Balanced Fertilization with Nitrogen, Molybdenum, and Zinc: Key to Optimizing Pecan Tree Yield and Quality of Western Schley Pecan Tree
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Location
2.2. Experimental Design and Treatments
2.3. Foliar Nutrition
2.4. Enzymatic Activity
- Endogenous NR: 100 mM potassium phosphate buffer (pH 7.5) + 1% propanol (J.T.Baker, Mexico City, State of Mexico, Mexico).
- NR + NO3: 100 mM potassium phosphate buffer (pH 7.5) with 50 mM potassium nitrate (KNO3) + 1% propanol.
- NR + Mo: 100 mM potassium phosphate buffer (pH 7.5) with 50 mM sodium molybdate (NaMoO4) + 1% propanol.
- NR + NO3 + Mo: 100 mM potassium phosphate buffer (pH 7.5) with 50 mM potassium nitrate (KNO3) and 50 mM sodium molybdate (NaMoO4) + 1% propanol.
2.5. Photosynthetic Pigments
- 470 nm (carotenoids)
- 653 nm (chlorophyll b)
- 666 nm (chlorophyll a)
2.6. Pomological Parameters
2.6.1. Yield
2.6.2. Number of Pecans per Kilogram
- Giant: 122 pecans or fewer per kilogram.
- Extra-large: 123–139 pecans per kilogram.
- Large: 140–170 pecans per kilogram.
- Medium: 171–210 pecans per kilogram.
- Small: 211 or more pecans per kilogram.
2.6.3. Kernel Percentage
- Quality I: Kernel percentage greater than 54%.
- Quality II: Kernel percentage between 50% and 54%.
2.7. Symptomatology and Foliar Development
2.8. Statistical Analysis
3. Results
3.1. Foliar Nutrition
3.2. Enzymatic Activity
3.3. Photosynthetic Pigments
3.4. Pomological Parameters
4. Discussion
4.1. Foliar Nutrition
4.2. Enzymatic Activity
4.3. Photosynthetic Pigments
4.4. Pomological Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moran-Duran, S.A.; Flynn, R.P.; Heerema, R.; VanLeeuwen, D. Leaf net photosynthesis, leaf greenness, and shoot lignin content of nonbearing pecan trees at two nitrogen and nickel application rates. HortScience 2020, 55, 231–236. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera. Producción Agrícola. Historico de Siembra y Cosecha. Available online: https://nube.agricultura.gob.mx/cierre_agricola/ (accessed on 22 September 2024).
- Wells, M.L. Pecan Response to Nitrogen Fertilizer Source. HortScience 2021, 56, 368–373. [Google Scholar] [CrossRef]
- Balandran-Valladares, M.I.; Cruz-Alvarez, O.; Jacobo-Cuellar, J.L.; Hernandez-Rodriguez, O.A.; Flores-Cordova, M.A.; Parra-Quezada, R.A.; Sanchez-Chavez, E.; Ojeda-Barrios, D.L. Changes in nutrient concentration and oxidative metabolism in pecan leaflets at different doses of zinc. Plant. Soil Environ. 2021, 67, 33–39. [Google Scholar] [CrossRef]
- Núñez-Moreno, J.H.; Walworth, J.; Pond, A.; Kilby, M. Effect of nitrogen rates on ‘Western’ pecan tree development. Acta Hortic 2019, 1217, 103–110. [Google Scholar] [CrossRef]
- Hounnou, L.; Wade-Brorsen, B.; Biermacher, J.T.; Rohla, C.T. Foliar applied zinc and the performance of pecan trees. J. Plant Nutr. 2019, 42, 512–516. [Google Scholar] [CrossRef]
- Walworth, J.L.; White, S.A.; Comeau, M.J.; Heerema, R.J. Soil-applied ZnEDTA: Vegetative growth, nut production, and nutrient acquisition of immature pecan trees grown in an alkaline, calcareous soil. HortScience 2017, 52, 301–305. [Google Scholar] [CrossRef]
- Ortega-Montes, F.I.; Rubio-Arias, H.O.; Clemente-Sanchez, F.; Uranga-Valencia, L.P. Cost-benefit analysis of the best combination of organic and inorganic sources to supply zinc deficiency in pecan (Carya illinoinensis [wangenh] k. Koch). ECORFAN J. 2023, 14, 55–59. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Confortin, T.C.; Todero, I.; Rodrigues, A.S.; Ribeiro, S.R.; Sasso, S.R.; Canabarro, N.I.; Wnager, R.; Cichoski, A.J.; Mazutti, M.A.; et al. Use of compressed fluids in the recovery of pecan nut cake oil: Influence of extraction conditions on yield and extract quality. J. Supercrit. Fluids 2020, 161, 104820. [Google Scholar] [CrossRef]
- Rana, M.S.; Bhantana, P.; Imran, M.; Saleem, M.H.; Moussa, M.G.; Khan, Z.; Imran, K.; Alam, M.; Abbas, M.; Binyamin, R.; et al. Molybdenum potential vital role in plants metabolism for optimizing the growth and development. Ann. Environ. Sci. Toxicol. 2020, 4, 32–44. [Google Scholar]
- Muñoz-Márquez, E.; Soto-Parra, J.M.; Noperi-Mosqueda, L.C.; Sánchez, E. Application of molybdenum nanofertilizer on the nitrogen use efficiency, growth and yield in green beans. Agronomy 2022, 12, 3163. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Orozco-Meléndez, L.R.; Hernández-Rodríguez, O.A.; Cruz-Alvarez, O.; Benavides-Mendoza, A.; Calderón-Jurado, M.; Ojeda-Barrios, D.L. Does the application of growth bioregulators improve the foliar concentration of nutrients, non-structural carbohydrates and yield in pecan? Ciênc. Agrotec. 2021, 45, e004721. [Google Scholar] [CrossRef]
- Torres-Beltran, N.G.; Yañez-Munoz, R.M.; Soto-Parra, J.M.; Noperi-Mosqueda, L.C. Nutritional standards through Integrated Differential Diagnosis (IDD) in pomegranate (Punica granatum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 12988. [Google Scholar] [CrossRef]
- Chávez, E.S. Caracterización del Estado Nutricional y Fisiológico en Plantas de Judía (Phaseolus vulgaris L. cv. Strike) Sometidas a un Estrés por Nitrógeno. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2006. Available online: https://digibug.ugr.es/bitstream/handle/10481/1348/16475185.pdf?sequence=1&isAllowed=y (accessed on 23 June 2025).
- Krywult, M.; Bielec, D. Method of measurement of nitrate reductase activity in field conditions. J. Ecol. Eng. 2013, 14, 1. [Google Scholar]
- NMX-FF-084-SCFI-2009. Norma Oficial Mexicana. Productos Alimenticios no Industrializados Para Consumo Humano—Fruto Fresco—Nuez Pecanera Carya illinoensis (Wangenh) K. Koch—Especificaciones y Métodos de Prueba. 2009. Available online: http://comenuez.com (accessed on 22 September 2024).
- Vargas, H.M.; Zarate, D.L.G.P.; Burguete, H.F. Factoriales fraccionados y superficie de respuesta, uso de paquetes estadísticos para microcomputadoras. Monogr. Man. Estadística Cómputo. 1991, 10, 1. [Google Scholar]
- Oviedo-Mireles, J.C.; Yáñez-Muñoz, R.M.; Soto-Parra, J.M.; Sanchez, E.; Perez-Leal, R.; Noperi-Mosqueda, L.C. Salicylic acid and nutrient sprays to improve apple fruit quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12841. [Google Scholar] [CrossRef]
- Smith, M.W.; Rohla, C.T.; Goff, W.D. Pecan leaf elemental sufficiency ranges and fertilizer recommendations. HortTechnology 2012, 22, 594–599. [Google Scholar] [CrossRef]
- Muñoz-Márquez, E.; Soto-Parra, J.M.; Pérez-Leal, R.; Sánchez-Chávez, E. New Nitrogen Use Efficiency Indices for Biomass Formation and Productivity in Green Beans Under Foliar Fertilization with Molybdenum Nanofertilizer. Nitrogen 2024, 5, 667–687. [Google Scholar] [CrossRef]
- Soto-Parra, J.M.; Piña-Ramírez, F.J.; Sánchez-Chávez, E.; Pérez-Leal, R.; Basurto-Sotelo, M. Alternativas orgánicas para disminuir la aplicación de nitrógeno en nogal pecanero. Nova Sci. 2016, 8, 140–161. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, Ch.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Lal, M.A. Nitrogen metabolism. In Plant physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer Nature: Singapore, 2018; pp. 295–334. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagoinism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Bonomelli, C.; de Freitas, S.T.; Aguilera, C.; Palma, C.; Garay, R.; Dides, M.; Brosssard, N.; O’Brien, J.A. Ammonium excess leads to Ca restrictions, morphological changes, and nutritional imbalances in tomato plants, which can be monitored by the N/Ca ratio. Agronomy 2021, 11, 1437. [Google Scholar] [CrossRef]
- Wang, J.; Geng, Y.; Zhang, J.; Li, L.; Guo, F.; Yang, S.; Yang, S.; Wan, S. Increasing Calcium and Decreasing Nitrogen Fertilizers Improves Peanut Growth and Productivity by Enhancing Photosynthetic Efficiency and Nutrient Accumulation in Acidic Red Soil. Agronomy 2023, 13, 1924. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Ju, C.; Wang, C. Calcium signaling in plant mineral nutrition: From uptake to transport. Plant Commun. 2023, 4, 100678. [Google Scholar] [CrossRef]
- Wöjcik, P. Effects of molybdenum sprays on the growth, yield and fruit quality of ‘Red Jonaprince’ apple trees. Sci. Hortic. 2020, 271, 109422. [Google Scholar] [CrossRef]
- De Sousa Ferreira, L.; De Souza Oliveira, V.; De Paula Marchiori, J.J.; Ferreira, T.C.; Bernabé, A.C.B.; Boone, G.T.F.; Do Santos Pereira, L.L.; Carriço, E. The Nutrient Magnesium in Soil and Plant: A Review. Int. J. Plant Soil Sci. 2023, 35, 136–144. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Wagle, P.; Smith, M.W.; Wood, B.W.; Rohla, C.T. Supplemental foliar nickel and copper applications do not reduce kernel necrosis in pecan trees receiving excess nitrogen. Commun. Soil Sci. Plant Anal. 2011, 42, 2219–2228. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein-Ibrahim, M.E.; Ibrahim-Salih, E.G. Effect of N on growth, antioxidant capacity, and chlorophyll content of sorghum. Agronomy 2022, 12, 501. [Google Scholar] [CrossRef]
- Hippler, F.W.; Boaretto, R.M.; Dovis, V.L.; Gomes, G.O.; Quaggio, J.A.; Quinones, A.; Mattos, D., Jr. Revisiting nutrient management for Citrus production: To what extent does molybdenum affect nitrogen assimilation of trees? Sci. Hortic. 2017, 225, 462–470. [Google Scholar] [CrossRef]
- Keshavarz, K.; Vahdati, K.; Samar, M.; Azadegan, B.; Brown, P.H. Foliar application of zinc and boron improves walnut vegetative and reproductive growth. HortTechnology 2011, 21, 181–186. [Google Scholar] [CrossRef]
- Márquez-Quiroz, C.; López-Espinosa, S.T.; Sánchez-Chávez, E.; García-Bañuelos, M.L.; la Cruz-Lazaro, D.; Reyes-Carrillo, J.L. Effect of vermicompost tea on yield and nitrate reductase enzyme activity in saladette tomato. J. Soil Sci. Plant Nutr. 2014, 14, 223–231. [Google Scholar] [CrossRef]
- Ponce-García, C.O.; Soto-Parra, J.M.; Sánchez-Chávez, E.; Muñoz-Márquez, E.; Piña-Ramírez, F.J.; Flores-Córdova, M.A.; Pérez-Leal, R.; Yáñez-Muñoz, R.M. Efficiency of nanoparticle, sulfate, and zinc-chelate use on biomass, yield, and nitrogen assimilation in green beans. Agronomy 2019, 9, 128. [Google Scholar] [CrossRef]
- Aitlessov, K.; Zhumabekova, B.; Sagyndykov, U.; Tuyakbayeva, A.; Bitkeyeva, A.; Bazarbaeva, K.Z.; Mukhtarov, A.; Nurbekova, Z.; Satkanov, M.; Kulatayeva, M.; et al. Foliar Fertilization with Molybdate and Nitrate Up-Regulated Activity of Nitrate Reductase in Lemon Balm Leaves. Horticulturae 2023, 9, 1325. [Google Scholar] [CrossRef]
- Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Saleem, M.H.; Moussa, M.G.; Hu, C.X. Molybdenum-induced effects on nitrogen metabolism enzymes and elemental profile of winter wheat (Triticum aestivum L.) under different nitrogen sources. Int. J. Mol. Sci. 2019, 20, 3009. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Qin, X.; Hu, Z.; Li, J.; Guo, Y.; Sun, G.; Chen, Z.; Huang, H.; Hu, C.; et al. Combined effect of molybdenum and nitrogen fertilization on nitrogen metabolism and amino acid content in tobacco leaves. Front. Agron. 2024, 6, 1427571. [Google Scholar] [CrossRef]
- Fu, Y.F.; Zhang, Z.W.; Yang, X.Y.; Wang, C.Q.; Lan, T.; Tang, X.Y.; Chen, G.D.; Zeng, J.; Yuan, S. Nitrate reductase is a key enzyme responsible for nitrogen-regulated auxin accumulation in Arabidopsis roots. Biochem. Biophys. Res. Commun. 2020, 532, 633–639. [Google Scholar] [CrossRef]
- Lu, S.; Zhuo, C.; Wang, X.; Guo, Z. Nitrate reductase (NR)-dependent NO production mediates ABA-and H2O2-induced antioxidant enzymes. Plant Physiol. Biochem. 2014, 74, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, K.; Tan, P.; Liu, J.; Xie, J.; Yao, X.; Chu, G.; Peng, F. Ammonia-nitrate mixture dominated by NH4±N promoted growth, photosynthesis and nutrient accumulation in pecan (Carya illinoinensis). Forests 2023, 12, 1808. [Google Scholar] [CrossRef]
- Escudero-Almanza, D.J.; Ojeda-Barrios, D.L.; Cruz-Alvarez, O.; Hernández-Rodríguez, O.A.; Ortiz-Rivera, Y. ZIP proteins related to zinc metabolism in plants. In Zinc in Plants: Current Knowledge and Recent Advances; Academic Press: Cambridge, MA, USA, 2025; pp. 173–192. [Google Scholar] [CrossRef]
- Rutkowski, K.; Lysiak, G.P. Effect of nitrogen fertilization on tree growth and nutrient content in soil and cherry leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Wang, Y.; Heerema, R.J.; Walworth, J.L.; Dungan, B.; VanLeeuwen, D.; Holguin, F.O. Nutraceutical properties of pecan kernels are affected by soil zinc fertilizer application. HortScience 2020, 55, 2001–2007. [Google Scholar] [CrossRef]
- Heerema, R.J.; VanLeeuwen, D.; Thompson, M.Y.; Sherman, J.D.; Comeau, M.J.; Walworth, J.L. Soil-application of Zinc-EDTA increases leaf photosynthesis of immature ‘Wichita’ pecan trees. J. Am. Soc. Hortic. Sci. 2017, 142, 27–35. [Google Scholar] [CrossRef]
- Noperi-Mosqueda, L.C.; Soto-Parra, J.M.; Sánchez- Chávez, E.; Navarro-León, E.; Pérez-Leal, R.; Flores-Córdova, M.A.; Salas-Salazar, N.A.; Yáñez-Muñoz, R.M. Yield, quality, alternate bearing and long-term yield index in pecan, as a response to mineral and organic nutrition. Not. Bot. Horti Agrobo. 2020, 48, 342–353. [Google Scholar] [CrossRef]
- Soto, J.M.; Sánchez, E.; Piña, F.J.; Basurto, M.; Yáñez, R.M.; Laos, T. Componentes del Rendimiento en Nogal Pecanero Bajo Fertilización Nitrogenada. In Proceedings of the XIV Simposio Hispamo-Luso de Nutrición Mineral de las Plantas, Madrid, Spain, 23–26 July 2012; pp. 104–110. [Google Scholar]
- Wood, B.W. Correction of zinc deficiency in pecan by soil banding. HortScience 2007, 42, 1554–1558. [Google Scholar] [CrossRef]



| Levels | Factors | |||||||
|---|---|---|---|---|---|---|---|---|
| Soil Application | Foliar Application | |||||||
| N | Mo | Zn | Zn-Mo | N | Mo | Zn | Zn-Mo | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 25.00 | 0.20 | 4.00 | 0.25 | 7.50 | 0.025 | 0.750 | 0.150 |
| 5 | 125.00 | 1.00 | 20.00 | 1.25 | 37.50 | 0.125 | 3.750 | 0.750 |
| 10 | 250.00 | 2.00 | 40.00 | 2.50 | 75.00 | 0.250 | 7.500 | 1.500 |
| Simple mean | 125.00 | 1.00 | 20.00 | 1.25 | 37.50 | 0.125 | 3.750 | 0.750 |
| Stock solution in mM | 500.00 | 50.00 | 52.74 | 52.74 | 500.00 | 50.00 | 52.74 | 52.74 |
| Treatment | Soil Application | Foliar Application | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mo | Zn | Zn-Mo | N | Mo | Zn | Zn-Mo | |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 |
| 2 | 0.00 | 0.20 | 4.00 | 0.25 | 0.00 | 0.025 | 0.750 | 0.150 |
| 3 | 0.00 | 1.00 | 20.00 | 1.25 | 0.00 | 0.125 | 3.750 | 0.750 |
| 4 | 0.00 | 2.00 | 40.00 | 2.50 | 0.00 | 0.250 | 7.500 | 1.500 |
| 5 | 25.00 | 0.00 | 4.00 | 1.25 | 7.500 | 0.00 | 0.750 | 0.750 |
| 6 | 25.00 | 0.20 | 0.00 | 2.50 | 7.500 | 0.025 | 0.000 | 1.500 |
| 7 | 25.00 | 1.00 | 40.00 | 0.00 | 7.500 | 0.125 | 7.500 | 0.000 |
| 8 | 25.00 | 2.00 | 20.00 | 0.25 | 7.500 | 0.250 | 3.750 | 0.150 |
| 9 | 125.00 | 0.00 | 20.00 | 2.50 | 37.500 | 0.00 | 3.750 | 1.500 |
| 10 | 125.00 | 0.20 | 40.00 | 1.25 | 37.500 | 0.025 | 7.500 | 0.750 |
| 11 | 125.00 | 1.00 | 0.00 | 0.25 | 37.500 | 0.125 | 0.000 | 0.150 |
| 12 | 125.00 | 2.00 | 4.00 | 0.00 | 37.500 | 0.250 | 0.750 | 0.000 |
| 13 | 250.00 | 0.00 | 40.00 | 0.25 | 75.000 | 0.00 | 7.500 | 0.150 |
| 14 | 250.00 | 0.20 | 20.00 | 0.00 | 75.000 | 0.025 | 3.750 | 0.000 |
| 15 | 250.00 | 1.00 | 4.00 | 2.50 | 75.000 | 0.125 | 0.750 | 1.500 |
| 16 | 250.00 | 2.00 | 0.00 | 1.25 | 75.000 | 0.250 | 0.000 | 0.750 |
| Treatment | Soil Application | Foliar Application | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mo | Zn | Zn-Mo | N | Mo | Zn | Zn-Mo | |
| 1 | 0.00 | 0.00 | 0.0000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 0.00 | 0.03 | 0.0007 | 0.41 | 0.00 | 0.01 | 0.34 | 0.24 |
| 3 | 0.00 | 0.15 | 0.0037 | 2.04 | 0.00 | 0.03 | 1.71 | 1.22 |
| 4 | 0.00 | 0.31 | 0.0074 | 4.08 | 0.00 | 0.06 | 3.41 | 2.45 |
| 5 | 1.60 | 0.00 | 0.0007 | 2.04 | 0.36 | 0.00 | 0.34 | 1.22 |
| 6 | 1.60 | 0.03 | 0.00 | 4.08 | 0.36 | 0.01 | 0.00 | 2.45 |
| 7 | 1.60 | 0.15 | 0.0074 | 0.00 | 0.36 | 0.03 | 3.41 | 0.00 |
| 8 | 1.60 | 0.31 | 0.0037 | 0.41 | 0.36 | 0.06 | 1.71 | 0.24 |
| 9 | 8.01 | 0.00 | 0.0037 | 4.08 | 2.40 | 0.00 | 1.71 | 2.45 |
| 10 | 8.01 | 0.03 | 0.0074 | 2.04 | 2.40 | 0.01 | 3.41 | 1.22 |
| 11 | 8.01 | 0.15 | 0.00 | 0.41 | 2.40 | 0.03 | 0.00 | 0.24 |
| 12 | 8.01 | 0.31 | 0.0007 | 0.00 | 2.40 | 0.06 | 0.34 | 0.00 |
| 13 | 16.02 | 0.00 | 0.0074 | 0.41 | 0.36 | 0.00 | 3.41 | 0.24 |
| 14 | 16.02 | 0.03 | 0.0037 | 0.00 | 0.36 | 0.01 | 1.71 | 0.00 |
| 15 | 16.02 | 0.15 | 0.0007 | 4.08 | 0.36 | 0.03 | 0.34 | 2.45 |
| 16 | 16.02 | 0.31 | 0.00 | 2.04 | 0.36 | 0.06 | 0.00 | 1.22 |
| Foliar Symptomatology and Development Parameters | ||||
|---|---|---|---|---|
| Percentage Levels | Zinc Deficiency | Leaf Size | Foliar Coverage | Fruiting Shoots |
| 0 | No deficiency | Very small | No leaves | Only vegetative shoots |
| 20 | Incipient | Small | Minimal coverage | Very few fruiting shoots |
| 40 | Low | Medium | Low coverage | Few fruiting shoots |
| 60 | Medium | Normal | Medium | Dense fruiting shoots |
| 80 | High | Large | High | Some fruiting shoots with nuts |
| 100 | Very high | Very large | Very high | Fruiting shoots with nuts |
| Factors [kg ha−1] | ||||||||
| N (A) | Mo (B) | Zn (D) | Zn-Mo (E) | |||||
| Eigenvalues | 162.500 T | 1.125 | 23.750 | 2.00 | Eigenvectors | +/− | ||
| Nitrogen R2 0.2019, µ 1.97% (1.69–2.31) VR (1.76–2.49) → 2.47% X | ||||||||
| 6.5 U | +1 | +3 | 4 | 4/0 | ||||
| −34.5(−22.8) | +4 V | (−2) | (+1) | 7 | 5/2 | |||
| [Frequency] | 4 | 3 | 1 | 3 | 11 W | |||
| Dose/response | 96.43 | 0.80 | 25.09 | 2.04 | Selection | ≥2 | ||
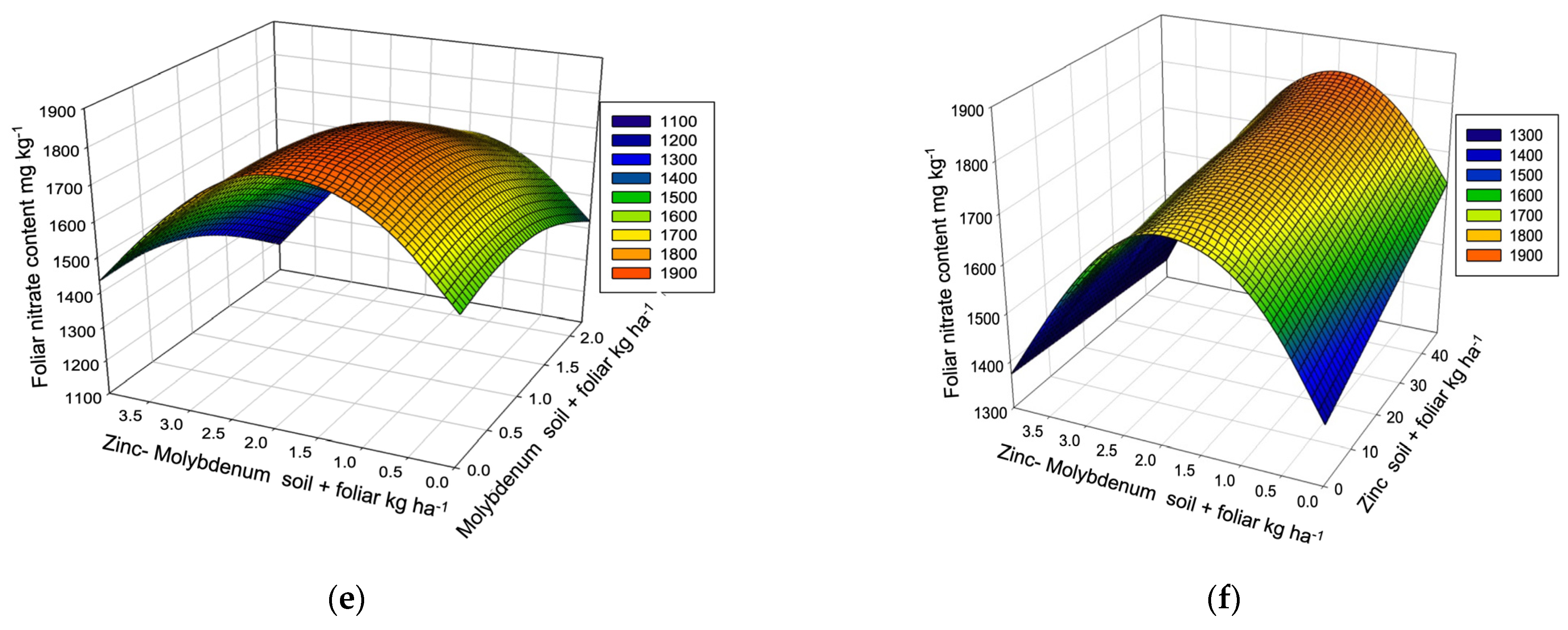
| Nitrates R2 0.6177, µ 1557.7 ppm (1095.5–2302.3) VR (1066.0–1510.0) LY, P → 1907.1 ppm | ||||||||
| [Frequency] | 4 L,C Y | 3 L,C,E | 3 L,C,E | 2 L,C | 12 | |||
| Dose/response | 202.92 ** | 1.15 | 27.71 ** | 2.00 ** | ≥2 | |||
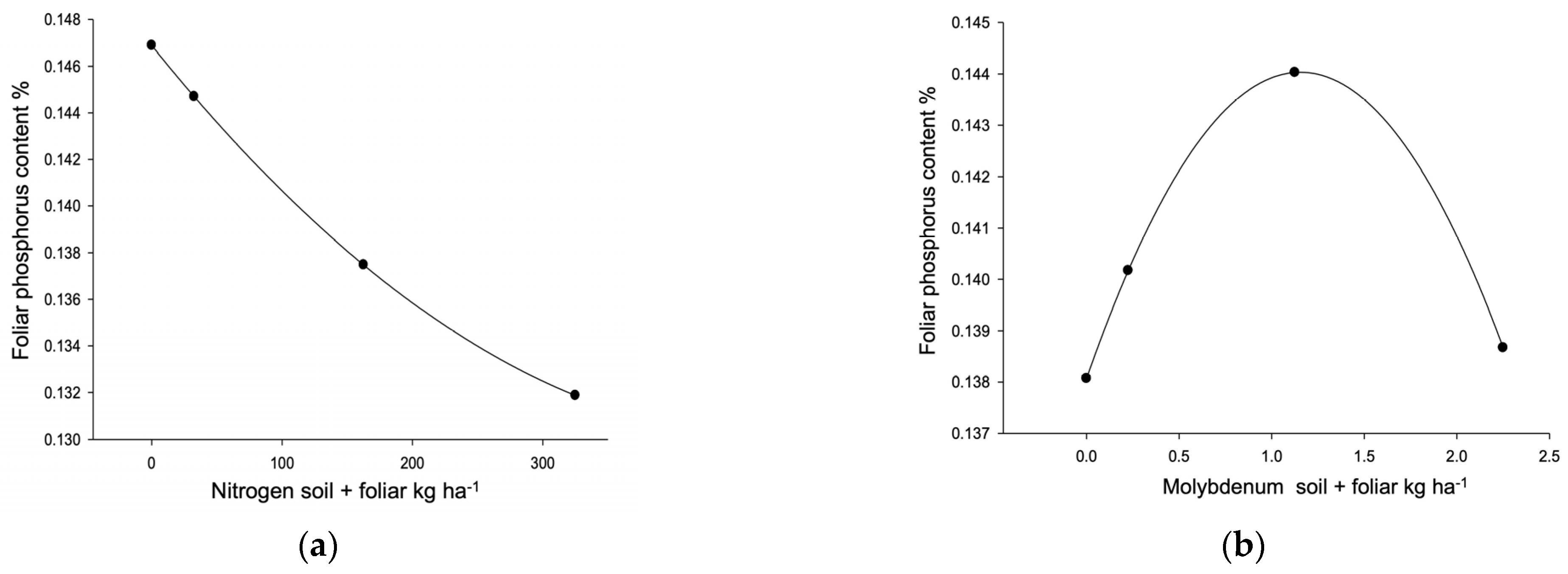
| Phosphorus R2 0.4518, µ 0.140% (0.111–0.183) VR (0.10–0.14) L, P → 0.185% | ||||||||
| [Frequency] | 4 L,C,E | 3 L,E | 3 L,E | 2 L,C | 12 | |||
| Dose/response | 285.35 ** | 1.16 * | 29.86 * | 1.93 ** | ≥2 | |||
| Potassium R2 0.2086, µ 1.08% (0.83–1.83) VR (0.87–1.23) L → 1.39% | ||||||||
| [Frequency] | 2 | 2 | 4 | 0 | 8 | |||
| Dose/response | 285.09 | 1.56 | 36.28 | 1.94 | ≥2 | |||
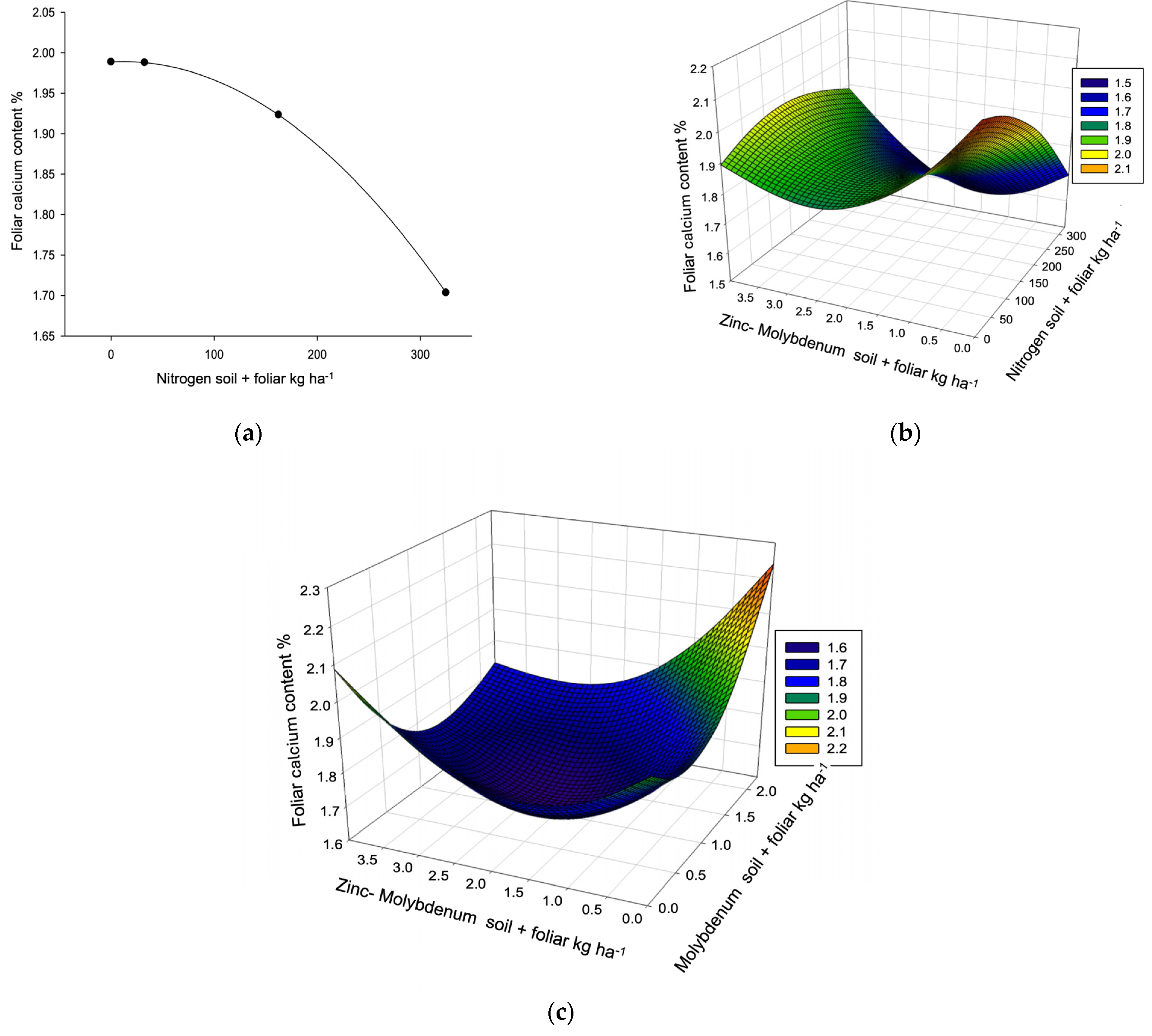
| Calcium R2 0.4106, µ 1.90% (1.02–2.60) VR (1.45–2.06) L, C, P. → 2.32% | ||||||||
| [Frequency] | 4 L,E | 3 E | 0 | 4 | 11 | |||
| Dose/response | 223.80 | 1.28 * | 30.82 | 1.94 * | ≥2 | |||
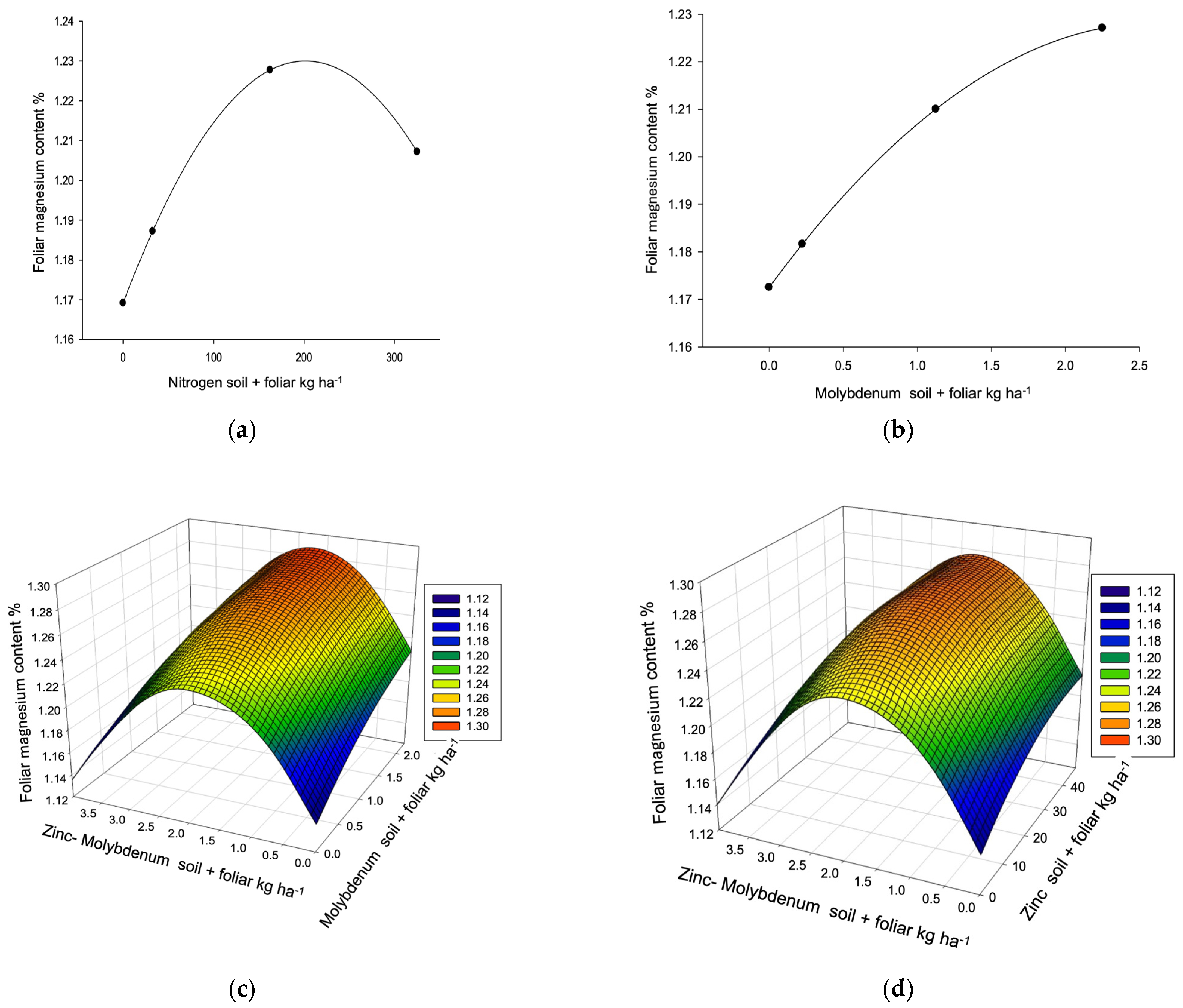
| Magnesium R2 0.3105, µ 1.20% (0.79–1.49) VR (1.09–1.55) P → 1.30% | ||||||||
| [Frequency] | 4 L,E | 3 L,E | 1 L,E | 3 C | 11 | |||
| Dose/response | 162.50 ** | 1.13 | 23.75 | 2.00 ** | ≥2 | |||
| Sodium R2 0.1894, µ 0.0154% (0.0040–0.0305) VR (0.028–0.039) → 0.0282% | ||||||||
| [Frequency] | 7 | 0 | 2 | 1 | 10 | |||
| Dose/response | 51.79 | 0.95 | 23.74 | 2.08 | ≥2 | |||
| Iron R2 0.7054, µ 71.2 ppm (50.0–137.5) VR (67.2–95.2) L, P → 126.8 ppm | ||||||||
| [Frequency] | 3 E | 3 | 3 | 0 | 9 | ≥2 | ||
| Dose/response | 177.27 * | 1.28 | 26.32 | 2.07 ** | ||||
| Manganese R2 0.1871, µ 244.9 ppm (151.5–294.5) VR (146.8–208.0) → 250.7 ppm | ||||||||
| [Frequency] | 4 | 2 | 2 | 4 | 12 | |||
| Dose/response | 228.05 | 1.12 | 30.72 | 2.07 | ≥2 | |||
| Zinc R2 0.5483, µ 21.4 ppm (14.0–30.0) VR (21.5–30.5) L, C, P → 40.7 ppm | ||||||||
| [Frequency] | 3 | 2 | 3 | 2 | 10 | |||
| Dose/response | 211.06 * | 2.10 * | 27.36 | 2.75 ** | ≥2 | |||
| Copper R2 0.3317, µ 7.0 ppm (4.5–10.0) VR (4.7–6.7) L → 7.9 ppm | ||||||||
| [Frequency] | 4 | 3 | 2 | 3 L | 12 | |||
| Dose/response | 9.14 | 0.96 | 29.27 | 2.37 * | ≥2 | |||
| Subtotal | Q43 | 27 | 24 | 24 | Total 118 Z | 110 Z/8 | ||
| Proportion +/− | 43/0 | 21/6 | 22/2 | 24/0 | ||||
| Selection | 7/11 | 7/11 | 4/11 | 7/11 | Factors/ | Variables | 22 Z/ | 11 |
| Factors [Mm ha−1] | N [285.35] > Mo [1.28] > Zn [30.82] = Zn-Mo [2.37] | |||||||
| Variables | Manganese (250.7 ppm) = Copper (7.9 ppm) = Phosphorus (0.185%) = Nitrates (1907.1 ppm) > Calcium (2.32%) = Nitrogen (2.47%) = Magnesium (1.30%) | |||||||
| % | mg kg−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Na | N-NO3 | Fe | Mn | Zn | Cu | |
| Regression Y | |||||||||||
| Linear | 0.2102 Z | 0.0016 | 0.0590 | 0.0141 | 0.6730 | 0.2316 | 0.0002 | <0.0001 | 0.3533 | 0.0003 | 0.0082 |
| Quadratic | 0.3131 | 0.6059 | 0.5173 | 0.0610 | 0.8563 | 0.7709 | 0.3055 | 0.1540 | 0.3034 | 0.0297 | 0.1875 |
| Interaction | 0.8166 | 0.0106 | 0.9791 | 0.0600 | 0.0092 | 0.5794 | <0.0001 | <0.0001 | 0.7727 | 0.0018 | 0.6256 |
| Total model | 0.4901 | 0.0016 | 0.4530 | 0.0062 | 0.0829 | 0.5598 | <0.0001 | <0.0001 | 0.5728 | <0.0001 | 0.0517 |
| Residual X | |||||||||||
| Lack of fit | 0.4840 | 0.1039 | 0.5226 | 0.1142 | 0.1848 | 0.9506 | 0.0520 | 0.1176 | 00.7044 | <0.0001 | 0.0150 |
| Parameters Y | |||||||||||
| Intercept | |||||||||||
| N | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0194 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| MO | 0.2612 | 0.0010 | 0.9395 | 0.0995 | 0.0066 | 0.3392 | <0.0001 | 0.6199 | 0.3890 | 0.5297 | 0.4750 |
| Zn | 0.7391 | 0.0009 | 0.9406 | 0.4855 | 0.0294 | 0.2106 | <0.0001 | 0.9901 | 0.4920 | 0.6035 | 0.4806 |
| ZnMo | 0.525 | 0.0032 | 0.7875 | 0.1032 | 0.0101 | 0.2552 | <0.0001 | 0.8594 | 0.4590 | 0.2443 | 0.5896 |
| N2 | 0.6296 | 0.0002 | 0.9267 | 0.3493 | 0.2828 | 0.5524 | <0.0001 | 0.6120 | 0.7090 | 0.1791 | 0.0428 |
| N*Mo | 0.3256 | 0.0332 | 0.9365 | 0.4060 | 0.1811 | 0.4266 | 0.0004 | 0.6186 | 0.5701 | 0.3786 | 0.5439 |
| Mo2 | 0.7600 | 0.7720 | 0.9857 | 0.7906 | 0.6327 | 0.7592 | 0.6001 | 0.6728 | 0.9488 | 0.6401 | 0.8474 |
| N*Zn | 0.7534 | 0.2444 | 0.9782 | 0.7846 | 0.3220 | 0.9435 | 0.0636 | 0.6410 | 0.8697 | 0.8909 | 0.9517 |
| Zn*Mo | 0.6817 | 0.9381 | 0.9280 | 0.8000 | 0.5930 | 0.8100 | 0.6237 | 0.6826 | 0.9924 | 0.6920 | 0.8902 |
| Zn2 | 0.7402 | 0.6667 | 0.9201 | 0.5898 | 0.4841 | 0.7620 | 0.2205 | 0.5956 | 0.8692 | 0.9068 | 0.9337 |
| N*ZnMo | 0.5409 | 0.3217 | 0.9675 | 0.7677 | 0.6167 | 0.4966 | 0.0697 | 0.6865 | 0.8117 | 0.4442 | 0.7565 |
| Mo*ZnMo | 0.6176 | 0.0020 | 0.7747 | 0.0965 | 0.0219 | 0.6482 | 0.5170 | 0.0049 | 0.4828 | 0.1590 | 0.3073 |
| Zn*ZnMo | 0.3288 | 0.0015 | 0.9409 | 0.0756 | 0.0196 | 0.4296 | <0.0001 | 0.6626 | 0.2776 | 0.4969 | 0.3984 |
| (ZnMo)2 | 0.3028 | 0.0017 | 0.9734 | 0.1335 | 0.0125 | 0.3116 | <0.0001 | 0.7898 | 0.2518 | 0.2933 | 0.4957 |
| Factors X | |||||||||||
| N | 0.5105 | 0.0016 | 0.6351 | 0.1267 | 0.0036 | 0.5169 | <0.0001 | 0.0242 | 0.6895 | 0.0440 | 0.5751 |
| Mo | 0.3748 | 0.0149 | 0.8582 | 0.0139 | 0.2792 | 0.6682 | <0.0001 | 0.2744 | 0.7682 | 0.0137 | 0.7280 |
| Zn | 0.7248 | 0.0490 | 0.9860 | 0.4485 | 0.1535 | 0.3728 | <0.0001 | 0.8054 | 0.5994 | 0.5625 | 0.9836 |
| ZnMo | 0.6372 | 0.0026 | 0.9953 | 0.0787 | 0.0036 | 0.7405 | <0.0001 | 0.0014 | 0.6340 | 0.0071 | 0.0588 |
| Mean | 1.97 | 0.140 | 1.08 | 1.90 | 1.20 | 0.0154 | 1157 | 71.17 | 244.90 | 21.38 | 7.00 |
| R2 | 0.2019 | 0.4518 | 0.2086 | 0.4106 | 0.3105 | 0.1894 | 0.6177 | 0.7054 | 0.1871 | 0.5483 | 0.3317 |
| CV | 8.55 | 10.22 | 17.29 | 14.62 | 13.49 | 61.79 | 11.08 | 14.99 | 12.37 | 11.88 | 19.78 |
| Factors [kg ha−1] | ||||||||
| N (A) | Mo (B) | Zn (D) | Zn-Mo (E) | |||||
| Eigenvalues | 162.500 T | 1.125 | 23.750 | 2.00 | Eigenvectors | +/− | ||
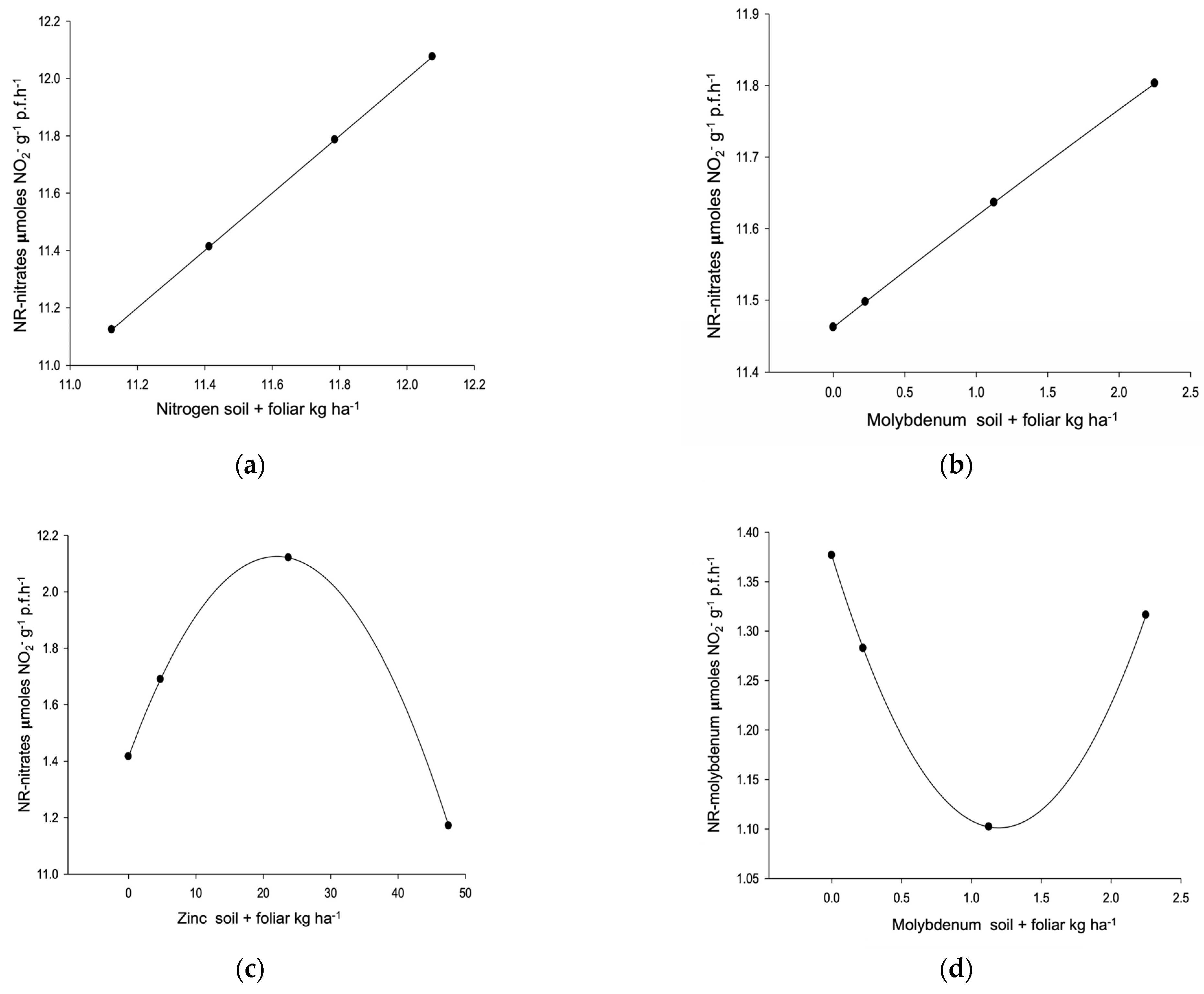
| Endogenous nitrate reductase R2 0.7756, µ 1.13 µmoles NO2 g−1 p.f.h−1 (0.42–2.21) L Y,C,P → 3.36 X | ||||||||
| 333.0 U | +3 V | 3 | 3/0 | |||||
| −345.9 | +3 | +3 | 3 | 3/0 | ||||
| [Frequency] | 3 L,C,B,E | 3 L,C,E | 0 L,E | 0 L,C | 6 | |||
| Dose/response | 196.52 ** | 1.23 ** | 28.33 ** | 1.96 ** | Selection | ≥2 | ||
| Nitrate reductase NO3 R2 0.5595, µ 11.60 µmoles NO2 g−1 p.f.h−1 (10.44–13.20) L,C,P → 13.62 | ||||||||
| [Frequency] | 3 L Y | 3 L,E | 3 L,E | 2 | 11 W | |||
| Dose/response | 141.87 ** | 1.16 ** | 20.49 ** | 2.13 ** | ≥2 | |||
| Nitrate reductase Mo. R2 0.5922, µ 1.27 µmoles NO2 g−1 p.f.h−1 (0.67–2.22) L,C,P → 2.84 | ||||||||
| [Frequency] | 4 B,D | 3 C,D | 3 C | 0 | 10 | |||
| Dose/response | 106.83 ** | 1.17 ** | 9.30 ** | 2.06 | ≥2 | |||
| Nitrate reductase NO3-Mo R2 0.3368, µ 2.17 µmoles NO2 g−1 p.f.h−1 (1.74–2.70) P → 2.82 | ||||||||
| [Frequency] | 4 L | 4 | 2 E | 1 | 11 | |||
| Dose/response | 17.85 * | 0.79 ** | 31.45 * | 2.25 | ≥2 | |||
| Endogenous urease R2 0.1705, µ 13.76 µmoles NH4 g−1 p.f. h−1 (12.19–16.39) → 14.67 | ||||||||
| [Frequency] | 3 | 4 | 3 | 0 | 10 | |||
| Dose/response | 4.26 | 0.96 | 27.85 | 2.01 | ≥2 | |||
| Urease NH4-Ni, R2 0.2597, µ 13.76 µmoles NH4 g−1 p.f. h−1 (42.1–64.36) → 58.44 | ||||||||
| [Frequency] | 4 | 4 | 3 C | 0 | 11 | |||
| Dose/response | 215.22 | 0.70 | 3.21 | 1.92 | ≥2 | |||
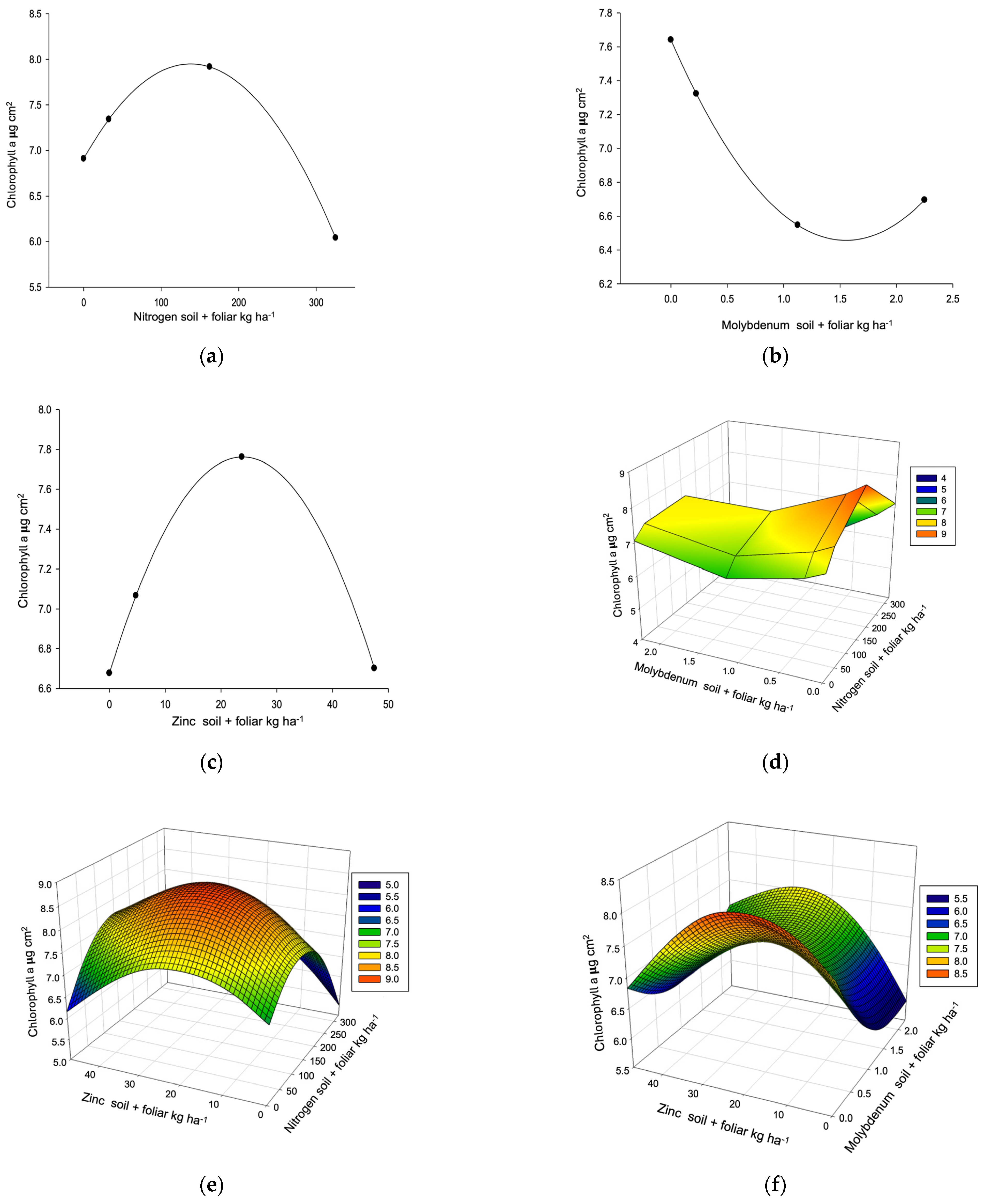
| Chlorophyll a R2 0.9949, µ 7.05 µg cm2 (4.12–10.22) L, C, P → 10.40 | ||||||||
| [Frequency] | 3 C,B,D,E | 4 C,D | 3 C | 0 | 10 | |||
| Dose/response | 166.90 * | 0.01 * | 22.91 * | 1.99 | ≥2 | |||
| Chlorophyll b R2 0.8244, µ 3.33 µg cm2 (2.42–4.63) → 5.17 | ||||||||
| [Frequency] | 4 | 3 | 2 | 3 | 12 | |||
| Dose/response | 143.92 | 1.19 | 18.33 | 2.30 | ≥2 | |||
| Chlorophyll a/b R2 0.8928, µ 2.16 µg cm2 (0.89–2.61) → 2.44 | ||||||||
| [Frequency] | 4 | 5 | 3 | 0 | 12 | |||
| Dose/response | 17.21 | 0.75 | 30.67 | 2.08 | ≥2 | |||
| Carotenoids R2 0.7487, µ 0.65 µg cm2 (0.42–0.86) → 0.83 | ||||||||
| [Frequency] | 4 | 2 | 2 | 3 | 11 | |||
| Dose/response | 275.10 | 0.80 | 17.15 | 2.01 | ≥2 | |||
| Chlorophyll a/Carotenoids R2 0.8194, µ 11.04 µg cm2 (7.80–16.09) → 14.02 | ||||||||
| [Frequency] | 4 | 4 | 2 | 0 | 10 | |||
| Dose/response | 5.89 | 0.93 | 28.35 | 2.09 | ≥2 | |||
| Subtotal | Q 49 | 39 | 26 | 9 | Total 114 Z | 91 Z/23 | ||
| Proportion +/− | 31/9 | 30/9 | 3/3 | 9/0 | ||||
| Selection | 10/11 | 10/11 | 10/11 | 7/11 | Factors/ | Variables | 18 Z/ | 9 |
| Factors [Mm ha−1] | N [275.10] = Mo [1.17] > Zn [31.45] | |||||||
| Variables | Chlorophyll a/b (2.44 µg cm2) = Chlorophyll b (5.17 µg cm2) > Nitrate reductase NO3 (13.62 µmoles NO2−) = Carotenoids (0.83 µg cm2) > Urease NH4-Ni (58.44 µmoles NH4) > Chlorophyll a (10.40 µg cm2) = Endogenous urease (14.67 µmoles NH4) = Chlorophyll a/Carotenoids (14.02 µg cm2) = Nitrate reductase Mo (2.84 µmoles NO2−) | |||||||
| Nitrate Reductase (µmoles NO2 g−1 p.f. h−1) | Urease µmoles (NH4 g−1 p.f. h−1) | Leaf Pigments µg cm2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression X | End. | NO3− | Mo | NO3−Mo | End. | NH4 | Chl a | Chlb | Chl tot | Chl (a/b) | Carot | Chl Tot/Carot |
| Linear | <0.0001 Z | 0.0022 | <.0001 | 0.4149 | 0.4763 | 0.0962 | 0.0559 | 0.0470 | 0.2636 | 0.5150 | 0.7630 | 0.3873 |
| Quadratic | <0.0001 | 0.0009 | 0.0007 | 0.6616 | 0.3968 | 0.4366 | 0.0283 | 0.4766 | 0.2227 | 0.3896 | 0.5996 | 0.6691 |
| Interaction | <0.0001 | 0.0019 | 0.0418 | 0.0082 | 0.7178 | 0.3934 | 0.0268 | 0.7759 | 0.2049 | 0.5327 | 0.9047 | 0.7517 |
| Total model | <0.0001 | <0.0001 | <0.0001 | 0.0459 | 0.6664 | 0.2178 | 0.0325 | 0.5787 | 0.2322 | 0.5316 | 0.8476 | 0.6210 |
| Residual X | ||||||||||||
| Lack of fit | 0.5515 | 0.0118 | 0.0007 | 0.4092 | 0.2244 | 0.3581 | . | . | . | . | . | . |
| Parameters Y | ||||||||||||
| Intercept | ||||||||||||
| N | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0016 | 0.0470 | 0.0091 | 0.0140 | 0.0613 | 0.0316 |
| MO | <0.0001 | 0.0335 | 0.4672 | 0.0886 | 0.4690 | 0.9417 | 0.1510 | 0.4766 | 0.3282 | 0.5873 | 0.5095 | 0.6313 |
| Zn | <0.0001 | 0.0302 | 0.6447 | 0.1136 | 0.3372 | 0.3924 | 0.9049 | 0.7759 | 0.8102 | 0.6963 | 0.6292 | 0.5726 |
| ZnMo | <0.0001 | 0.0002 | 0.9125 | 0.4319 | 0.4076 | 0.6401 | 0.2559 | 0.5787 | 0.4513 | 0.6201 | 0.5653 | 0.6181 |
| N2 | <0.0001 | 0.2680 | 0.5234 | 0.3724 | 0.7832 | 0.5035 | 0.4577 | 0.6408 | 0.9074 | 0.4681 | 0.5918 | 0.5513 |
| N*Mo | 0.0077 | 0.1864 | 0.1592 | 0.1478 | 0.2140 | 0.1492 | 0.0441 | 0.7091 | 0.4159 | 0.2718 | 0.5721 | 0.4045 |
| Mo2 | 0.0624 | 0.5836 | 0.0079 | 0.6862 | 0.3580 | 0.1096 | 0.0187 | 0.8666 | 0.1570 | 0.3368 | 0.9198 | 0.5361 |
| N*Zn | 0.0122 | 0.2450 | 0.0181 | 0.9691 | 0.5558 | 0.1838 | 0.0210 | 0.8900 | 0.1739 | 0.3606 | 0.9639 | 0.6856 |
| Zn*Mo | 0.2109 | 0.6576 | 0.0118 | 0.5553 | 0.3657 | 0.1021 | 0.0182 | 0.9156 | 0.1594 | 0.3119 | 0.8740 | 0.5117 |
| Zn2 | 0.0761 | 0.2890 | 0.0052 | 0.5517 | 0.4349 | 0.1195 | 0.0181 | 0.8673 | 0.1529 | 0.3326 | 0.9905 | 0.5915 |
| N*ZnMo | 0.4766 | 0.2889 | 0.0118 | 0.3955 | 0.3000 | 0.0992 | 0.0277 | 0.8603 | 0.2606 | 0.2788 | 0.7518 | 0.4941 |
| Mo*ZnMo | <0.0001 | 0.6771 | 0.5111 | 0.8904 | 0.9092 | 0.2919 | 0.0288 | 0.7720 | 0.2934 | 0.2694 | 0.8235 | 0.8879 |
| Zn*ZnMo | <0.0001 | 0.0025 | 0.4237 | 0.1465 | 0.3144 | 0.5085 | 0.9745 | 0.5256 | 0.6314 | 0.4347 | 0.5341 | 0.5433 |
| (ZnMo)2 | <0.0001 | 0.0024 | 0.3620 | 0.0753 | 0.2837 | 0.6894 | 0.6295 | 0.6307 | 0.8298 | 0.4700 | 0.5871 | 0.5061 |
| Factors X | ||||||||||||
| N | <0.0001 | <0.0001 | 0.0004 | 0.0676 | 0.8300 | 0.2545 | 0.0308 | 0.8278 | 0.2625 | 0.4789 | 0.8711 | 0.8505 |
| Mo | <0.0001 | 0.0011 | 0.0050 | 0.0071 | 0.6878 | 0.5897 | 0.0553 | 0.6986 | 0.3382 | 0.5511 | 0.9307 | 0.6718 |
| Zn | <0.0001 | 0.0005 | 0.0021 | 0.0156 | 0.1902 | 0.3896 | 0.0329 | 0.6716 | 0.2070 | 0.6697 | 0.8620 | 0.4847 |
| ZnMo | <0.0001 | 0.0007 | 0.8641 | 0.2149 | 0.6123 | 0.2841 | 0.0870 | 0.6846 | 0.5236 | 0.4833 | 0.9027 | 0.873 |
| Mean | 1.13 | 11.60 | 1.27 | 2.17 | 13.76 | 53.04 | 7.05 | 3.33 | 10.38 | 2.16 | 0.65 | 16.42 |
| R2 | 0.7756 | 0.6349 | 0.5922 | 0.3368 | 0.1705 | 0.2597 | 0.9949 | 0.8244 | 0.9602 | 0.8928 | 0.7487 | 0.8613 |
| CV | 25.43 | 29.62 | 27.13 | 19.66 | 20.76 | 18.13 | 4.33 | 23.13 | 10.23 | 15.45 | 27.87 | 23.11 |
| Factors [kg ha−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| N (A) | Mo (B) | Zn (D) | Zn-Mo (E) | |||||
| Eigenvalues | 162.500 T | 1.125 | 23.750 | 2.00 | Eigenvectors | +/− | ||
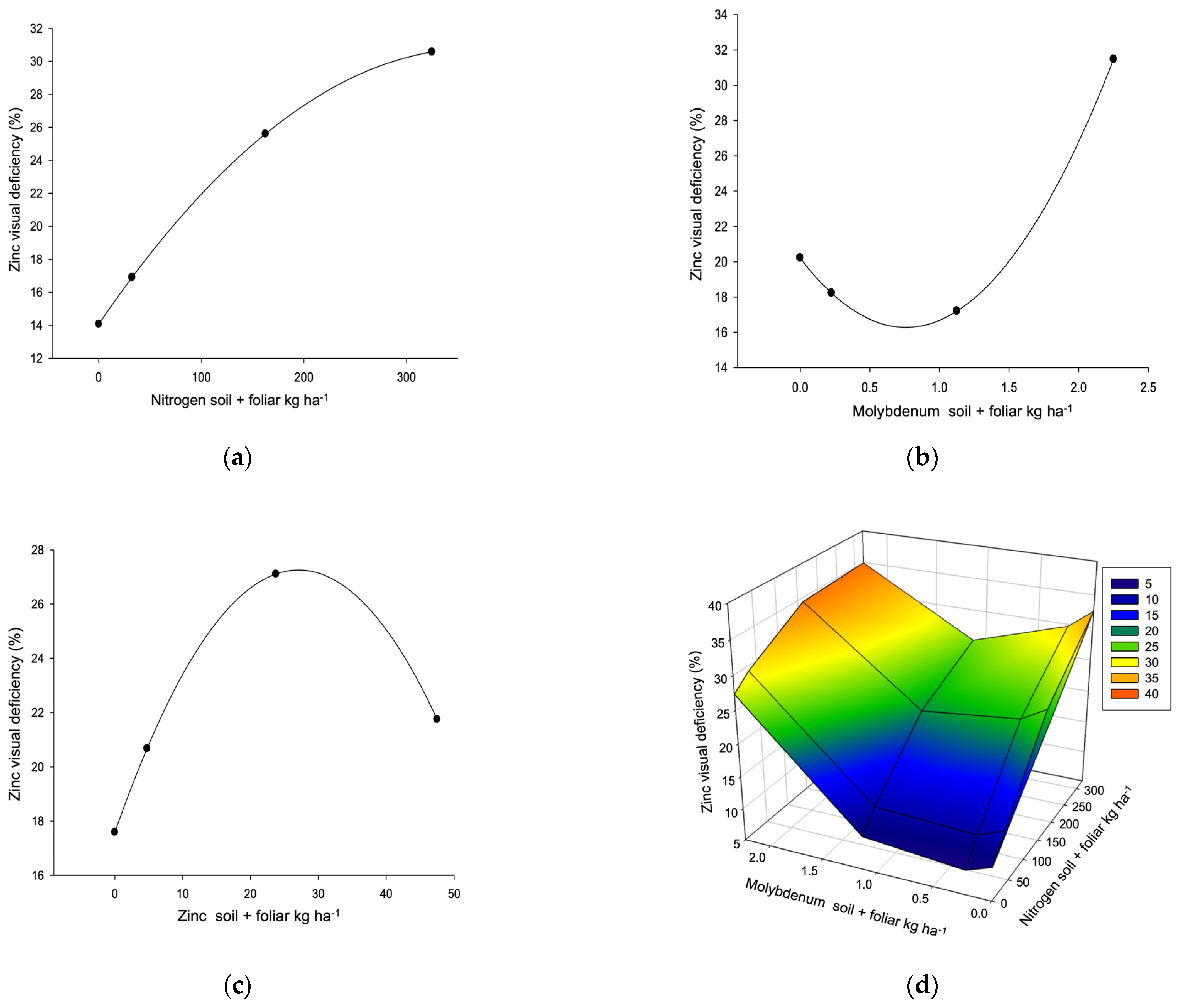
| Zinc deficiency R2 0.3293, µ 21.8% (3.3–46.7) L Y → 5.9% X | ||||||||
| 485.4 U | +1 V | +2 | +1 | 4 | 4/0 | |||
| −1346(−1109) | +3 | (−3) | −1(+1) | 8 | 4/4 | |||
| [Frequency] | 4 C Y,B,D | 5 C,D | 3 C | 0 | 12 W | |||
| Dose/response | 6.55 * | 0.91 * | 28.61 | 2.01 | Selection | ≥2 | ||
| Leaflet size R2 0.1889, µ 55.4% (46.7–63.3) → 71.9% | ||||||||
| [Frequency] | 4 | 4 | 3 | 0 | 11 | |||
| Dose/response | 112.47 | 0.20 | 28.32 | 2.08 | ≥2 | |||
| Foliar cover R2 0.2369, µ 61.5% (46.7–70.0) → 82.1% | ||||||||
| [Frequency] | 2 | 3 | 2 | 0 | 7 | ≥1 | ||
| Dose/response | 118.41 | 1.73 | 6.68 | 2.71 | ||||
| Percentage of fruitful shoots R2 0.4416,12.3% (6.7–26.7) L, C, P → 22.9% | ||||||||
| [Frequency] | 5 | 3 | 3 | 0 | 11 | |||
| Dose/response | 110.71 | 1.60 | 4.17 | 2.40 | ≥2 | |||
| Percentage of kernel R2 0.2651, µ 57.1% (52.5–60.7) → 60.4% | ||||||||
| [Frequency] | 4 | 4 | 3 C | 0 | 11 | |||
| Dose/response | 215.22 | 0.70 | 3.21 | 1.92 | ≥2 | |||
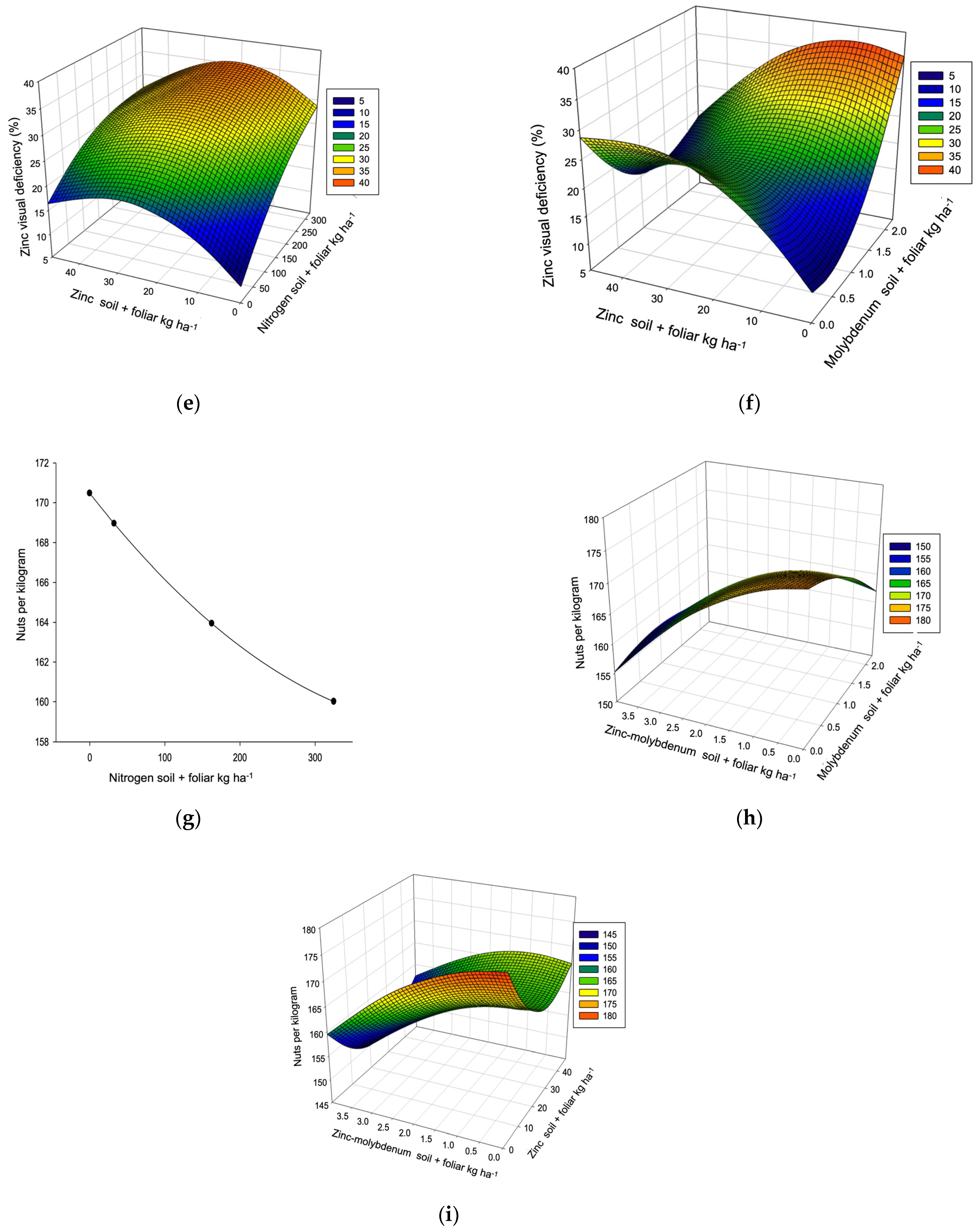
| Nuts per kilogram R2 0.4704, µ 166 (135–203) L, P → 155 | ||||||||
| [Frequency] | 4 L | 3 E | 1 E | 4 L,C | 12 | |||
| Dose/response | 186.56 * | 1.16 * | 26.84 ** | 1.99 * | ≥2 | |||
| Nut weight R2 0.4900, µ 6.1 g (4.92–7.01) L, P → 7.63 g | ||||||||
| [Frequency] | 4 L | 2 E | 1 E | 4 L,C | 11 | |||
| Dose/response | 323.28 * | 1.00 * | 25.91 ** | 2.07 * | ≥2 | |||
| Yield R2 0.3373 µ 121.0 kg ha−1 (7.9–425.4) L → 464.3 kg ha−1 | ||||||||
| [Frequency] | 4 | 3 | 3 | 0 L | 10 | |||
| Dose/response | 152.67 | 1.07 | 22.32 | 2.00 | ≥2 | |||
| Subtotal | Q31 | 26 | 19 | 10 | Total 86 Z | 71 Z/15 | ||
| Proportion +/− | 26/5 | 20/6 | 15/4 | 10/0 | ||||
| Selection | 8/8 | 8/8 | 6/8 | 8/8 | Factors/ | Variables | 14 Z/ | 7 |
| Factors [Mm ha−1] | N [323.28] = Mo [1.73] > Zn [28.61] | |||||||
| Variables | Percentage of kernel (60.4) = Zinc deficiency (5.9%) = Nuts per kilogram (155) > Nut weight (7.63 g) > Percentage of fruitful shoots (22.9%) = Leaflet size (71.9%) > Yield (464.3 kg ha−1) > Foliar cover (82.1%) | |||||||
| Yield | Development % | |||||||
|---|---|---|---|---|---|---|---|---|
| Regression Y | Production kg ha−1 | Percentage of Kernel | Nuts kg−1 | Nut Weight g | Zinc Deficiency | Leaf Size | Canopy Cover | Fruiting Shoots |
| Linear | 0.0047 Z | 0.1992 | 0.0005 | 0.0003 | 0.0263 | 0.6123 | 0.3311 | 0.0024 |
| Quadratic | 0.2585 | 0.1949 | 0.2294 | 0.1517 | 0.2490 | 0.3620 | 0.2179 | 0.0620 |
| Interaction | 0.6548 | 0.3469 | 0.0247 | 0.0222 | 0.2444 | 0.4711 | 0.4145 | 0.0706 |
| Total model | 0.0454 | 0.1987 | 0.0008 | 0.0004 | 0.0546 | 0.5627 | 0.3114 | 0.0023 |
| Residual X | ||||||||
| Lack of fit | 0.2686 | 0.1017 | <0.0001 | <0.0001 | 0.1768 | 0.5562 | 0.5129 | 0.4160 |
| Parameters Y | ||||||||
| Intercept | ||||||||
| N | 0.0131 | <0.0001 | <0.0001 | <0.0001 | 0.3136 | <0.0001 | <0.0001 | 0.0005 |
| MO | 0.1816 | 0.2892 | 0.0195 | 0.0422 | 0.2623 | 0.7741 | 0.4315 | 0.4879 |
| Zn | 0.3506 | 0.1149 | 0.0903 | 0.1741 | 0.1662 | 0.9636 | 0.5611 | 0.2242 |
| ZnMo | 0.4196 | 0.1093 | 0.1403 | 0.2530 | 0.2109 | 0.7179 | 0.2195 | 0.2152 |
| N2 | 0.0490 | 0.1797 | 0.0044 | 0.0084 | 0.4519 | 0.7761 | 0.9605 | 0.7425 |
| N*Mo | 0.8259 | 0.3440 | 0.1731 | 0.1302 | 0.0097 | 0.1944 | 0.8684 | 0.7134 |
| Mo2 | 0.3620 | 0.9258 | 0.5903 | 0.9361 | 0.0170 | 0.1460 | 0.7084 | 0.3402 |
| N*Zn | 0.3052 | 0.6477 | 0.4339 | 0.8110 | 0.0593 | 0.1470 | 0.691 | 0.1820 |
| Zn*Mo | 0.4153 | 0.6813 | 0.8016 | 0.8257 | 0.0150 | 0.1507 | 0.6374 | 0.2498 |
| Zn2 | 0.3645 | 0.8818 | 0.5277 | 0.8921 | 0.0203 | 0.1327 | 0.6462 | 0.2554 |
| N*ZnMo | 0.6086 | 0.5108 | 0.8076 | 0.5776 | 0.0063 | 0.1224 | 0.9764 | 0.5350 |
| Mo*ZnMo | 0.2307 | 0.5604 | 0.6354 | 0.8167 | 0.6917 | 0.2217 | 0.7101 | 0.8061 |
| Zn*ZnMo | 0.3398 | 0.1069 | 0.0137 | 0.0246 | 0.2103 | 0.7491 | 0.5179 | 0.3829 |
| (ZnMo)2 | 0.3937 | 0.0859 | 0.0203 | 0.0385 | 0.2584 | 0.6457 | 0.3081 | 0.1883 |
| Factors X | ||||||||
| N | 0.6671 | 0.3144 | 0.0182 | 0.0177 | 0.0872 | 0.6507 | 0.7020 | 0.1388 |
| Mo | 0.5419 | 0.0673 | 0.0371 | 0.0318 | 0.0353 | 0.5972 | 0.9796 | 0.3193 |
| Zn | 0.8570 | 0.4773 | 0.0044 | 0.0040 | 0.1574 | 0.4847 | 0.4807 | 0.4049 |
| ZnMo | 0.1252 | 0.5308 | 0.0266 | 0.0378 | 0.6388 | 0.6217 | 0.4166 | 0.1202 |
| Mean | 120.9 | 57.1 | 166 | 6.09 | 21.78 | 55.42 | 61.47 | 12.30 |
| R2 | 0.3373 | 0.2651 | 0.4704 | 0.4900 | 0.3293 | 0.1889 | 0.2369 | 0.4416 |
| CV | 72.83 | 3.05 | 8.28 | 7.87 | 86.40 | 19.44 | 18.57 | 48.78 |
| Factors [kg ha−1] | ||||||
|---|---|---|---|---|---|---|
| N (A) | Mo (B) | Zn (D) | Zn-Mo (E) | |||
| Eigenvalues | 162.500 T | 1.125 | 23.750 | 2.00 | Eigenvectors | +/− |
| Foliar nutritional content (11 variables) | ||||||
| Subtotal | Q43 | 27 | 24 | 24 | 118 | |
| Proportion +/− | 43/0 | 21/6 | 22/2 | 24/0 | 110/8 | |
| Selection | 7/11 | 7/11 | 4/11 | 7/11 | 22/11 | |
| Factors [Mm ha−1] | N [285.35] > Mo [1.28] > Zn [30.82] = Zn-Mo [2.37] | |||||
| Variables | Manganese (250.7 ppm) = Copper (7.9 ppm) = Phosphorus (0.185%) = Nitrates (1907.1ppm) > Calcium (2.32%) = Nitrogen (2.47%) = Magnesium (1.30%) | |||||
| Enzymatic activity and leaf pigments (10 variables) | ||||||
| Subtotal | Q40 | 39 | 26 | 9 | 114 | |
| Proportion +/− | 31/9 | 30/9 | 23/3 | 9/0 | 91/23 | |
| Selection | 10/11 | 10/11 | 10/11 | 18/9 | ||
| Factors [Mm ha−1] | N [275.10] = Mo [1.17] > Zn [31.45] | |||||
| Variables | Chlorophyll a/b (2.44 µg cm2) = Chlorophyll b (5.17 µg cm2) > Nitrate reductase NO3 (2.82 µmoles NO2−) = Carotenoids (0.83 µg cm2) > Urease NH4-Ni (58.44 µmoles NH4) > Chlorophyll a (10.40 µg cm2) = Endogenous urease (14.67 µmoles NH4) = Chlorophyll a/Carotenoids (14.02 µg cm2) = Nitrate reductase Mo (2.84 µmoles NO2−) | |||||
| Pomological parameters (8 variables) | ||||||
| Subtotal | Q31 | 26 | 19 | 10 | 86 | |
| Proportion +/− | 26/5 | 20/6 | 15/4 | 10/0 | Z 71/15 | |
| Selection | 8/8 | 8/8 | 6/8 | 8/8 | 14/7 | |
| Factors [Mm ha−1] | N [319.6] = Mo [1.73] > Zn [28.61] | |||||
| Variables | Percentage of kernel (60.4) = Zinc deficiency (5.9%) = Nuts per kilogram (155) > Nut weight (7.63 g) > % Percentage of fruitful shoots (22.9%) = Leaflet size (71.9%) > Yield (464.3 kg ha−1) > Foliar cover (82.1%) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Meléndez, L.R.; Noperi-Mosqueda, L.C.; Oviedo-Mireles, J.C.; Torres-Beltrán, N.G.; Yáñez-Muñoz, R.M.; Soto-Parra, J.M. Balanced Fertilization with Nitrogen, Molybdenum, and Zinc: Key to Optimizing Pecan Tree Yield and Quality of Western Schley Pecan Tree. Horticulturae 2025, 11, 741. https://doi.org/10.3390/horticulturae11070741
Orozco-Meléndez LR, Noperi-Mosqueda LC, Oviedo-Mireles JC, Torres-Beltrán NG, Yáñez-Muñoz RM, Soto-Parra JM. Balanced Fertilization with Nitrogen, Molybdenum, and Zinc: Key to Optimizing Pecan Tree Yield and Quality of Western Schley Pecan Tree. Horticulturae. 2025; 11(7):741. https://doi.org/10.3390/horticulturae11070741
Chicago/Turabian StyleOrozco-Meléndez, Laura R., Linda C. Noperi-Mosqueda, Julio C. Oviedo-Mireles, Nubia G. Torres-Beltrán, Rosa M. Yáñez-Muñoz, and Juan M. Soto-Parra. 2025. "Balanced Fertilization with Nitrogen, Molybdenum, and Zinc: Key to Optimizing Pecan Tree Yield and Quality of Western Schley Pecan Tree" Horticulturae 11, no. 7: 741. https://doi.org/10.3390/horticulturae11070741
APA StyleOrozco-Meléndez, L. R., Noperi-Mosqueda, L. C., Oviedo-Mireles, J. C., Torres-Beltrán, N. G., Yáñez-Muñoz, R. M., & Soto-Parra, J. M. (2025). Balanced Fertilization with Nitrogen, Molybdenum, and Zinc: Key to Optimizing Pecan Tree Yield and Quality of Western Schley Pecan Tree. Horticulturae, 11(7), 741. https://doi.org/10.3390/horticulturae11070741






