Grapevine Berry Inner Necrosis Virus (GINV) and Grapevine Yellow Speckle Viroid 1 (GYSVd1) Exhibit Different Regulatory Effects on Soluble Sugars and Acids in ‘Welschriesling’ Grape Berries and Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Virus Detection
2.2. Determination of Soluble Sugar Fractions
2.3. Determination of Organic Acid Fractions
2.4. Measurement of Sugar Metabolism Essential Enzyme Activity
2.5. Measurement of Acid Metabolism Essential Enzyme Activity
2.6. Analysis of the Relative Gene Expression
2.7. Statistical Analysis
3. Results
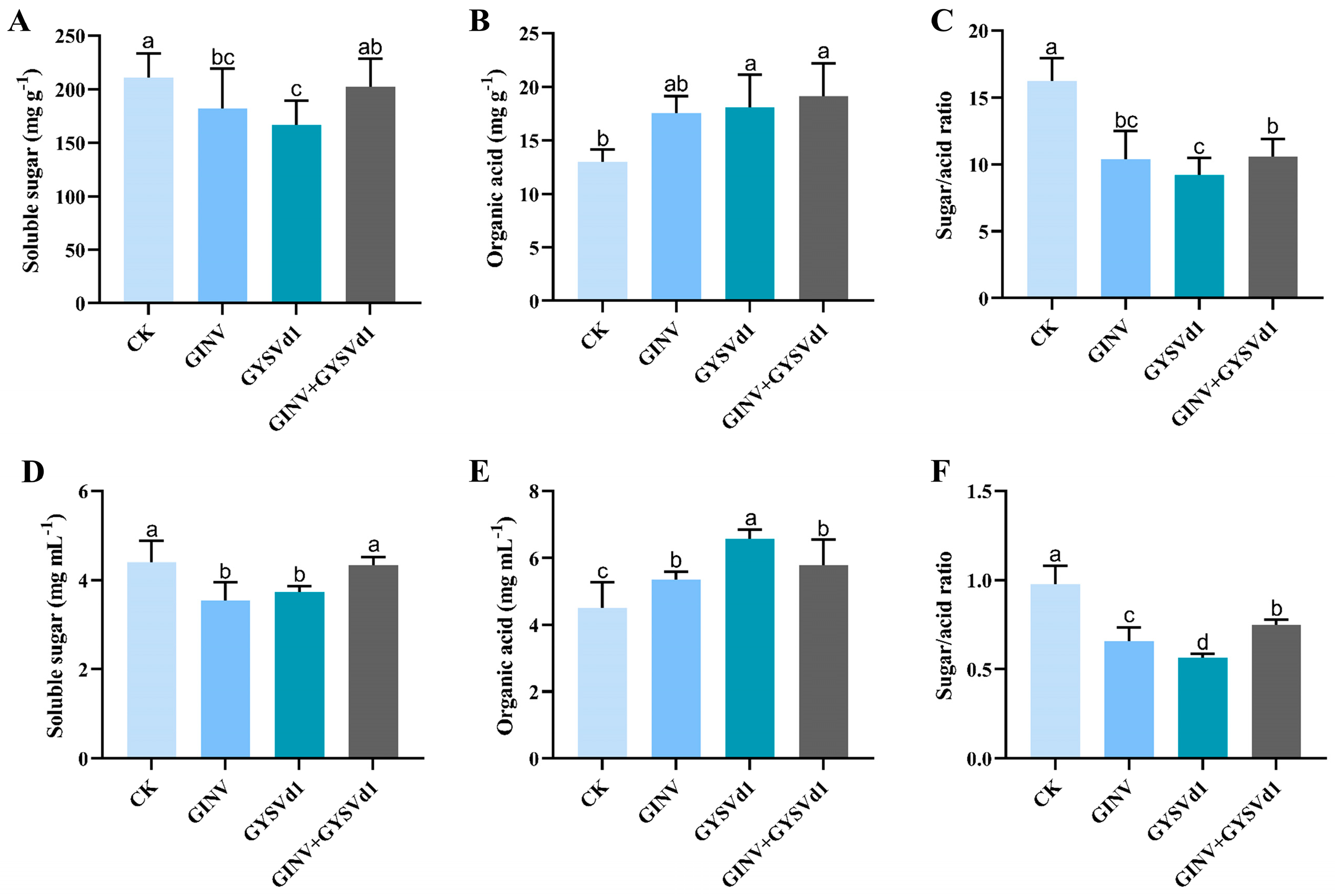
3.1. GINV, GYSVd1, and Mixed Infection Regulated Soluble Sugar and Organic Acid Content
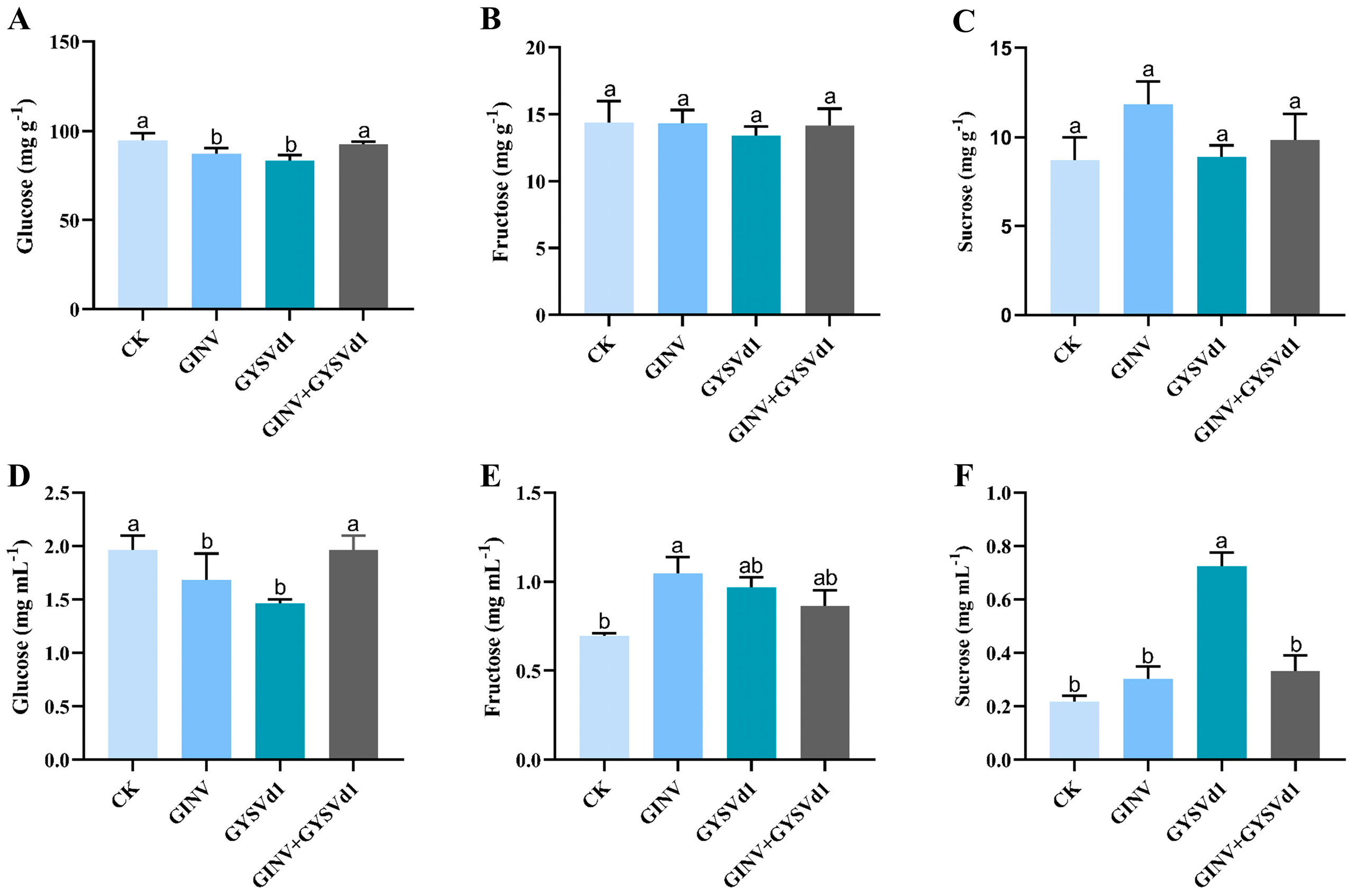
3.2. GINV, GYSVd1, and Mixed Infection Regulated Soluble Sugar Fraction Content
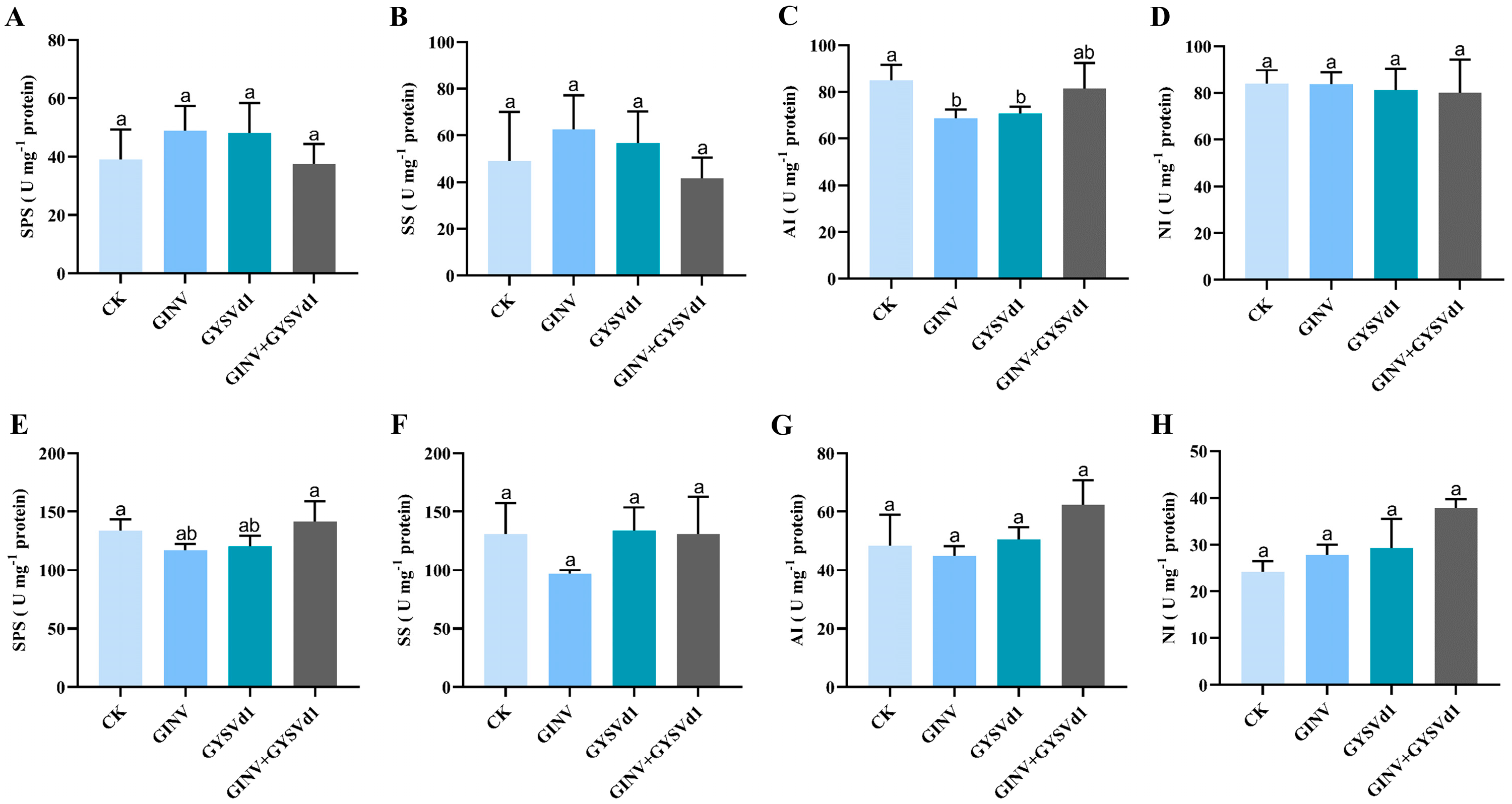
3.3. GINV, GYSVd1, and Mixed Infection Regulated Activity of Enzymes for Sugar Metabolism
3.4. GINV, GYSVd1, and Mixed Infection Regulated Expression Levels of Genes for Sugar Metabolism
3.5. GINV, GYSVd1, and Mixed Infection Regulated Organic Acid Fraction Content
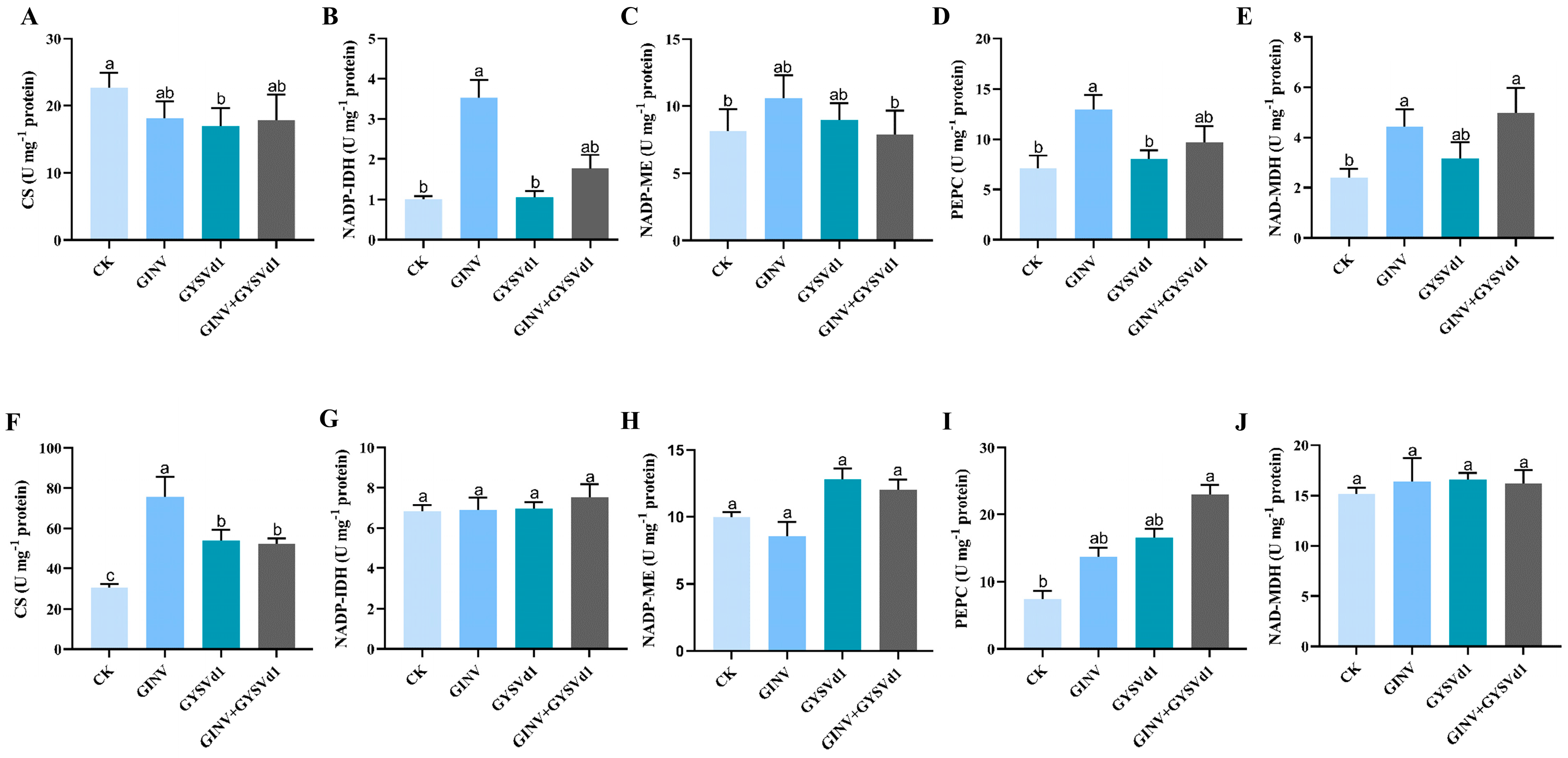
3.6. GINV, GYSVd1, and Mixed Infection Regulated Activity of Enzymes for Acid Metabolism
3.7. GINV, GYSVd1, and Mixed Infection Regulated Expression Levels of Genes for Acid Metabolism
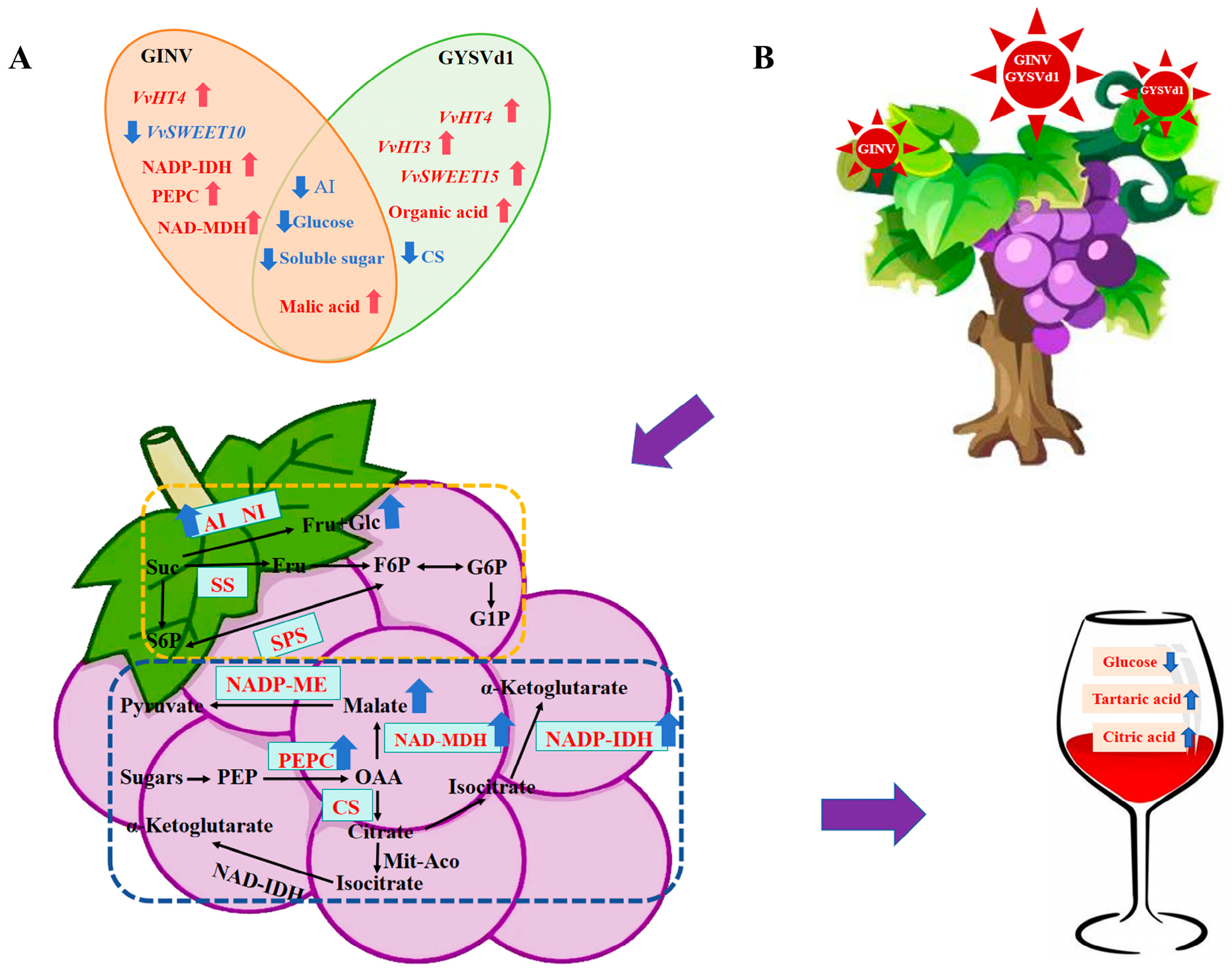
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bester, R.; Burger, J.T.; Maree, H.J. Transcriptome analysis reveals differentially expressed small RNAs and genes associated with grapevine leafroll-associated virus 3 infections. Physiol. Mol. Plant Pathol. 2017, 100, 220–236. [Google Scholar] [CrossRef]
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Pathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Vega, A.; Gutiérrez, R.A.; Peña, N.A.; Cramer, G.R.; Arce, J.P. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 2011, 77, 261–274. [Google Scholar] [CrossRef]
- Zhu, Y.; Zong, Y.; Wang, X.; Gong, D.; Zhang, X.; Zhang, F.; Bi, Y. Regulation of sucrose metabolism, sugar transport and pentose phosphate pathway by PacC in apple fruit colonized by Penicillium expansum. Food Chem. 2024, 461, 140863. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rennaker, C.D.; Thompson, B.D.; Karasev, A.V. Influence of grapevine red blotch virus (GRBV) on Idaho ‘Syrah’ grape composition. Sci. Hortic. 2021, 282, 110055. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.D.; Thompson, B.D.; Dahan, J.; Karasev, A.V. Idaho ‘Cabernet Sauvignon’ grape composition altered by grapevine leafroll-associated virus 3. NFS J. 2023, 31, 1–6. [Google Scholar] [CrossRef]
- Pereira, G.E.; Padhi, E.M.T.; Sudarshana, M.R.; Fialho, F.B.; Medina, P.C.; Girardello, R.C.; Oberholster, A. Impact of grapevine red blotch disease on primary and secondary metabolites in ‘Cabernet Sauvignon’ grape tissues. Food Chem. 2021, 342, 128312. [Google Scholar] [CrossRef]
- Malagnini, V.; de Lillo, E.; Saldarelli, P.; Beber, R.; Duso, C.; Raiola, A.; Gualandri, V. Transmission of grapevine Pinot gris virus by Colomerus vitis (Acari: Eriophyidae) to grapevine. Arch. Virol. 2016, 161, 2595–2599. [Google Scholar] [CrossRef]
- Saldarelli, P.; Giampetruzzi, A.; Morelli, M.; Malossini, U.; Pirolo, C.; Bianchedi, P.; Gualandri, V. Genetic Variability of Grapevine Pinot gris virus and Its Association with Grapevine Leaf Mottling and Deformation. Phytopathology 2015, 105, 555–565. [Google Scholar] [CrossRef]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A History of Grafting. Hortic. Rev. 2009, 35, 437–493. [Google Scholar]
- Wang, X.; Liu, Y.; Guo, L.; Shen, J.; Hu, H.; Zhou, R. Transcriptome analysis of Crimson seedless grapevine (Vitis vinifera L.) infected by grapevine berry inner necrosis virus. Curr. Res. Virol. Sci. 2022, 3, 100024. [Google Scholar] [CrossRef]
- Fan, X.; Hong, N.; Zhang, Z.; Yang, Z.; Ren, F.; Hu, G.; Wang, G. Identification of a divergent variant of grapevine berry inner necrosis virus in grapevines showing chlorotic mottling and ring spot symptoms. Arch. Virol. 2016, 161, 2025–2027. [Google Scholar] [CrossRef]
- Morgan, S.W.; Read, D.A.; Burger, J.T.; Pietersen, G. Diversity of viroids infecting grapevines in the South African Vitis germplasm collection. Virus Genes 2023, 59, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, X.Y.; Zhao, Y.W.; Wang, C.K.; Sun, Q.; Hu, D.G. Optimization of apple fruit flavor by MdVHP1-2 via modulation of soluble sugar and organic acid accumulation. Plant Physiol. Biochem. 2024, 206, 108227. [Google Scholar] [CrossRef]
- Zerihun, A.; McClymont, L.; Lanyon, D.; Goodwin, I.; Gibberd, M. Deconvoluting effects of vine and soil properties on grape berry composition. J. Sci. Food Agric. 2015, 95, 193–203. [Google Scholar] [CrossRef]
- Wei, L.; Wang, W.; Gao, X.; Yao, S.; Deng, L.; Zeng, K. Alterations in glucose metabolism and shikimate pathway affected by transient overexpression of CsPAL gene in postharvest citrus fruit. Postharvest Biol. Technol. 2024, 213, 112936. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Cao, S.; Di, H.; Zheng, Y. Effects of benzothiadiazole on disease resistance and soluble sugar accumulation in grape berries and its possible cellular mechanisms involved. Postharvest Biol. Technol. 2015, 102, 51–60. [Google Scholar] [CrossRef]
- Hedrich, R.; Sauer, N.; Neuhaus, H.E. Sugar transport across the plant vacuolar membrane: Nature and regulation of carrier proteins. Curr. Opin. Plant Biol. 2015, 25, 63–70. [Google Scholar] [CrossRef]
- Chen, Y.; Miller, A.J.; Qiu, B.; Huang, Y.; Zhang, K.; Fan, G.; Liu, X. The role of sugar transporters in the battle for carbon between plants and pathogens. Plant Biotechnol. J. 2024, 22, 2844–2858. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Hu, X.; Chao, P.; Ali, S.; Akram, M.T.; Naveed, W.A.; Chen, F. Magnesium’s impact on fruit quality of loquat: Insights into sugar and acid dynamics. Sci. Hortic. 2024, 328, 11297. [Google Scholar] [CrossRef]
- Simkova, K.; Veberic, R.; Hudina, M.; Grohar, M.C.; Pelacci, M.; Smrke, T.; Jakopic, J. Non-destructive and destructive physical measurements as indicators of sugar and organic acid contents in strawberry fruit during ripening. Sci. Hortic. 2024, 327, 112843. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.; Jin, Y.; Liu, J.; Wang, M.; Guo, Y.; Ge, Y. Phenyllactic acid regulated salicylic acid biosynthesis and organic acids metabolism in Zaosu pear fruit during storage. Sci. Hortic. 2024, 329, 112983. [Google Scholar] [CrossRef]
- Rajendar, B.; Mulagalapati, R.; Reddy, M.V.N.J.; Patri, S.; Karthik, Y.K.; Matur, R.V. 2-Phenoxyethanol: A novel reagent for improved sensitivity of carbohydrate quantification. Anal. Biochem. 2020, 595, 113624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Samkumar, A.; Karppinen, K.; Dhakal, B.; Martinussen, I.; Jaakola, L. Insights into sugar metabolism during bilberry (Vaccinium myrtillus L.) fruit development. Physiol. Plant. 2022, 174, 13657. [Google Scholar] [CrossRef]
- Elsa, D.Y.; Valentina, B.; Véronique, S.; Michel, G.; Bénédicte, Q.T. Profiling sugar metabolism during fruit development in a peach progeny with different fructose-to-glucose ratios. BMC Plant Biol. 2014, 14, 336–342. [Google Scholar]
- Gerós, H.; Alabi, O.J.; Casassa, L.F.; Gutha, L.R.; Larsen, R.C.; Henick, K.T.; Naidu, R.A. Impacts of grapevine leafroll disease on fruit yield and grape and wine chemistry in a wine grape (Vitis vinifera L.) cultivar. PLoS ONE 2016, 11, 149666. [Google Scholar]
- Shimizu, Y.H.; Tsujimoto, N.; Naka, T. Acid invertase activities of dahlia ‘Kokucho’ petals during flower opening and following cutting and treatment with 6-benzylaminopurine. Sci. Hortic. 2020, 272, 10952. [Google Scholar] [CrossRef]
- Xu, F.; Xi, Z.M.; Zhang, H.; Zhang, C.J.; Zhang, Z.W. Brassinosteroids are involved in controlling sugar unloading in Vitis vinifera ‘Cabernet Sauvignon’ berries during véraison. Plant Physiol. Biochem. 2015, 94, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Vignault, C.; Vachaud, M.; Cakir, B.; Glissant, D.; Dédaldéchamp, F.; Büttner, M.; Delrot, S. VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J. Exp. Bot. 2005, 56, 1409–1418. [Google Scholar] [CrossRef]
- Chen, L.Q.; Lin, I.W.; Qu, X.Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Frommer, W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the arabidopsis embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X.; Lin, Q.; Chen, J.; Grierson, D.; Yin, X.; Chen, K. Differential expression of organic acid degradation-related genes during fruit development of navel oranges (Citrus sinensis) in two habitats. Plant Mol. Biol. Report. 2013, 31, 1131–1140. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbegui, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Ma, F.F.; Hao, Y.J. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol. J. 2020, 19, 285–299. [Google Scholar] [CrossRef]
- Liu, H.F.; Wu, B.H.; Fan, P.G.; Xu, H.Y.; Li, S.H. Inheritance of sugars and acids in berries of grape (Vitis vinifera L.). Euphytica 2006, 153, 99–107. [Google Scholar] [CrossRef]
- Tollenaere, C.; Susi, H.; Laine, A.L. Evolutionary and epidemiological implications of multiple infection in plants. Trends Plant Sci. 2016, 21, 80–97. [Google Scholar] [CrossRef]
- Buckling, A.; Brockhurst, M.A. Kin selection and the evolution of virulence. Heredity 2008, 100, 484–488. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host–multi-pathogen warfare: Pathogen interactions in Co-infected plants. Front. Plant Sci. 2017, 8, 1086. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef] [PubMed]
- Barbu, S.P.; Bălănescu, I.; Tudor, G. Influence of viral infection on the quality of grapes at harvest. Hortic. Res. 2022, 54, 25–30. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Liu, S.; Wang, P.; Li, X.; Du, Y.; Zhu, S. Grapevine Berry Inner Necrosis Virus (GINV) and Grapevine Yellow Speckle Viroid 1 (GYSVd1) Exhibit Different Regulatory Effects on Soluble Sugars and Acids in ‘Welschriesling’ Grape Berries and Wine. Horticulturae 2025, 11, 879. https://doi.org/10.3390/horticulturae11080879
Wu M, Liu S, Wang P, Li X, Du Y, Zhu S. Grapevine Berry Inner Necrosis Virus (GINV) and Grapevine Yellow Speckle Viroid 1 (GYSVd1) Exhibit Different Regulatory Effects on Soluble Sugars and Acids in ‘Welschriesling’ Grape Berries and Wine. Horticulturae. 2025; 11(8):879. https://doi.org/10.3390/horticulturae11080879
Chicago/Turabian StyleWu, Menghuan, Shuo Liu, Ping Wang, Xin Li, Yejuan Du, and Shuhua Zhu. 2025. "Grapevine Berry Inner Necrosis Virus (GINV) and Grapevine Yellow Speckle Viroid 1 (GYSVd1) Exhibit Different Regulatory Effects on Soluble Sugars and Acids in ‘Welschriesling’ Grape Berries and Wine" Horticulturae 11, no. 8: 879. https://doi.org/10.3390/horticulturae11080879
APA StyleWu, M., Liu, S., Wang, P., Li, X., Du, Y., & Zhu, S. (2025). Grapevine Berry Inner Necrosis Virus (GINV) and Grapevine Yellow Speckle Viroid 1 (GYSVd1) Exhibit Different Regulatory Effects on Soluble Sugars and Acids in ‘Welschriesling’ Grape Berries and Wine. Horticulturae, 11(8), 879. https://doi.org/10.3390/horticulturae11080879





