Ozone Treatment Modulates Reactive Oxygen Species Metabolism Regulation and Enhances Storage Quality of Kiwifruit During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material and Ozone Treatment
2.2. Determination of Firmness and Rate of Weight Loss
2.3. Determination of TSS and TA Content
2.4. Determination of MDA Content and the Cell Membrane Permeability
2.5. Determination of Total Phenolic and Flavonoid Content
2.6. Determination of O2−, H2O2, VC, and GSH Content
2.7. Determination of Enzyme Activities Related to ROS Metabolism
2.8. Statistical Analysis
3. Results and Discussion
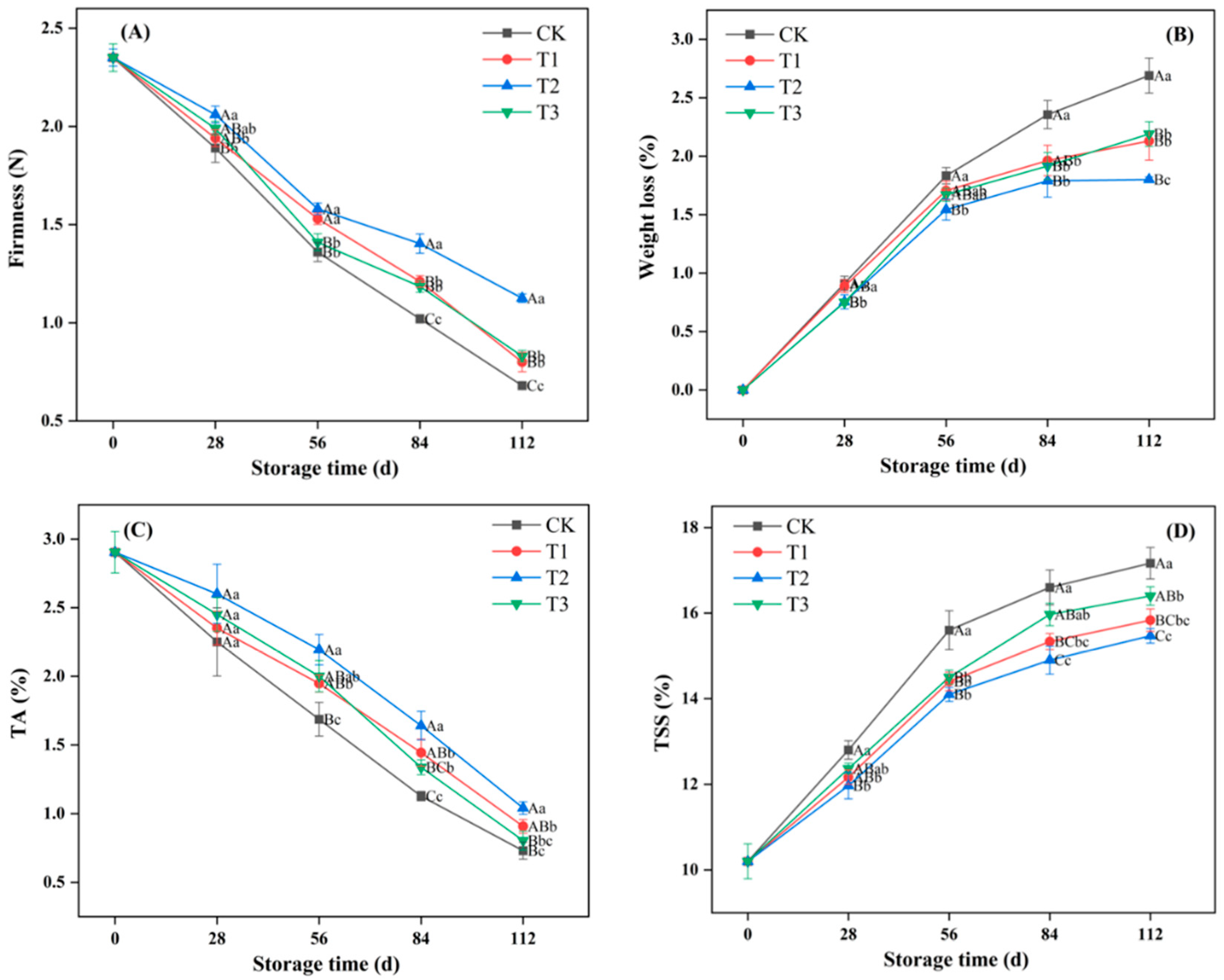
3.1. Effect of Ozone Treatment on Firmness, Weight Loss, TSS, and TA Content
3.2. Effect of Ozone Treatment on MDA Content, Cell Membrane Permeability and LOX Activity
3.3. Effect of Ozone Treatment on Total Phenol and Flavonoid Content
3.4. Effect of Ozone Treatment on VC and GSH Content
3.5. Effect of Ozone Treatment on O2− and H2O2 Content
3.6. Effect of Ozone Treatment on Enzyme Activities Related to ROS Metabolism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Liu, L.; McClements, D.J.; Liu, Z.D.; Liu, X.B.; Liu, F.G. A Review of the Bioactive Compounds of Kiwifruit: Bioactivity, Extraction, Processing and Challenges. Food Rev. Int. 2024, 40, 996–1027. [Google Scholar] [CrossRef]
- Wang, S.N.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.F.; Liao, G.L.; Ye, B.; Zhong, M.; Huang, C.H.; Xu, X.B. Changes in fruit quality, phenolic compounds, and antioxidant activity of kiwifruit (Actinidia eriantha) during on-vine ripening. Lwt-Food Sci. Technol. 2024, 206, 116564. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Xiao, X.; Cao, S.F.; Chen, W.; Yang, Z.F.; Shi, L.Y. Effect of 1-MCP on the regulation processes involved in ascorbate metabolism in kiwifruit. Postharvest Biol. Technol. 2021, 179, 111563. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Foggia, M.; Fasano, A.; Baraldi, E. Heat treatment effect on Cadophora luteo-olivacea of kiwifruit. Plant Pathol. 2022, 71, 644–653. [Google Scholar] [CrossRef]
- Yang, S.X.; Li, R.J.; Wang, D.; Liang, J.; Huang, T.Z.; Zhang, L.; Luo, A.W. Effect of low-dose high-energy electron beam irradiation on postharvest storage quality of Actinidia arguta. J. Food Process. Preserv. 2022, 46, e16761. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Y.X.; Yang, S.H.; Li, C.; Li, L.; Gao, S.Y.; Wu, Z.X. Mechanism of ozone treatment in delayed softening of fresh-cut kiwifruit during storage. Postharvest Biol. Technol. 2023, 204, 112469. [Google Scholar] [CrossRef]
- Glowacz, M.; Colgan, R.; Rees, D. The use of ozone to extend the shelf-life and maintain quality of fresh produce. J. Sci. Food Agric. 2015, 95, 662–671. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.Y.; Wang, T.Y.; Li, C.; Wu, Z.X. Effects of ozone treatment on pesticide residues in food: A review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Ceciu, S.; Lazar, V.; Mantzourani, I.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Evaluation of ozone efficacy on the reduction of microbial population of fresh cut lettuce (Lactuca sativa) and green bell pepper (Capsicum annuum). Food Control 2013, 30, 491–496. [Google Scholar] [CrossRef]
- Li, L.L.; Meng, Z.Y.; Liang, Y.Y.; Gong, S.; Wang, Z.L.; Xu, X.; Wang, S.F. Future directions for multifunctional preservation technologies in food preservation: A review. J. Stored Prod. Res. 2025, 114, 102712. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Sunoj, S.; Manikantan, M.R.; Kothakota, A.; Hebbar, K.B. Application and Kinetics of Ozone in Food Preservation. Ozone-Sci. Eng. 2017, 39, 115–126. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Priya, E.P.B.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone-Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.M.; Brandao, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Shezi, S.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, K.L.; Zhang, X.J.; Dong, C.H.; Ji, H.P.; Ke, R.H.; Ban, Z.J.; Hu, Y.F.; Lin, S.H.; Chen, C.K. Effects of ozone treatment on the antioxidant capacity of postharvest strawberry. Rsc Adv. 2020, 10, 38142–38157. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhang, H.J.; Zhang, N.; Dong, C.H.; Ji, H.P.; Yu, J.Z.; Ban, Z.J.; Yan, R.X.; Zhang, T.; Chen, C.K.; et al. Effects of ozone treatment on gene profiling involved in ASA-GSH cycle in postharvest cantaloupe. Sci. Hortic. 2023, 312, 111843. [Google Scholar] [CrossRef]
- Lin, S.H.; Zhang, X.J.; Li, M.; Zhang, N.; Dong, C.H.; Ji, H.P.; Zheng, P.F.; Ban, Z.J.; Mei, X.; Gu, C.Y.; et al. Analysis of the Antioxidant Mechanism of Ozone Treatment to Extend the Shelf Life and Storage Quality of ‘Korla’ Fragrant Pears Based on Label-Free Proteomics. Horticulturae 2024, 10, 424. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, W.H.; Zhai, X.Q.; Li, X.H. Combination of precooling with ozone fumigation or low fluctuation of temperature for the quality modifications of postharvest sweet cherries. J. Food Process. Preserv. 2021, 45, e15504. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Y.X.; Ye, L.X.; Shi, Y.B.; Luo, A.W. Transcriptomic analysis reveals ozone treatment delays kiwifruit postharvest softening by modulating cell wall metabolism. J. Food Sci. 2024, 89, 2001–2016. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, C.; Cheng, S.C.; Wei, B.D.; Liu, X.Y.; Ji, S.J. Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D.; Boldingh, H. Fruit maturation and the soluble solids harvest index for ‘Hayward’ kiwifruit. Sci. Hortic. 2016, 213, 193–198. [Google Scholar] [CrossRef]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D. Softening of ‘Hayward’ kiwifruit on the vine and in storage: The effects of temperature. Sci. Hortic. 2017, 220, 176–182. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.B.; Cao, J.K.; Ma, L. A combination of 1-methylcyclopropene treatment and intermittent warming alleviates chilling injury and affects phenolics and antioxidant activity of peach fruit during storage. Sci. Hortic. 2018, 229, 175–181. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lin, H.T.; Zhang, S.; Lin, Y.F.; Chen, Y.H.; Lin, Y.X. Effects of Adenosine Triphosphate (ATP) Treatment on Postharvest Physiology, Quality and Storage Behavior of Longan Fruit. Food Bioprocess Technol. 2015, 8, 971–982. [Google Scholar] [CrossRef]
- Gutierrez, D.R.; Chaves, A.R.; Rodriguez, S.D. UV-C and ozone treatment influences on the antioxidant capacity and antioxidant system of minimally processed rocket (Eruca sativa Mill.). Postharvest Biol. Technol. 2018, 138, 107–113. [Google Scholar] [CrossRef]
- Venkatachalam, K. Exogenous nitric oxide treatment impacts antioxidant response and alleviates chilling injuries in longkong pericarp. Sci. Hortic. 2018, 237, 311–317. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Su, Z.H.; Hu, M.J.; Gao, Z.Y.; Li, M.; Yun, Z.; Pan, Y.G.; Zhang, Z.K.; Jiang, Y.M. Apple polyphenols delay senescence and maintain edible quality in litchi fruit during storage. Postharvest Biol. Technol. 2019, 157, 110976. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lin, X.C.; Dou, Y.; Zhang, W.; Du, H.Y.; Wan, C.P.; Chen, J.Y.; Zhang, L.L.; Zhu, L.Q. Transcriptome profiling of postharvest kiwifruit in response to exogenous nitric oxide. Sci. Hortic. 2021, 277, 109788. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Balderas, C.; Sánchez-Moreno, C.; De Ancos, B. Impact of an in vitro dynamic gastrointestinal digestion on phenolic compounds and antioxidant capacity of apple treated by high-pressure processing. Innov. Food Sci. Emerg. Technol. 2020, 66, 102486. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Teng, J.C.; Liang, J.Y.; Gibson, B.W.; Zhang, W.D.; Li, A.; Cheng, Y.J.; Zeng, Y.L. Effect of different pre-cooling strategies on the storage quality of kiwifruit. Sci. Hortic. 2025, 344, 114105. [Google Scholar] [CrossRef]
- Chen, C.K.; Zhang, H.J.; Zhang, X.J.; Dong, C.H.; Xue, W.T.; Xu, W.T. The effect of different doses of ozone treatments on the postharvest quality and biodiversity of cantaloupes. Postharvest Biol. Technol. 2020, 163, 111124. [Google Scholar] [CrossRef]
- Yang, W.T.; Liu, Y.X.; Sang, Y.Y.; Ma, Y.Y.; Guo, M.R.; Bai, G.R.; Cheng, S.B.; Chen, G.G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 111614. [Google Scholar] [CrossRef]
- Vlassi, E.; Vlachos, P.; Kornaros, M. Effect of ozonation on table grapes preservation in cold storage. J. Food Sci. Technol. 2018, 55, 2031–2038. [Google Scholar] [CrossRef]
- Choi, H.R.; Baek, M.W.; Tilahun, S.; Jeong, C.S. Long-term cold storage affects metabolites, antioxidant activities, and ripening and stress-related genes of kiwifruit cultivars. Postharvest Biol. Technol. 2022, 189, 111912. [Google Scholar] [CrossRef]
- Luo, A.W.; Bai, J.Q.; Li, R.; Fang, Y.M.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.Z.; Kou, L.P. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Ge, M.J.; Zhang, L.Y.; Ai, J.Y.; Ji, R.; He, L.; Liu, C.H. Effect of heat shock and potassium sorbate treatments on gray mold and postharvest quality of ‘XuXiang’ kiwifruit. Food Chem. 2020, 324, 126891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, Y.X.; Ye, L.X.; Shi, Y.B.; Luo, A.W. Ozone treatment modulates reactive oxygen species levels in kiwifruit through the antioxidant system: Insights from transcriptomic analysis. J. Plant Physiol. 2023, 291, 154135. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Liu, Y.P.; Di, J.B.; Ren, G.; Wang, W.; He, W.C.; Wang, Y. Effects of 1-MCP Treatment on Physiology and Storage Quality of Root Mustard at Ambient Temperature. Foods 2022, 11, 2978. [Google Scholar] [CrossRef] [PubMed]
- Modesti, M.; Petriccione, M.; Forniti, R.; Zampella, L.; Scortichini, M.; Mencarelli, F. Methyl jasmonate and ozone affect the antioxidant system and the quality of wine grape during postharvest partial dehydration. Food Res. Int. 2018, 112, 369–377. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Ma, X.W.; Wu, H.X.; Liu, L.Q.; Yao, Q.S.; Wang, S.B.; Zhan, R.L.; Xing, S.S.; Zhou, Y.G. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Zhang, J.R.; Xiang, M.L.; Zeng, J.K.; Chen, J.Y.; Chen, M. Exogenous melatonin treatment affects ascorbic acid metabolism in postharvest ‘Jinyan’ kiwifruit. Front. Nutr. 2022, 9, 1081476. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.Q.; Wu, Z.X. Gaseous ozone regulates reactive oxygen species metabolism and ascorbate-glutathione cycle to delay the senescence of fresh-cut red pitaya (Selenicereus undatus) fruit. Sci. Hortic. 2023, 312, 111839. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Y.X.; Yang, S.H.; Wu, Z.X.; Shen, Y.X. Gaseous ozone treatment prolongs the shelf-life of fresh-cut kiwifruit by maintaining its ascorbic acid content. Lwt-Food Sci. Technol. 2022, 172, 114196. [Google Scholar] [CrossRef]
- Wang, Y.J.; Rong, L.Y.; Wang, T.Y.; Gao, S.Y.; Zhang, S.Y.; Wu, Z.X. Transcriptome analysis reveals ozone treatment maintains ascorbic acid content in fresh-cut kiwifruit by regulating phytohormone signalling pathways. Food Res. Int. 2024, 191, 114699. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.C.; Chen, T.; Li, B.Q.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit-Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.F.; Xu, Y.; Li, H.; Chen, X.; Song, Z.F. Antioxidant activation, cell wall reinforcement, and reactive oxygen species regulation promote resistance to waterlogging stress in hot pepper (Capsicum annuum L.). Bmc Plant Biol. 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Hou, F.; Li, G.K.; Sang, N. Effects of nitrogen dioxide and its acid mist on reactive oxygen species production and antioxidant enzyme activity in Arabidopsis plants. J. Environ. Sci. 2015, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Grzelak-Blaszczyk, K.; Sojka, M.; Balawejder, M. One-time ozone treatment improves the postharvest quality and antioxidant activity of Actinidia arguta fruit. Phytochemistry 2022, 203, 113393. [Google Scholar] [CrossRef]
- Liu, J.; Chang, M.C.; Meng, J.L.; Liu, J.Y.; Cheng, Y.F.; Feng, C.P. Effect of ozone treatment on the quality and enzyme activity of Lentinus edodes during cold storage. J. Food Process. Preserv. 2020, 44, e14557. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Ji, L.L.; Chen, C.K.; Dong, C.H.; Wang, C.R. Effects of Ozone Treatment on the Storage Quality of Post-Harvest Tomato. Int. J. Food Eng. 2018, 14, 20180012. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Balawejder, M. Ozone Treatment Induces Changes in Antioxidative Defense System in Blueberry Fruit During Storage. Food Bioprocess Technol. 2020, 13, 1240–1245. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Tan, J.; Zhang, X.; Li, X.; Guan, W.; Liu, P.; Chen, A. Ozone Treatment Modulates Reactive Oxygen Species Metabolism Regulation and Enhances Storage Quality of Kiwifruit During Cold Storage. Horticulturae 2025, 11, 911. https://doi.org/10.3390/horticulturae11080911
Jin Z, Tan J, Zhang X, Li X, Guan W, Liu P, Chen A. Ozone Treatment Modulates Reactive Oxygen Species Metabolism Regulation and Enhances Storage Quality of Kiwifruit During Cold Storage. Horticulturae. 2025; 11(8):911. https://doi.org/10.3390/horticulturae11080911
Chicago/Turabian StyleJin, Ziyu, Jin Tan, Xinyu Zhang, Xin Li, Wenqiang Guan, Pu Liu, and Aiqiang Chen. 2025. "Ozone Treatment Modulates Reactive Oxygen Species Metabolism Regulation and Enhances Storage Quality of Kiwifruit During Cold Storage" Horticulturae 11, no. 8: 911. https://doi.org/10.3390/horticulturae11080911
APA StyleJin, Z., Tan, J., Zhang, X., Li, X., Guan, W., Liu, P., & Chen, A. (2025). Ozone Treatment Modulates Reactive Oxygen Species Metabolism Regulation and Enhances Storage Quality of Kiwifruit During Cold Storage. Horticulturae, 11(8), 911. https://doi.org/10.3390/horticulturae11080911





