Mitigating Water Stress and Enhancing Aesthetic Quality in Off-Season Potted Curcuma cv. ‘Jasmine Pink’ via Potassium Silicate Under Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Preparation
2.2. Phenological Shift and Plant Growth Parameters
2.3. Photosynthetic Parameters
2.4. Data Analysis
3. Results
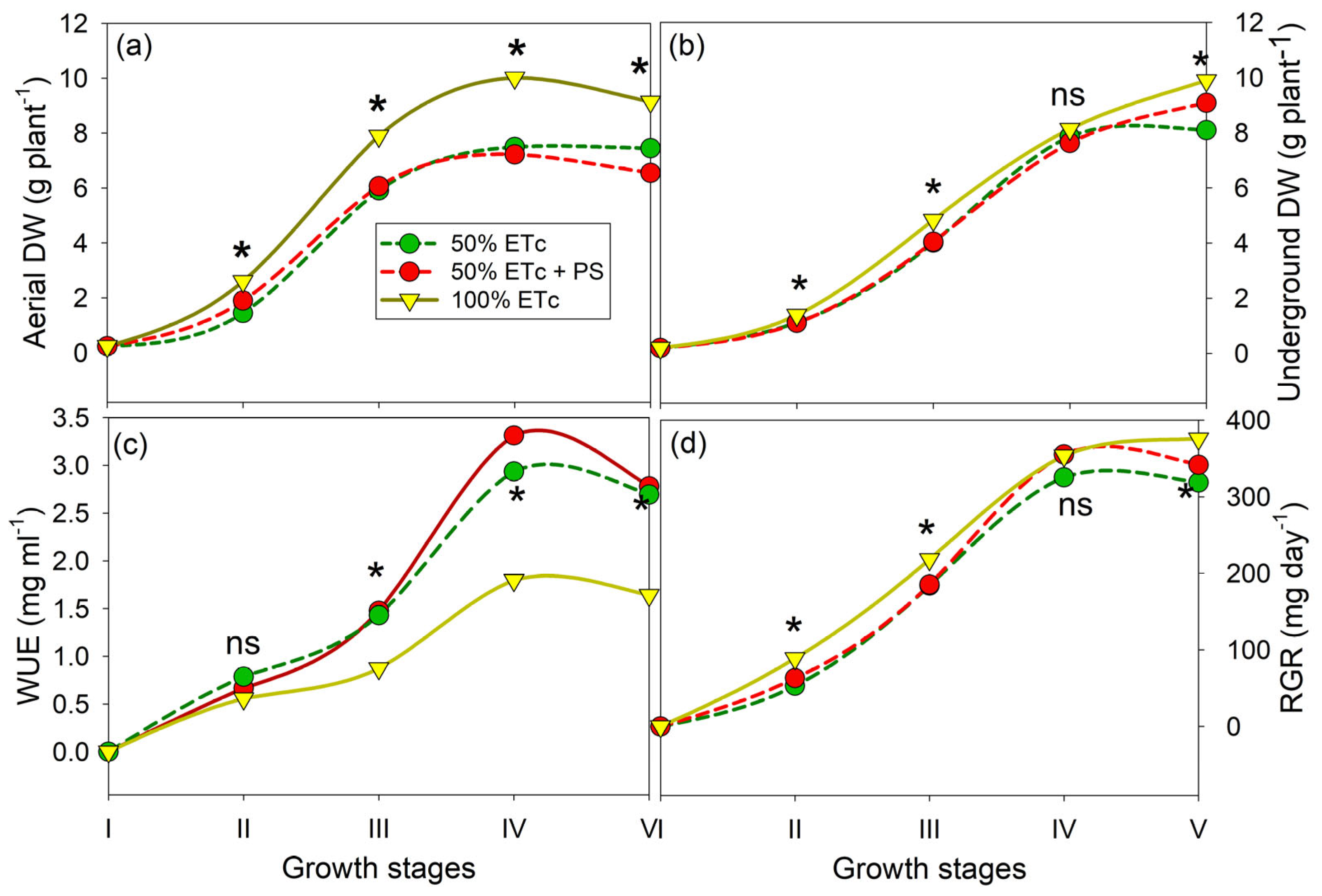
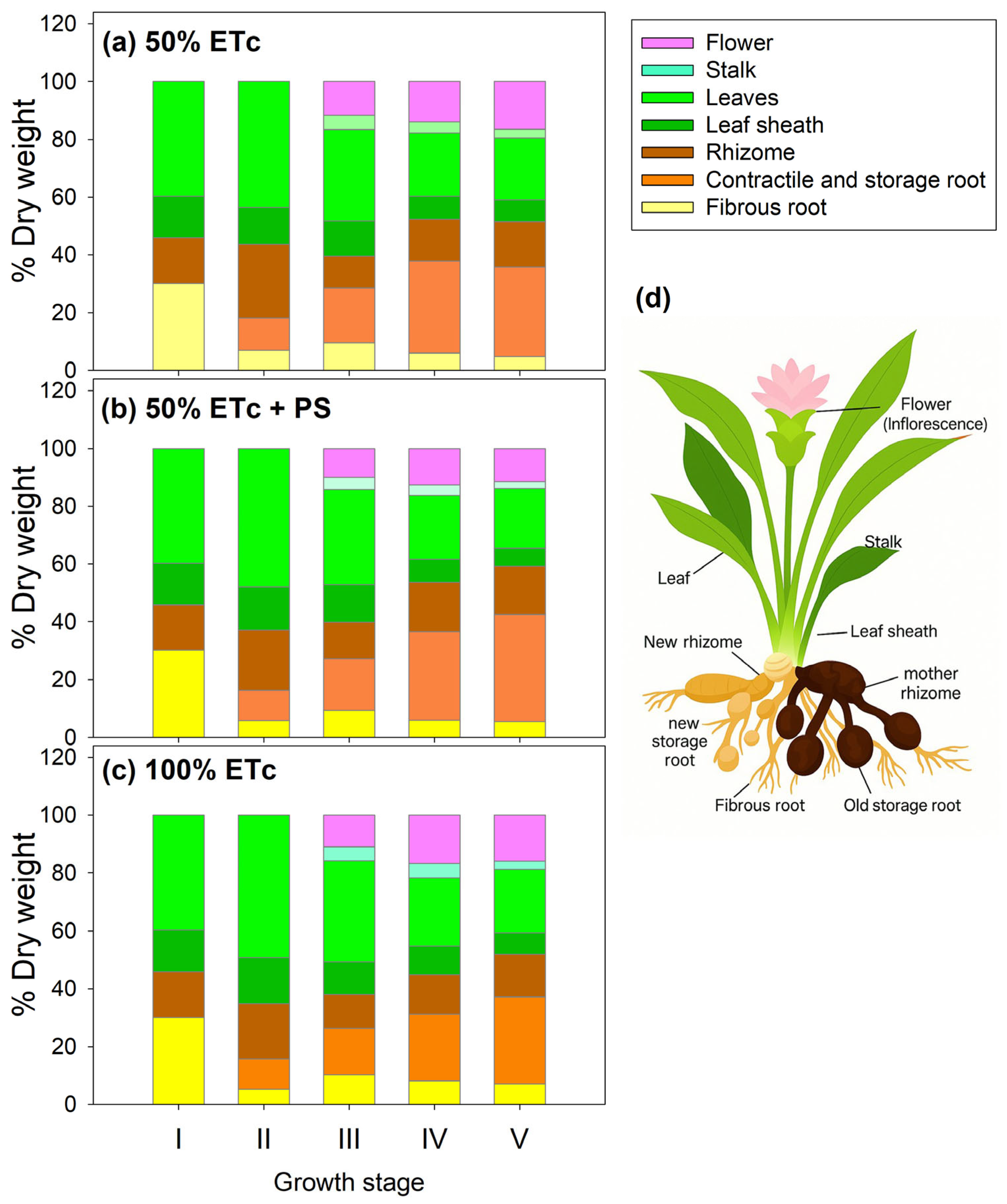
3.1. Phenological Shift, Plant Growth, and Yields
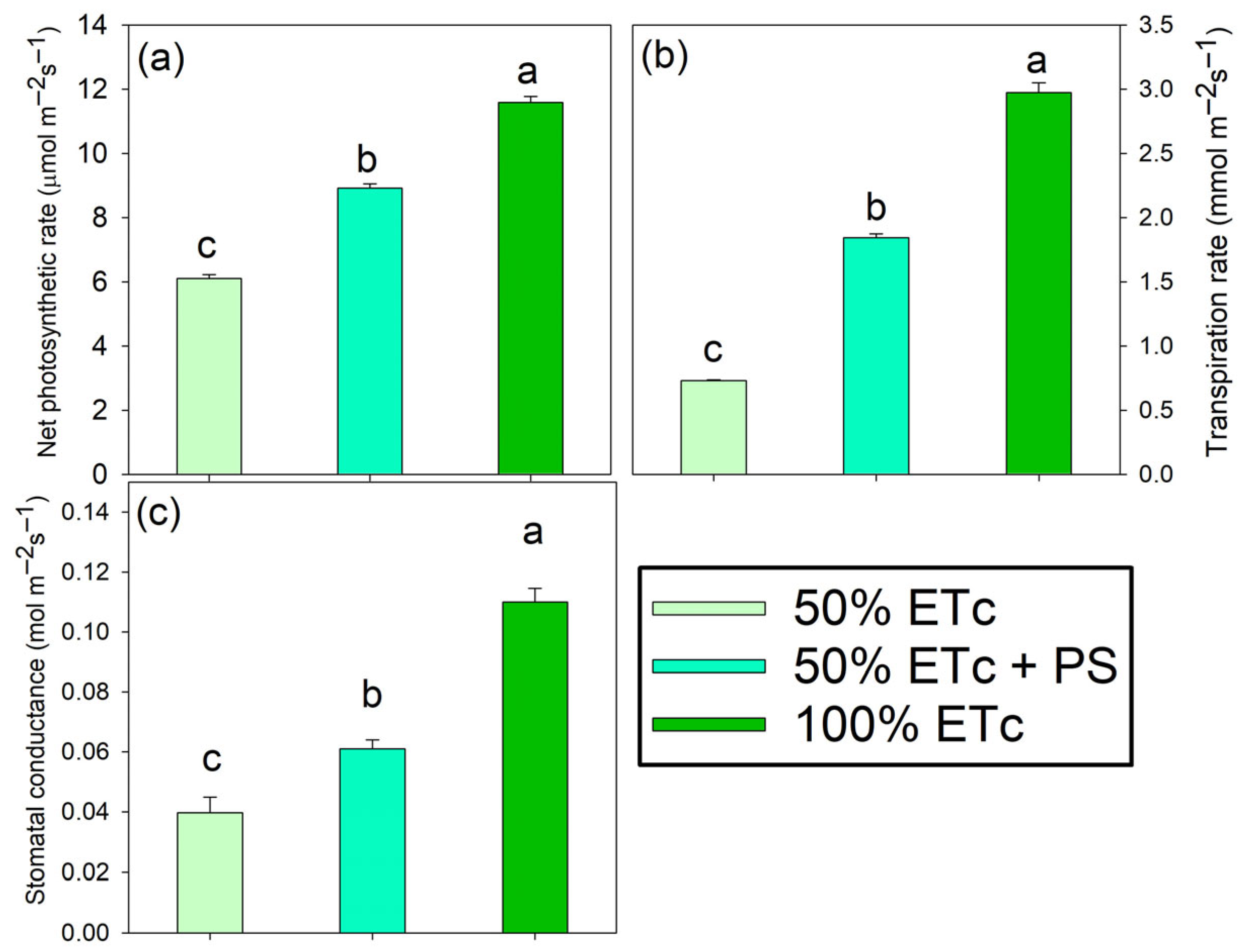
3.2. Photosynthetic Parameters
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhat, C.; Paschapur, A. Urban agriculture: The saviour of rapid urbanization. Indian Farmer 2020, 7, 01–09. [Google Scholar]
- Mordor Intelligence. Flower Pots and Planters Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/flower-pots-and-planters-market (accessed on 22 May 2025).
- Prasadini, W.; Rosairo, R. Consumer preferences for selected exotic flowering pot plants: A market assessment study. Sri Lanka J. Food Agric. 2017, 3, 21. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Karimi, M.; Salimi, F.; Pakdin Parizi, A. The Effect of different concentrations of uniconazole and cycocel and their application method on vegetative growth and biochemical properties of Lilium Hybrids (Longiflorum × Asiatic cv. Eyeliner). J. Hortic. Sci. 2023, 36, 937–948. [Google Scholar][Green Version]
- Kishore, K.; Singh, H.; Kurian, R. Role of paclobutrazol in fruit crops and its residual fate. In Plant Growth Regulators in Tropical and Sub-Tropical Fruit Crops; Ghosh, S.S., Tarai, R.K., Ahlawat, T.R., Eds.; CRC Press (Taylor & Francis Group): Oxfordshire, UK, 2022; pp. 35–51. [Google Scholar]
- Ruamrungsri, S. The physiology of Curcuma alismatifolia Gagnep. as a basis for the improvement of ornamental production. Eur. J. Hortic. Sci. 2015, 80, 316–321. [Google Scholar] [CrossRef]
- Ruamrungsri, S.; Uthai-Butra, J.; Wichailux, O. Off-Season Flowering in Curcuma alismatifolia Gagnep. Gard. Bull. Singapore 2007, 59, 173–182. [Google Scholar]
- Seelatlao, C.; Dongsansuk, A.; Isarangkool Na Ayutthaya, S.; Hongpakdee, P. Evaluated crop water requirement of flowering potted Curcuma hybrid cv. “Doi Tung Red”. Acta Hortic. 2024, 1404, 157–162. [Google Scholar] [CrossRef]
- Hongpakdee, P.; Ruamrungsri, S. Water use efficiency, nutrient leaching, and growth in potted marigolds affected by coconut coir dust amended in substrate media. Hortic. Environ. Biotechnol. 2015, 56, 27–35. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Reimer, J.E.; Maas, C.M.; Galella, J.G.; Utz, R.M.; Duan, S.; Kryger, J.R.; Yaculak, A.M. Freshwater salinization syndrome: From emerging global problem to managing risks. Biogeochemistry 2021, 154, 255–292. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Kabir, M.; Zuhara, F.T.; Mehjabin, A.; Tasannum, N.; Hoang, A.T.; Kabir, Z.; Mofijur, M. Threats, challenges and sustainable conservation strategies for freshwater biodiversity. Environ. Res. 2022, 214, 113808. [Google Scholar] [CrossRef] [PubMed]
- Mircea, D.M.; Estrelles, E.; Al Hassan, M.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Effect of water deficit on germination, growth and biochemical responses of four potentially invasive ornamental grass species. Plants 2023, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Ferrante, A.; Romano, D. Response of mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Finke, D. Plant-vector-pathogen interactions in the context of drought stress. Front. Ecol. Evol. 2019, 7, 262. [Google Scholar] [CrossRef]
- El Khoumsi, W.; Essiba, W.; Basir, I.; Harrouni, C.; Redouane, C.; Bourziza, R. Effect of treated wastewater irrigation on ornamental plants: Case study of Lantana and Hibiscus, Morocco. World Water Policy 2024, 10, 280–296. [Google Scholar] [CrossRef]
- Hinsley, A.; Hughes, A.C.; van Valkenburg, J.; Stark, T.; van Delft, J.; Sutherland, W.; Petrovan, S.O. Understanding the environmental and social risks from the international trade in ornamental plants. BioScience 2025, 75, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Zuazo, V.H.D.; García-Tejero, I.F.; Rodríguez, B.C.; Tarifa, D.F.; Ruiz, B.G.; Sacristán, P.C. Deficit irrigation strategies for subtropical mango farming. A review. Agron. Sustain. Dev. 2021, 41, 13. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.; Ortuño, M.; Bañon, S.; Álvarez, S. Deficit irrigation as a strategy to control growth in ornamental plants and enhance their ability to adapt to drought conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 137–150. [Google Scholar] [CrossRef]
- Chand, J.; Hewa, G.; Hassanli, A.; Myers, B. Evaluation of deficit irrigation and water quality on production and water productivity of tomato in greenhouse. Agriculture 2020, 10, 297. [Google Scholar] [CrossRef]
- Inkham, C.; Panjama, K.; Ruamrungsri, S. Deficit irrigation and fertilizer rates affecting growth, flower quality and physiological changes in tuberous begonia grown in plant factory. Acta Hortic. 2023, 1404, 1365–1370. [Google Scholar] [CrossRef]
- Álvarez, S.; Navarro, A.; Bañón, S.; Sánchez-Blanco, M.J. Regulated deficit irrigation in potted Dianthus plants: Effects of severe and moderate water stress on growth and physiological responses. Sci. Hortic. 2009, 122, 579–585. [Google Scholar] [CrossRef]
- Vandegeer, R.K.; Zhao, C.; Cibils-Stewart, X.; Wuhrer, R.; Hall, C.R.; Hartley, S.E.; Tissue, D.T.; Johnson, S.N. Silicon deposition on guard cells increases stomatal sensitivity as mediated by K+ efflux and consequently reduces stomatal conductance. Physiol. Plantarum 2021, 171, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, A.H.; Mir, S.H.; Rehman, I.U.; Tahir, I.; Ahmad, P.; Rashid, I. Elucidating the role of silicon in drought stress tolerance in plants. Plant Physiol. Biochem. 2021, 165, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Saja-Garbarz, D.; Libik-Konieczny, M.; Fellner, M.; Jurczyk, B.; Janowiak, F. Silicon-induced alterations in the expression of aquaporins and antioxidant system activity in well-watered and drought-stressed oilseed rape. Plant Physiol. Biochem. 2011, 174, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Oraee, A.; Tehranifar, A.; Ghorbani, Z.; SayadAmin, P. Potassium silicate enhances drought tolerance of Bellis perennis by improving antioxidant activity and osmotic regulators. Adv. Hortic. Sci. 2023, 37, 377–389. [Google Scholar] [CrossRef]
- Zhang, X.; Roberson, T.; Goatley, M.; Flanary, T.; McCall, D. Silicon Enhances Antioxidant Capacity and Photochemical Efficiency in Drought-Stressed Creeping Bentgrass (Agrostis stolonifera L.) Putting Greens. Horticulturae 2025, 11, 664. [Google Scholar] [CrossRef]
- Aziz, H.A.; Sharaf, M.; Omar, M.; El-Yazied, A.A.; AlJwaizea, N.I.; Ismail, S.; Omar, M.M.; Alharbi, K.; Elkelish, A.; Tawfik, M. Improvement of selected morphological, physiological, and biochemical parameters of banana (Musa acuminata L.) using potassium silicate under drought stress condition grown in vitro. Phyton Int. Exp. Botany 2023, 92, 1019–1036. [Google Scholar]
- NSTDA. Curcuma sp.: The queen of wild flower of rainy forest. Available online: https://www.nstda.or.th/agritec/wp-content/uploads/2021/07/siam-tulip-resize.pdf (accessed on 22 May 2025).
- Allen, R.G.; Pereira, L.S.; Smith, M.; Raes, D.; Wright, J.L. FAO-56 dual crop coefficient method for estimating evaporation from soil and application extensions. J. Irrig. Drain. Eng. 2005, 131, 2–13. [Google Scholar] [CrossRef]
- Vudhivanich, V. Monthly potential evaporation of Thailand. Kasetsart J. (Nat. Sci.) 1996, 30, 392–399. [Google Scholar]
- Yang, T.; Zhao, J.; Fu, Q. Quantitative relationship of plant height and leaf area index of spring maize under different water and nitrogen treatments based on effective accumulated temperature. Agronomy 2024, 14, 1018. [Google Scholar] [CrossRef]
- Chandarak, N.; Somjinda, P.; Phoncharoen, P.; Banterng, P.; Taratima, W.; Theerakulpisut, P.; Dongsansuk, A. Booting heat stress alters leaf photosynthesis, growth rate, phenology and yield in rice. Plant Stress 2023, 10, 100226. [Google Scholar] [CrossRef]
- Chungloo, D.; Tisarum, R.; Pinruan, U.; Sotesaritkul, T.; Saimi, K.; Praseartkul, P.; Himanshu, S.K.; Datta, A.; Cha-Um, S. Alleviation of water-deficit stress in turmeric plant (Curcuma longa L.) using phosphate solubilizing rhizo-microbes inoculation. 3 Biotech 2024, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. Cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, A.; Zhang, Y.; Yang, X.; Yang, S.; Zhao, K. Effects of progressive drought stress on the growth, ornamental values, and physiological properties of begonia semperflorens. Horticulturae 2024, 10, 405. [Google Scholar] [CrossRef]
- Hongpakdee, P.; Siritrakulsak, P.; Ohtake, N.; Sueyoshi, K.; Ohyama, T.; Ruamrungsri, S. Changes in endogenous abscisic acid, trans-zeatin riboside, indole-3-acetic acid levels and the photosynthetic rate during the growth cycle of Curcuma alismatifolia Gagnep. In different production seasons. Eur. J. Hortic. Sci. 2010, 75, 204–213. [Google Scholar] [CrossRef]
- Yang, X.-L.; An, T.; Ye, Z.-W.-Y.; Kang, H.-J.; Robakowski, P.; Ye, Z.-P.; Wang, F.-B.; Zhou, S.-X. Modeling light response of effective quantum efficiency of photosystem II for C3 and C4 crops. Front. Plant Sci. 2025, 16, 1478346. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yu, W.; Yang, X.; Liang, J.; Sun, X.; Sun, M.; Xiao, Y.; Peng, F. Silicon enhances the drought resistance of peach seedlings by regulating hormone, amino acid, and sugar metabolism. BMC Plant Biol. 2022, 22, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goatley, M.; Wang, K.; Goddard, B.; Harvey, R.; Brown, I.; Kosiarski, K. Silicon improves heat and drought stress tolerance associated with antioxidant enzyme activity and root viability in creeping bentgrass (Agrostis stolonifera L.). Agronomy 2024, 14, 1176. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Zhang, A.; Lu, C. Non-photochemical quenching: From light perception to photoprotective gene expression. Int. J. Mol. Sci. 2022, 23, 687. [Google Scholar] [CrossRef] [PubMed]
- Nosalewicz, A.; Okoń, K.; Skorupka, M. Non-photochemical quenching under drought and fluctuating light. Int. J. Mol. Sci. 2022, 23, 5182. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cresswell, T.; Johansen, M.P.; Harrison, J.J.; Jiang, Y.; Keitel, C.; Cavagnaro, T.R.; Dijkstra, F.A. Reallocation of nitrogen and phosphorus from roots drives regrowth of grasses and sedges after defoliation under deficit irrigation and nitrogen enrichment. J. Ecol. 2021, 109, 4071–4080. [Google Scholar] [CrossRef]
- Ashfaq, W.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Silicon improves root system and canopy physiology in wheat under drought stress. Plant Soil 2024, 502, 279–296. [Google Scholar] [CrossRef]
- Gomaa, M.; Kandil, E.E.; El-Dein, A.A.Z.; Abou-Donia, M.E.; Ali, H.M.; Abdelsalam, N.R. Increase maize productivity and water use efficiency through application of potassium silicate under water stress. Sci. Rep. 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Delta, A.K.; Kaushik, P. Effects of Funneliformis mosseae and potassium silicate on morphological and biochemical traits of onion cultivated under water stress. Horticulturae 2022, 8, 663. [Google Scholar] [CrossRef]
- Chalki, A.B.; Shoor, M.; Kakhki, S.F.F.; Abedi, B. Evaluation of potassium silicate applying to reduce adverse effects of salinity on marigold plant (Tagetes erecta L.‘Nana’). J. Ornam. Plants 2022, 12, 157–166. [Google Scholar]
- Eyni-Nargeseh, H.; Shirani Rad, A.H.; Shiranirad, S. Does potassium silicate improve physiological and agronomic traits and oil compositions of rapeseed genotypes under well-watered and water-limited conditions? Gesunde Pflanzen 2022, 74, 801–816. [Google Scholar] [CrossRef]
- Chao, X.; Zhang, T.; Lyu, G.; Liang, Z.; Chen, Y. Sustainable application of sodium removal from red mud: Cleaner production of silicon-potassium compound fertilizer. J. Clean. Prod. 2022, 352, 131601. [Google Scholar] [CrossRef]
- Waly, A.; Abd El-Fattah, Y.; Hassan, M.; El-Ghadban, E.-M.; Abd Alla, A.S. Enhancing growth, productivity and essential oil percentage of Thymus vulgaris L. plant using seaweeds extract, chitosan and potassium silicate in sandy soil. Sci. J. Flowers Ornam. Plants 2020, 7, 549–562. [Google Scholar] [CrossRef]
- Rasouli, M.; Bayanati, M.; Tavakoli, F. Improving quantitative and qualitative traits of grapes cv.‘Fakhri’of iran with foliar application of potassium silicate and humic acid. Russ. J. Plant Physiol. 2024, 71, 86. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; El-Razek, U.A.A.; Elbagory, M.; Omara, A.E.-D.; Eid, M.A.; Gowayed, S.M. Foliar-applied potassium silicate coupled with plant growth-promoting rhizobacteria improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water in salt-affected soil. Plants 2021, 10, 894. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Water Application (mL pot−1day−1) | ||
|---|---|---|---|
| Vegetative Stage | Flowering Stage | Pre-Dormancy Stage | |
| 50% ETc | 80 | 125 | 130 |
| 50% ETc + PPA | 80 | 125 | 130 |
| 100% ETc | 160 | 250 | 260 |
| Phenological Shift (DAP) | Irrigation Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50% ETc | 50% ETc + PS | 100% ETc | |||||||
| Complete expanded leaves | 73.11 | ±0.86 | b | 72.33 | ±0.83 | b | 69.44 | ±0.58 | a |
| First emergence of floral bud | 96.89 | ±3.45 | b | 90.00 | ±1.15 | a | 90.89 | ±2.91 | a |
| First bloom of true flower | 109.00 | ±3.17 | b | 104.56 | ±1.45 | a | 100.55 | ±0.61 | a |
| Fully inflorescent ceasing | 147.00 | ±1.17 | a | 142.78 | ±1.12 | b | 139.33 | ±0.77 | b |
| Floral display period (DAP) ns | 38.00 | ±2.60 | - | 38.22 | ±0.98 | - | 38.78 | ±0.29 | - |
| Parameters | Irrigation Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50% ETc | 50% ETc + PS | 100% ETc | |||||||
| Height (cm) | 45.81 | ±0.79 | b | 44.93 | ±0.84 | b | 49.47 | ±0.80 | a |
| Total LF Area (cm2) | 429.32 | ±8.98 | c | 467.22 | ±13.98 | b | 621.25 | ±12.41 | a |
| Shoot number ns | 1.44 | ±0.11 | - | 1.67 | ±0.19 | - | 2.00 | ±0.00 | - |
| Leaves number ns | 6.25 | ±0.07 | - | 7.00 | ±0.34 | - | 7.53 | ±0.75 | - |
| New rhizome number ns | 1.44 | ±0.11 | - | 1.67 | ±0.19 | - | 2.00 | ±0.00 | - |
| New storage root number | 8.67 | ±0.57 | b | 8.56 | ±0.98 | b | 11.11 | ±0.72 | a |
| New rhizome size (cm) | 1.89 | ±0.03 | a | 1.87 | ±0.03 | a | 1.66 b | ±0.05 | b |
| New storage root size (cm) ns | 1.36 | ±0.11 | - | 1.50 | ±0.04 | - | 1.31 | ±0.13 | - |
| Total DW (g) | 10.04 | ±0.17 | b | 10.11 | ±0.32 | b | 12.73 | ±0.12 | a |
| Root/shoot ratio ns | 1.55 | ±0.03 | - | 1.54 | +0.04 | - | 1.65 | ±0.12 | - |
| Compactness index (cm2 cm−1) | 9.38 | ±0.17 | c | 10.40 | ±0.21 | b | 12.57 | ±0.20 | a |
| Parameters | Irrigation Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50% ETc | 50% ETc + PS | 100% ETc | |||||||
| Flower number | 1.33 | ±0.19 | b | 1.44 | ±0.25 | b | 2.11 | ±0.10 | a |
| Stalk length (cm) | 21.38 | ±0.45 | c | 23.11 | ±0.18 | b | 24.51 | ±0.20 | a |
| Inflorescence length (cm) | 11.00 | ±0.00 | b | 10.83 | ±0.10 | b | 12.15 | ±0.02 | a |
| Inflorescence diameter (cm) | 6.56 | ±0.20 | b | 6.55 | ±0.19 | b | 7.69 | ±0.04 | a |
| Coma bract number | 9.22 | ±0.11 | b | 9.443 | ±0.05 | b | 11.44 | ±0.19 | a |
| Green bract number | 9.33 | ±0.33 | b | 10.11 | ±0.10 | ab | 10.77 | ±0.25 | a |
| Parameters | Irrigation Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50% ETc | 50% ETc + PS | 100% ETc | |||||||
| SPAD unit | 39.70 | ±0.93 | b | 39.05 | ±0.45 | b | 43.96 | ±0.36 | a |
| Fv/Fm ns | 0.72 | ±0.02 | - | 0.77 | ±0.01 | - | 0.76 | ±0.01 | - |
| ΔF/Fm′ | 0.57 | ±0.01 | b | 0.66 | ±0.02 | a | 0.66 | ±0.00 | a |
| ETR (µmol em−2s−1) | 229.44 | ±2.14 | b | 254.76 | ±5.49 | a | 261.27 | ±2.53 | a |
| qP ns | 0.95 | ±0.01 | - | 0.98 | ±0.00 | - | 0.97 | ±0.01 | - |
| qN | 0.563 | ±0.01 | a | 0.47 | ±0.01 | b | 0.31 | ±0.00 | c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sour, V.; Dongsansuk, A.; Isarangkool Na Ayutthaya, S.; Ruamrungsri, S.; Hongpakdee, P. Mitigating Water Stress and Enhancing Aesthetic Quality in Off-Season Potted Curcuma cv. ‘Jasmine Pink’ via Potassium Silicate Under Deficit Irrigation. Horticulturae 2025, 11, 856. https://doi.org/10.3390/horticulturae11070856
Sour V, Dongsansuk A, Isarangkool Na Ayutthaya S, Ruamrungsri S, Hongpakdee P. Mitigating Water Stress and Enhancing Aesthetic Quality in Off-Season Potted Curcuma cv. ‘Jasmine Pink’ via Potassium Silicate Under Deficit Irrigation. Horticulturae. 2025; 11(7):856. https://doi.org/10.3390/horticulturae11070856
Chicago/Turabian StyleSour, Vannak, Anoma Dongsansuk, Supat Isarangkool Na Ayutthaya, Soraya Ruamrungsri, and Panupon Hongpakdee. 2025. "Mitigating Water Stress and Enhancing Aesthetic Quality in Off-Season Potted Curcuma cv. ‘Jasmine Pink’ via Potassium Silicate Under Deficit Irrigation" Horticulturae 11, no. 7: 856. https://doi.org/10.3390/horticulturae11070856
APA StyleSour, V., Dongsansuk, A., Isarangkool Na Ayutthaya, S., Ruamrungsri, S., & Hongpakdee, P. (2025). Mitigating Water Stress and Enhancing Aesthetic Quality in Off-Season Potted Curcuma cv. ‘Jasmine Pink’ via Potassium Silicate Under Deficit Irrigation. Horticulturae, 11(7), 856. https://doi.org/10.3390/horticulturae11070856







