Relationship Between Volatile Aroma Components and Amino Acid Metabolism in Crabapple (Malus spp.) Flowers, and Development of a Cultivar Classification Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Standards
2.3. Sensory Evaluation
2.4. Collection and Determination of Volatile Compounds
2.5. Determination of AA Content
2.6. Statistical Analysis
3. Results
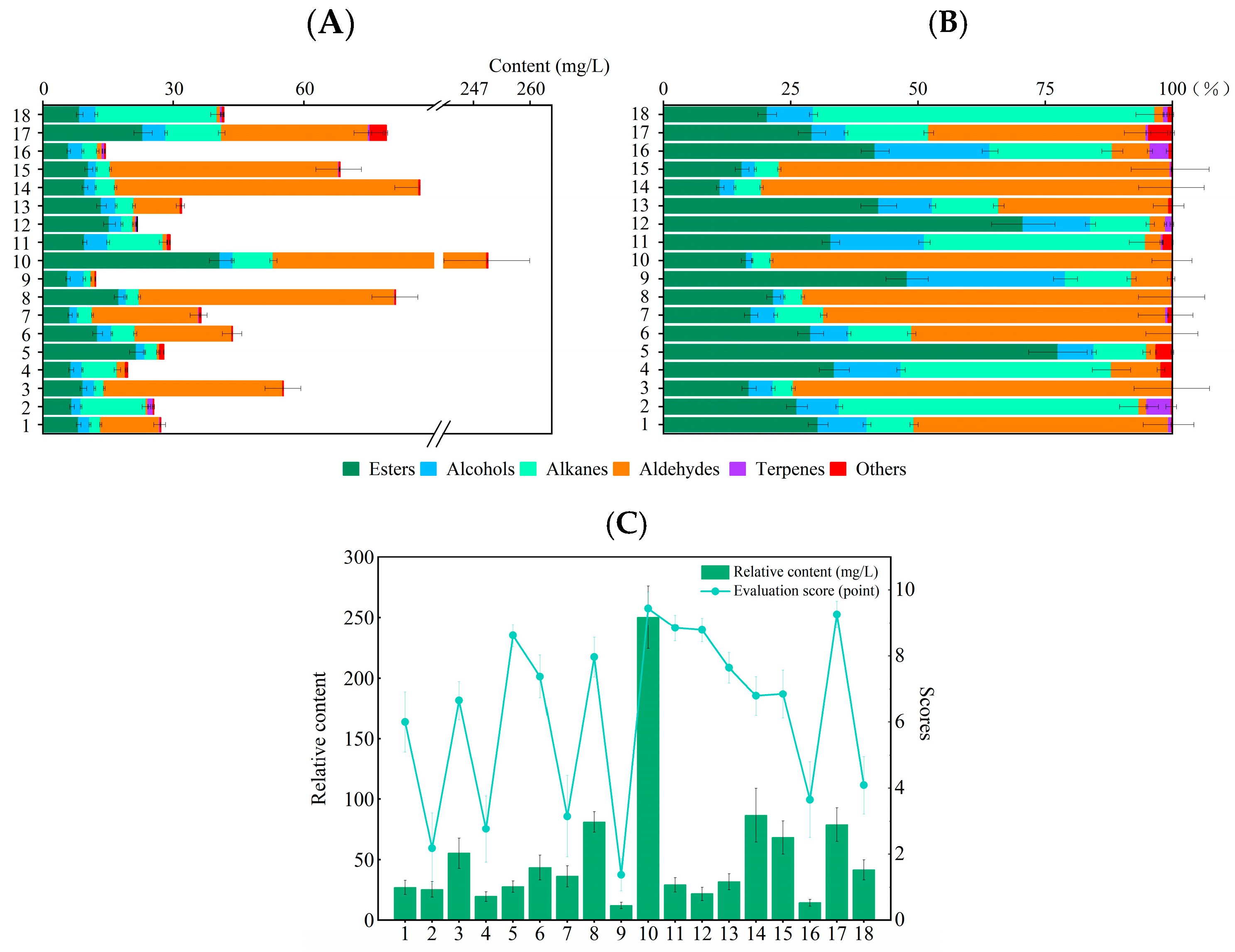
3.1. Classification of Volatile Component
3.2. Analysis of Major Volatile Components in Different Cultivars
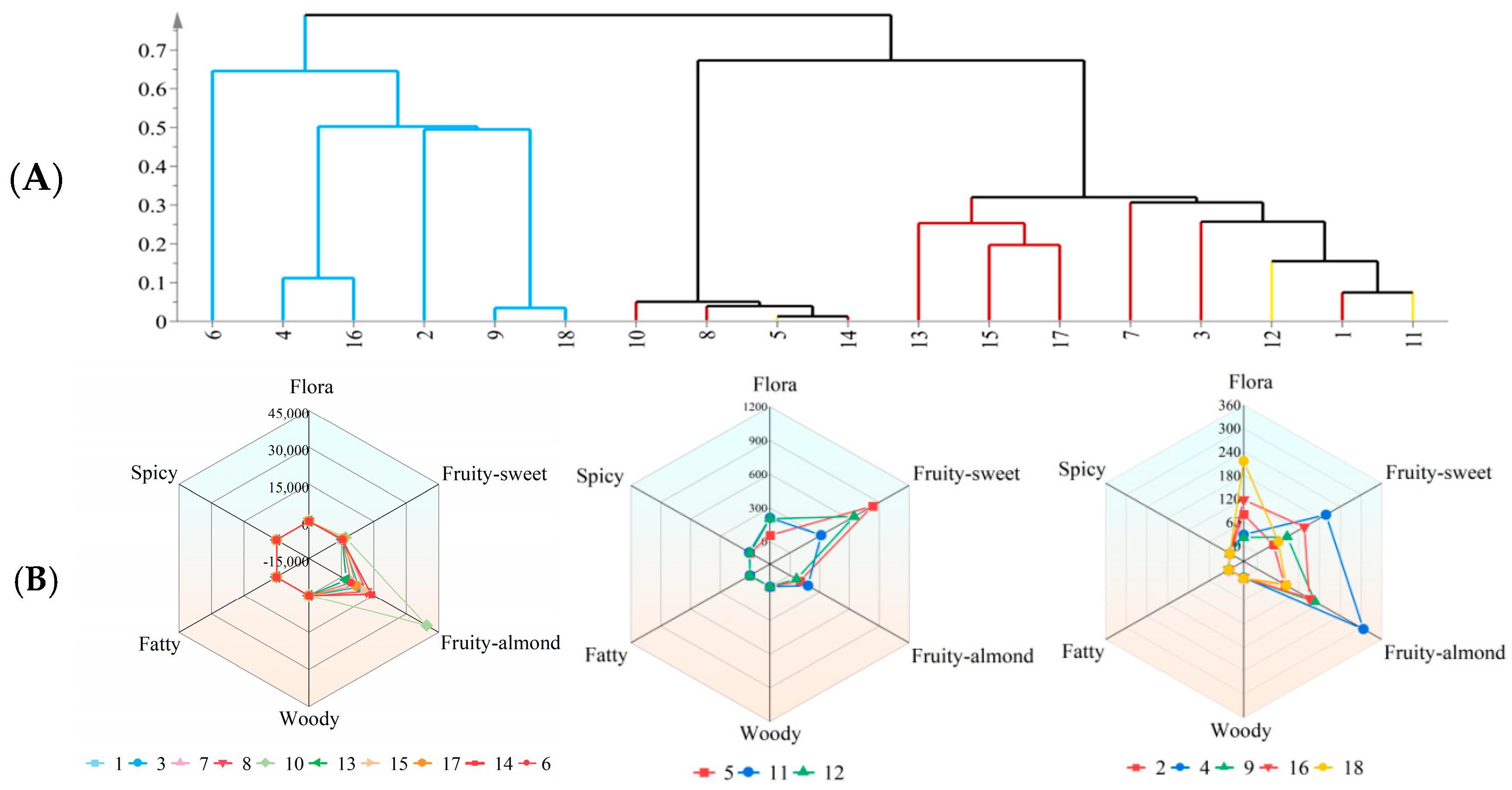
3.3. Odor-Based Classification of Volatiles and OAV Analysis
3.4. Classification of Crabapple Cultivars Based on Fragrance Profiles
- Fruit-Almond Type: This classification includes the cultivars ‘Tamlin’, ‘Harvest Gold’, ‘Donald Wyman’, ‘Prairifire’, ‘Royal Raindrops’, ‘Indian Magic’, ‘Profusion’, ‘Red Jewel’, and ‘Adams’. The predominant aromatic driver in these cultivars is benzaldehyde, which constitutes over 80% of the total OAV. Benzaldehyde imparts an almond-like scent, stemming from its molecular structure that combines a benzene ring with a formyl substituent. This compound is prevalent in plants of the Rosaceae family and is typically produced through the deamination of Phe via the shikimate pathway [20]. The scent profile of this group aligns closely with that of the tropical water lily Nymphaea ‘Eldorado’ at full bloom, which is also dominated by benzaldehyde [29].
- Fruit-Sweet Type: This group comprises ‘Kelsey’, ‘Radiant’, and ‘Purple Prince’. Their fragrance is primarily characterized by ethyl benzoate, accounting for over 65% of the total OAV. Ethyl benzoate delivers a distinct fruity sweet aroma. Biologically, the synthesis of ethyl benzoate is generally facilitated by BAHD acyltransferases, such as LoAAT1, which utilize benzoyl-CoA and ethanol as substrates to form this ester aroma molecule [30].
- Mixed Type: The cultivars ‘Purple Classic’, ‘Flame’, ‘Toringo’, ‘Tang-2’, ‘Sparkler’, ‘Spring Snow’, and ‘Royalty’ fall under the mixed type category. The fragrance of these cultivars is defined by a co-dominance of benzaldehyde, ethyl benzoate, and benzyl alcohol, with their contributions to the OAV being relatively balanced. This results in a complex and layered aroma profile. These cultivars are often referred to as having a “mixed” fragrance type [31], displaying diverse and challenging-to-classify sensory characteristics during evaluations.
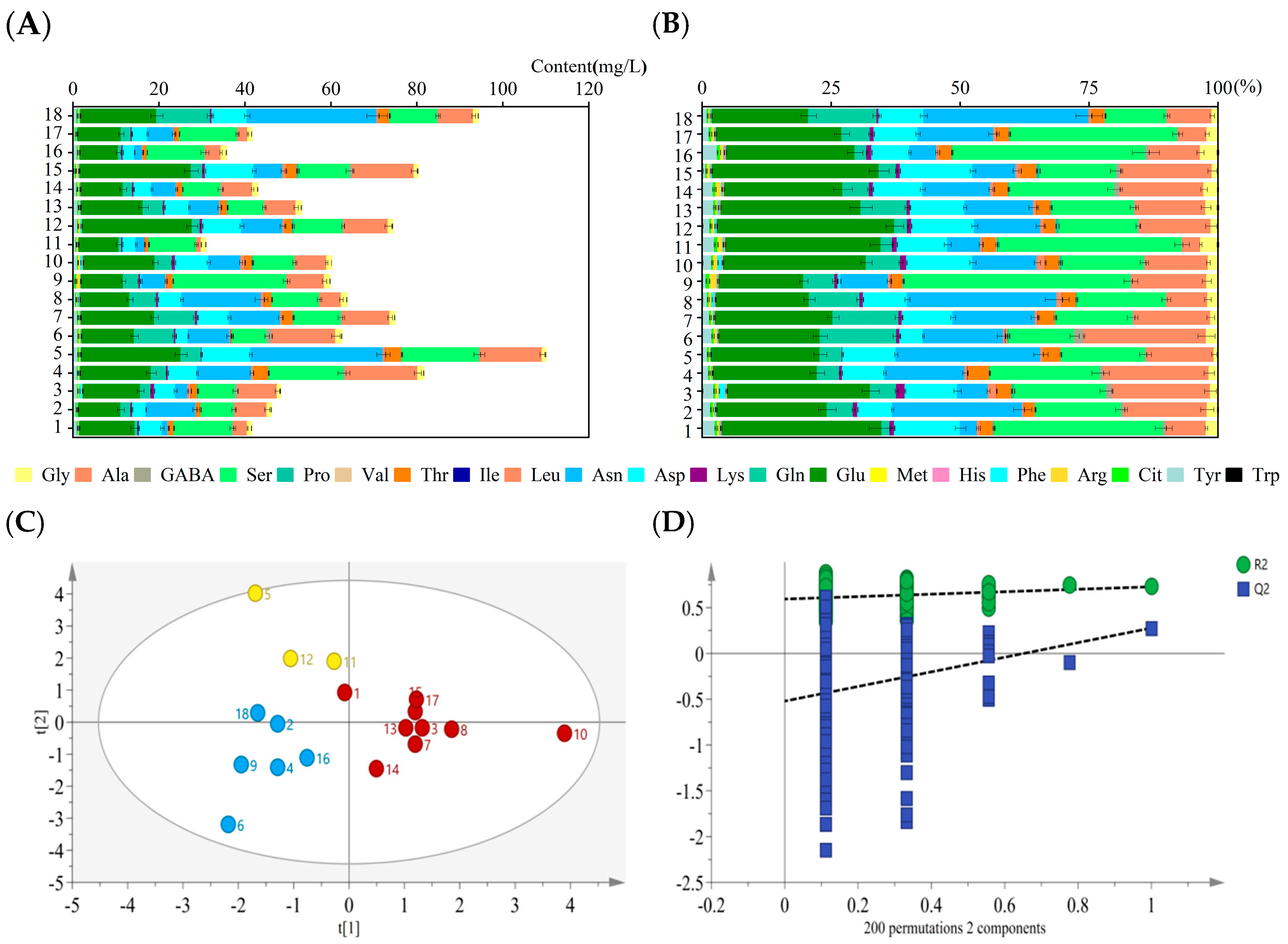
3.5. AA Analysis
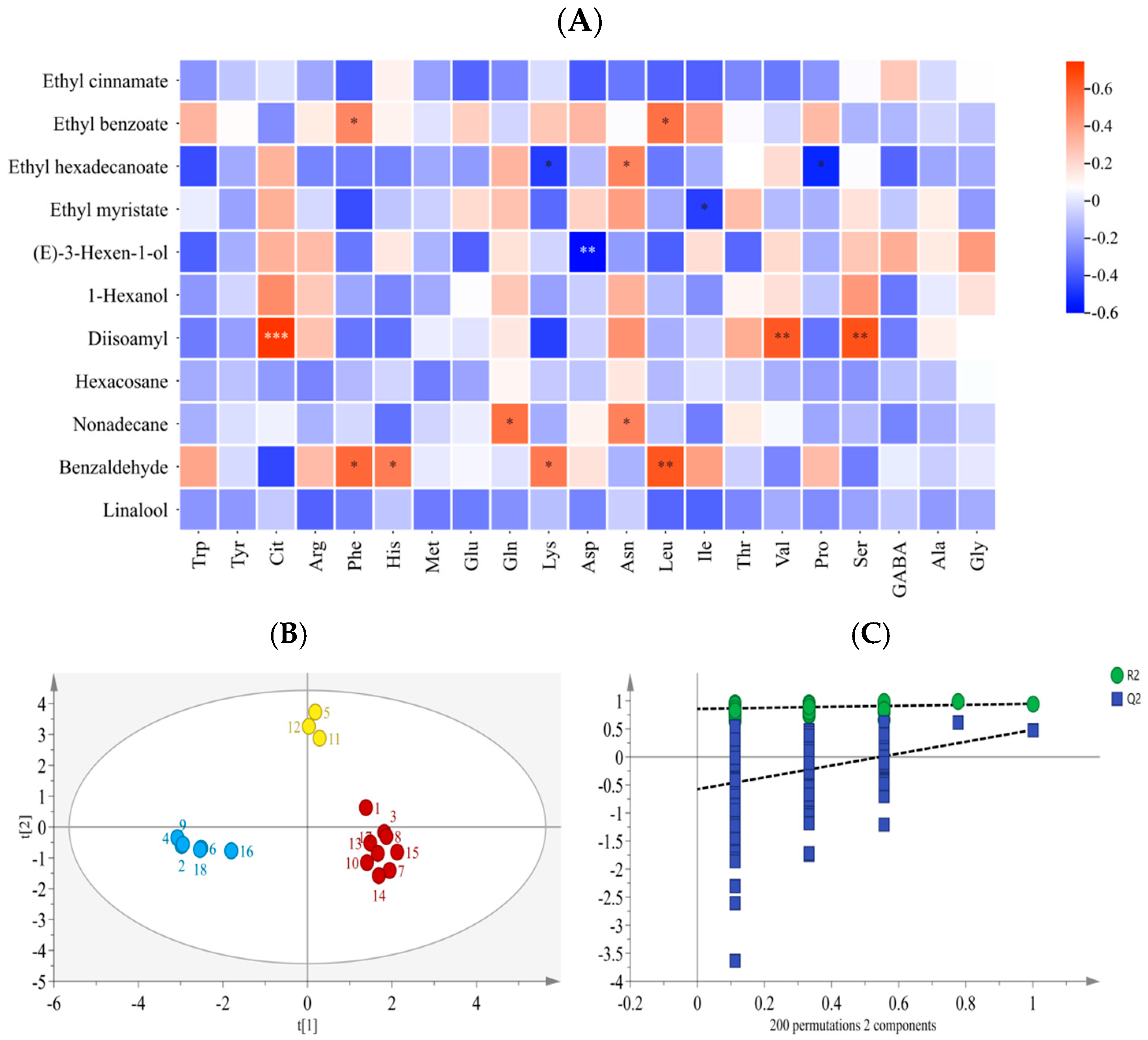
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Liu, J.; Zhang, H.; Jia, L.; Liu, Y.; Li, J.; Zhou, S.; Wang, P.; Tan, M.; Shao, J. Volatile Metabolome and Floral Transcriptome Analyses Reveal the Volatile Components of Strongly Fragrant Progeny of Malus × Robusta. Front. Plant Sci. 2023, 14, 1065219. [Google Scholar] [CrossRef] [PubMed]
- Csrl, E. Biosynthesis, Composition and Sources of Floral Scent in Ornamental Crops: A Review. Chem. Sci. Rev. Lett. 2017, 6, 1502–1509. [Google Scholar]
- Ghissing, U.; Mitra, A. Biology of Floral Scent Volatiles in Ornamental Plants. In Floriculture and Ornamental Plants; Springer: Singapore, 2021; pp. 1–41. ISBN 9789811515545. [Google Scholar]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral Scents and Fruit Aromas: Functions, Compositions, Biosynthesis, and Regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef]
- Ma, B.; Li, Z.; Li, R.; Xu, M.; Wang, Z.; Leng, P.; Hu, Z.; Wu, J. Functional Analysis of PsHMGR1 and PsTPS1 Related to Floral Terpenoids Biosynthesis in Tree Peony. Int. J. Mol. Sci. 2024, 25, 12247. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, S.; Yuan, M.; Li, B.; Niu, L.; Shi, Q. Transcriptome and Volatile Compounds Profiling Analyses Provide Insights into the Molecular Mechanism Underlying the Floral Fragrance of Tree Peony. Ind. Crops Prod. 2021, 162, 113286. [Google Scholar] [CrossRef]
- Duan, Y.; Lin, H.; Chen, Z.; Liu, C.; Yuan, Y.; Gao, Y.; Lei, M.; Wang, M. Parallel Floral Evolution of Lilium Species Pollinated by Pierid Butterflies. Flora 2025, 326, 152713. [Google Scholar] [CrossRef]
- Chen, J.; Luo, W.; Li, W.; Zheng, Y.; Zheng, X.; Lu, J.-L.; Liang, Y.-R.; Ye, J.-H. Effect of Scenting Process on the Taste Profile of Jasmine Green Tea and the Potent Bitterness-Enhancing Compound in Jasmine Flowers. Food Res. Int. 2025, 204, 115927. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Ming, F.; Liu, M.; Wang, X.; Zhang, Y. Correlation between Floral Color Attributes and Volatile Components among 10 Fragrant Phalaenopsis Cultivars. Phyton 2025, 94, 379–391. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, J.; Niu, Y.; Liu, Q.; Liu, J. Verification of Key Odorants in Rose Oil by Gas Chromatography-Olfactometry/Aroma Extract Dilution Analysis, Odour Activity Value and Aroma Recombination. Nat. Prod. Res. 2017, 31, 2294–2302. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Q.; Wang, L.; Lv, M.; Hou, Y.; Li, S. Linalool: A Ubiquitous Floral Volatile Mediating the Communication between Plants and Insects. J. Sytematics Evol. 2023, 61, 538–549. [Google Scholar] [CrossRef]
- Kumar, V.; Elazari, Y.; Ovadia, R.; Bar, E.; Nissim-Levi, A.; Carmi, N.; Lewinsohn, E.; Oren-Shamir, M. Phenylalanine Treatment Generates Scent in Flowers by Increased Production of Phenylpropanoid-Benzenoid Volatiles. Postharvest Biol. Technol. 2021, 181, 111657. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, R.; Huang, C.; Mao, Z.; Guo, L.; Shen, X. Taxonomic Analysis of Volatiles Emitted by Ornamental Crabapple Flowers. Acta Ecol. Sin. 2014, 34, 213–218. [Google Scholar] [CrossRef]
- Rong, H.; Huang, B.; Han, X.; Wu, K.; Xu, M.; Zhang, W.; Yang, F.; Xu, L.-A. Pedigree Reconstruction and Genetic Analysis of Major Ornamental Characters of Ornamental Crabapple (Malus spp.) Based on Paternity Analysis. Sci. Rep. 2022, 12, 14093. [Google Scholar] [CrossRef]
- Chen, X.; Shi, J.; Yan, Y.; Zhang, G.; Peng, L.; Yang, B.; Hu, B. Detection of Volatile Organic Compounds in Farfarae Flos Using HS-GC-IMS across Different Flower Colors. Food Chem. X 2025, 26, 102227. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, L.; Xiong, Q.; Yu, F.; Zhang, W.; Fan, J. Revealing the Key Aromatic Compounds in Malus ’Lollipop’ Flowers by Transcriptome and Metabolome. Gene 2025, 951, 149371. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, S.; Wei, J.; Zhou, Y. Volatile Metabolomics and Chemometric Study Provide Insight into the Formation of the Characteristic Cultivar Aroma of Hemerocallis. Food Chem. 2023, 404, 134495. [Google Scholar] [CrossRef]
- Arsa, S.; Theerakulkait, C.; Cadwallader, K.R. Quantitation of Three Strecker Aldehydes from Enzymatic Hydrolyzed Rice Bran Protein Concentrates as Prepared by Various Conditions. J. Agric. Food Chem. 2019, 67, 8205–8211. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Formation of Phenylacetic Acid and Benzaldehyde by Degradation of Phenylalanine in the Presence of Lipid Hydroperoxides: New Routes in the Amino Acid Degradation Pathways Initiated by Lipid Oxidation Products. Food Chem. X 2019, 2, 100037. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, X.; He, Z.; Su, H.; Li, W.; Ren, X. Amino Acid and Microbial Community Dynamics during the Fermentation of Hong Qu Glutinous Rice Wine. Food Microbiol. 2020, 90, 103467. [Google Scholar] [CrossRef]
- Qin, L.; Huang, M.; Ma, Y.; Zhang, D.; Cui, Y.; Kang, W. Effects of Two Saccharomyces cerevisiae Strains on Physicochemical and Oenological Properties of Aranèle White Wine. Food Biosci. 2023, 52, 102495. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z. Genetic and Biochemical Aspects of Floral Scents in Roses. Int. J. Mol. Sci. 2022, 23, 8014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gu, L.; Li, Y.; Zhi, H.; Luo, J.; Zhang, Y. Volatile Composition and Classification of Paeonia iactiflora Flower Aroma Types and Identification of the Fragrance-Related Genes. Int. J. Mol. Sci. 2023, 24, 9410. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, D.; Zhu, B.; Wu, G.; Duan, C. Rapid HPLC Analysis of Amino Acids and Biogenic Amines in Wines during Fermentation and Evaluation of Matrix Effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef]
- Xie, J.; Liao, B.; Tang, R. Functional Application of Sulfur-Containing Spice Compounds. J. Agric. Food Chem. 2020, 68, 12505–12526. [Google Scholar] [CrossRef]
- Yao, L.; Mo, Y.; Chen, D.; Feng, T.; Song, S.; Wang, H.; Sun, M. Characterization of Key Aroma Compounds in Xinjiang Dried Figs (Ficus carica L.) by GC–MS, GC–Olfactometry, Odor Activity Values, and Sensory Analyses. LWT 2021, 150, 111982. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhi, H.; Qu, L.; Su, D.; Luo, J. Analysis of Flower Volatile Compounds and Odor Classification of 17 Tree Peony Cultivars. Sci. Hortic. 2024, 338, 113665. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, F.; Shi, M.; Zhang, H.; Zhu, Z. Variation in the Floral Scent Chemistry of Nymphaea ’Eldorado’, a Valuable Water Lily, with Different Flowering Stages and Flower Parts. Plants 2024, 13, 939. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, L.; Li, M.; Liu, F.; Yin, J.; Huang, L.; Zhou, B.; Li, X.; Yu, Y.; Chen, F.; et al. A BAHD Acyltransferase Contributes to the Biosynthesis of Both Ethyl Benzoate and Methyl Benzoate in the Flowers of Lilium Oriental Hybrid “Siberia”. Front. Plant Sci. 2023, 14, 1275960. [Google Scholar] [CrossRef]
- Du, F.; Wang, T.; Fan, J.; Liu, Z.; Zong, J.; Fan, W.; Han, Y.; Grierson, D. Volatile Composition and Classification of Lilium Flower Aroma Types and Identification, Polymorphisms, and Alternative Splicing of Their Monoterpene Synthase Genes. Hortic. Res. 2019, 6, 110. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Sharma, N.R. Fragrance Stimulation Mechanisms of Flowers and Their Regulation under Environmental Constraints. J. Plant Growth Regul. 2022, 42, 60–82. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Wen, B.; Tian, J.; Cheng, Z.; Tang, S.; Nie, Y.; Wu, X.; Guo, X.; Li, B. Characterization and Metabolism Pathway of Volatile Compounds in Blueberries of Different Varieties and Origins Analyzed via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2025, 480, 143813. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Dong, J.; Wang, Q.; Yan, F.; Tan, J.; Xu, Y.; Zhu, G.; Fan, Y.; Ye, Y. Postharvest Fragrance Dynamics of Different Scented Cut Lilies: Insights from HS–SPME–GC–MS and Electronic Nose. Postharvest Biol. Technol. 2025, 222, 113378. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, X.; Lu, Y.; Wang, H.; Chen, D.; Luo, C.; Liu, H.; Gao, S.; Lei, T.; Huang, C.; et al. Gas Chromatography-Mass Spectrometry Analysis of Floral Fragrance-Related Compounds in Scented Rose (Rosa hybrida) Varieties and a Subsequent Evaluation on the Basis of the Analytical Hierarchy Process. Plant Physiol. Biochem. 2022, 185, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Kopriva, S. Transporters in Plant Sulfur Metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Sanad, M.H.; El-Tawoosy, M.; Ibrahim, I.T. Preparation and Biological Evaluation of 99m Tc-Timonacic Acid as a New Complex for Hepatobiliary Imaging. Radiochemistry 2017, 59, 92–97. [Google Scholar] [CrossRef]
- Qiu, Y.; An, K.-L.; Sun, J.; Chen, X.; Gong, X.; Ma, L.; Wu, S.; Jiang, S.; Zhang, Z.; Wang, Y. Investigating the Effect of Methyl Jasmonate and Melatonin on Resistance of Malus Crabapple ‘Hong Jiu’ to Ozone Stress. Environ. Sci. Pollut. Res. 2019, 26, 27761–27768. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Huang, Z.; He, L.; Wu, L.; Yu, L.; Zhu, W. Characterisation of the Volatile Compounds in Nine Varieties and Three Breeding Selections of Celery Using GC–IMS and GC–MS. Food Chem. X 2024, 24, 101936. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, G.; Xie, H.; Zhang, J.; Huang, J.; Liu, Z.; Wang, C. Characterization of the Key Differential Aroma Compounds in Five Dark Teas from Different Geographical Regions Integrating GC–MS, ROAV and Chemometrics Approaches. Food Res. Int. 2024, 194, 114928. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Kuang, H.; Zhang, J.; Wang, B.; Wang, S. Transcriptomic and Physiological Analysis Reveals the Possible Mechanism of Inhibiting Strawberry Aroma Changes by Ultrasound after Harvest. Foods 2024, 13, 2231. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Qiang, W.; Kou, X.; Chen, F.; Ke, Q.; He, M.; Meng, Q. Differences in Volatile Composition and Expression of Genes Involved in Terpenoids Biosynthesis in Chrysanthemum indicum Var. Aromaticum. Sci. Hortic. 2024, 337, 113461. [Google Scholar] [CrossRef]
- Dönmez, A.; Demirci, F.; Demirci, B.; Baser, K.; Taşdemir, D.; Rüedia, P. Analysis of the Volatile Components of Five Turkish Rhododendron Species by Headspace Solid-Phase Microextraction and GC-MS (HS-SPME-GC-MS). Z. Für Naturforschung C 2003, 58, 797–803. [Google Scholar] [CrossRef]
- Shang, C.; Hu, Y.; Deng, C.; Hu, K. Rapid Determination of Volatile Constituents of Michelia alba Flowers by Gas Chromatography–Mass Spectrometry with Solid-Phase Microextraction. J. Chromatogr. A 2002, 942, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of Key Odor Volatile Compounds in the Essential Oil of Nine Peach Accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Maoz, I.; Lewinsohn, E.; Gonda, I. Amino Acids Metabolism as a Source for Aroma Volatiles Biosynthesis. Curr. Opin. Plant Biol. 2022, 67, 102221. [Google Scholar] [CrossRef]
- Cordente, A.G.; Schmidt, S.; Beltran, G.; Torija, M.J.; Curtin, C.D. Harnessing Yeast Metabolism of Aromatic Amino Acids for Fermented Beverage Bioflavouring and Bioproduction. Appl. Microbiol. Biotechnol. 2019, 103, 4325–4336. [Google Scholar] [CrossRef]
- Reitzer, L. Biosynthesis of Glutamate, Aspartate, Asparagine, l-Alanine, and d-Alanine. EcoSal Plus 2004, 1. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.; Li, X.; Xu, H.; Liang, Y.; Ma, N.; Fei, Z.; Gao, J.; Jiang, C.-Z.; Ma, C. Transcriptome Profiling of Petal Abscission Zone and Functional Analysis of an Aux/IAA Family Gene rhIAA16 Involved in Petal Shedding in Rose. Front. Plant Sci. 2016, 7, 1375. [Google Scholar] [CrossRef]
- Liao, H.; Chung, Y.; Hsieh, M. Glutamate: A Multifunctional Amino Acid in Plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.-J. Multifaceted Regulations of Gateway Enzyme Phenylalanine Ammonia-Lyase in the Biosynthesis of Phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Ardö, Y. Flavour Formation by Amino Acid Catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Lu, X.; Yang, C.; Yang, Y.; Peng, B. Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae. Molecules 2023, 28, 3100. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Yao, Y.; Kang, W.; Wang, Y.; Li, J.; Wang, H.; Qin, L. Relationship Between Volatile Aroma Components and Amino Acid Metabolism in Crabapple (Malus spp.) Flowers, and Development of a Cultivar Classification Model. Horticulturae 2025, 11, 845. https://doi.org/10.3390/horticulturae11070845
Han J, Yao Y, Kang W, Wang Y, Li J, Wang H, Qin L. Relationship Between Volatile Aroma Components and Amino Acid Metabolism in Crabapple (Malus spp.) Flowers, and Development of a Cultivar Classification Model. Horticulturae. 2025; 11(7):845. https://doi.org/10.3390/horticulturae11070845
Chicago/Turabian StyleHan, Jingpeng, Yuxing Yao, Wenhuai Kang, Yang Wang, Jingchuan Li, Huizhi Wang, and Ling Qin. 2025. "Relationship Between Volatile Aroma Components and Amino Acid Metabolism in Crabapple (Malus spp.) Flowers, and Development of a Cultivar Classification Model" Horticulturae 11, no. 7: 845. https://doi.org/10.3390/horticulturae11070845
APA StyleHan, J., Yao, Y., Kang, W., Wang, Y., Li, J., Wang, H., & Qin, L. (2025). Relationship Between Volatile Aroma Components and Amino Acid Metabolism in Crabapple (Malus spp.) Flowers, and Development of a Cultivar Classification Model. Horticulturae, 11(7), 845. https://doi.org/10.3390/horticulturae11070845




