Abstract
Flavonoids serve as crucial plant antioxidants in drought tolerance, yet their antioxidant regulatory mechanisms within mycorrhizal plants remain unclear. In this study, using a two-factor design, trifoliate orange (Poncirus trifoliata (L.) Raf.) seedlings in the four-to-five-leaf stage were either inoculated with Funneliformis mosseae or not, and subjected to well-watered (70–75% of field maximum water-holding capacity) or drought stress (50–55% field maximum water-holding capacity) conditions for 10 weeks. Plant growth performance, photosynthetic physiology, leaf flavonoid content and their antioxidant capacity, reactive oxygen species levels, and activities and gene expression of key flavonoid biosynthesis enzymes were analyzed. Although drought stress significantly reduced root colonization and soil hyphal length, inoculation with F. mosseae consistently enhanced the biomass of leaves, stems, and roots, as well as root surface area and diameter, irrespective of soil moisture. Despite drought suppressing photosynthesis in mycorrhizal plants, F. mosseae substantially improved photosynthetic capacity (measured via gas exchange) and optimized photochemical efficiency (assessed by chlorophyll fluorescence) while reducing non-photochemical quenching (heat dissipation). Inoculation with F. mosseae elevated the total flavonoid content in leaves by 46.67% (well-watered) and 14.04% (drought), accompanied by significantly enhanced activities of key synthases such as phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), 4-coumarate:coA ligase (4CL), and cinnamate 4-hydroxylase (C4H), with increases ranging from 16.90 to 117.42% under drought. Quantitative real-time PCR revealed that both mycorrhization and drought upregulated the expression of PtPAL1, PtCHI, and Pt4CL genes, with soil moisture critically modulating mycorrhizal regulatory effects. In vitro assays showed that flavonoid extracts scavenged radicals at rates of 30.07–41.60% in hydroxyl radical (•OH), 71.89–78.06% in superoxide radical anion (O2•−), and 49.97–74.75% in 2,2-diphenyl-1-picrylhydrazyl (DPPH). Mycorrhizal symbiosis enhanced the antioxidant capacity of flavonoids, resulting in higher scavenging rates of •OH (19.07%), O2•− (5.00%), and DPPH (31.81%) under drought. Inoculated plants displayed reduced hydrogen peroxide (19.77%), O2•− (23.90%), and malondialdehyde (17.36%) levels. This study concludes that mycorrhizae promote the level of total flavonoids in trifoliate orange by accelerating the flavonoid biosynthesis pathway, hence reducing oxidative damage under drought.
1. Introduction
Drought is one of the most severe abiotic stresses confronting global agricultural production in the 21st century, further threatening global food security [1]. Under drought stress, plants reduce water loss by closing stomata, while this process simultaneously decouples light and dark reactions in photosynthesis and activates enzymes such as NADPH oxidase, triggering excessive accumulation of reactive oxygen species (ROS)—including superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and singlet oxygen (1O2) [2,3]. Drought-induced ROS overaccumulation attacks unsaturated fatty acids in cell membranes, initiating lipid peroxidation, protein denaturation, and DNA damage, ultimately leading to programmed cell death [4]. This oxidative damage progressively impairs plant growth and development, and even causes plant death in severe cases.
Plants have evolved a complex antioxidant defense system to counteract drought-induced ROS accumulation, comprising enzymatic and non-enzymatic components. The enzymatic system includes superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase [5]; the non-enzymatic system encompasses antioxidants such as ascorbic acid, glutathione, tocopherols, carotenoids, and flavonoids [6]. Among them, flavonoids, as an important class of secondary metabolites in plants predominantly accumulating in aerial tissues (e.g., leaves and flowers), play a pivotal role in antioxidant defense of plants against drought stress due to their unique chemical structures (e.g., phenolic hydroxyl and catechol groups) and potent ROS scavenging capabilities [7,8]. Flavonoids derive from phenylalanine through a cascade catalyzed by phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), chalcone synthase (CHS), chalcone isomerase (CHI), and 4-coumaroyl CoA ligase (4CL), enabling stepwise conversion into chalcones and downstream derivatives [8]. Notably, drought upregulates the expression of flavonoid biosynthesis-related genes to enhance its accumulation, as demonstrated in grape berries in which elevated flavonoids improved drought tolerance [9].
Arbuscular mycorrhizal (AM) fungi, as crucial symbiotic partners of plant roots, play a key role in plant environmental adaptation [10,11]. AM fungi significantly enhance host plant tolerance to abiotic stress by expanding the root surface area and directly participating in the uptake of nutrients and water [11,12]. Flavonoid compounds secreted by host plant roots act as key signaling molecules, driving early recognition and development during the establishment of AM symbiosis by inducing AM fungal spore germination, hyphal growth, and branching [13,14]. The application of AM fungi can more than double the total flavonoid content in plants such as Calendula officinalis, Origanum majorana, and Melissa officinalis [15]. In Libidibia ferrea, AM fungal inoculation significantly increased leaf total flavonoid content (17%) and concurrently enhanced total antioxidant activity, an effect independent of phosphorus fertilizer application [16]. Another study showed that AM fungi positively regulated the flavonoid biosynthesis pathway by upregulating the expression of CHS gene [17]. Similarly, under moderate drought stress, a mixed AM fungal inoculation significantly increased total flavonoid levels in Fagopyrum esculentum leaves, which were positively correlated with plant antioxidant activity [18]. This reveals that mycorrhizae improve host drought tolerance by enhancing the total flavonoid-mediated antioxidant defense system. It is worth noting that AM regulation of flavonoid metabolism exhibits environmental response patterns. Under combined elevated CO2 and Cd pollution, an AM fungus (Funneliformis mosseae) downregulated PAL and CHS gene expression levels and corresponding enzyme activities to reduce flavonoid synthesis in Robinia pseudoacacia leaves, as mycorrhizal hyphae directly adsorbed Cd, reducing the plant’s need for endogenous flavonoid defense [19]. Conversely, under Cd stress, Rhizophagus irregularis inoculation specifically promoted the accumulation of flavonoids such as eriodictyol and genistein in Broussonetia papyrifera, while components like rutin, quercetin, myricetin, and kaempferol remained stable, accompanied by upregulated expression of flavonoid synthesis genes such as BpC4H2, BpCHS8, and BpCHI5 [20]. This demonstrates that AM fungi regulate flavonoid accumulation to meet specific environmental demands by influencing the expression levels of specific genes within the flavonoid synthesis pathway.
Citrus is one of the most economically important fruit trees globally. However, in citrus-producing regions, soil drought has become a key factor limiting the high yield and quality of citrus due to insufficient annual rainfall, seasonal drought, steep mountainous terrain, and a lack of irrigation facilities [3]. It has been confirmed that AM fungi can enhance citrus drought tolerance [5,14], but the underlying mechanisms remain unclear. We hypothesized that AM fungi promote total flavonoid levels in citrus by accelerating the flavonoid biosynthetic pathway to reduce oxidative damage under drought. The objective of this study was to analyze whether AM fungi alleviate oxidative damage in drought-stressed trifoliate orange (Poncirus trifoliata (L.) Raf., a citrus rootstock) by modulating flavonoid synthesis and accumulation.
2. Materials and Methods
2.1. Preparation of AMF Inoculum
Funneliformis mosseae (Nicol. & Gerd.) Schüßler & Walker, strain BGC XZ02A, was obtained from the Bank of Glomeromycota in China (BGC). This strain was cultivated in sterilized substrates using white clover (Trifolium repens L.) as the host plant under controlled greenhouse conditions. The resulting inoculum consisted of a mixture containing hyphae, colonized root fragments, and spores at a density of 22 spores per gram.
2.2. Plant Culture and Treatments
Trifoliate orange seeds underwent initial processing by immersion in 1 mol/L NaOH to remove pectins, followed by surface disinfection in 75% ethanol for 5 min. After thorough rinsing with sterile water, seeds were germinated in autoclaved river sand within a growth chamber at 28 °C (day)/20 °C (night), 68% relative humidity, a 16 h photoperiod, and light intensity of 1250 Lux. Seedlings exhibiting uniform growth at the 4–5-leaf stage were then transferred to plastic pots. Each pot contained 2.38 kg of an autoclaved soil–sand substrate (3:1, v/v). The physicochemical characteristics of the substrate were pH 6.2, ammonium nitrogen at 45.73 mg/kg, nitrate nitrogen at 15.36 mg/kg, Olsen-P at 49.23 mg/kg, and soil organic carbon at 9.86 g/kg.
At transplantation, the AM fungus was introduced. Pots designated for the mycorrhizal treatment (+AMF) received 120 g of F. mosseae inoculum. Control pots (-AMF) received the same quantity of autoclaved F. mosseae inoculum, amended with 3 mL of a filtrate (25 μm) from the live inoculum to maintain a comparable microflora background (excluding viable AMF propagules) between treatments. All plants were subsequently maintained under well-watered (WW) conditions (70–75% of field maximum water-holding capacity) for an 8-week pre-culture period. Following this, a randomized selection of half the plants from both the +AMF and -AMF groups were subjected to drought stress (DS; 50–55% field maximum water-holding capacity) for 10 weeks. Substrate moisture content was regulated daily by gravimetric measurement (pot weighing) and water replenishment.
2.3. Experimental Design
The experiment comprised four treatments: WW + AMF, WW-AMF, DS + AMF, and DS-AMF. Each treatment included six biological replicates (pots), with three seedlings maintained per pot. This resulted in a total of 24 pots and 72 seedlings across the experiment. Pots were regularly moved to minimize microenvironmental heterogeneity.
2.4. Variable Measurement
After completing the 10-week DS treatment, plants underwent harvest. Prior to harvest, leaf photosynthetic parameters including net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) were quantified using a Li-6400 portable photosynthesis system. Concurrently, chlorophyll fluorescence parameters, including maximum photochemical efficiency (QY_max), steady-state quantum efficiency (QY_Lss), and steady-state non-photochemical quenching (NPQ_Lss), were recorded with a FluorCam instrument (Photon Systems Instruments, Drásov, Czech Republic).
At harvest, plants were dissected into leaves, stems, and roots for biomass measurement. Root morphology was analyzed by scanning with an Epson J221A root scanner; WinRHIZO software (2007v, Regent Instruments Company, Quebec, QC, Canada) was then employed to determine root total surface area and average diameter.
Root mycorrhizal colonization was stained with trypan blue [21], calculating the colonization rate (%) as the proportion of infected root segment length relative to the total observed root length. Additionally, soil hyphal length density was measured following the protocol established by Bethlenfalvay and Ames [22].
Leaf H2O2 content was measured following Velikova et al. [23], O2•− content using the method of Wang and Luo [24], and malondialdehyde (MDA) content according to Sudhakar et al. [25].
Total flavonoid content in leaves was extracted and determined using the method of Sharma and Janmeda [26], with rutin as the standard. For scavenging rate assays of •OH, O2•−, and DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals, 0.1 g of dry leaf samples was extracted with 100 mL of 70% ethanol in the dark for 24 h. •OH scavenging was measured by using the salicylic acid method [27], O2•− scavenging by using the pyrogallol method [14], and DPPH scavenging by using the method of Torey et al. [28].
For enzyme activity assays, 0.5 g of fresh leaf samples was ground in 5 mL of 0.1 mol/L phosphate buffer (pH 7.6) on ice, centrifuged at 10,000× g/min for 10 min at 4 °C, and the supernatant was used as the crude extract. PAL activity was determined following Wang and Huang [29]; CHS and CHI activities were measured via ELISA (kits from Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China); 4CL activity was assessed according to Knoblock and Hahlbrock [30]; C4H activity was assayed according to Lamb and Rubery [31].
Total RNA isolation was performed using a TaKaRa extraction kit, followed by assessment of RNA concentration and purity. cDNA was subsequently generated using the PrimeScript™ RT reagent kit (Takara Bio Inc., Shiga, Japan). Nucleotide sequences for key flavonoid biosynthesis genes (PtPAL1, PtCHI, Pt4CL, and PtC4H) were identified through alignment against the NCBI database and the Citrus Pan-Genome Breeding Database (http://citrus.hzau.edu.cn, accessed on 10 August 2022). Gene-specific primers (Supplementary Material Table S1) were designed with Primer Premier 5.0 software. Quantitative reverse transcription PCR (qRT-PCR) analysis was conducted on three biological replicates per treatment group. Reactions utilized 2× AceQ qPCR SYBR Green Master Mix (Aidlab), with β-actin serving as the reference gene. Relative transcript levels were computed via the comparative threshold cycle (2−ΔΔCt) method [32], using the WW-AMF treatment as the calibrator for normalization.
2.5. Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) in SAS software (8.1v). Prior to ANOVA, assumptions of normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) were confirmed. Post hoc pairwise comparisons of treatment means were conducted using Duncan’s New Multiple Range Test at a 5% level. Additionally, linear relationships between variables were assessed for significance using Pearson’s correlation coefficient (r) with t-tests (p < 0.05).
3. Results
3.1. Changes in Mycorrhizal Status
F. mosseae colonized the roots of trifoliate orange (Figure 1a), with root colonization rates (Figure 1b) of 38.51–58.74% and soil hyphal length (Figure 1c) ranging from 22.28 cm/g to 25.84 cm/g. However, 10-week DS treatment significantly reduced both root mycorrhizal colonization rates and soil hyphal length by 34.44% and 13.78%, respectively, compared with WW conditions. A significant interaction effect between DS and AM fungal inoculation was observed on the root colonization rate and soil hyphal length (Table 1).
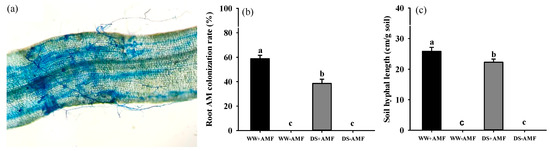
Figure 1.
Root mycorrhizal colonization (a) and changes in root colonization rate (b) and soil hyphal length (c) of Funneliformis mosseae-colonized trifoliate orange seedlings exposed to well-watered and drought stress. Data (means ± SD, n = 4) followed by different letters above bars indicate significant (p < 0.05) differences between treatments. Abbreviations: +AMF, inoculation with F. mosseae; -AMF, inoculation without F. mosseae; WW, well-watered; DS, drought stress.

Table 1.
Significance of variables between arbuscular mycorrhizal and non-arbuscular mycorrhizal trifoliate orange seedlings grown in well-watered and drought stress conditions.
3.2. Changes in Plant Growth Performance
DS did not significantly affect biomass accumulation in non-colonized plants compared to WW conditions (Table 2). However, colonized plants under DS versus WW conditions exhibited significant reductions in leaf (15.69%), stem (21.05%), and root biomass (20.17%) and root total surface area (6.57%). Regardless of soil moisture status, F. mosseae-colonized plants exhibited superior growth performance compared to non-colonized ones. Compared with non-inoculated treatment, AMF inoculation significantly promoted leaf, stem, and root biomass, as well as root total surface area and average diameter, with increases of 155.00%, 156.76%, 97.46%, 8.60%, and 36.00% under WW conditions and 168.75%, 158.62%, 84.16%, 7.50%, and 52.38% under DS conditions, respectively. Only one significant interaction effect was observed in root biomass (Table 2).

Table 2.
Effects of Funneliformis mosseae on plant growth of trifoliate orange seedlings exposed to well-watered and drought stress conditions.
3.3. Changes in Leaf Photosynthetic Physiology
Both DS and AM fungal inoculation significantly influenced leaf photosynthetic parameters (Table 3). Compared with WW conditions, DS distinctly reduced Pn, gs, and Tr in colonized plants by 37.60%, 28.95%, and 33.58%, respectively, while decreasing Ci in non-colonized plants by 14.03%. Inoculation with F. mosseae significantly enhanced Pn, gs, Ci, and Tr under WW conditions by 273.13%, 111.11%, 14.83%, and 470.83%, respectively, and under DS by 205.88%, 68.75%, 24.94%, and 405.56%, respectively, compared with non-inoculated treatment. Under WW conditions, the inoculation increased QY_max and QY_Lss by 70.59% and 228.57%, respectively, but decreased NPQ_Lss by 68.18%; under DS, the inoculation also triggered 73.33% and 480.00% increases in QY_max and QY_Lss, with a 62.34% decrease in NPQ_Lss. Drought treatment significantly raised QY_Lss (26.09%) in colonized plants and NPQ_Lss (16.67%) in non-colonized plants compared with WW controls. There was a significant interaction effect between DS and AM fungal inoculation on Pn, gs, Tr, and QY_Lss (Table 3).

Table 3.
Effects of Funneliformis mosseae on leaf photosynthetic physiology of trifoliate orange seedlings exposed to well-watered and drought stress conditions.
3.4. Changes in Leaf Total Flavonoid Content
Both DS treatment and AM fungal inoculation significantly altered the total flavonoid content in trifoliate orange leaves (Figure 2). Compared to non-AM fungal colonization treatment, inoculation with F. mosseae significantly increased leaf total flavonoid content by 26.65% and 23.34% under WW and DS conditions, respectively. DS treatment reduced leaf total flavonoid content in both colonized and non-colonized plants, with decreases of 14.36% and 12.06%, respectively. The two treatments did not show a significant interaction effect on total flavonoid content (Table 1).
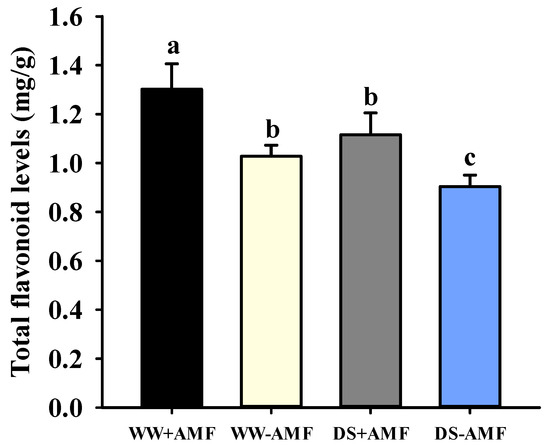
Figure 2.
The effects of Funneliformis mosseae on leaf total flavonoid levels in trifoliate orange seedlings exposed to well-watered and drought stress conditions. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences between treatments. Abbreviations: +AMF, inoculation with Funneliformis mosseae; -AMF, inoculation without F. mosseae; WW, well-watered; DS, drought stress.
3.5. Changes in the Antioxidant Capacity of Leaf Total Flavonoid Compounds
AMF inoculation and DS treatment differentially affected the capacity of flavonoid extracts from trifoliate orange leaves to scavenge •OH, O2•−, and DPPH (Figure 3). Specifically, the scavenging rates of flavonoid extracts ranged from 30.07% to 41.60% for •OH, from 71.89% to 78.06% for O2•−, and from 49.97% to 74.75% for DPPH. DS treatment did not exhibit a significant effect on the O2•− scavenging capacity of flavonoid extracts, regardless of AM fungal inoculation status. However, DS treatment significantly inhibited the •OH scavenging capacity of flavonoid extracts, reducing it by 13.93% in colonized plants and 17.64% in non-colonized plants. Conversely, compared to WW conditions, DS treatment increased the DPPH scavenging capacity of flavonoid extracts by 6.88% in colonized plants and 13.48% in non-colonized plants. Furthermore, compared to non-AM treatment, inoculation with F. mosseae significantly enhanced the scavenging capacity of leaf flavonoid extracts for •OH, O2•−, and DPPH by 13.93%, 5.94%, and 39.96% under WW conditions, respectively, and by 19.07%, 5.00%, and 31.81% under DS, respectively. The scavenging capacity of leaf flavonoid extracts for •OH, O2•−, and DPPH was not significantly affected by the interaction between DS and AM fungal inoculation (Table 1).
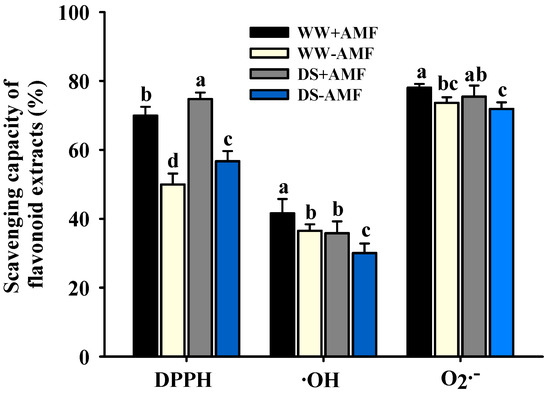
Figure 3.
The effects of Funneliformis mosseae on the •OH, O2•−, and DPPH scavenging rates of flavonoid extracts in leaves of trifoliate orange seedlings exposed to well-watered and drought stress conditions. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences between treatments. Abbreviations: +AMF, inoculation with Funneliformis mosseae; -AMF, inoculation without F. mosseae; WW, well-watered; DS, drought stress; •OH, hydroxyl radical; O2•−, superoxide radical anion; DPPH, 2,2-diphenyl-1-picrylhydrazyl.
3.6. Changes in Leaf ROS and MDA Levels
Compared to WW conditions, DS treatment significantly increased H2O2 accumulation in trifoliate orange leaves (Figure 4b). Specifically, H2O2 levels increased by 20.65% in colonized plants and by 25.57% in non-colonized plants. However, DS treatment significantly increased O2•− levels only in colonized plants by 31.12%, with no significant change observed in non-colonized plants (Figure 4a). Compared to inoculation without F. mosseae, inoculation with F. mosseae significantly reduced leaf H2O2 and O2•− levels, with reductions of 16.50% (H2O2) and 40.09% (O2•−) under WW conditions and 19.77% and 23.90% under DS, respectively. Similarly, F. mosseae inoculation significantly reduced leaf MDA levels compared to non-inoculated controls, with reductions of 41.17% under WW conditions and 17.36% under DS (Figure 4c). DS treatment significantly increased leaf MDA levels in trifoliate orange seedling leaves compared to WW conditions, with increases of 36.15% in colonized plants and 13.18% in non-colonized plants. A significant interaction effect was observed only in leaf O2•− levels (Table 1).
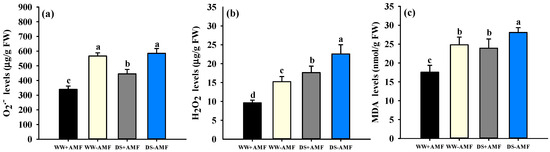
Figure 4.
The effects of Funneliformis mosseae on O2•− (a), H2O2 (b), and MDA (c) levels in leaves of trifoliate orange seedlings exposed to well-watered and drought stress. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences between treatments. Abbreviations: +AMF, inoculation with Funneliformis mosseae; -AMF, inoculation without F. mosseae; WW, well-watered; DS, drought stress; O2•−, superoxide radical anion; H2O2, hydrogen peroxide; MDA, malondialdehyde.
3.7. Changes in Key Flavonoid Biosynthesis Enzyme Activities in Leaves
Both DS treatment and AM fungal inoculation differentially altered the activities of key enzymes involved in flavonoid biosynthesis in trifoliate orange leaves (Figure 5). DS treatment significantly inhibited the activities of CHS and CHI in colonized plants, reducing them by 17.76% and 29.09%, respectively, compared to WW conditions. The activities of PAL, C4H, and 4CL in colonized plants were not significantly affected under DS versus WW conditions. In non-colonized plants, DS treatment significantly inhibited the activities of PAL, CHS, and CHI, reducing them by 10.37%, 26.67%, and 54.01%, respectively, compared with WW treatment. Compared to non-colonized plants, F. mosseae-colonized plants exhibited significantly higher activities of PAL, CHS, CHI, 4CL, and C4H, with increases of 13.59%, 37.69%, 41.03%, 26.40%, and 20.34% under WW conditions and increases of 28.46%, 54.42%, 117.42%, 29.22%, and 16.09% under DS, respectively. No significant interaction effect was observed on these enzyme activities (Table 1).
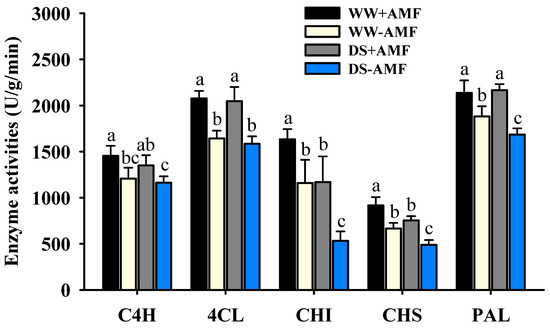
Figure 5.
The effects of Funneliformis mosseae on C4H, 4CL, CHI, CHS, and PAL activities in leaves of trifoliate orange seedlings exposed to well-watered and drought stress conditions. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences between treatments. Abbreviations: +AMF, inoculation with Funneliformis mosseae; -AMF, inoculation without F. mosseae; WW, well-watered; DS, drought stress; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:coA ligase; CHI, chalcone isomerase; CHS, chalcone synthase; PAL, phenylalanine ammonia-lyase.
3.8. Changes in Expression Levels of Key Flavonoid Biosynthesis Enzyme Genes in Leaves
The expression levels of key flavonoid biosynthesis enzyme genes in trifoliate orange leaves were influenced by both drought and AM fungal inoculation treatments (Figure 6). Compared to WW conditions, DS conditions significantly upregulated the relative expression of PtPAL1 (2.43-fold), PtCHI (1.05-fold), Pt4CL (1.04-fold), and PtC4H (0.42-fold) in colonized plants. Meanwhile, DS treatment significantly upregulated only PtC4H expression (0.65-fold) and had no significant effect on PtPAL1, PtCHI, or Pt4CL expression in non-colonized plants. Under DS, inoculation with F. mosseae significantly upregulated PtPAL1 expression 0.95-fold compared to non-inoculated plants, but significantly downregulated its expression 0.46-fold under WW conditions. The effect of AM fungal inoculation on PtCHI expression was soil moisture-dependent: there was no significant effect under WW conditions and but it did significantly upregulate expression (3.11-fold) under DS. Similarly, the effect of AM fungal inoculation on Pt4CL expression was strongly modulated by soil moisture: 0.34-fold downregulation under WW conditions and significant upregulation (0.73-fold) under DS. AMF inoculation did not significantly alter the relative expression of PtC4H, regardless of soil moisture conditions. A significant interaction effect was observed on the expression of PtPAL1, PtCHI, and Pt4CL (Table 1).
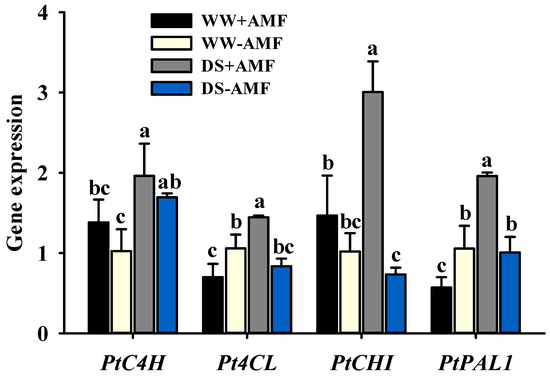
Figure 6.
The effects of Funneliformis mosseae on the expression of PtC4H, Pt4CL, PtCHI, and PtPAL1 genes in leaves of trifoliate orange seedlings exposed to well-watered and drought stress conditins. Data (means ± SD, n = 4) followed by different letters above the bars indicate significant (p < 0.05) differences between treatments. Abbreviations: +AMF, inoculation with Funneliformis mosseae; -AMF, inoculation without F. mosseae; WW, well-watered; DS, drought stress; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:coA ligase; CHI, chalcone isomerase; PAL1, phenylalanine ammonia-lyase 1.
3.9. Correlation Analysis
The correlation analysis among variables revealed that leaf total flavonoid levels exhibited significantly positive correlations with both root mycorrhizal colonization rate (r = 0.80, p < 0.01) and soil hyphal length (r = 0.83, p < 0.01) (Table 4). Additionally, leaf total flavonoid levels, root mycorrhizal colonization rate, and soil hyphal length showed significantly positive correlations with the •OH, O2•−, and DPPH scavenging rates of flavonoid extracts. Conversely, the three variables exhibited significantly negative correlations with O2•−, H2O2, and MDA levels.

Table 4.
Pearson’s correlation coefficient for the selected variables (n = 16).
4. Discussion
This study showed that F. mosseae colonized trifoliate orange roots, but DS restricted its colonization in roots and hyphal formation in soil. The dual negative effects likely originated from (i) drought-induced reduction in host photosynthates, limiting carbon supply to mycorrhizae [33], (ii) decreased soil water potential directly impairing hyphal growth and branching [34], and (iii) altered root exudate composition under DS weakening chemotactic signals for fungal recruitment [35].
The present results show that F. mosseae inoculation significantly improved various organ biomass and root morphological parameters (e.g., total surface area and average diameter) under drought, aligning with known AM fungal roles in water/nutrient uptake through hyphal networks [36,37] and drought buffering by regulating host carbon allocation (e.g., prioritizing photosynthates to mycorrhizae) [38,39]. Additionally, the 52.38% increase in root average diameter of colonized plants under drought suggests improved hydraulic efficiency in roots [40], though prolonged drought may elevate metabolic costs from mycorrhizal carbon demands. In addition, this study demonstrates that DS treatment significantly inhibited Pn, Gs, and Tr in mycorrhizal plants but only lowered Ci in non-mycorrhizal plants, indicating stomatal limitations (reduced CO2 supply due to lower Gs) versus metabolic limitations (e.g., reduced Rubisco enzyme activity), respectively. Notably, AM fungal inoculation significantly mitigated the DS-induced suppression of photosynthesis in trifoliate orange seedlings, elevating Pn by 205.88% and enhancing PSII efficiency (QY_max and QY_Lss) while reducing non-photochemical quenching (NPQ_Lss) under drought. This suggests that mycorrhizal plants maintained a higher proportion of open PSII reaction centers under light-adapted conditions, directing more energy towards photochemistry [41]. The reduced NPQ_Lss reflects a diminished thylakoid proton gradient, potentially resulting from AM-assisted mitigation of photoinhibition [42]. Moreover, DS increased QY_Lss in mycorrhizal plants (26.09%) but increased NPQ_Lss in non-mycorrhizal plants (16.67%), further confirming the role of AM fungi in photoprotection under drought. In summary, F. mosseae enhanced host drought tolerance through a dual mechanism: (1) alleviating stomatal limitations through water acquisition, and (2) optimizing light energy utilization efficiency to minimize photodamage.
This study demonstrates a significant positive correlation of soil hyphal length and root mycorrhizal colonization rate with leaf total flavonoid content in trifoliate orange, suggesting that AM symbiosis stimulates flavonoid biosynthesis. Additionally, DS treatment led to a significant reduction in leaf total flavonoid content in both colonized and non-colonized plants, likely due to drought-induced carbon reallocation away from flavonoid synthesis [43]. Notably, the reduction in leaf total flavonoid content under drought was less pronounced in colonized plants, suggesting that the symbiosis alleviates drought-induced suppression of flavonoid synthesis. This protective effect may occur through several interconnected mechanisms: (i) AM fungi enhance water uptake, thus maintaining cell turgor and metabolic activity [34]; (ii) AM fungi reduce ROS accumulation, thereby diminishing the consumption of flavonoid antioxidants [44]; and (iii) AM fungi may directly regulate flavonoid biosynthetic gene expression, potentially via fungal hyphal transport of signaling molecules like lysophosphatidic acid or through the upregulation of key pathway genes such as CHS [45,46]. Modulation of plant hormone signaling, such as jasmonic acid (JA) pathways, is another potential mechanism [47]. Consistent with these mechanisms, inoculation with F. mosseae significantly increased leaf total flavonoid content in trifoliate orange, irrespectively of soil moisture conditions, an effect observed in other species like Libidibia ferrea and linked to the upregulation of CHS genes independent of nutrient status [16]. Conversely, the magnitude of the AM-induced increase in leaf total flavonoid content was less pronounced under DS compared to WW conditions, indicating that drought partially counteracts the ability of AM fungi to stimulate flavonoid synthesis.
The antioxidant activity of flavonoids is closely associated with their capacity to scavenge free radicals [48]. In this study, drought treatment differentially affected the antioxidant capacity of flavonoids: it reduced the •OH scavenging capacity of flavonoids in all plants, had no significant effect on the O2•− scavenging capacity of flavonoids (likely due to SOD dominance), but distinctly increased the DPPH scavenging capacity of flavonoids, possibly through structural changes enhancing reducing power [49,50]. Crucially, inoculation with F. mosseae significantly enhanced the scavenging efficiency of the flavonoid extracts against all three radicals (•OH, O2•−, and DPPH) regardless of soil moisture. In addition, root colonization rate, soil hyphal length, and leaf total flavonoid content were all significantly and positively correlated with the •OH, O2•−, and DPPH scavenging rates of flavonoids. This suggests that AM plants can enhance ROS scavenging via flavonoids and mycorrhizal symbiosis under DS, thereby mitigating oxidative damage and burst [44]. The mechanism likely involves AM fungi promoting both the synthesis of highly active flavonoid compounds in the host plant and their targeted compartmentalization towards ROS generation sites like chloroplasts [51]. As a result, F. mosseae inoculation acts as a “biological amplifier” of flavonoid functionality, with root colonization and hyphal proliferation serving as biomarkers for predicting antioxidant gains. This synergy underscores AM’s role beyond nutrient acquisition—as a key regulator of plant metabolic resilience.
This study demonstrates that DS treatment triggered significant oxidative burst in trifoliate orange, evidenced by elevated leaf H2O2, O2•−, and MDA levels. Furthermore, AM fungal inoculation significantly reduced leaf H2O2 and O2•− levels under two soil moistures, indicating enhanced ROS scavenging and membrane stability in AM plants during drought. These findings align with previous studies on lavender [52], Dracocephalum moldavica [53], Bombax ceiba [54], and olive (Olea europaea) [55]. There was a significantly negative correlation of root AM fungal colonization rate, soil hyphal length, and leaf total flavonoid content with leaf H2O2, O2•−, and MDA levels, revealing that AM symbiosis and flavonoids collectively mitigate oxidative burst and damage [44]. This phenomenon is due to multiple interconnected mechanisms, including elevated activity and gene expression of antioxidant enzymes and higher accumulation of antioxidants (flavonoids, polyamines, and unsaturated fatty acids) [5,10,52,56,57,58]. In addition, AM fungi may optimize carbon allocation efficiency, reducing ROS leakage from photorespiration and mitochondrial electron transport chain under drought conditions [59].
In this study, DS treatment selectively inhibited key flavonoid biosynthetic enzyme activities, with effects modulated by AM symbiosis. In colonized plants, DS treatment suppressed only the activities of midstream enzymes, namely CHS and CHI, likely due to their redox sensitivity and pathway position [60]. In non-colonized plants, DS treatment additionally inhibited upstream PAL activity, with CHI showing greater suppression, suggesting that AM fungal inoculation partially protects enzyme function under drought. Crucially, AM fungal inoculation significantly enhanced the activity of all tested enzymes (PAL, CHS, CHI, 4CL, and C4H), but the magnitude increased along the pathway: minimal increase in upstream enzymes (PAL and C4H) and a strong increase in mid/downstream enzymes (CHS, CHI, and 4CL). This demonstrates that mycorrhizas primarily promote flavonoid synthesis by enhancing the activity of mid- to downstream enzymes in the flavonoid pathway. Strong positive correlations linked AM fungal colonization rate and soil hyphal length to all tested enzyme activities, confirming direct regulation by AM fungi. This finding aligns with the AM-induced increase in PAL, C4H, and 4CL activities and total flavonoid levels in Lycium barbarum under Fusarium solani infection [61]. This suggests a conserved strategy, namely that AM fungi enhance host stress resilience via targeted activation of flavonoid pathway enzymes, leading to elevated antioxidant (e.g., total flavonoid) production across stressors.
On the other hand, DS treatment differentially regulated the expression of flavonoid pathway genes in trifoliate orange leaves, with AM-colonized plants showing broader transcriptional sensitivity: DS upregulated PtPAL1, PtCHI, Pt4CL, and PtC4H expression in colonized plants, whereas only PtC4H was induced in non-colonized plants. The regulatory effect of AM treatment on the expression levels of PtPAL1, PtCHI, and Pt4CL was influenced by soil moisture, demonstrating context-dependent specificity. Specifically, under DS, inoculation with F. mosseae significantly upregulated PtPAL1 and PtCHI expression, while for Pt4CL, AM inoculation downregulated its expression under WW conditions but upregulated it under DS. This moisture-responsive pattern aligns with studies showing AM-induced upregulation of flavonoid genes in Broussonetia papyrifera under Cd stress [20] but suppression in Robinia pseudoacacia leaves under combined elevated CO2 and Cd pollution [19]. This further confirms the stress-context dependency of AM fungal gene regulation. Notably, an inconsistency emerged between gene expression and corresponding enzyme activity: under WW conditions, AM fungal inoculation significantly enhanced all five key enzyme activities despite suppressing PtPAL1 and Pt4CL transcripts and not altering PtC4H or PtCHI expression. This decoupling phenomenon highlights the multi-tiered regulatory complexity governing the flavonoid pathway, involving potential contributions from DNA methylation, post-translational modification, substrate availability, and enzyme activity hysteresis beyond transcriptional control [50,62].
5. Conclusions
This study conclusively demonstrates that F. mosseae significantly enhanced drought tolerance in trifoliate orange through multifaceted mechanisms, as evidenced by photosynthesis protection via stomatal limitation alleviation and optimized photochemistry (reduced NPQ and increased PSII efficiency), as well as mitigation of oxidative damage through flavonoid biosynthesis stimulation—particularly via upregulation of mid-pathway enzymes (CHS, CHI, and 4CL). AM fungi operate as a “biological amplifier” of flavonoid functioning to enhance ROS scavenging capacity and possibly promoting compartmentalization. This underscores AM symbiosis as a key regulator of plant metabolic quality and stress resilience. These mechanistic insights refine models of AM-mediated stress resilience. Future research should identify AM-induced bioactive flavonoid isoforms and their mechanisms, decipher hyphal-transported signaling molecules regulating flavonoid pathways, resolve gene–enzyme decoupling via post-transcriptional/post-translational analyses, and validate field efficacy across citrus rootstocks.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11080910/s1: Table S1: The primer sequences of genes used in the present study for qRT-PCR.
Author Contributions
Conceptualization, H.-N.M.; data curation, L.L.; investigation, L.L. and H.-N.M.; methodology, H.-N.M.; supervision, H.-N.M.; writing—original draft, L.L.; writing—review and editing, H.-N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research Project of Education Department of Hubei Province (B2019367) and the Ministry of Education Industry-University Collaborative Education Project (230828530707297). This work was also supported by the Joint Fund in Hubei Provincial Natural Science Foundation (JCZRLH202500740).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaffai, R.; Ganesan, M.; Cherif, A. Global drought threat: Impact on food security. In Plant Adaptation to Abiotic Stress: From Signaling Pathways and Microbiomes to Molecular Mechanisms; Chaffai, R., Ganesan, M., Cherif, A., Eds.; Springer Nature: Singapore, 2024; pp. 61–82. [Google Scholar]
- Rehaman, A.; Khan, S.; Rawat, B.; Gaira, K.S.; Asgher, M.; Semwal, P.; Tripathi, V. Mechanistic insights into plant drought tolerance: A multi-level perspective. J. Crop Health 2025, 77, 53. [Google Scholar] [CrossRef]
- Cao, J.L.; He, W.X.; Zou, Y.N.; Wu, Q.S. An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiol. 2023, 43, 452–466. [Google Scholar] [CrossRef]
- Zheng, F.L.; Tan, Z.P.; Zhang, Y.; Xu, X.H.; Hashem, A.; Debnath, A.; Wu, Q.S. Enhancing walnut growth and drought tolerance through Serendipita indica: Focus on mitochondrial antioxidant defense. Plant Growth Regul. 2024, 104, 1697–1706. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, M.A.; Kuca, K.; Alqahtani, M.D.; Muthuramalingam, P.; Wu, Q.S. Cloning of CAT genes in Satsuma mandarin and their expression characteristics in response to environmental stress and arbuscular mycorrhizal fungi. Plant Cell Rep. 2024, 43, 123. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Ma, D.; Guo, Y.; Ali, I.; Lin, J.; Xu, Y.; Yang, M. Accumulation characteristics of plant flavonoids and effects of cultivation measures on their biosynthesis: A review. Plant Physiol. Biochem. 2024, 215, 108960. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Ma, W.Y.; Qin, Q.Y.; Zou, Y.N.; Kuča, K.; Giri, B.; Wu, Q.S.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; Abd_Allah, E.F.; et al. Arbuscular mycorrhiza induces low oxidative burst in drought-stressed walnut through activating antioxidant defense systems and heat shock transcription factor expression. Front. Plant Sci. 2022, 13, 1089420. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, D.P.; Salwan, R. Surviving the stress: Understanding the molecular basis of plant adaptations and uncovering the role of mycorrhizal association in plant abiotic stresses. Microb. Pathog. 2024, 193, 106772. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef]
- Larose, G.; Chênevert, R.; Moutoglis, P.; Gagné, S.; Piché, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cheng, S.; Aroca, R.; Zou, Y.N.; Wu, Q.S. Arbuscular mycorrhizal fungi induce flavonoid synthesis for mitigating oxidative damage of trifoliate orange under water stress. Environ. Exp. Bot. 2022, 204, 105089. [Google Scholar] [CrossRef]
- Engel, R.; Szabó, K.; Abrankó, L.; Rendes, K.; Füzy, A.; Takács, T. Effect of arbuscular mycorrhizal fungi on the growth and polyphenol profile of marjoram, lemon balm, and marigold. J. Agric. Food Chem. 2016, 64, 3733–3742. [Google Scholar] [CrossRef]
- da Silva, F.A.; Sampaio, E.V.S.B.; da Silva, F.S.B.; Maia, L.C. Use of arbuscular mycorrhizal fungi and phosphorus for increase in the concentration of compounds with antioxidant activity in Libidibia ferrea. Res. Soc. Dev. 2021, 10, e13010413827. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Zhu, H.H.; Zhao, H.Q.; Yao, Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 2013, 170, 74–79. [Google Scholar] [CrossRef]
- Mohammadi, E.; Fattahi, M.; Barin, M.; Ashrafi-Saeidlou, S. Arbuscular mycorrhiza and vermicompost alleviate drought stress and enhance yield, total flavonoid concentration, rutin content, and antioxidant activity of buckwheat (Fagopyrum esculentum Moench). S. Afr. J. Bot. 2022, 148, 588–600. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, X.; Zhao, Y.; Wang, L.; Wang, Y. Adaptive response of flavonoids in Robinia pseudoacacia L. affected by the contamination of cadmium and elevated CO2 to arbuscular mycorrhizal symbiosis. Ecotoxicol. Environ. Saf. 2023, 263, 115379. [Google Scholar] [CrossRef]
- Deng, S.; Pan, L.; Ke, T.; Liang, J.; Zhang, R.; Chen, H.; Tang, M.; Hu, W. Rhizophagus irregularis regulates flavonoids metabolism in paper mulberry roots under cadmium stress. Mycorrhiza 2024, 34, 317–339. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Wang, A.G.; Luo, G.H. Quantitative relation between the reaction of hydroxylamin and superoxide anion radicals in plants. Plant Physiol. Commun. 1996, 32, 55–57. [Google Scholar]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Sharma, V.; Janmeda, P. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arab. J. Chem. 2014, 10, 509–514. [Google Scholar] [CrossRef]
- Su, S.G.; Wei, Y.L.; Huang, Y.J.; Huang, Y.Q.; Luo, G.X. Study on the antioxidant and free radical scavenging effects of total flavonoids from Rosa laevigata. J. Guangxi Univ. Chin. Med. 2015, 18, 47–48. [Google Scholar]
- Torey, A.; Sasidharan, S.; Latha, L.Y.; Sudhakaran, S.; Ramanathan, S. Antioxidant activity and total phenolic content of methanol extracts of Ixora coccinea. Pharm. Biol. 2010, 48, 1119–1123. [Google Scholar] [CrossRef]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Knoblock, K.H.; Hahlbrock, K. Isoenzymes of p-Coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur. J. Biochem. 1975, 52, 311–320. [Google Scholar] [CrossRef]
- Lamb, C.J.; Rubery, P.H. A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal. Biochem. 1975, 68, 554–561. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, B.; Man, J.; Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal fungi attenuate negative impact of drought on soil functions. Global Chang. Biol. 2024, 30, e17409. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, X.F.; Zou, Y.N.; Srivastava, A.K.; Alqahtani, M.D.; Wu, Q.S. Negotiating soil water deficit in mycorrhizal trifoliate orange plants: A gibberellin pathway. Environ. Exp. Bot. 2024, 219, 105658. [Google Scholar] [CrossRef]
- Yang, C.X.; Chen, S.J.; Hong, X.Y.; Wang, L.Z.; Wu, H.M.; Tang, Y.Y.; Hao, G.F. Plant exudates driven microbiome recruitment and assembly facilitates plant health management. FEMS Microbiol. Rev. 2025, 49, fuaf008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.L.; Wang, Y.J.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Mycorrhizae with Funneliformis mosseae regulate the trehalose synthesis and sucrose cleavage for enhancing drought tolerance in trifoliate orange. Sci. Hortic. 2023, 337, 113486. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated enhanced biomass, root morphological traits and nutrient uptake under drought stress: A meta-analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef]
- Nair, R.S.; Raju, S.; More, S.J.; Puthur, J.T.; Makasana, J.; Ravi, V. Evaluating non-photochemical quenching (NPQ) kinetics and photosynthetic efficiency in cassava (Manihot esculenta) subjected to variable high light conditions. Funct. Plant Biol. 2024, 51, FP24118. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Liu, S.N.; An, X.J.; Xu, C.Q.; He, D.M.; Li, X.N.; Chen, C.X.; Guo, B.L.; Xu, D.; Huang, J. Integrative transcriptomic-physiological analysis deciphers nitrogen-mediated carbon reallocation balancing growth and flavonoid metabolism in Epimedium pubescens. Front. Plant Sci. 2025, 16, 1539445. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Singla, P.; Garg, N. Plant flavonoids: Key players in signaling, establishment, and regulation of rhizobial and mycorrhizal endosymbioses. In Mycorrhiza-Function, Diversity, State of the Art; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 133–176. [Google Scholar]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or facilitators? Roles of lipids in plant root–microbe interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. In vivo antioxidant activity of common dietary flavonoids: Insights from the yeast model Saccharomyces cerevisiae. Antioxidants 2024, 13, 1103. [Google Scholar] [CrossRef]
- Huang, S.; Ke, W.; Lu, Q.; Gao, L.; Zhou, X.; Ma, C. Effects of total flavonoids from Taraxacum mongolicum Hand.-Mazz. on fermentation quality, antioxidant status and microbial community of Caragana korshinskii Kom. silage. Fermentation 2023, 9, 949. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Marulanda, A.; Porcel, R.; Barea, J.M.; Azcón, R. Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb. Ecol. 2007, 54, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Hoseinzadeh, M.; Mafakheri, S. Enhancing drought resistance in Dracocephalum moldavica L. through mycorrhizal fungal inoculation and melatonin foliar application. Sci. Rep. 2025, 15, 10051. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Liu, C.; Gao, Y.; Han, L.; Chu, H. Arbuscular mycorrhizal fungi contribute to reactive oxygen species homeostasis of Bombax ceiba L. under drought stress. Front. Microbiol. 2022, 13, 991781. [Google Scholar] [CrossRef]
- Fouad, M.O.; Essahibi, A.; Benhiba, L.; Qaddoury, A. Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Span. J. Agric. Res. 2014, 12, 763–771. [Google Scholar] [CrossRef]
- Wu, Q.S.; He, J.D.; Srivastava, A.K.; Zou, Y.N.; Kuca, K. Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol. 2019, 39, 1149–1158. [Google Scholar] [CrossRef]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ. Exp. Bot. 2020, 171, 103962. [Google Scholar] [CrossRef]
- Bücking, H.; Kafle, A. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Sun, T.; Zhang, C.; Liu, X.; Li, Y. Genome-wide classification and evolutionary analysis reveal diverged patterns of chalcone isomerase in plants. Biomolecules 2022, 12, 961. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Wang, B.; Zhang, C.; Wang, Y.; Li, R.; Yan, Y.K.; He, J. Arbuscular mycorrhizal fungi improve the disease resistance of Lycium barbarum to root rot by activating phenylpropane metabolism. Front. Plant Sci. 2024, 15, 1459651. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, H. Deciphering the intricate hierarchical gene regulatory network: Unraveling multi-level regulation and modifications driving secondary cell wall formation. Hortic. Res. 2024, 11, uhad281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).