Joint Transcriptomic and Metabolomic Analysis of Molecular Physiological Mechanisms of Tea Tree Roots in Response to pH Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Sampling
2.2. Determination of Tea Plant Root Indexes
2.3. Transcriptome Sequencing Analysis of the Tea Plant Root System
2.4. Determination of Metabolites in Tea Plant Roots
2.5. Statistical Analysis
3. Results
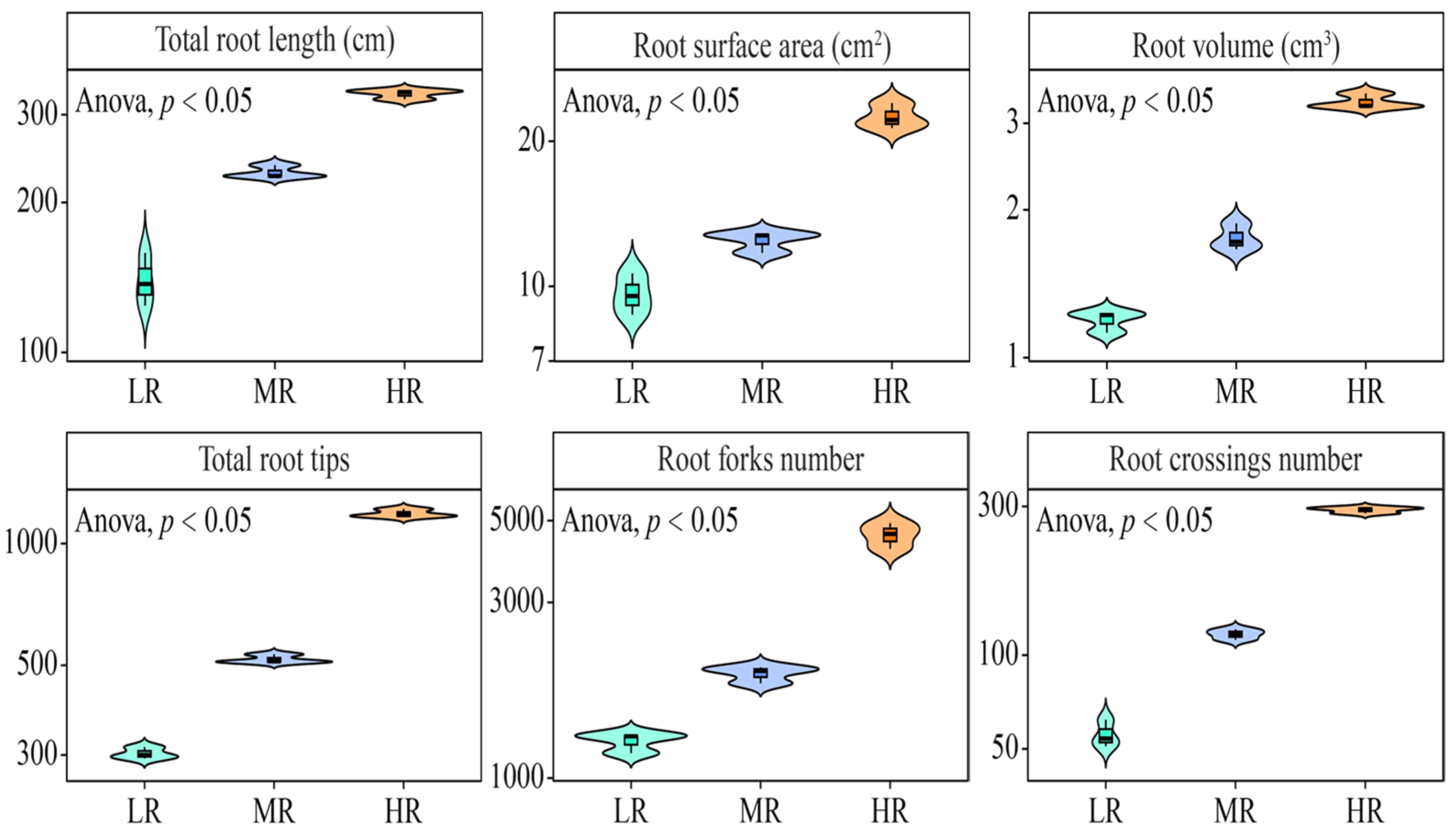
3.1. Effect of pH on the Growth of Tea Plant Roots
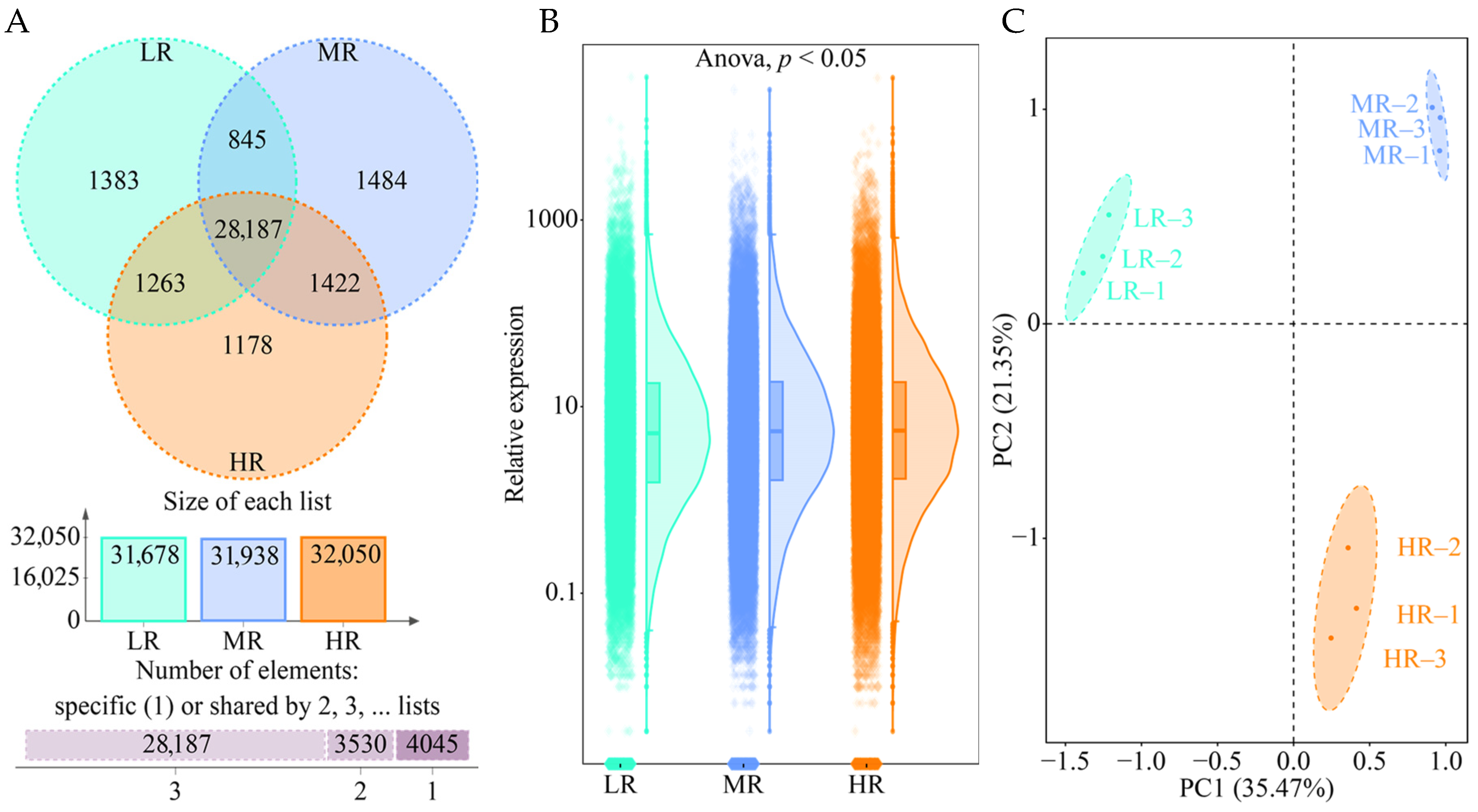
3.2. Effect of pH on Gene Expression in Tea Plant Roots
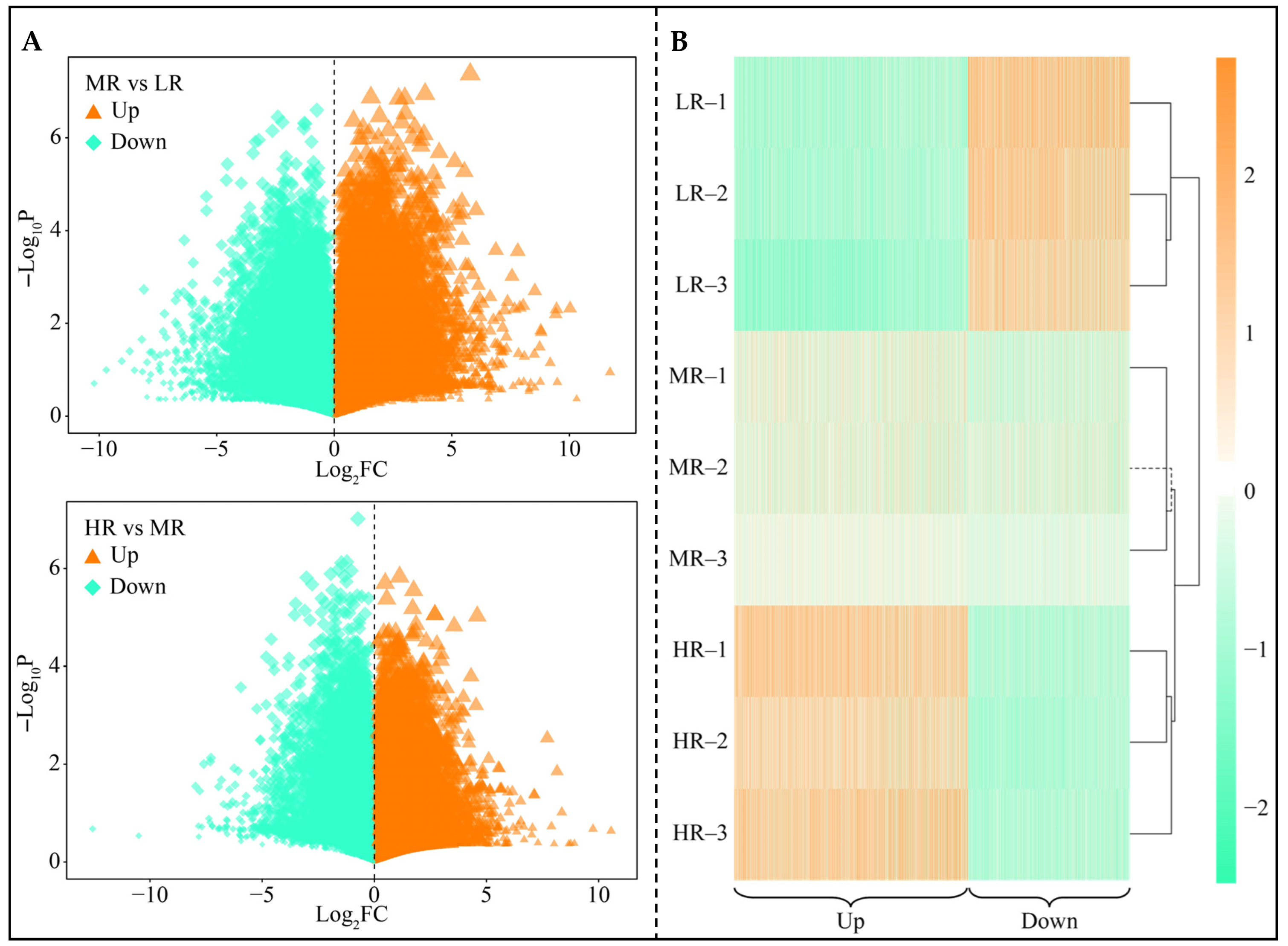
3.3. Screening for Differential Genes in the Root System of Tea Plant in Response to pH Regulation
3.4. Screening for Characteristic Genes in the Root System of Tea Plant in Response to pH Regulation
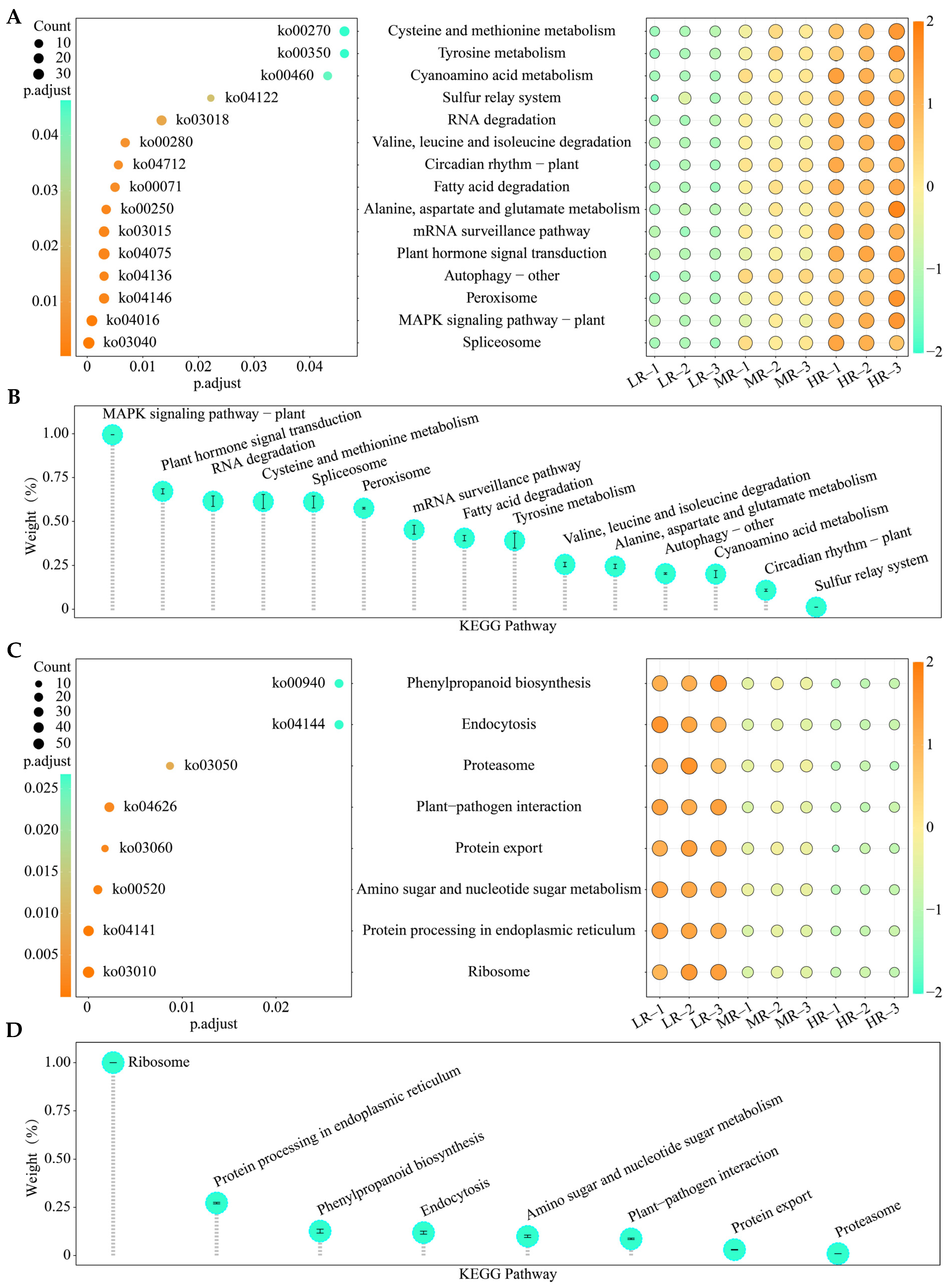
3.5. KEGG Pathway Enrichment Analysis of Characteristic Genes
3.6. Effect of pH on Metabolites of Tea Plant Roots
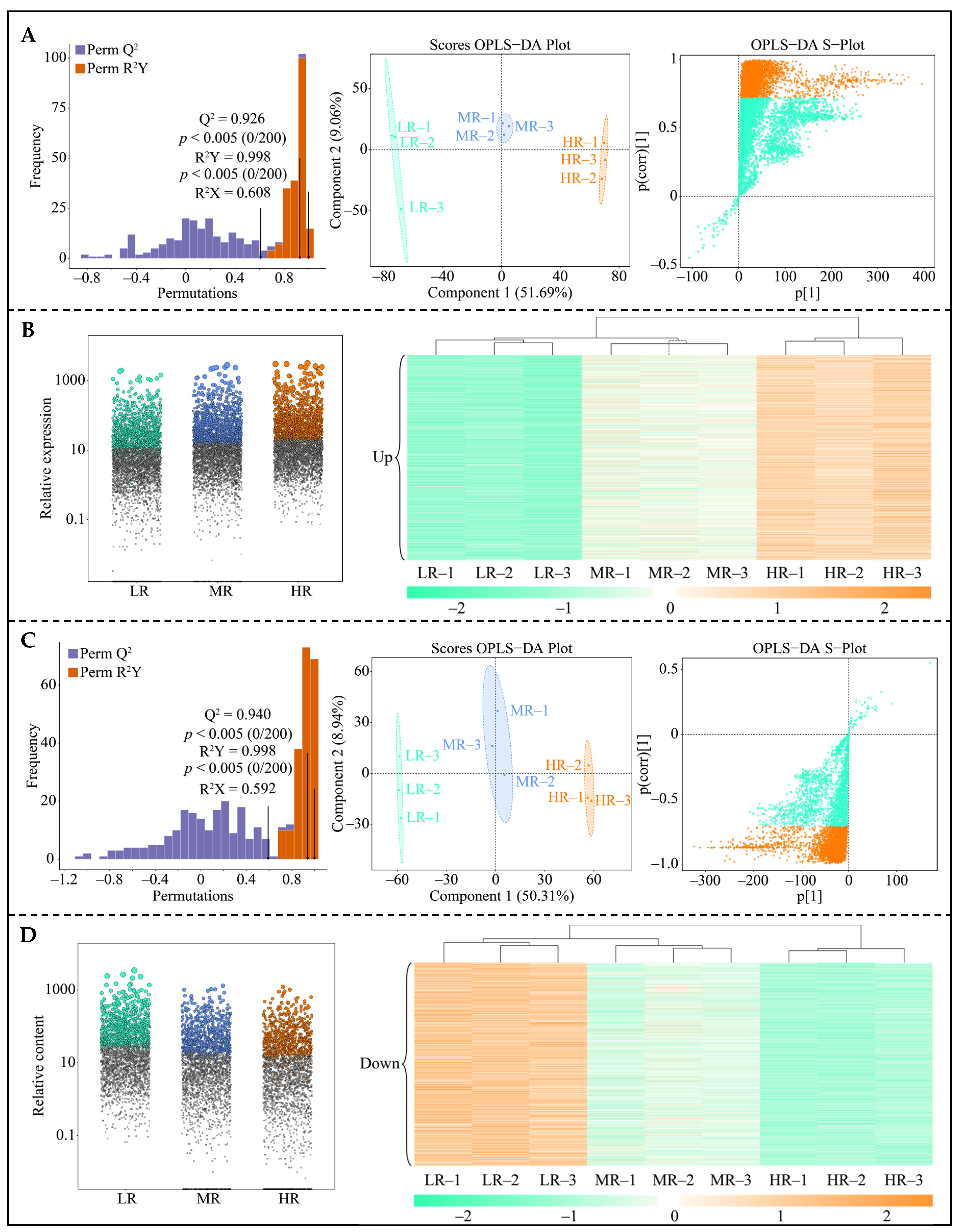
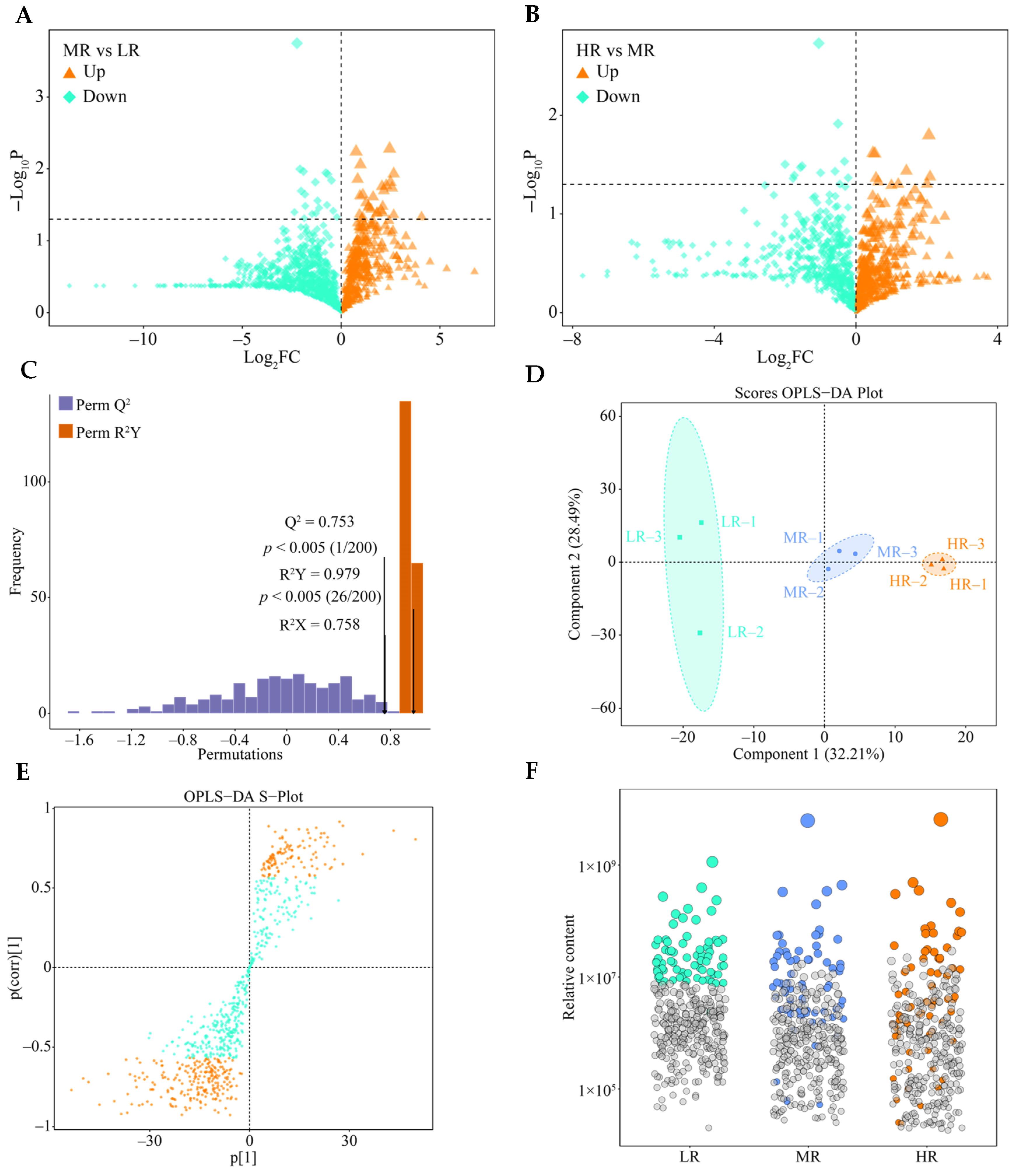
3.7. Screening for Characteristic Metabolites in the Root System of Tea Plant in Response to pH Regulation
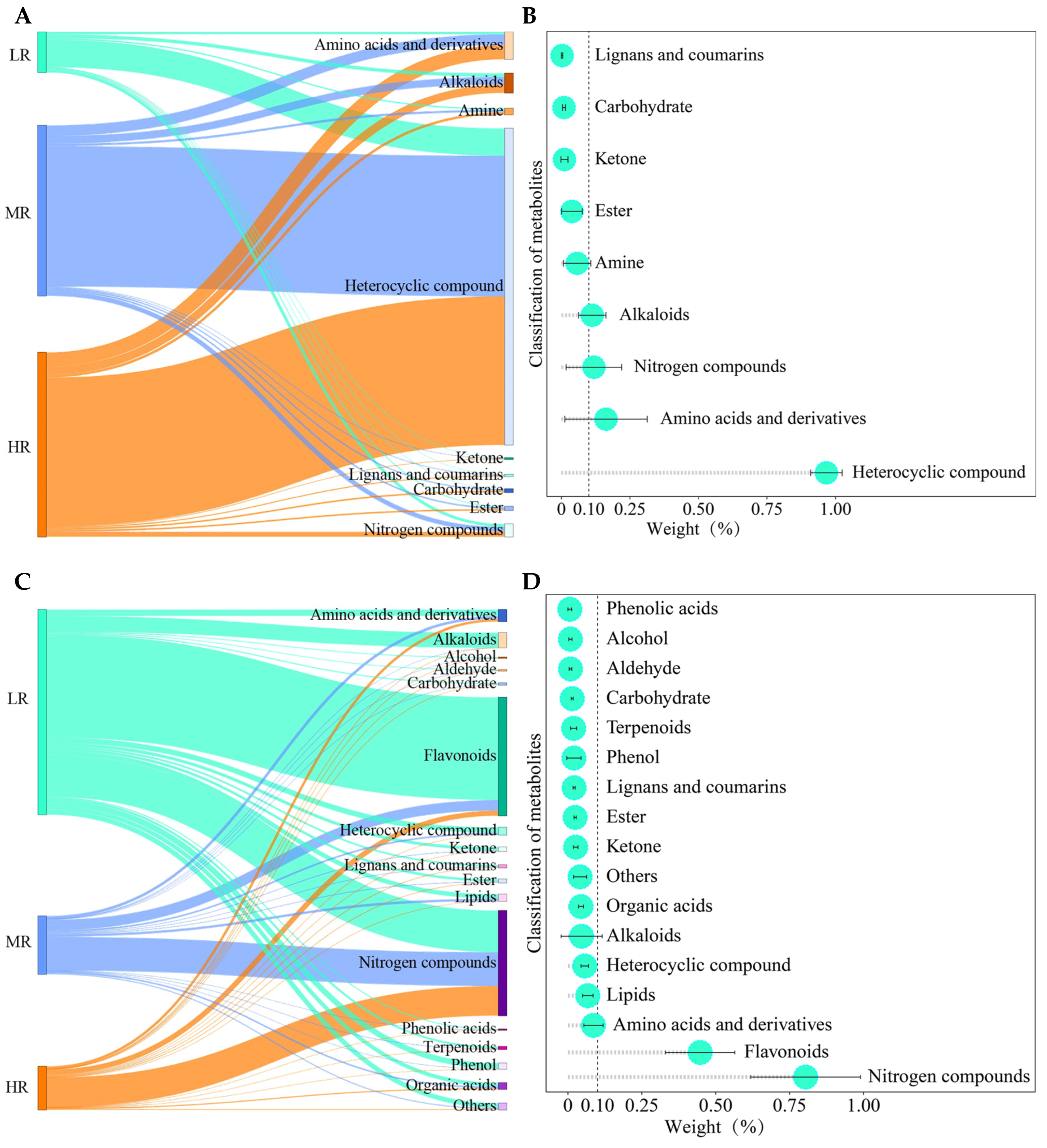
3.8. Classification Analysis of Characteristic Metabolites in the Root System of Tea Plant in Response to pH Regulation
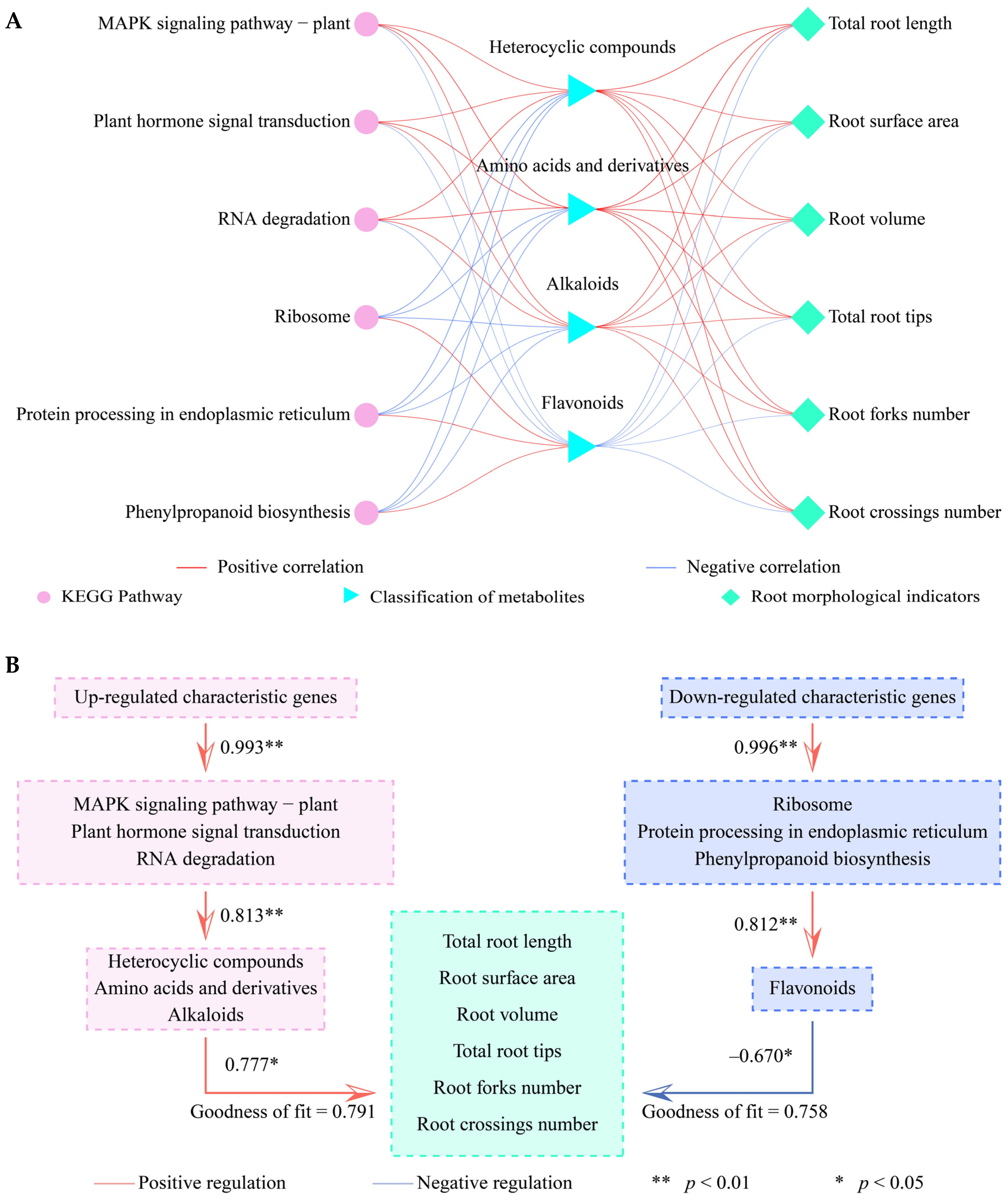
3.9. Analysis of the Interactions of Different Indexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef] [PubMed]
- Hartemink, A.E.; Barrow, N.J. Soil pH-nutrient relationships: The diagram. Plant Soil 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Zhang, Q.; Lin, S.; Zhang, Q.; Chen, Y.; Hong, L.; Jia, X.; Ye, J.; Wang, H. Effect of soil pH on the uptake of essential elements by tea plant and subsequent impact on growth and leaf quality. Agronomy 2024, 14, 1338. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Li, Y.; Zhang, Y.; Huang, X.; Yang, Y.; Zhu, Y.; Xiong, H.; Jiang, T. Influence of nitrogen fertilizer application on soil acidification characteristics of tea plantations in karst areas of southwest China. Agriculture 2023, 13, 849. [Google Scholar] [CrossRef]
- Ramachandran, P.; Ramirez, A.; Dinneny, J.R. Rooting for survival: How plants tackle a challenging environment through a diversity of root forms and functions. Plant Physiol. 2025, 197, kiae586. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shen, R.F. Towards sustainable use of acidic soils: Deciphering aluminum-resistant mechanisms in plants. Fundamental Res. 2024, 4, 1533–1541. [Google Scholar] [CrossRef]
- Zou, Z.; Mi, W.; Li, X.; Hu, Q.; Zhang, L.; Zhang, L.; Fu, J.; Li, Z.; Han, W.; Yan, P. Biochar application method influences root growth of tea (Camellia sinensis L.) by altering soil biochemical properties. Sci. Hortic. 2023, 315, 111960. [Google Scholar] [CrossRef]
- Manzoor; Ma, L.; Ni, K.; Ruan, J. Influence of organic and inorganic fertilizers on tea growth and quality and soil properties of tea qrchards’ top rhizosphere soil. Plants 2024, 13, 207. [Google Scholar] [CrossRef]
- Huang, F.; Lei, Y.; Duan, J.; Kang, Y.; Luo, Y.; Ding, D.; Chen, Y.; Li, S. Investigation of heat stress responses and adaptation mechanisms by integrative metabolome and transcriptome analysis in tea plants (Camellia sinensis). Sci. Rep. 2024, 14, 10023. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Q.; Shi, L.; Wang, Y.; Li, M.; Chen, Y.; Zhang, M.; Chen, J.; Chen, M.; Jia, X.; et al. Joint analysis of transcriptome and hormone metabolome on the mechanism of pruning effect on tea tree (Camellia sinensis) growth. Ind. Crops Prod. 2024, 218, 118929. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Z.; Wu, W.; Zhou, Z.; Deng, X.; Chen, Z.; Sun, W. Transcriptome and metabolome reveal the effects of ABA promotion and inhibition on flavonoid and amino acid metabolism in tea plant. Tree Physiol. 2024, 44, tpae065. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, J.; Zhang, L.; Zhang, X.; Zhao, H.; Guo, F.; Wang, Y.; Wang, P.; Chen, Y.; Ni, D.; et al. Metabolome and RNA-seq analysis of responses to nitrogen deprivation and resupply in tea plant (Camellia sinensis) roots. Front. Plant Sci. 2022, 13, 932720. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, J.; Lv, Z.; Shao, C.; Chen, Z.; Zhou, Y.; Shen, C. Tea-derived polyphenols enhance drought resistance of tea plants (Camellia sinensis) by alleviating jasmonate–isoleucine pathway and flavonoid metabolism flow. Int. J. Mol. Sci. 2024, 25, 3817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, C.; Zhao, J.; Xia, E.; Wen, W.; Zeng, L.; Benedito, V.A. Secondary metabolites and metabolism in tea plants. Front. Plant Sci. 2023, 14, 1143022. [Google Scholar] [CrossRef]
- Huang, W.; Lin, M.; Liao, J.; Li, A.; Tsewang, W.; Chen, X.; Sun, B.; Liu, S.; Zheng, P. Effects of potassium deficiency on the growth of tea (Camelia sinensis) and strategies for optimizing potassium levels in soil: A critical review. Horticulturae 2022, 8, 660. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, D.; Zhang, G.; Zheng, L.; Chen, C.; Sun, X.; Yu, F. Effects of soil physical and chemical properties on the quality of Nanjing ‘Yuhua’ Tea, a type of famous green tea. Horticulturae 2023, 9, 189. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, J.; Yao, X.; Wang, X.; Huang, J.; Xiong, D.; Chen, G. Fine root nutrient foraging ability in relation to carbon availability along a chronosequence of Chinese fir plantations. Forest Ecol. Manag. 2022, 507, 120003. [Google Scholar] [CrossRef]
- Cheng, Y.; Mao, J.; Cao, L.; Wang, H.; Li, S.; Jin, X.; Zhang, X.; Li, Y. Molecular basis of flavonoid accumulation in tea leaves grafted with Camellia sinensis var. assamica cv.“Yinghong9” as rootstock based on multi-omics analysis. Sci. Hortic. 2023, 321, 112290. [Google Scholar] [CrossRef]
- Robinson, J.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.; Getz, G.; Mesirov, J. Integrative genomics viewer. Nat. biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, L.; Hoang, N.H.; Thanh, T.L.; Zhou, C.; Mei, X.; Buensanteai, K. The changes in metabolites, quality components, and antioxidant activity of tea (Camellia sinensis) infected with exobasidium vexans by applying UPLC-MS/MS-based widely targeted metabolome and biochemical analysis. Phytopathology 2024, 114, 164–176. [Google Scholar] [CrossRef]
- Li, X.; Jia, J.; Li, T.; Zhao, Z.; Liu, H.; Song, N.; Pei, J. Metabolomics analysis of Ligustri Lucidi Fructus at different harvest times during the whole growing period based on ultra-high-performance liquid chromatography with mass spectrometry. J. Sep. Sci. 2023, 46, 2300196. [Google Scholar] [CrossRef] [PubMed]
- Wen, O.; Ning, J.; Zhu, X.; Jiang, Y.; Wang, J.; Yuan, H.; Hua, J. UPLC-ESI-MS/MS analysis revealed the dynamic changes and conversion mechanism of non-volatile metabolites during green tea fixation. LWT 2024, 198, 116010. [Google Scholar] [CrossRef]
- Wickham, H. Advanced R, 2nd ed.; Chapman and Hall/CRC The R Series; Taylor and Francis: Milton Park, UK, 2019. [Google Scholar]
- Wang, Y.; Wang, J.; Guo, D.; Zhang, H.; Che, Y.; Li, Y.; Tian, B.; Wang, Z.; Sun, G.; Zhang, H. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant Physiol. Bioch. 2021, 167, 140–152. [Google Scholar] [CrossRef]
- Le, V.S.; Herrmann, L.; Hudek, L.; Nguyen, T.B.; Bräu, L.; Lesueur, D. How application of agricultural waste can enhance soil health in soils acidified by tea cultivation: A review. Environ. Chem. Lett. 2022, 20, 813–839. [Google Scholar] [CrossRef]
- Ding, Z.J.; Shi, Y.Z.; Li, G.X.; Harberd, N.P.; Zheng, S.J. Tease out the future: How tea research might enable crop breeding for acid soil tolerance. Plant Commun. 2021, 2, 100182. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annual Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Guo, L.; Zhou, T.; Shan, Y.; Xia, Z.; Li, X.; An, M.; Wu, Y. Antiviral modes of action of the novel compound GLY-15 containing pyrimidine heterocycle and moroxydine skeleton against tobacco mosaic virus. Pest Manag. Sci. 2022, 78, 5259–5270. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, J.; Wu, K.X.; Yang, N.; Pan, L.B.; Song, Y.; Liu, Y.; Tang, Z.H. Comparative analysis of endogenous hormones and metabolite profiles in early-spring flowering plants and unflowered plants revealing the strategy of blossom. J. Plant Growth Regul. 2022, 41, 2421–2434. [Google Scholar] [CrossRef]
- Carlos, E.; Lerma, T.A.; Martínez, J.M. Phytohormones and plant growth regulators—A review. J. Sci. Technol. Appl. 2021, 10, 27–65. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and mechanisms of microRNA-mediated gene regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef]
- Robles, P.; Quesada, V. Unveiling the functions of plastid ribosomal proteins in plant development and abiotic stress tolerance. Plant Physiol. Biochem. 2022, 189, 35–45. [Google Scholar] [CrossRef]
- Liu, J.X.; Howell, S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Boil. 2021, 63, 180–209. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, M.; Jia, M.; Zhou, Z.; Wang, Y.; Liao, Y.; Jia, X.; Wang, T.; Wang, H.; Ye, J. Joint Transcriptomic and Metabolomic Analysis of Molecular Physiological Mechanisms of Tea Tree Roots in Response to pH Regulation. Horticulturae 2025, 11, 821. https://doi.org/10.3390/horticulturae11070821
Zhang Q, Li M, Jia M, Zhou Z, Wang Y, Liao Y, Jia X, Wang T, Wang H, Ye J. Joint Transcriptomic and Metabolomic Analysis of Molecular Physiological Mechanisms of Tea Tree Roots in Response to pH Regulation. Horticulturae. 2025; 11(7):821. https://doi.org/10.3390/horticulturae11070821
Chicago/Turabian StyleZhang, Qi, Mingzhe Li, Miao Jia, Zewei Zhou, Yulin Wang, Yankun Liao, Xiaoli Jia, Tingting Wang, Haibin Wang, and Jianghua Ye. 2025. "Joint Transcriptomic and Metabolomic Analysis of Molecular Physiological Mechanisms of Tea Tree Roots in Response to pH Regulation" Horticulturae 11, no. 7: 821. https://doi.org/10.3390/horticulturae11070821
APA StyleZhang, Q., Li, M., Jia, M., Zhou, Z., Wang, Y., Liao, Y., Jia, X., Wang, T., Wang, H., & Ye, J. (2025). Joint Transcriptomic and Metabolomic Analysis of Molecular Physiological Mechanisms of Tea Tree Roots in Response to pH Regulation. Horticulturae, 11(7), 821. https://doi.org/10.3390/horticulturae11070821





