience Store Supply Chain |
| Logistics turnover (15–30 DAS,15–30 °C) | Moderate concentration (2–4 μL·L−1) | E-commerce supply chain and Food Delivery Platform Supply Chain |
| Cellar storage and Large storage facilities (>30 DAS, 15 °C) | Moderate concentration (1 μL·L−1) | Off-season Supply, Feedstock Reserves, and Raw Material Stockpile |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Hu, C.; Wei, S.; Wei, J.; Zhu, Y.; Wang, Z.; Xiang, C.; Lv, Z.; Lu, G. Effects of 1-Methylcyclopropene Fumigant on Texture and Nutritional Quality of ‘Yanshu 25’ Sweet Potato During Shelf-Life and Long-Term Storage at Room Temperature. Horticulturae 2025, 11, 936. https://doi.org/10.3390/horticulturae11080936
Xu X, Hu C, Wei S, Wei J, Zhu Y, Wang Z, Xiang C, Lv Z, Lu G. Effects of 1-Methylcyclopropene Fumigant on Texture and Nutritional Quality of ‘Yanshu 25’ Sweet Potato During Shelf-Life and Long-Term Storage at Room Temperature. Horticulturae. 2025; 11(8):936. https://doi.org/10.3390/horticulturae11080936
Chicago/Turabian StyleXu, Ximing, Chengyuan Hu, Shixiang Wei, Jingwen Wei, Yueming Zhu, Zhoumin Wang, Chao Xiang, Zunfu Lv, and Guoquan Lu. 2025. "Effects of 1-Methylcyclopropene Fumigant on Texture and Nutritional Quality of ‘Yanshu 25’ Sweet Potato During Shelf-Life and Long-Term Storage at Room Temperature" Horticulturae 11, no. 8: 936. https://doi.org/10.3390/horticulturae11080936
APA StyleXu, X., Hu, C., Wei, S., Wei, J., Zhu, Y., Wang, Z., Xiang, C., Lv, Z., & Lu, G. (2025). Effects of 1-Methylcyclopropene Fumigant on Texture and Nutritional Quality of ‘Yanshu 25’ Sweet Potato During Shelf-Life and Long-Term Storage at Room Temperature. Horticulturae, 11(8), 936. https://doi.org/10.3390/horticulturae11080936








