Abstract
Research on young trees’ adaptation to shade has predominantly focused on leaf-level responses, overlooking critical structural and functional adaptations in branch systems. In this study, we address this gap by investigating hierarchical branch morphology–physiology integration in 20-year-old Pinus koraiensis specimens across four distinct light conditions classified by photosynthetic photon flux density (PPFD): three in the understory (low light, LL: 0–25 μmol/m2/s; moderate light, ML: 25–50 μmol/m2/s; and high levels of light, HL: 50–100 μmol/m2/s) and one under full light as a control (FL: 1300–1700 μmol/m2/s). We measured branch base diameter, length, and angle as well as chlorophyll and NSCs content in branches and needles. Branch base diameter and length were more than 1.5-fold higher in the FL Korean pine trees compared to the understory-grown ones, while the branching angle and ratio in the LL Korean pine trees were more than two times greater than those in the FL trees. As light levels increased, Chlorophyll a and b and total chlorophyll (Chla, Chlb, and Chl) concentrations in the needles all significantly decreased. Starch, glucose, and NSC (Starch + Soluble Sugars) concentrations in both needles and branches were the highest in the trees under FL and lowest under ML (except for soluble sugars in branches). Understory young P. koraiensis trees morphologically and physiologically adapt to limited light conditions, growing to be more horizontal, synthesizing more chlorophyll in needles, and attempting to increase their light-foraging ability. We recommend gradually expanding growing spaces to increase light availability for 20-year-old Korean pine trees grown under canopy level.
1. Introduction
Mixed forests exhibit higher productivity and greater resilience to global climate change than pure forests [1]. Replanting trees in selectively harvested sites with abundant pre-regenerated young trees is a common and effective way to establish mixed forests [2]. However, trees that have regenerated naturally or been replanted often find themselves under shaded conditions beneath the canopy.
Plants growing under the canopy always show plastic phenotypic responses to different light conditions [3]. The plasticity exhibited by plants during shoot development is remarkable, as it allows them to adapt to various harmful external and internal conditions in order to survive and thrive. Moreover, shoot branching has been shown to be significantly affected by phytohormones, nitrogen, light, and sugars [4]. The pattern exhibited during shoot branching is controlled by axillary bud development, which is also significantly reliant on environmental conditions, especially light [5]. For instance, far-red light inhibits lateral bud growth in tomato [6], and this form of light is much more abundant under the canopy [7]. Branching frequency, branch base diameter, the physiological and biochemical properties of plant branches, and leaf morphology are all affected by light intensity [8]. The live crown ratio and maximum and average branch diameters of spruce (Picea glauca) mixed with aspen (Populus tremuloides) significantly decreases with an increase in aspen density (implying a decrease in light intensity) [9]. Plants modulate branch growth in response to light availability. However, quantitative studies on light-mediated branch development remain limited, despite the critical role of non-vertical growth angles in roots and shoots for efficient above- and below-ground resource capture [10]. Furthermore, it remains unclear how young conifers growing in the field change their branching patterns to adapt to a constantly changing light environment.
Alterations in plant branching patterns subsequently affect the leaves growing on these branches. Shifts in branch angle alter light interception by leaves, thereby driving plasticity in chlorophyll content. Chlorophyll is an important pigment in leaves that is involved in photosynthesis and plays a crucial role in absorbing, transmitting, and converting solar energy into electrochemical energy. Furthermore, optimal photosynthesis and proper leaf development rely on the spatiotemporal regulation of chlorophyll metabolism [11]. In many species, it has been found that shaded leaves have higher chlorophyll concentrations than those grown under sunlight [12]; however, the relationship between chlorophyll composition (e.g., the ratio of chlorophyll a to b) and light gradients in relation to young conifers is not well-defined.
NSCs, mainly composed of starch and soluble sugar, are important energy supply materials during plant growth and metabolism. Shade significantly impacts NSCs concentrations in woody species’ leaves, so NSCs storage may help trees survive long periods in low-light environments [13,14,15]. Additionally, regarding Cunninghamia lanceolata and Schima superba, it has been found that both species show a decrease in NSCs, soluble sugar, and starch content with an increase in shade [16]. These research results indicate that plants in shaded environments are subject to various light conditions generated by surrounding plants, leading to diverse morphological and physiological responses [10,17,18]. In addition, research has revealed that a novel rice tiller angle mutant could regulate starch biosynthesis in gravity-sensing cells [19], indicating that starch is related to branch architecture. Understanding these response patterns and their biological mechanisms is crucial for promoting the growth of understory trees and precisely regulating the relationship between the target trees and their surrounding plants and habitat factors.
The Korean pine (Pinus koraiensis Sieb. et Zucc.) is a valuable evergreen arbor species prized for its high-quality timber, nutritious edible nuts, and ecological significance as the climax species in zonal mixed forests containing broad-leaved species in the humid areas of the middle temperate zone in China [2,20,21,22]. However, the primary Korean pine and broad-leaved mixed forests have been seriously damaged and almost entirely transformed into secondary forests lacking Korean pine seed sources or overharvested forests, with fewer—and younger—Korean pine trees compared to the original forests [22,23,24]. These secondary forests can be transformed into near-climax mixed forests again by introducing Korean pine trees through the “planting conifers and preserving broad-leaved trees” approach, especially in areas lacking Korean pine seed sources [23,25,26].
Commonly, the Korean pine trees in primary mixed forests undergo a lengthy growth period of 80–120 years under the canopy before reaching the upper layer. The planted Korean pine trees in secondary forests and the naturally regenerated Korean pine trees in overharvested forests can also undergo an under-canopy growth period before ascending to the upper canopy [21]. Throughout this process, under-canopy light conditions can significantly affect the growth of the planted or naturally regenerated Korean pine trees until they become the upper-layer trees. A light environment created by surrounding trees has been proven to be the critical ecological factor influencing the growth of Korean pine trees before they ascend to the upper layer [27,28].
Korean pine seedlings display considerable plasticity in needle morphology, tree height, and crown growth in response to varying light intensities, with needle chlorophyll content increasing with canopy openness and needle NSC levels decreasing as light intensity decreases [29].
While previous research has yielded valuable insights, three main limitations exist: (1) the focus has been on using seedlings as testing materials; (2) predominantly stand-level research scales have been employed; and (3) there is a scarcity of data obtained from trees that have undergone long-term field acclimation to realistic light regimes. Consequently, linkages between branch architecture, light-harvesting efficiency, and downstream physiological processes (chlorophyll metabolism, NSCs allocation, etc.) remain unresolved at the individual-tree level, hindering precision silviculture.
In this study, we aimed to address these limitations by selecting young Korean pine specimens (about 20 years old) that had grown for a long period under the canopy of a secondary forest composed of naturally regenerating broadleaved trees as the target trees; selecting scarcely researched morphological and physiological indices that have not yet been proven to be important; and elucidating the response mechanisms of the developmental and physiological characteristics of P. koraiensis trees in adapting to different light environments. Our main aim was to clarify the following issue: How does an individual 20-year-old Korean pine’s branching architecture adapt to understory low light? Will this architecture, in turn, influence the light-foraging strategy for needles through chlorophyll synthesis, NSCs content, and storage? We hypothesized that young conifers growing in the understory would exhibit a different branching architecture and coordinated set of chlorophyll and NSCs production adjustments in response to variations in light availability. This work will provide further theoretical references and a scientific basis for determining the optimal light environment for the growth of P. koraiensis trees under the canopy and addressing issues related to the sluggish growth and slow regeneration of P. koraiensis trees under deciduous broad-leaved canopies.
2. Materials and Methods
2.1. Study Site
The Maoershan Experimental Forestry Farm of Northeast Forestry University (45°23′~45°26′ N, 127°34′~127°39′ E) is situated in Maoershan Town, Shangzhi City, Heilongjiang Province. This region has a temperate continental monsoon climate with a growing season spanning from May to September. The annual mean air temperature is 3 °C, ranging from −19.7 °C in January to 22 °C in July. There are around 120–140 days with no frost on an annual basis, and the average amount of precipitation is between 600 and 800 mm, primarily (80%) falling from May to September, with 2471.3 h of annual sunshine [2,21,23].
2.2. Research Design and Sampling
This study focused on understory Korean pine trees obtained from permanent plots established in 2012. The Korean pine trees were planted in 1989 and replenished in 2003 at micro-sites where Korean pine trees were scarce [23]. The trees used in this experiment were the ones that had been replenished and were 20 years old when they were sampled with three replicates. Plot information is given in Table 1.

Table 1.
Location and community composition of sampling sites.
Based on the photosynthetic photon flux density (PPFD) measured using a dual radiometer (Spectrum Technologies, Inc., Aurora, IL, USA), we categorized under-canopy light environments into three levels, namely, low light (<25 μmol/m2/s, LL), moderate light (25–50 μmol/m2/s, ML), and high light levels (50–100 μmol/m2/s, HL), employing full light (1300–1700 μmol/m2/s, FL) as a control. Under each light condition, three Korean pine trees exhibiting vigorous growth that were mutually similar in height and diameter at breast height (DBH) were selected as sample trees. We investigated branch morphology, chlorophyll content, and NSCs concentrations using these sample trees. In mid-June, during the peak growth period, healthy branches were selected from the upper half of the mid-third of the crown of trees to standardize developmental stage and light exposure history. These branches were cut and placed in sterile polythene bags before being transported to the laboratory in a box with ice bags for a laboratory experiment.
2.3. Measurement of Branch Morphology and Needle Chlorophyll Concentrations
The DBH, height, and health statuses were similar among the sampled trees (for more information, please refer to Table 2). We measured the morphological traits of the P. koraiensis branches and needles of the topmost whorl (with current-year and 2-year-old branches and needles), including the number of branches, branch base diameter, branch length in two years, and branching angle. The branching ratio (OBR) can be used to quantify topological hierarchy, as it correlates strongly with carbon allocation efficiency in conifers; it was calculated using Formula (1).
where Nt is the total number of branches in all branch-class levels; Ns is the number of branches at the last branch-class level; and N1 is the number of branches at the first branch-class level. The first-class branches are attached to the main stem, while the secondary class branches are attached to the first branch, and the rest of the branches are attached in the same manner; i.e., branches directly attached to the main stem were considered class 1 branches, and lateral branches attached to class 1 branches were considered level 2 branches (this scheme is clearer in Figure 1).
OBR = (Nt − Ns)/(Nt − N1)

Table 2.
Basic information on the sampled trees (n = 3).
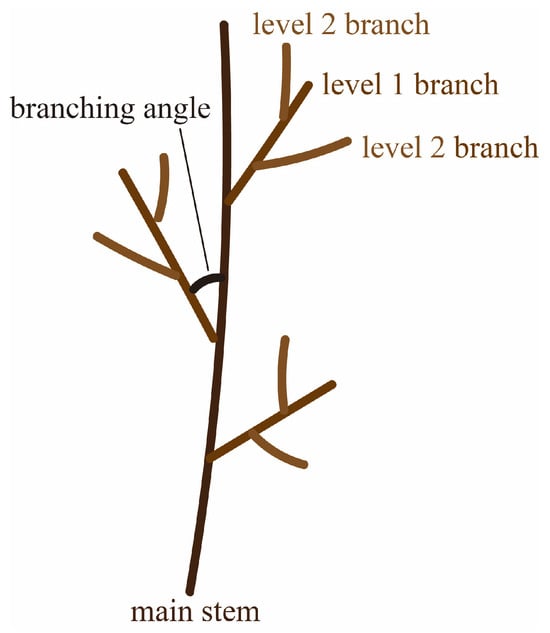
Figure 1.
Simple schematic figure depicting the branch system of Korean pines, considering branch level and branch angle. This system has more than 2 levels, but for illustrative purposes, we have depicted only two levels of branches.
The quantities of level 1, level 2, and total branches of the sampled P. koraiensis trees under FL were 111, 479, and 590, and those of the P. koraiensis trees under LL, ML, and HL were 98, 213, and 311; 88, 215, and 303; and 108, 251, and 359, respectively. Branch base diameter, branch length, and branching angle were measured for the main stem tip branch and top whorl branches.
Needles were sampled from the upper one-third of the crown of the young P. koraiensis trees, mainly in the morning from 8:00 to 11:00 on 18 June 2021. Fresh annual needles were cleaned and weighed to 0.2 g. Then, they were cut up, placed in a 50 mL centrifuge tube, extracted with 40 mL of a 1:1 mixture of 99.5% acetone and 95% ethanol by volume at 10–25 °C, and protected from light until the material decolorized and turned white. The absorbance value was measured using a spectrophotometer, and the chlorophyll content was calculated according to the method described by He J et al. [30].
2.4. Measurement of Non-Structural Carbohydrate Concentrations
The samples were oven-dried to a constant weight at 65 °C for at least 72 h, ground several times, sieved through a 100-mesh (0.15 mm aperture) sieve, pulverized using a SCIENTZ-48L pulverizer (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China), and stored in plastic bags for further measurement. The non-structural carbohydrate (NSCs) content was determined as the sum of soluble sugars (SSs) and starch (ST) content, which, in turn, was determined using the anthrone colorimetric method according to Hansen and Møller [31].
2.5. Statistical Analysis
All the measurements were conducted using three biological replicates. All the data were subjected to ANOVA to search for statistically significant differences between different light conditions. We used the Least-Significant Difference test as a post hoc test (p < 0.05). Pearson’s correlation matrix was used to find the correlation coefficients (r) of all pairwise combinations. Data analysis and graph generation were conducted using R 4.2.2 software.
3. Results
3.1. Branch Morphology and Needle Chlorophyll Content of Pinus koraiensis Under Different Light Conditions
3.1.1. Branch Characteristics of Pinus koraiensis
There was no significant difference in the branch base diameters of the Korean pine trees at different light levels in the understory (p > 0.05), but the branch base diameters under HL were relatively higher than those under ML and LL (18% and 19% higher, respectively), whereas they were almost the same under LL and ML. Notably, under FL, the branch base diameter was significantly higher than that under the canopy (p < 0.05—61%, 90%, and 92% higher than that under HL, ML, and LL) (Figure 2A).
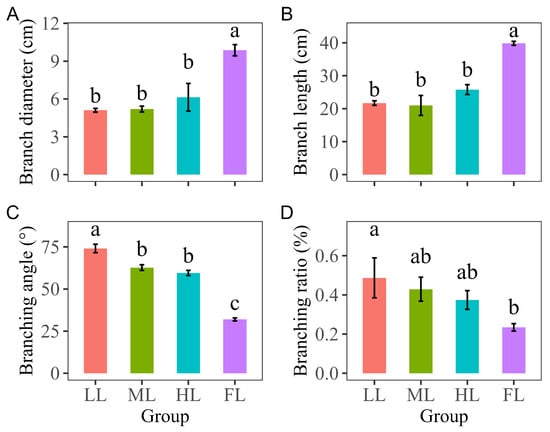
Figure 2.
Differences in the branch traits of level 1 branches of Pinus koraiensis under different light intensities: (A) differences in branch diameter, (B) branch length, (C) branching angle, and (D) branching ratio of level 1 branches of Pinus koraiensis under different light intensities. LL, low light; ML, moderate light; HL, high light levels; FL, full light. Different lowercase letters indicate significant differences among different light conditions, with p < 0.05 according to one-way ANOVA and an LSD post hoc test; non-significant results were omitted. Values are means ± SE, with n = 3 biological replicates.
The branches of the Korean pine trees under FL were significantly longer than those under the canopy (p < 0.05).
However, compared to what was observed for branch size, the pattern in the response of the branching angles of the Korean pine trees was different (Figure 2C): the branches of the Korean pine trees under LL exhibited a near-flat angle, reaching 74°, which is significantly higher than the angles of the trees under ML, HL, and FL (11°, 15°, and 42° higher, respectively). Additionally, different branching patterns were observed between under-canopy light conditions and full-light conditions. The top whorl branching angles of the Korean pine trees that had adapted to under-canopy light environments over the long term were greater than 45° and tended towards a flat pattern, whereas the angles under full-light conditions were less than 45° and tended towards an erect pattern.
The branching ratios of the low-light Korean pine trees were significantly higher than those of the full-light trees (p < 0.05, Figure 2D).
3.1.2. Needle Chlorophyll Concentrations of Pinus koraiensis
The Chla, Chlb, and Chl concentrations in the Korean pine needles all significantly decreased as light conditions improved (p < 0.05). Chla/Chlb (Chlorophyll a/Chlorophyll b) levels in needles were the highest under FL and significantly higher than that under ML (p < 0.05). As light conditions improved, Chla/Chlb initially declined and then increased (Figure 3D).
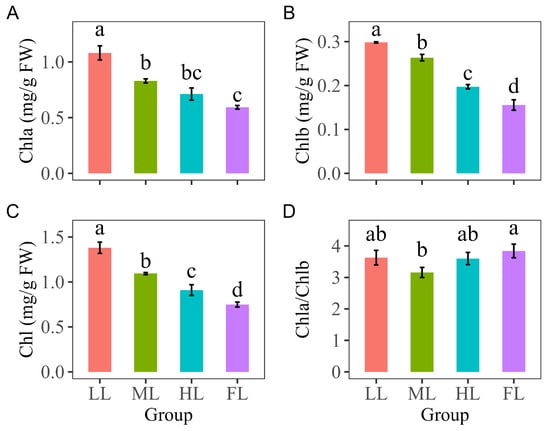
Figure 3.
Comparisons of chlorophyll concentrations in Pinus koraiensis needles under different light intensities: (A) chlorophyll a (Chla), (B) chlorophyll b (Chlb), (C) total chlorophyll (Chl), and (D) chlorophyll a/chlorophyll b (Chla/Chlb) concentrations in Pinus koraiensis needles under different light intensities. LL, low light; ML, moderate light; HL, high light levels; FL, full light. Different lowercase letters indicate significant differences among different light conditions, with p < 0.05 according to one-way ANOVA and an LSD post hoc test; non-significant results were omitted. Values are means ± SE, with n = 3 biological replicates.
3.2. Needle and Branch NSCs of Pinus koraiensis Under Different Light Conditions and Their Relationships
The highest starch, soluble sugars, and NSC concentrations were all found in the Korean pine trees under FL, both in branches and needles (Figure 4). The soluble sugars, starch, NSC, and starch/NSC levels in the needles all decreased and then increased as light conditions improved, with the lowest readings obtained from the needles under ML. An “S” shaped curve was observed for the starch/NSC ratio in branches (Figure 4D).
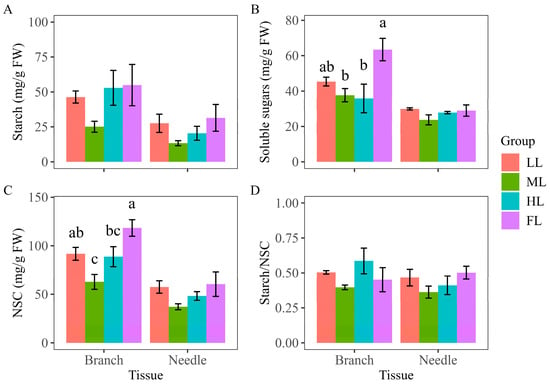
Figure 4.
Comparison of non-structural carbohydrates in branches and needles of Pinus koraiensis under different light intensities: (A) starch, (B) soluble sugars, (C) NSC (starch + soluble sugars), and (D) starch/NSC concentrations in branches and needles of Pinus koraiensis under different light intensities. LL, low light; ML, moderate light; HL, high light levels; FL, full light. Different lowercase letters indicate significant differences between different light conditions, with p < 0.05 according to one-way ANOVA and an LSD post hoc test; non-significant results were omitted. Values are means ± SE, with n = 3 biological replicates.
There were no significant relationships between light and NSC concentrations in needles and branches (Figure 5, p > 0.05). However, light conditions, branch diameter, and branch length were significantly and negatively related to Chla, Chlb, Chl, and Chla/Chlb concentrations (Figure 5, p < 0.05). Branch soluble sugars and NSC concentrations were negatively related to branching ratio but positively related to branch diameter and branch length. Overall, the Chla, Chlb, and Chl concentrations in the Korean pine needles were all negatively and significantly related to light conditions (Figure 5, p < 0.05). Generally, tree morphology and physiology are closely related to light conditions.
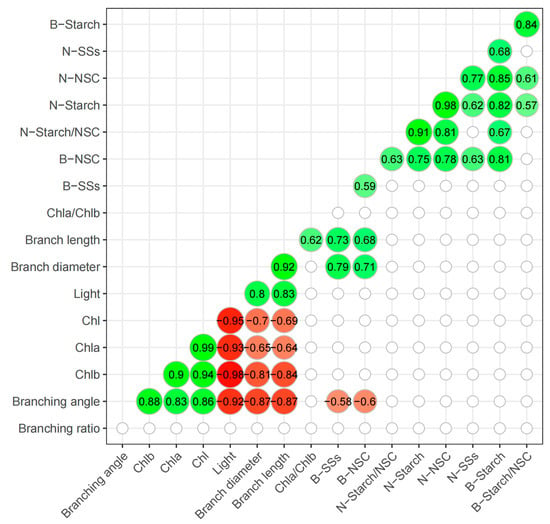
Figure 5.
Pearson correlation matrix of the correlation between different parameters (p < 0.05). Non-significant correlations were omitted (p > 0.05). Negative and positive correlations are given in red and green, respectively; the color gradient shows the strength of the correlation. N indicates needle traits, while B indicates branch traits.
4. Discussion
4.1. There Is Some Evidence Showing That Branch Morphological Traits Are Linked with Light-Foraging Strategies
The P. koraiensis trees grown under FL tended to grow better than those in the understory. Our findings also revealed that the branch production rate of first- and second-order branches was greater for the full-light trees than those grown in the understory, a finding consistent with the observations made by Kwon et al. [32] regarding Platycodon grandiflora. The number of branches as well as leaf length and width decreased significantly under low light-intensity conditions. According to Casal and Fankhauser [18], one of a plant’s tactics for avoiding shadow consists of inhibiting branching at the base of the plant and improving elongation of the stem.
In this study, we investigated the morphological indicators of branches of young P. koraiensis trees in different light environments. It was found that the branch angles of the young P. koraiensis branches in shaded environments were significantly higher than those in the full-light environment, suggesting that in environments with insufficient light, young Pinus koraiensis trees can expand the total area of light acquisition by increasing the branch angle and thus obtaining more light. This adaptive response highlights the ability of young P. koraiensis trees to adjust to varying light conditions. Branch growth is phototropic, resulting in different branch patterns in different light environments [33]. In a study on spruce (Picea asperata), it was found that the number of spruce branches decreased significantly with an increase in light intensity after thinning mixed forests [34]. Our results also align with this finding. As light conditions improved, the branches of young P. koraiensis trees gradually changed from being scarce and elongated to numerous and shorter [2,35]. P. koraiensis’s strategy for adapting to changes in external light environments consists of harvesting light by expanding its light interception ability to efficiently use light energy. However, increased horizontal branching may not fully compensate for receiving more light, resulting in shorter and thinner branches. While the sample size used (n = 3 per light treatment) limits the generalizability of some of our statistical inferences, the observed trends align with the findings of previous studies. In future studies, we will prioritize expanded replication.
4.2. Weak Light Use by Needles Could Compensate for Less Light Foraging via Variation in Branch Traits
Chlorophyll, indispensable in the process of photosynthesis, can be used to measure the strength of plant photosynthesis, and chlorophyll synthesis is mainly affected by light [36]. In this study, we observed a gradual decrease in chlorophyll content with an increase in light intensity, a result consistent with the findings reported by Minotta and Pinzauti [37]. Chlorophyll concentrations play a crucial role in the light-capturing ability of leaves, indicating that leaves of understory trees need more chlorophyll to capture light in order to support their growth owing to the limited amount of light available to them [29]. Leaves of shade-tolerant plants, such as Alocasia macrorrhiza, undergo changes in photosynthetic structures, including increases in chlorophyll concentrations, to optimize efficiency in low light [38]. It was also found that the chlorophyll content in A. thaliana plants grown under low light decreased, whereas this decrease was attenuated in C. hirsuta plants [39,40]. Chla/Chlb levels in many species have been found to be higher in leaves exposed to higher levels of light. However, in Arabidopsis thaliana (L.) Heynh. cv. Landsberg erecta, Chla/Chlb levels increase in low and high irradiance ranges and plateau at intermediate irradiance, reflecting the complexity of photosynthetic acclimation to different light levels, a result that aligns with our findings [41]. Furthermore, Chla/Chlb levels in the all-light environment were higher than those in the other light environments, indicating that Chlb predominantly drives chlorophyll changes under varying light conditions, a common adaptation of plants in low-light habitats [42]. Under insufficient light conditions, the proportion of blue-violet light increases, leading to a higher content of chlorophyll b, which absorbs blue-violet light and facilitates the absorption and utilization of weak light by leaves [10]. Research indicates that P. koraiensis seedlings exhibit lower photosynthetic rates in the understory [29], resulting in reduced net assimilation and growth rates under the canopy [43,44].
NSCs fulfil distinct functional roles, including transport, energy metabolism, and osmoregulation, and provide substrates for the synthesis of defense compounds or exchange with symbionts involved in nutrient acquisition or defense. Branches and roots usually contain storage pools that may be mobilized only in small proportions during normal functioning but can make up most of the C source during catastrophic events that occur on decadal/centennial timescales, like severe and repeated herbivory, wind and ice-storm damage (leading to branch breakage), or fire [45]. Other than being one of the photosynthetic products of plants, NSCs also reflects their survival strategies [46]. NSCs levels in branches and needles varied under different light conditions, with the levels of starch, soluble sugars, and NSC in the trees under FL being higher than those in the trees in the understory. In contrast, among the Korean pine trees grown in the understory, the trees under ML had lower NSC concentrations than those under LL and HL, possibly because the active radial growth and construction of photosynthetic organs in the Korean pine trees grown under ML result in difficulties in accumulating NSC. In contrast, because of environmental constraints in LL conditions, the trees likely reduced their energy consumption to conserve resources, which might be one reason why their NSC levels were higher than those under ML. Carbon storage is important for species that regenerate in persistently shady habitats [47], and carbohydrate storage in stems and roots enhances long-term survival in the shade by enabling seedlings to cope with periods of biotic and abiotic stress [48]. Without root NSCs measurements, we cannot draw more comprehensive conclusions regarding whole-tree NSCs dynamics in P. koraiensis. Furthermore, light duration and tree age would also affect the experimental outcomes. In future work, we will focus more on conducting controlled experiments on whole-tree NSCs dynamics. Moreover, NSC also functions as a source of stored energy and carbon for biosynthesis [49]. Furze et al. [50] showed that branches were the largest reservoir of total NSC. In our study, branches had higher NSC content than needles since NSCs are used to provide C skeletons for structural growth [45].
Furthermore, numerous studies have provided experimental evidence that C storage is an active process that allows the buffering of environmental fluctuations and supports long-term plant growth [49]. Under FL, P. koraiensis displayed greater vitality and put more resources into active development rather than storage, as evident from the higher soluble sugars and NSC content in the branches and the lower starch/NSC ratio. Starch is inactive and accumulates as a storage compound when photosynthetic supply exceeds growth demand [13,51]. The Korean pines under FL had higher starch/NSC levels in their needles, indicating their higher photosynthetic rates served to address more than growth demand, allowing for starch accumulation for future active growth. In contrast, the Korean pines under ML and HL had lower starch/NSC ratios than those under LL, indicating more active growth since higher light intensity usually brings vigorous growth [52]. Plants rely on both newly assimilated carbon and stored reserves of NSCs for growth and other physiological functions, such as respiration, osmotic regulation, and defense [53]. Previous research has shown that NSCs are transferred from mature bamboo to young shoots, facilitating their fast growth [54]. Light is one indispensable factor for NSCs content, composition, and distribution. As research has shown, as canopy openness increases, NSC levels in Korean pine needles also increases [29]. Based on the NSCs results, we recommend changing the growth environment (to increase PPFD over 50 μmol/m2/s) to ensure Korean pine trees receive more light under moderate light conditions.
5. Conclusions
In general, young P. koraiensis trees growing under full-light conditions have thicker, longer branches and a more horizontal branching pattern than those growing under a canopy. Besides having a flatter branching pattern, Korean pine trees that grow in the understory also have higher Chla, Chlb, and Chl content; lower Chla/Chlb levels; and lower NSCs content than those grown under full-light conditions. Relying on these morphological and physiological changes, understory Korean pine trees can attempt to grow better under a light-limited environment. Due to insufficient light, the Korean pines under both ML and LL conditions exhibited growth limitation. Based on our findings, we recommend gradually improving light conditions for 20-year-old understory Korean pines through forest management. However, given the limitations of the current experimental results, the development of more specific management protocols still requires further supporting data, including physiological indicators across different age groups under varying forest canopies and in distinct functional organs of Korean pines.
Author Contributions
W.L., X.M., B.L., and H.S. conceived the entire study; B.L., X.M., Y.L., and W.L. performed the experiment and performed the statistical analyses, created figures, and wrote the entire manuscript; S.S. revised the manuscript; S.S., H.W., P.Z., and H.S. conducted the final revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (Grant No. 2022YFD2201002), the Fundamental Research Funds for the Central Universities (Grant No. 2572023CT02), and the National Natural Science Foundation of China (Grant No. 31972950 and No. 32271857).
Data Availability Statement
The datasets generated during and/or analyzed in this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank Maoershan Experimental Forest Farm of Northeast Forestry University for providing access to the study area for this research.
Conflicts of Interest
The authors declare there are no conflicts of interest.
References
- Giberti, G.S.; von Arx, G.; Giovannelli, A.; du Toit, B.; Unterholzner, L.; Bielak, K.; Carrer, M.; Uhl, E.; Bravo, F.; Tonon, G.; et al. The admixture of Quercus sp. in Pinus sylvestris stands influences wood anatomical trait responses to climatic variability and drought events. Front. Plant Sci. 2023, 14, 1213814. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, P.; Shen, H. Competition intensity affects growing season nutrient dynamics in Korean pine trees and their microhabitat soil in mixed forest. For. Ecol. Manag. 2023, 539, 121018. [Google Scholar] [CrossRef]
- Valladares, F.; Arrieta, S.; Aranda, I.; Lorenzo, D.; Sánchez-Gómez, D.; Tena, D.; Suárez, F.; Pardos, J.A. Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental Mediterranean sites. Tree Physiol. 2005, 25, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Khourchi, S.; Li, S.; Du, Y.; Delaplace, P. Unlocking the Multifaceted Mechanisms of Bud Outgrowth: Advances in Understanding Shoot Branching. Plants 2023, 12, 3628. [Google Scholar] [CrossRef] [PubMed]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2014, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gu, X.; Chen, S.; Qi, Z.; Yu, J.; Zhou, Y.; Xia, X. Far-red light inhibits lateral bud growth mainly through enhancing apical dominance independently of strigolactone synthesis in tomato. Plant Cell Environ. 2023, 47, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Murchie, E.H.; Burgess, A.J. Casting light on the architecture of crop yield. Crop Environ. 2022, 1, 74–85. [Google Scholar] [CrossRef]
- Comeau, P.G. Effects of Aspen and Spruce Density on Size and Number of Lower Branches 20 Years after Thinning of Two Boreal Mixedwood Stands. Forests 2021, 12, 211. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Shoot and root branch growth angle control-the wonderfulness of lateralness. Curr. Opin. Plant Biol. 2015, 23, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Grimm, B. Connecting Chlorophyll Metabolism with Accumulation of the Photosynthetic Apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Jin, Z.; Yang, Z.; Li, Y. Leaf anatomy, photosynthesis, and chloroplast ultrastructure of Heptacodium miconioides seedlings reveal adaptation to light environment. Environ. Exp. Bot. 2022, 195, 1–11. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Wang, N.; Ji, T.; Liu, X.; Li, Q.; Sairebieli, K.; Wu, P.; Song, H.; Wang, H.; Du, N.; Zheng, P.; et al. Defoliation Significantly Suppressed Plant Growth Under Low Light Conditions in Two Leguminosae Species. Front. Plant Sci. 2022, 12, 777328. [Google Scholar] [CrossRef] [PubMed]
- Veneklaas, E.J.; den Ouden, F. Dynamics of non-structural carbohydrates in two Ficus species after transfer to deep shade. Environ. Exp. Bot. 2005, 54, 148–154. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Z.; Wang, Z.; Chen, Y.; Wen, Z.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Fankhauser, C. Shade avoidance in the context of climate change. Plant Physiol. 2023, 191, 1475–1491. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, W.; Zhang, N.; Cai, Y.; Liang, Y.; Meng, X.; Yuan, Y.; Li, J.; Wu, D.; Wang, Y. LAZY2 controls rice tiller angle through regulating starch biosynthesis in gravity-sensing cells. New Phytol. 2021, 231, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.G.; Zhang, T.; Yuan, J.; Yu, L.; Zhu, J.; Yan, Q. Use of direct seeding and seedling planting to restore Korean pine (Pinus koraiensis Sieb. Et Zucc.) in secondary forests of Northeast China. For. Ecol. Manag. 2021, 493, 119243. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Ma, X.; Saha, S.; Wu, H.; Zhang, P.; Shen, H. Physiological and biochemical traits of needles imply that understory light conditions in the growing season may be favorable to Pinus koraiensis trees. Forests 2023, 14, 1333. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, K.; Sun, Y.; Yan, Q. Response of Pinus koraiensis seedling growth to different light conditions based on the assessment of photosynthesis in current and one-year-old needles. J. For. Res. 2014, 25, 53–62. [Google Scholar] [CrossRef]
- Shen, H.L.; Cong, J.; Zhang, P.; Zhang, Q.; Fan, S.H.; Yang, W.H.; Liu, S.R. Effect of opening degree regulation on diameter and height increment and aboveground biomass of Korean pine trees planted under secondary forest. Chin. J. Appl. Ecol. 2011, 22, 2781–2791. [Google Scholar] [CrossRef]
- Yu, D.-P.; Zhou, W.-M.; Zhou, L.; Dai, L.-M. Exploring the history of the management theory and technology of broad—Leaved Korean pine (Pinus koraiensis Sieb. et Zucc.) forest in Changbai Mountain Region, Northeast China. Chin. J. Appl. Ecol. 2019, 30, 1426–1434. [Google Scholar]
- Chen, D.-K.; Zhou, X.-F.; Ding, B.-Y.; Hu, Z.-C.; Zhu, N.; Wang, Y.-H.; Zhao, H.-X.; Ju, Y.-G.; Jin, Y.-Y. Research on natural secondary forest in Heilongjiang province-the management approach of planting conifers and conservating deciduous trees. J. North-East. For. Inst. 1984, 12, 1–12. [Google Scholar] [CrossRef]
- Chen, D.-K.; Zhou, X.; Ding, B.; Hu, Z.-C.; Zhu, N.; Wang, Y.-H.; Zhao, H.-X.; Ju, Y.-G.; Jin, Y.-Y. Research on natural secondary forest in Heilongjiang province-the dynamic management system. J. North-East. For. Inst. 1985, 13, 1–18. [Google Scholar]
- Zhang, Q.; Fan, S.-H.; Shen, H.-L. Research and development on the growth environment of the young tree of Pinus koraiensis in Pinus koraiensis-broadleaved mixed forest. For. Res. 2003, 16, 216–224. [Google Scholar]
- Zhou, G.; Xu, W.-Z.; Wan, J.; Wang, Y.-N.; Liu, L.-T.; Liu, Q.-J. Seasonal dynamics of energy and nutrients of Pinus koraiensis seedlings in different successional stages of broadleaved Korean pine forest in Changbai Mountain, China. Chin. J. Appl. Ecol. 2021, 32, 1663–1672. [Google Scholar]
- Sun, Y.; Zhu, J.; Sun, O.J.; Yan, Q. Photosynthetic and growth responses of Pinus koraiensis seedlings to canopy openness: Implications for the restoration of mixed-broadleaved Korean pine forests. Environ. Exp. Bot. 2016, 129, 118–126. [Google Scholar] [CrossRef]
- He, J.; Xü, X.; Li, S.H.; Mi, H.L.; Zhang, Y.P.; Zhao, T.C.; Ma, Y.M. Effects of water stress on photosynthetic pigment in leaves and chlorophyll fluorescence of Cynanchum komarovii. Acta Bot. Boreali-Occident. Sin. 2004, 24, 1594–1598, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-J.; Kim, H.-R.; Roy, S.K.; Kim, H.-J.; Boo, H.-O.; Woo, S.-H.; Kim, H.-H. Effects of Temperature, Light Intensity and DIF on Growth Characteristics in Platycodon grandiflorum. J. Crop Sci. Biotechnol. 2019, 22, 379–386. [Google Scholar] [CrossRef]
- Duchemin, L.; Eloy, C.; Badel, E.; Moulia, B. Tree crowns grow into self-similar shapes controlled by gravity and light sensing. J. R. Soc. Interface 2018, 15, 20170976. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Z.; Ma, X.; Wang, Z.; Xing, X.; Liu, B. Changes of seediling frowth and C, N, P stochiometric characteristics in Chinese fir under shading. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 74–82, (In Chinese with English Abstract). [Google Scholar]
- Li, Y.; Zhang, X.; Cai, K.; Zhang, Q.; Jiang, L.; Li, H.; Lv, Y.; Qu, G.; Zhao, X. Comparative Transcriptomic and Metabolic Analyses Reveal the Coordinated Mechanisms in Pinus koraiensis under Different Light Stress Conditions. Int. J. Mol. Sci. 2022, 23, 9556. [Google Scholar] [CrossRef] [PubMed]
- Giglou, R.H.; Giglou, M.T.; Estaji, A.; Bovand, F.; Ghorbanpour, M. Light-emitting diode irradiation and glycine differentially affect photosynthetic performance of black henbane (Hyoscyamus niger L.). S. Afr. J. Bot. 2023, 155, 230–240. [Google Scholar] [CrossRef]
- Minotta, G.; Pinzauti, S. Effects of light and soil fertility on growth, leaf chlorophyll content and nutrient use efficiency of beech (Fagus syluatica L.) seedlings. For. Ecol. Manag. 1996, 86, 61–71. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Paulisic, S.; Qin, W.; Iglesias-Sanchez, A.; Roig-Villanova, I.; Florez-Sarasa, I.; Rodriguez-Concepcion, M.; Martinez-Garcia, J.F. Light signals generated by vegetation shade facilitate acclimation to low light in shade-avoider plants. Plant Physiol. 2021, 186, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Molina-Contreras, M.J.; Paulisic, S.; Then, C.; Moreno-Romero, J.; Pastor-Andreu, P.; Morelli, L.; Roig-Villanova, I.; Jenkins, H.; Hallab, A.; Gan, X.; et al. Photoreceptor Activity Contributes to Contrasting Responses to Shade in Cardamine and Arabidopsis Seedlings. Plant Cell 2019, 31, 2649–2663. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Walters, R.G.; Jansson, S.; Horton, P. Acclimation of Arabidopsis thaliana to the light environment: The existence of separate low light and high light responses. Planta 2001, 213, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Nezhad, M.; Alibeigi, B.; Estaji, A.; Gruda, N.S.; Aliniaeifard, S. Growth, Biomass Partitioning, and Photosynthetic Performance of Chrysanthemum Cuttings in Response to Different Light Spectra. Plants 2022, 11, 3337. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.M.; Gardiner, E.S.; Vaughn, K.C. High-light acclimation in Quercus robur L. seedlings upon over-topping a shaded environment. Environ. Exp. Bot. 2012, 78, 25–32. [Google Scholar] [CrossRef]
- Poorter, L. Growth responses of 15 rain-forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 1999, 13, 396–410. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, G.; Liu, Z. Dynamic variation of non-structural carbohydrates in branches and leaves of temperate broad-leaved tree species over a complete life history. Front. For. Glob. Change 2023, 6, 1130604. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Zepeda, A.C.; Heuvelink, E.; Marcelis, L.F.M.; Hammer, G. Carbon storage in plants: A buffer for temporal light and temperature fluctuations. In Silico Plants 2023, 5, diac020. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Schulze, E.-D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Beaudet, M.; Messier, C. Growth and morphological responses of yellow birch, sugar maple, and beech seedlings growing under a natural light gradient. Can. J. For. Res. 1998, 28, 1007–1015. [Google Scholar] [CrossRef]
- Signori-Mueller, C.; Oliveira, R.S.; Barros, F.d.V.; Tavares, J.V.; Gilpin, M.; Carvalho Diniz, F.; Marca Zevallos, M.J.; Salas Yupayccana, C.A.; Acosta, M.; Bacca, J.; et al. Non-structural carbohydrates mediate seasonal water stress across Amazon forests. Nat. Commun. 2021, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, M.; Song, Y.C.; Zargar, M.; Chen, M.X.; Lin, S.Y.; Zhu, F.Y.; Song, T. Unlocking bamboo’s fast growth: Exploring the vital role of non-structural carbohydrates (NSCs). Plant J. 2025, 122, e70147. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).