Influence of Mineral Fertilizers and Application Methods on Raspberry Composition Cultivated in an Acid Soil
Abstract
1. Introduction
2. Materials and Methods
- T1: 1 t/ha NPK, applied on the entire planting area, standard fertilization practice;
- T2: 1 t/ha NPK + 400 kg/ha urea applied on the entire planting area, (urea-P);
- T3: 1 t/ha NPK + 400 kg/ha urea applied in rows, (urea-R);
- T4: 2 t/ha NPK applied on the entire planting area, (NPK-P);
- T5: 2 t/ha NPK applied in rows, (NPK-R).
2.1. Soil Samples and Plan Samples Analyses
2.2. Statistical Analysis
3. Results
3.1. Soil Characteristics
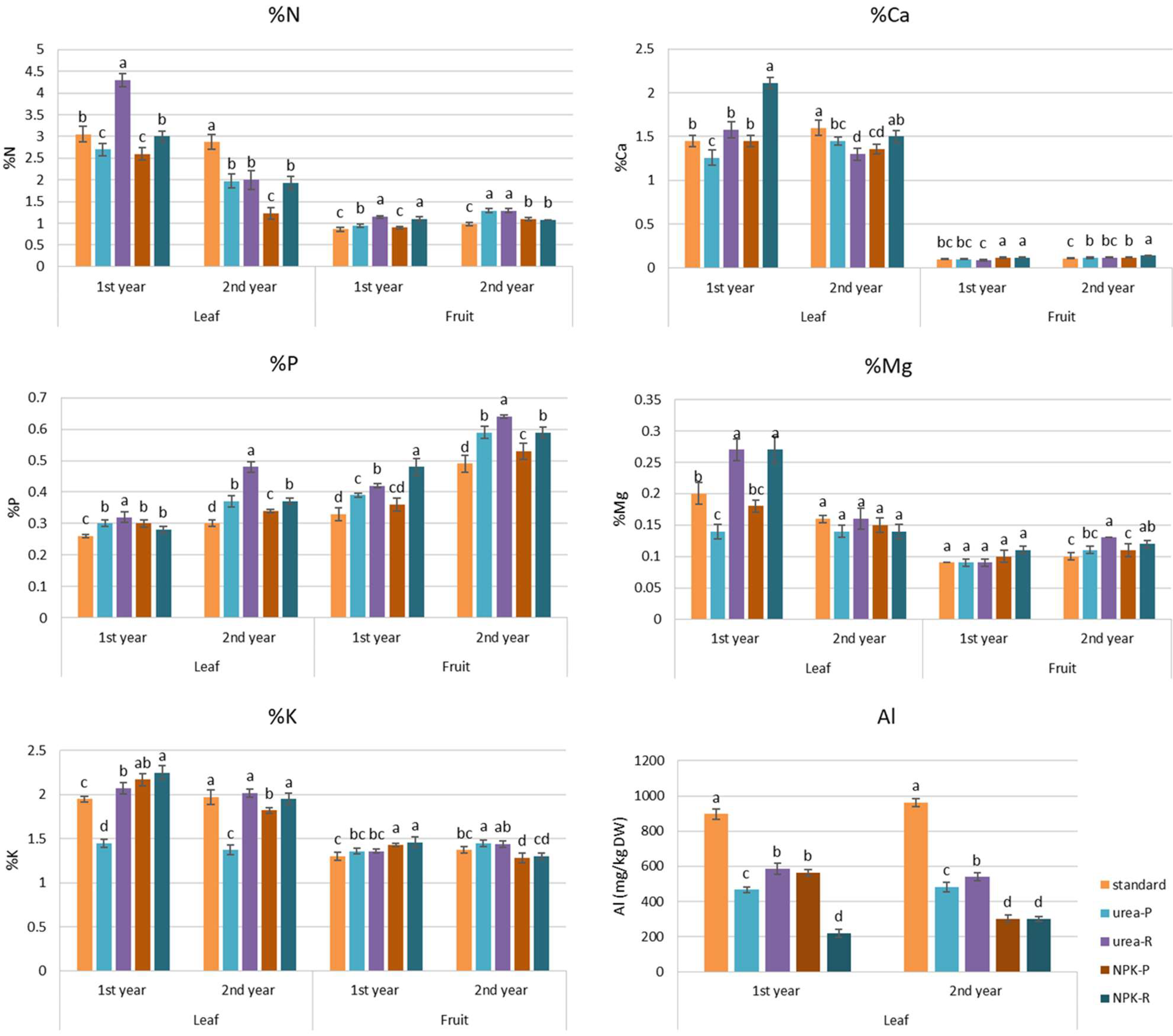
3.2. Content of Elements in Raspberry Leaves and Fruits
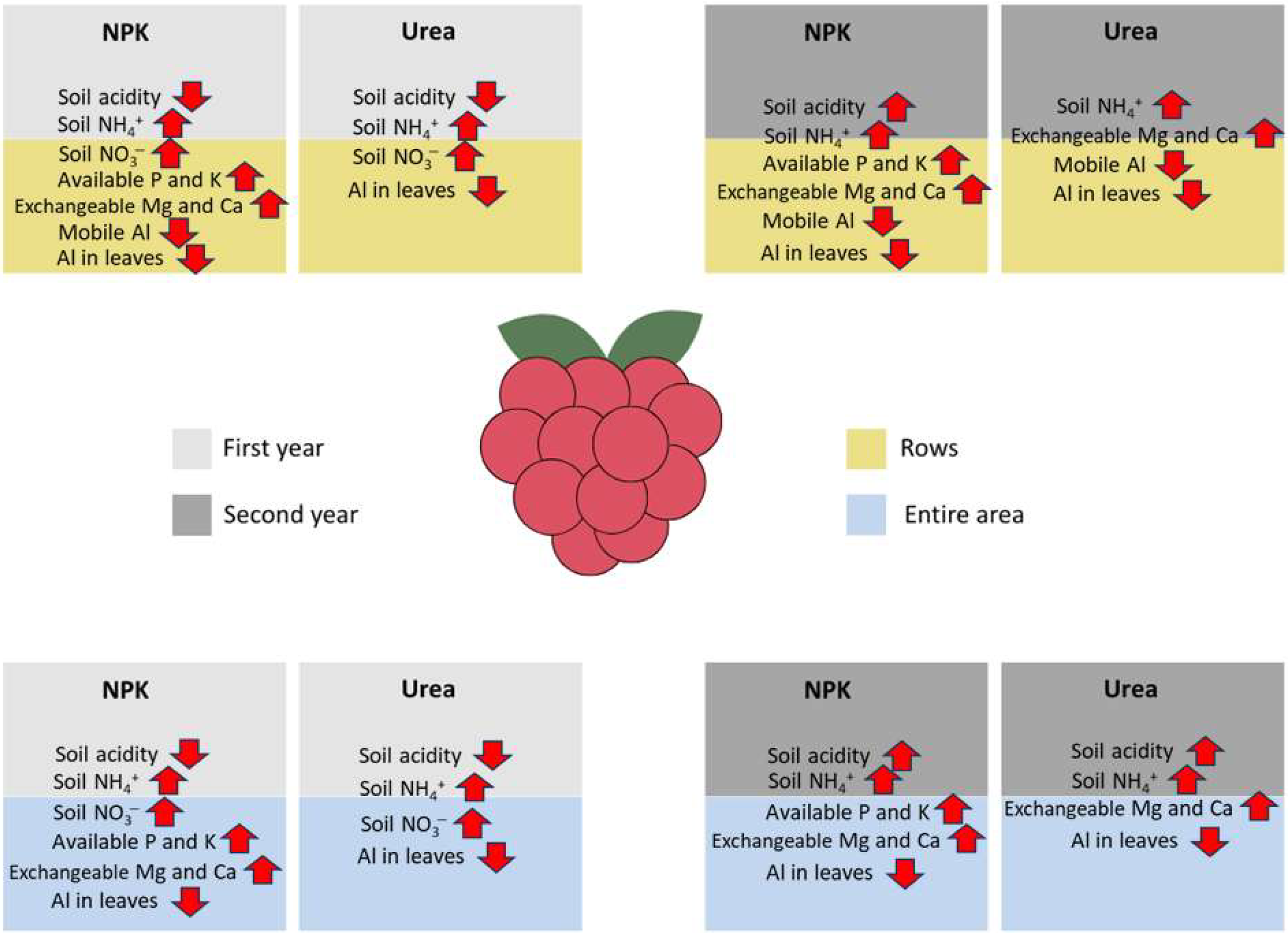
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fageria, N.K.; Nascente, A.S. Management of Soil Acidity of South American Soils for Sustainable Crop Production. Adv. Agron. 2014, 128, 221–275. [Google Scholar]
- Buskienė, L.; Uselis, N. The influence of nitrogen and potassium fertilizers on the growth and yield of raspberries cv. ‘Polana’. Agron. Res. 2008, 6, 27–35. [Google Scholar]
- Leposavić, A.; Đurović, D.; Keserović, Z.; Popović, B.; Mitrović, O.; Miletić, N.; Magazin, N. Evaluation of raspberry cultivars grown in the western Serbia region. Hortic. Sci. 2013, 40, 1–7. [Google Scholar] [CrossRef]
- Stevanović, D.; Martinović, L.; Šalipurović, B. Stanje i promene agrohemijskih karakteristika zemljišta u malinjacima na području Arilja. Jugosl. Vocar. 2003, 147–148, 135–141. [Google Scholar]
- Voigt, P.W.; Staley, T.E. Selection for aluminium and acid–soil resistance in white clover. Crop Sci. 2004, 44, 38–48. [Google Scholar]
- Von Uexküll, H.R.; Mutert, E. Global Extent, Development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Sikirić, B.B.; Stajković-Srbinović, O.S.; Saljnikov, E.R.; Litvinovich, A.V.; Jovković, M.V.; Mrvić, V.V. Microelements changes in leaves and fruits of raspberry (Rubus idaeus L.) under the influence of ameliorative measures. Int. J. Fruit Sci. 2022, 22, 358–369. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Bošković Rakočević, L.; Bokan, N.; Ubavić, M. The effects of amelioration measures on the yield of maize grown on acid soil. Acta Agric. Serb. 2000, 5, 29–36. [Google Scholar]
- Koković, N.; Jaćimović, G.; Sikirić, B.; Čirić, V.; Ugrenović, V.; Zhapparova, A.; Saljnikov, E. Changes in Eutric Cambisol due to long-term mineral fertilization: A case study in Serbia. Ital. J. Agron. 2022, 17, 2029. [Google Scholar] [CrossRef]
- Danilov, D.; Yakovleva, L.; Nikolaeva, E. Efficiency of Lime Application on Sod-Podzolic Soil in the North-Western Region of Russia. BIO Web Conf. 2020, 17, 00129. [Google Scholar] [CrossRef][Green Version]
- Hu, W.; Cheng, W.; Wen, S.; Rahman, M.M. Effects of chemical contamination on microscale structural characteristics of intact loess and resultant macroscale mechanical properties. Catena 2021, 203, 105361. [Google Scholar] [CrossRef]
- Li, Q.; Li, S.; Xiao, Y.; Zhao, B.; Wang, C.; Li, B.; Gao, X.; Li, Y.; Bai, G.; Wang, Y. Soil Acidification and its influencing factors in the purple hilly area of Southwest China from 1981 to 2012. Catena 2019, 175, 278–285. [Google Scholar] [CrossRef]
- Yadav, D.S.; Jaiswal, B.; Gautam, M.; Agrawal, M. Soil acidification and its impact on plants. In Plant Responses to Soil Pollution, 1st ed.; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 1–26. [Google Scholar]
- Rahman, M.A.; Lee, S.H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, M. Aluminum Uptake and Migration from the Soil Compartment into Betula pendula for two different environments: A polluted and environmentally protected area of Poland. Environ. Sci. Pollut. Res. Int. 2016, 23, 1398–1407. [Google Scholar] [CrossRef]
- Foy, C.D.; Lafever, H.N.; Schwartz, J.W.; Fleming, A.L. Aluminum tolerance of wheat cultivars as related to regions of Origin. Agron. J. 1974, 66, 751–758. [Google Scholar] [CrossRef]
- Dugalić, M.; Bošković-Rakočević, L.j.; Dugalić, G. Change in acidity and mobile aluminium levels in forest, meadow and arable land pseudogley soils. In Proceedings of the X International Scientific Symposium “Agrosym 2019”, Jahorina, Bosnia and Herzegovina, 3–6 October 2019. [Google Scholar]
- Čakmak, D.; Saljnikov, E.; Mrvić, V.; Jakovljević, M.; Marjanović, Z.; Sikirić, B.; Maksimović, S. Soil properties and trace elements contents following 40 years of phosphate fertilization. J. Environ. Qual. 2010, 39, 541–547. [Google Scholar] [CrossRef] [PubMed]
- WRB. World Reference Base for Soil Resources 2014; World Soil Resources Reports No. 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; p. 113. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 9 April 2025).
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1958. [Google Scholar]
- Bremner, J.M. Inorganic forms of nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.E., Eds.; ASA and SSSA: Madison, WI, USA, 1965; pp. 1179–1237. [Google Scholar]
- Egner, H.A.N.S.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Barnhisel, R.; Bertsch, P.M. Aluminium. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; SSSA: Madison, WI, USA, 1982; pp. 275–300. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soil, Plants and Water; University of California: Berkeley, CA, USA, 1961. [Google Scholar]
- Džamić, R.; Stevanović, D. Agrochemistry; Partenon: Belgrade, Serbia, 2000. (In Serbian) [Google Scholar]
- Bergmann, W. Farbatlas—Ernährungsstörungen bei Kulturpflanzen: Visuelle und Analytische Diagnose; VEB Gustav Fischer Verlag: Jena, Germany, 1986. [Google Scholar]
- Sikirić, B.; Stajković-Srbinović, O.; Čakmak, D.; Delić, D.; Koković, N.; Kostić-Kravljanac, L.J.; Mrvić, V. Macronutrient contents in the leaves and fruits of red raspberry as affected by liming in an extremely acid soil. Plant Soil Environ. 2015, 61, 23–28. [Google Scholar] [CrossRef]
- Watanabe, T.; Osaki, M.; Tadano, T. Al uptake kinetics in roots of Melastoma malabathricum L. an Al accumulator plant. Plant Soil. 2001, 231, 283–291. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Krasilnikov, P.; Taboada, M.A.; Amanullah. Fertilizer Use, Soil Health and Agricultural Sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Alves, I.M.D.; Soares, J.R.; Montezano, Z.F.; DelGrosso, S.; Vitti, A.C.; Rossetto, R.; Cantarella, H. Nitrogen sources and application rates affect emissions of N2O and NH3 in sugarcane. Nutr. Cycl. Agroecosystems 2020, 116, 329–344. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Wang, C.; Huang, J.; Feng, Y.; Shen, W.; Zhou, M.; Yang, L. Ammonia volatilization mitigation in crop farming: A review of fertilizer amendment technologies and mechanisms. Chemosphere 2022, 303, 134944. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hedley, M.J. Role of carbon and nitrogen cycles in soil acidification. In Handbook of Soil Acidity; Rengel, Z., Ed.; Marcel Dekker: New York, NY, USA, 2003; pp. 47–48. [Google Scholar]
- Tkaczyk, P.; Mocek-Płóciniak, A.; Skowrońska, M.; Bednarek, W.; Kuśmierz, S.; Zawierucha, E. The Mineral Fertilizer-Dependent Chemical Parameters of Soil Acidification under Field Conditions. Sustainability 2020, 12, 7165. [Google Scholar] [CrossRef]
- Kissel, D.E.; Bock, B.R.; Ogles, C.Z. Thoughts on acidification of soils by nitrogen and sulfur fertilizers. Agrosystems Geosci. Environ. 2020, 3, e20060. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hedley, M.J.; White, R.E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 1991, 134, 53–63. [Google Scholar] [CrossRef]
- Malhi, S.S.; Harapiak, J.T.; Nyborg, M.; Gill, K.S. Effects of long-term applications of various nitrogen sources on chemical soil properties and composition of bromegrass hay. J. Plant Nutr. 2000, 23, 903–912. [Google Scholar] [CrossRef]
- Tang, C.; Conyers, M.K.; Nuruzzaman, M.; Poile, G.J.; Liu, D.L. Biological amelioration of subsoil acidity through managing nitrate uptake by wheat crops. Plant Soil 2011, 338, 383–397. [Google Scholar] [CrossRef]
- Skowronska, M.; Filipek, T. Life cycle assessment of fertilizers: A review. Int. Agrophys. 2014, 28, 101–110. [Google Scholar] [CrossRef]
- Dawar, K.; Zaman, M.; Rowarth, J.S.; Blennerhassett, J.; Turnbull, M.H. Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agric. Ecosyst. Environ. 2011, 144, 41–50. [Google Scholar] [CrossRef]
- Klimczyk, M.; Siczek, A.; Schimmelpfennig, L. Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total Environ. 2021, 771, 145483. [Google Scholar] [CrossRef] [PubMed]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Sukor, A.S.A.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Fu, W.; Xu, C.; Zhang, H.; Xu, X.; Ma, H.; Wang, J.; Zhang, Y. Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato–Wheat Rotation System. Plants 2024, 13, 1740. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Chien, S.H.; Gearhart, M.M.; Collamer, D.J. The effect of different ammonical nitrogen sources on soil acidification. Soil Sci. 2008, 173, 544–551. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; de Vries, W. Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef]
- Russell, A.E.; Laird, D.A.; Mallarino, A.P. Nitrogen fertilization and cropping system impacts on soil quality in Midwestern Mollisols. Soil Sci. Soc. Am. J. 2006, 70, 249–255. [Google Scholar] [CrossRef]
- Koković, N.; Saljnikov, E.; Dinić, Z.; Sikirić, B.; Mrvić, V.; Nerandžić, B. Hemijske osobine zemljišta posle 50 godišnjeg đubrenja zemljišta mineralnim đubrivima. Zemlj. Biljka 2018, 67, 1–9. [Google Scholar]
- Marschner, H. Plant-Soil Interactions at Low pH; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 683–702. [Google Scholar]
- Osawa, H.; Ikeda, S.; Tange, T. The rapid accumulation of aluminum is ubiquitous in both the evergreen and deciduous leaves of Theaceae and Ternstroemiaceae plants over a wide pH range in acidic soils. Plant Soil 2013, 363, 49–59. [Google Scholar] [CrossRef]
- Sierra, J.; Noel, C.; Dufour, L.; Ozier-Lafontaine, H.; Welcker, C.; Desfontaines, L. Mineral nutrition and growth of tropical maize as affected by soil acidity. Plant Soil 2003, 252, 215–226. [Google Scholar] [CrossRef]
- Su, C.; Ewans, L. Soil solution chemistry and alfalfa response to CaCO3 and MgCO3 on an acid Gleysol. Can. J. Soil Sci. 1996, 796, 41–47. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dalls Agnol, M.; Bouton, J.H.; Parott, W.A. Screening methods to develop alfalfa populations tolerant to acid, aluminum toxic soils. Crop Sci. 1996, 36, 64–70. [Google Scholar] [CrossRef]
- Das, S.; Mohapatra, A.; Sahu, K.; Panday, D.; Ghimire, D.; Maharjan, B. Nitrogen dynamics as a function of soil types, compaction, and moisture. PLoS ONE 2024, 19, e0301296. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.; Baleani, D.; Casado-Murillo, N.; Noe, L. Effect of wheat crop fertilization on nitrogen dynamics and balance in the Humid Pampas, Argentina. Agric. Ecosyst. Environ. 2007, 119, 171–176. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Hong, Y.; Wang, F.; Mao, X.; Yi, J. Controlled-release urea application and optimized nitrogen applied strategy reduced nitrogen leaching and maintained grain yield of paddy fields in Northwest China. Front. Plant Sci. 2023, 14, 1033506. [Google Scholar] [CrossRef]
- Mandal, A.; Patra, A.; Singh, D.; Swarup, A.; Ebhinmasto, R. Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour. Technol. 2007, 96, 3585–3592. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Chen, X.Q.; Zhou, J.M.; Liu, D.H.; Wang, H.Y.; Du, C.W. Influence of humic acid on interaction of ammonium and potassium ions on clay minerals. Pedosphere 2013, 23, 493–502. [Google Scholar] [CrossRef]
- Hoopen, F.T.; Cuin, T.A.; Pedas, P.; Hegelund, J.N.; Shabala, S.; Schjørring, J.K.; Jahn, T.P. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar] [CrossRef]
- Zhen, R.G.; Leigh, R.A. Nitrate accumulation by wheat (Triticum aestivum) in relation to growth and tissue N concentrations. Plant Soil 1990, 124, 157–160. [Google Scholar] [CrossRef]
- Dresler, S.; Bednarek, W.; Tkaczyk, P.; Hawrylak-Nowak, B. Estimation of the macro- and micronutrient status of raspberries grown in the Lublin region. Folia Hortic. 2015, 27, 53–62. [Google Scholar] [CrossRef]
- Stecker, J.; Nathan, M.; Errin, E.; Jett, L. Lawn and Garden Soil Test Interpretations and Fertilizer Recommendation Guide; MU Extension publication MP 733; University of Missauri-Columbia: Columbia, MO, USA, 2003. [Google Scholar]
- Clark, R.B. Plant genotype differences in the uptake, translocation, accumulation, and use of mineral element required for plant growth. Plant Soil 1983, 72, 175–196. [Google Scholar] [CrossRef]
- Lefever, H.N. Genetic differences in plant response to soil nutrient stress. J. Plant Nutr. 1981, 4, 89–109. [Google Scholar] [CrossRef]
| Treatments | pH KCl | H cmol/kg | S cmol/kg | V % | Al mg/100 g | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | |
| Standard fertilization (1 t/ha NPK) | 3.50 bc (±0.01) | 3.50 b (±0.02) | 14.7 b (±0.36) | 14.5 b (±0.30) | 3.0 b (±0.26) | 3.0 c (±0.00) | 20.4 c (±0.53) | 17.2 b (±0.40) | 10.84 a (±1.62) | 9.86 b (±0.37) |
| 400 kg/ha urea-P | 3.45 c (±0.01) | 3.38 c (±0.02) | 16.8 a (±0.62) | 14.9 b (±0.26) | 2.6 c (±0.15) | 2.2 d (±0.17) | 13.4 e (±0.36) | 13.1 d (±0.56) | 9.10 a (±0.44) | 11.93 a (±0.67) |
| 400 kg/ha urea-R | 3.65 a (±0.05) | 3.55 a (±0.02) | 13.3 c (±0.46) | 13.2 c (±0.62) | 4.4 a (±0.06) | 5.6 b (±0.23) | 24.8 b (±0.55) | 30.0 a (±0.53) | 9.79 a (±0.78) | 7.33 c (±0.98) |
| 2 t/ha NPK-P | 3.55 b (±0.03) | 3.32 d (±0.01) | 14.0 c (±0.50) | 16.1 a (±0.30) | 3.0 b (±0.10) | 3.0 c (±0.17) | 18.3 d (±0.70) | 15.7 c (±0.70) | 9.68 a (±1.28) | 11.21 a (±0.96) |
| 2 t/ha NPK-R | 3.70 a (±0.03) | 3.40 c (±0.02) | 9.1 d (±0.38) | 14.7 b (±0.44) | 4.6 a (±0.06) | 5.8 a (±0.21) | 27.1 a (±0.56) | 29.3 a (±0.62) | 2.55 b (±0.18) | 5.32 d (±0.24) |
| Treatments | NH4+ mg/kg | NO3− mg/kg | NH4++NO3− mg/kg | |||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | |
| Standard fertilization (1 t/ha NPK) | 15.1 d (±1.01) | 31.5 e (±1.44) | 18.2 e (±1.11) | 26.3 a (±1.83) | 33.3 | 57.8 |
| 400 kg/ha urea-P | 23.0 c (±1.81) | 38.5 c (±1.93) | 54.3 c (±1.11) | 23.5 b (±1.40) | 77.3 | 63.0 |
| 400 kg/ha urea-R | 59.9 a (±4.37) | 63.0 a (±2.11) | 88.6 a (±1.91) | 22.8 b (±0.70) | 148.4 | 87.5 |
| 2 t/ha NPK-P | 35.0 b (±1.97) | 42.0 b (±1.50) | 41.3 d (±2.93) | 15.8 c (±1.49) | 76.3 | 57.8 |
| 2 t/ha NPK-R | 58.1 a (±1.85) | 35.5 d (±0.98) | 65.1 b (±2.09) | 23.5 b (±1.40) | 123.2 | 60.4 |
| Treatments | P | K | Ca mg/kg | Mg | Ca/Mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | 1st Year | 2nd Year | |
| Standard fertilization (1 t/ha NPK) | 14.1 d (±0.65) | 37.0 c (±0.98) | 119 e (±3.17) | 152 c (±4.88) | 890 c (±120.0) | 890 c (±70.0) | 117 c (±5.20) | 90 e (±2.00) | 4.6:1 | 06:01 |
| 400 kg/ha urea-P | 18.5 c (±0.34) | 37.8 c (±1.53) | 129 d (±2.71) | 146 c (±3.73) | 780 c (±70.0) | 1800 a (±111.4) | 97 d (±3.00) | 278 b (±6.80) | 4.6:1 | 3.9:1 |
| 400 kg/ha urea-R | 14.1 d (±0.90) | 28.6 d (±1.53) | 150 c (±1.80) | 201 b (±5.93) | 920 c (±72.0) | 2010 a (±115.3) | 118 c (±4.60) | 292 a (±2.10) | 5.2:1 | 4.2:1 |
| 2 t/ha NPK-P | 23.8 b (±1.47) | 52.8 b (±1.71) | 176 b (±1.39) | 216 b (±13.02) | 1130 a (±36.1) | 1400 b (±137.5) | 158 a (±8.30) | 255 c (±5.60) | 4.3:1 | 3.4:1 |
| 2 t/ha NPK-R | 25.5 a (±0.91) | 67.8 a (±2.22) | 214 a (±2.83) | 233 a (±13.75) | 1090 b (±37.9) | 1250 b (±132.3) | 132 b (±4.40) | 205 d (±5.00) | 5.0:1 | 3.8:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikirić, B.; Mrvić, V.; Koković, N.; Tošić Jojević, S.; Pešić, M.; Prekop, N.; Stajković-Srbinović, O. Influence of Mineral Fertilizers and Application Methods on Raspberry Composition Cultivated in an Acid Soil. Horticulturae 2025, 11, 914. https://doi.org/10.3390/horticulturae11080914
Sikirić B, Mrvić V, Koković N, Tošić Jojević S, Pešić M, Prekop N, Stajković-Srbinović O. Influence of Mineral Fertilizers and Application Methods on Raspberry Composition Cultivated in an Acid Soil. Horticulturae. 2025; 11(8):914. https://doi.org/10.3390/horticulturae11080914
Chicago/Turabian StyleSikirić, Biljana, Vesna Mrvić, Nikola Koković, Sonja Tošić Jojević, Mila Pešić, Nenad Prekop, and Olivera Stajković-Srbinović. 2025. "Influence of Mineral Fertilizers and Application Methods on Raspberry Composition Cultivated in an Acid Soil" Horticulturae 11, no. 8: 914. https://doi.org/10.3390/horticulturae11080914
APA StyleSikirić, B., Mrvić, V., Koković, N., Tošić Jojević, S., Pešić, M., Prekop, N., & Stajković-Srbinović, O. (2025). Influence of Mineral Fertilizers and Application Methods on Raspberry Composition Cultivated in an Acid Soil. Horticulturae, 11(8), 914. https://doi.org/10.3390/horticulturae11080914





