Abstract
Calcium (Ca2+) is a macronutrient essential for the growth, development, yield, and quality of vegetables and fruits. It performs structural, enzymatic, and signaling functions in plants. This review examines Ca2+ translocation from soil to the fruit via the plant xylem network, emphasizing the importance of Ca2+ compartmentalization within fruit cell organelles in the development of calcium deficiency disorders such as blossom-end rot (BER). The underlying causes of BER and potential control measures are also discussed. Soil-available Ca2+, transported by water flow, enters the root apoplast through membrane channels and moves toward the xylem via apoplastic or symplastic routes. The transpiration force and the growth of organs determine the movement of Ca2+-containing xylem sap to aerial plant parts, including fruits. At the fruit level, the final step of Ca2+ regulation is intracellular partitioning among organelles and cellular compartments. This distribution ultimately determines the fruit’s susceptibility to Ca2+-deficiency disorders such as BER. Excessive sequestration of Ca2+ into organelles such as vacuoles may deplete cytosolic and apoplastic Ca2+ pools, compromising membrane integrity and leading to BER, even when overall Ca2+ levels are adequate at the blossom end. Effective BER management requires cultural and physiological practices that promote Ca2+ uptake, translocation to the fruit, and appropriate intracellular distribution. Additionally, the use of BER-resistant and Ca2+-efficient cultivars can help mitigate this disorder. Therefore, a comprehensive understanding of Ca2+ dynamics in plants is critical for managing BER, minimizing production loss and environmental impacts, and maximizing overall crop productivity.
1. Introduction
Calcium (Ca2+) as a macronutrient is essential for plant growth and development [1,2,3] and is found in higher quantities (0.5%) than any other divalent inorganic cations [4]. When hydrated, Ca2+ is a big cation with a 41.2 Å ionic radius [5]. Among the macronutrients, Ca2+ is third after nitrogen (N) and potassium (K) in terms of quantity in plant tissue, which reflects its indispensability for the plant. The plant requires 1–3 mM Ca2+ for proper growth and development [6]. It plays roles in membrane and cell wall stabilization, cell function, signal transduction, growth and development, gene expression, and stress resistance [7,8,9,10,11,12,13,14,15]. Ca2+ concentration varies from 10−7 M to 10−3 M across the cell organelles (Figure 1). Though the cytoplasm contains 10−7 M calcium at the resting stage, it increases to 10−5 to 10−4 M in the storage organelles and 10−3 M in the extracellular milieu [16,17]. The Ca2+ content of mitochondrial and nuclear matrices is similar to that of the cytosol. The free Ca2+ content in cytosol and vacuoles is 100–200 nM and 1–10 mM, respectively [18,19], and 60% of the plant Ca2+ remains as calcium pectate. Ca2+ content in plants varies greatly; shoot-Ca2+ ranges from 0.1–5%, while fruit-Ca2+ varies from 0.2–0.3% of total dry mass [11]. The proportion of Ca2+ in specific tissues can be more than 10% without affecting plant growth and development [4].
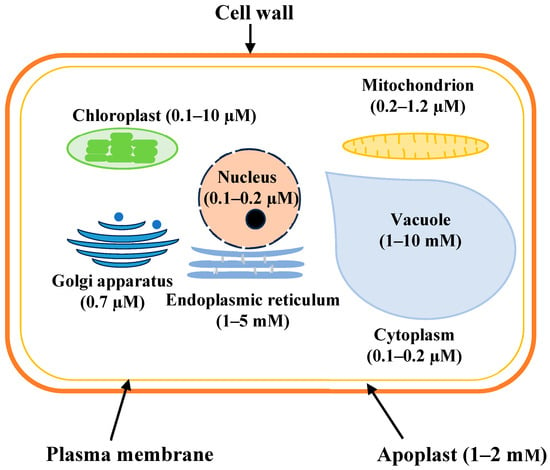
Figure 1.
Calcium compartmentalization within a plant cell. Calcium (Ca2+) is an essential macronutrient involved in diverse structural, regulatory, and signaling processes within plant cells. Its structural role is most evident in the cell wall, where Ca2+ cross-links pectic polysaccharides to maintain cell wall stability, and in the plasma membrane, where it contributes to membrane integrity and modulates permeability. In the cytosol, calcium functions as a fundamental second messenger, mediating intracellular signaling cascades in response to both biotic and abiotic stimuli. Although transient increases in cytosolic Ca2+ concentrations are critical for signaling, the bulk of cellular calcium is sequestered in the vacuole. This compartment serves as the principal intracellular calcium reservoir, contributing to the regulation of ion homeostasis and buffering cytoplasmic pH. The tight spatial and temporal regulation of calcium distribution is essential for maintaining cellular function and avoiding calcium toxicity, given the cytotoxic potential of sustained elevated cytosolic Ca2+ levels.
The available Ca2+, carried by water flow, enters the root apoplast through membrane channels [20], and moves toward the xylem, following either apoplastic or symplastic routes [21]. Along the xylem water flow, Ca2+ is transported to the leaves and fruits based on their transpiration strength during the daytime, while at night, root pressure does the job [22]. Poor Ca2+ translocation to the fruit or leaf tip can result in Ca2+ deficiency disorders, e.g., blossom-end rot (BER), tip burn, blackheart, brown heart, bitter pit, empty pod, and fruit cracking (Table 1) [21,23,24,25,26,27].
Among numerous Ca2+-deficiency disorders, BER is most prevalent and causes up to 50% economic loss in extreme conditions [25]. BER often initiates at the fruit blossom part (away from the peduncle) during early fruit growth stages (2–3 weeks following anthesis) [28,29,30,31,32,33,34,35,36,37,38]. Insufficient Ca2+ supply to the rapidly growing fruit tissue causes the disintegration of the plasma membrane and lysis of the middle lamella, resulting in cell plasmolysis and a water-soaked appearance. Subsequent drying develops sunken, brown, and black spots that are limited to the fruit blossom end or can encompass the entire fruit [39]. BER incidence is positively correlated with root relative water content, fruit number per plant, potassium (K+) and vitamin C levels of fruits, and negatively correlated with plant height, leaf chlorophyll content, total yield, and fruit Ca2+ content in tomato [40,41].
Though agricultural soils are not usually low in Ca2+, Ca2+-deficiency disorders are numerous and cause significant economic losses worldwide. Ca2+ deficiency is usually not manifested by the unavailability of soil Ca2+; instead, it is the soil’s inability to supply sufficient Ca2+ to the affected plant parts [24]. By being phloem-immobile, Ca2+ cannot be translocated from the available sources (mature leaves and the peduncle end of the fruit) to the deficient sinks (young-growing leaves and the blossom end of the fruit). Therefore, Ca2+ fertilization generally does not overcome these physiological disorders, and thus, these disorders (e.g., BER) are complex and challenging to solve. Though the genes linked to calcium deficiency disorders are not well-documented, expression of Ca2+ /H+ antiporters (CAXs), such as CAX1 and sCAX1 [42,43,44,45,46], and calreticulin (CRT) [45,47] may play a role in this regard [48]. There is no straightforward solution for these disorders. Moreover, the uncertainty of the onset of these deficiency disorders complicates the issues regarding their workable solutions [24]. Although calcium’s dual role as a nutrient and signal transducer is well established, significant knowledge gaps persist. The molecular identity and regulation of many calcium channels, pumps, and sensors remain uncharacterized [49]. Moreover, the interconnectedness of calcium’s roles in nutrient uptake versus signaling pathways is poorly understood. It remains unclear whether distinct or overlapping sets of genes mediate these functions, such as those involved in calcium uptake in roots versus those regulating signal transduction in leaves. Therefore, the present study discusses the route of calcium translocation within plants, the causes and control of BER, and recent advances and future directions in calcium research.
2. Function of Calcium in Plants
The roles of Ca2+ in cellular integrity and growth are well documented [50,51].
2.1. Structural Role of Calcium
Calcium maintains cell wall integrity [11,52], cell division and elongation [53,54,55], cell expansion [55], membrane permeability and membrane stability [52,56], and assembly of microtubules [57].
The plant cell wall contains carbohydrates (cellulose, hemicellulose, pectin), proteins, particularly structural ones, and lignin (secondary cell wall) [58]. Cell wall Ca2+ mainly represents Ca2+ bindings in the middle lamella that glue adjacent cells and maintain cell wall integrity [59]. Ca2+ is unique among other inorganic elements, which are not usually integral components of cell walls except nitrogen (N). Cell wall Ca2+ generally binds with pectin, a polymer of a diverse group of pectic polysaccharides, including homogalacturonans, rhamnogalacturonan I, and rhamnogalacturonan II. Homogalacturonans are polymers of galacturonic acid in a fashion of α(1–4) linkage. Ca2+ forms a tight linkage between the charged carboxyl (COO−) group of galacturonic acid [4], through which it provides cell wall strength. Pectin forms a gel-like structure by binding with Ca2+ molecules in a reversible fashion that aids in tightening (binding with Ca2+) and loosening (Ca2+ removal) of the cell wall. During the biosynthesis of sugar residues in the Golgi apparatus, the charged carboxyl group can be esterified with methyl, acetyl, or unknown groups that prevent the binding of Ca2+ [4] and keep the cell wall loose. Cell walls are also loosened by the degradation of Ca2+-pectate by polygalacturonase (PG), and the activity of PG is inhibited by high Ca2+ concentration [60]. The PG activity is increased in Ca2+-deficient tissue, leading to middle lamella disintegration, primary cell wall degradation, and cell death [61]. Ca2+ cannot bind to methylated pectin residue. Pectin methyl esterase (PME) removes the methyl group from methylated pectin and opens up free binding sites for Ca2+, where Ca2+ binds to form a strong electrovalent bond [62]. Demethylation of pectin by PME action favors the further degradation of pectin by enzymes like endo-polygalacturonase, exo-polygalacturonase, β-galactosidases, and pectate lyases [63,64].
Besides strengthening the cell wall, Ca2+ plays a role in stabilizing and functioning of the plasma membrane. The plasma membrane is composed of phospholipid bilayers held together by proteins. Ca2+, on the apoplast side of the plasma membrane, binds to the carboxylic group of protein and the phosphate group of phospholipid and thus stabilizes the membrane, allowing proper membrane selective permeability. The requirement of Ca2+ increases due to an increase in heavy metals [65], aluminum (Al3+), sodium (Na+) [66], and protons (H+) in the external environment. Other cations can replace the Ca2+, but their role is not in proper membrane functioning. Ca2+ in high concentration is required to restrain the unfavorable effects. For plants growing in soil with a higher concentration of other cations, the Ca2+ requirement increases substantially to ensure optimum plant growth and development [67]. Replacement of Ca2+ with Na+, heavy metals, or Al3+ can cause salinity, heavy metal, or aluminum toxicity, respectively [68,69]. Membrane instability is prevalent under freezing, low temperature, and anaerobiosis [5]. Unstable membranes are prone to loss of low molecular weight solutes, such as potassium (K+) and sugars. It can also cause an influx of toxic ions (e.g., the heavy metal Al3+) into the cytosol. A high concentration of free Ca2+ in the apoplast prevents the loss of solutes and helps to avoid potential toxicity from toxic elements. Lack of Ca2+ results in a leaky membrane that causes loss of cell material, impairment of cell metabolism, and subsequent cell death.
Ca2+ stabilizes the cell wall by binding with pectin, the cell membrane, and the proteins and lipids at the membrane surfaces [50,70]. Ca2+ influences vesicles—full of materials and enzymes for cell wall and membrane construction—in their incorporation into the plasma membrane [55]. Moreover, Ca2+ is required for regulating ion uptake, pH, carbohydrate translocation, the activity of the oxygen-evolving complex, and as a counteraction in the vacuoles for all types of anions [5,12].
2.2. Enzymatic Role of Calcium
Ca2+ can promote or demote enzyme activity essential for cell growth and development. The activity of α-amylase is stimulated by high Ca2+ concentration during starch breakdown in germinating cereal seeds, in which the Ca2+ ion stabilizes amylase [71]. However, high Ca2+ concentrations may inhibit enzyme activity [24], as has been shown with cytosolic enzyme fructose-1,6-bisphosphatase (FBPase) (Figure 2), which regulates sucrose synthesis from triosephosphate (TP) in the cytosol. Ca2+ is also a cofactor of several enzymes, e.g., 1,4-lactonase, phosphoinositide phospholipase C, N-acetylgalactoseaminyltransferase, and affects the synthesis and transport of those enzymes [72].
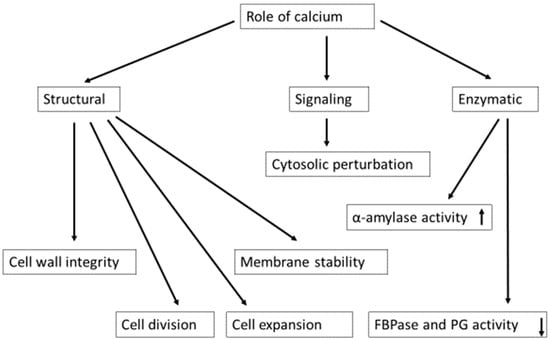
Figure 2.
Calcium plays structural, enzymatic, and signaling functions. Alpha-amylase works during seed germination; fructose 1,6 bisphosphatase (FBPase) is a regulatory enzyme in the sucrose biosynthesis pathway; polygalacturonase (PG) hydrolyzes the alpha-1,4 glycosidic bonds between galacturonic acid residues of pectin. An upward arrow (↑) indicates an increase, and a downward arrow (↓) shows a decrease in enzyme activity.
2.3. Calcium and Signal Transduction
The interest in Ca2+ in recent years has gained momentum due to its role as a secondary messenger, particularly for developmental and environmental cues [5]. It plays a crucial role as a signaling molecule in signaling pathways [73]. Ca2+ acts as a universal signaling molecule [74], and plays a role in plants’ growth, development, and stress management. Environmental stressors initiate cytosolic Ca2+ spikes, activating downstream gene expression and adaptation in adverse conditions [74]. Understanding Ca2+ dynamics may help develop and engineer climate-smart crop varieties [74]. Ca2+ is a stress-response element. Upon sensing stresses, it conveys signals to the downstream protein kinases, leading to phenotypic responses that may result in stress tolerance [75,76,77,78]. It also contributes to immunity by activating immune responses [79]. Characterizing the Ca2+ channels, pumps, and binding proteins is required to comprehend the role of stress signals on Ca2+ homeostasis and adaptive responses [80]. It will improve understanding of how specific stress signals modulate Ca2+ homeostasis to orchestrate adaptive responses [80].
In response to stimuli, Ca2+ transduces signals to the other end upon binding with calmodulin, a calcium protein in the cytosol [13,81,82,83] (Figure 3). Plants maintain a very low (100 to 200 nM) cytosolic [Ca2+], which can rise to 2 µM in the stimulated state [84]. Plants maintain very low cytosolic [Ca2+] to serve as a messenger, prevent precipitation of inorganic phosphate, and minimize competition for binding sites with magnesium [5]. The role of Ca2+ as a messenger is possibly due to this low cytosolic [Ca2+] concentration and its chemistry [21]. Any signal—changes in light intensity, day length, temperature, salinity, drought, osmotic and oxidative stresses, aluminum toxicity, mechanical injury, anoxia, nodulation, and pathogen attack—exerts an abrupt change in cytosolic [Ca2+] and initiates a Ca2+-signaling pathway [21,82,85]. Additionally, the pathway is also activated by various developmental cues, such as germination, cell division and elongation, circadian rhythms, tropic responses, senescence, and apoptosis [4]. The Ca2+-signaling event is location- and time-specific and vital for encoding specific cellular responses [86]. This signaling is subject to strict regulations as a marked increase in Ca2+-concentration activates Ca+-dependent enzymes, which are harmful to a cell. Therefore, very tight regulation is in place for Ca2+-signaling processes through the coordinated activities of calcium proteins, calcium channels, and efflux systems.
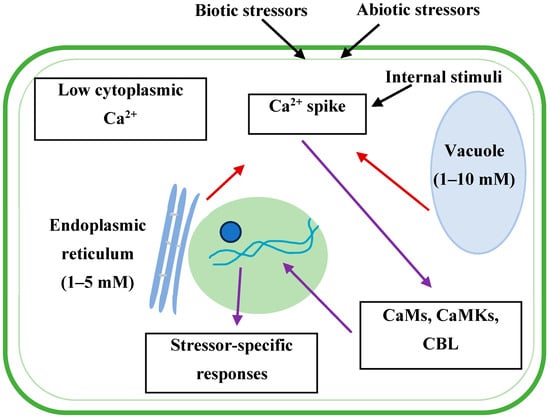
Figure 3.
Though cytoplasm maintains a low state of Ca2+, the cytoplastic Ca2+ spikes due to the release of more Ca2+ principally from the vacuoles and endoplasmic reticulum—the large storage of Ca2+ (dark red arrows). Ca2+ binds with calmodulins (CaMs), Ca2+/calmodulin-dependent protein kinases (CaMKs), or calcineurin B-like (CBL) proteins, which cause stressor-specific responses through binding to the transcription factors (purple arrows).
2.3.1. Calcium Proteins
Changes in cytosolic [Ca2+] are detected by specific proteins that either relay or respond to messages. Upon binding with Ca2+, relay proteins such as calmodulin undergo conformational changes that enable them to interact with a target protein to regulate its function [87,88]. Response proteins such as Ca2+-dependent protein kinases (CDPKs) bind with Ca2+, followed by a conformational change that initiates their intrinsic kinase activity. Cytosolic Ca2+-binding proteins include calmodulins (CaMs), CaM-like proteins, annexins, calcineurin B-like (CBL) proteins, and CDPKs. Calmodulins bind with CaM-binding transcription activators (CAMTAs) and are responsible for gene expression [89,90]. Calmodulins and similar proteins initiate responses to developmental or environmental cues and pathogen attack; CBL to cold, drought, salinity, and wounding; and CDPKs to various stimuli [5]. Plant annexins are associated with cell elongation, membrane repair, the secretory process, salinity, and drought stresses [91]. Several Ca2+-binding proteins, e.g., calreticulin, calnexin, calsequestrin, and BiP (binding immunoglobulin protein), are found in the ER and are responsible for protein folding, Ca2+ homeostasis, and modifications at the post-translational stage [5].
2.3.2. Calcium Channels
The membrane-bound calcium channels move Ca2+ into the cell cytosol from the apoplast space, vacuoles, and ER. The channels are voltage-sensitive and are called depolarization-activated calcium channels (DACCs), hyperpolarization-activated calcium channels (HACCs), and voltage-insensitive calcium channels (VICCs) [88,92,93]. The membrane-bound K+ channel [outward-rectifying (Ca2+-permeable) K+ channel, KORC] is also considered a calcium-permeable DACC [94]. Calcium channels are activated and perform specific roles to different environmental and developmental signals, such as DACCs, which are activated by stresses such as low temperatures [21,95]; HACCs by pathogen attack, oxidative stresses, cell elongation, and tropism [21,82,96]; and VICCs maintains steady-state cytosolic Ca2+ at the cells’ resting stage [21]. Ca2+ channels are also found in the tonoplast and ER membrane, allowing Ca2+ to enter the cytosol. Tonoplast-bound channels include HACC, SV (slow-vacuolar), inositol phosphates (IP3, inositol-1,4,5-triosephosphate; IP6), and cADPR (cyclic ADP-ribose). Tonoplast IP3 may be involved in turgor regulation, cell elongation, tropism, salt stress, and hyperosmotic stress [21,97,98,99], and cADPR in cold adaptation, desiccation tolerance, stomatal behavior, circadian rhythms, and pathogen attack [5]. The IP3, cADPR, and NAADP receptors are also found in the membrane of the ER [21].
2.3.3. Calcium Efflux Systems
Plant cells tightly regulate cytosolic [Ca2+]. Therefore, excess Ca2+ is expelled out to the vacuoles, apoplast, ER, and plastids through active transporters such as Ca+-ATPases and H+/ Ca2+-antiporters (CAX) [21] to aid proper metabolism in the cytoplasm, to restore intra- and extracellular Ca2+-stores, and to remove divalent cations [100,101,102,103,104,105]. As Ca2+ transporters, Ca2+-ATPases have high-affinity but low-capacity attributes, and H+/Ca+-antiporters are the opposite [106]. Ca2+-ATPases are located in organelle membranes such as vacuolar membranes (tonoplast), ER membranes, plastids, and cell [107,108,109,110,111,112,113], and ER-type calcium ATPases are found in the Golgi, ER, and endosomes [114,115,116]. CAX proteins are found in the plasma membrane and the vacuolar membrane (tonoplast) [88,106,117,118,119]. Ca2+ serves as the coordinator for cell wall and cytoplasm communication [120].
3. Plant Calcium Uptake by the Root System
The soil Ca2+ may not ensure its availability for the plant unless it has a vigorous system to take up available Ca2+. Ca2+ enters the root apoplast of young, unsuberized roots through the membrane channels. Ca2+ generally enters through the root tip, where the suberized endodermis (Casparian strip) [121] is absent and where the suberized endodermis is broken due to new root growth [56,122]. The Casparian strip is a barrier to apoplastic solute movement, while suberization blocks Ca2+ transfer in endodermal cells [56,122,123]. Thus, the root is the first gateway for Ca2+ entry from the soil solution into the plant system. Intact roots with profuse new growth (volume and size) may exploit more soil volume, which favors higher Ca2+ uptake. Impaired root systems due to hard soil, waterlogged conditions, pathogen attacks, damage by insects and animals, and nematode infestation can reduce Ca2+ uptake.
4. Calcium Uptake Through Foliar Application
Foliar application of Ca2+ can increase leaf and fruit Ca2+ and reduce deficiency disorders. Foliar application of Ca2+ strengthens cell walls [124], and tomato leaves absorb 90% of foliar calcium chloride application [125]. Foliar application of Ca2+ at 0.9% increases fruit Ca2+ and decreases BER in tomatoes [126]. Foliar application of 150% of the recommended dose of calcium nitrate decreases cabbage tip burn [127]. Tip burn of two mini Chinese cabbages (QYH and HN) disappears due to the applications of 4–6 mmol·L−1 Ca2+ [128]. Foliar spray of eggshell solutions increases the Ca2+ content on the aerial part of tomato plants and decreases BER in fruits [129]. Calcium foliar application increases defense mechanisms against diseases [124]. Spraying with CaCl2 or Ca(NO3)2 controls blackheart, a Ca2+ deficiency disorder in celery [130,131]. Ca2+-spraying in the form of ‘Calciogreen’ or ‘CaCl2’ or with other calcium formulations either decreases or effectively controls deficiency disorders, including BER in tomato and bell pepper [132,133,134]. However, Ca2+ has minimal mobility within the phloem [135], and thus foliar Ca2+-application may not improve fruit-Ca2+ status [136]. Therefore, foliar absorption and Ca2+ transport are yet to be clarified [125].
5. Calcium Uptake Through the Fruit
One of the leading causes of Ca2+ application is to increase fruit Ca2+ content to reduce deficiency disorders. Soil or foliar applications, decreasing competition at the root zone, and ameliorating plant and environmental issues are all indirect approaches to increasing fruit Ca2+ content. Applying Ca2+ directly to the fruit surface can be another approach. However, it is complex to maintain sufficient fruit Ca2+ [137]. It is noteworthy to recall a four-decade-old comment about the BER complexity—‘‘the number of possible interactions that can affect Ca2+ uptake and distribution is so great that in the near future, we are unlikely to see the development of cultural practices that will eliminate Ca2+ deficiency, without a direct application of Ca2+ to the susceptible organ’’ [138]. Ca2+ applied to the apple fruit surface may penetrate the fruit epidermis [139,140], preferably at 40–50 days after full bloom [141], probably through trichomes and stomata. Young apple fruitlets take up less exogenous Ca2+ than mature fruit [142]; penetration of Ca2+ into the fruit depends on the retention time of the solution on the fruit surface and the concentration of the applied solution [141]. Applying 1.33 g calcium nitrate/polybag decreases BER in tomatoes [143]. Ca2+ at 100 mg L−1 reduces BER incidence in susceptible tomato accession (Solanum lycopersicum lycopersicum) by 5–11% [41]. Bone meal decreases BER in tomato ‘Cobra F1’ by increasing fruit Ca2+ content [144]. Ca2+ application improves the quality of cucumber, Ca2+ content in leaves and peels in pomegranate, and reduces phenolics and flavonoids in cherries [145,146,147,148]. Instead of fruit calcium (pedicel, proximal half, and distal half) content, Ca2+/K+ and their relative % in the pedicel are associated with the BER in peppers [148]. BER-resistant peppers express defense responses against calcium-deficient stressors [148]. However, direct Ca2+ application can decrease BER symptoms but cannot control the disorder completely; moreover, exogenous Ca2+ may leak out easily [149,150]. Detection of BER at early stages may lead to appropriate control measures to minimize postharvest losses, as the success rate of detecting BER by fluorescence and image analysis at this stage is above 86% [151].
6. Calcium Translocations
6.1. Calcium Translocations Within the Plant
The solution Ca2+ enters the root apoplast through membrane channels and moves through the xylem following apoplastic or symplastic routes [20,21]. In the apoplastic route, Ca2+ travels following water potential gradients, and in the symplastic route, Ca2+ moves through plasmodesmata [54,152]. Though the apoplastic route is considered the principal pathway of Ca2+ translocation, there are debates about it [152,153]. The Casparian strip along this route restricts further Ca2+ movement to the xylem. Once Ca2+ is in the xylem sap, its further movement within the shoot is directed by the xylem flow of water, xylem water potential [154,155,156], and cation exchange capacity (CEC) of the xylem cell wall. The canopy’s transpiration force and plant growth drive the xylem water flow. Along the flow of xylem water, Ca2+ is transported to the fruits and storage tissues.
6.2. Leaf or Fruit?
What determines whether the xylem sap containing Ca2+ will move toward the leaf or the fruit? It is the transpiration force and organ growth [154,155,156]. Transpiration from leaf and fruit surfaces triggers water flow toward them. The leaf, being a strong transpiring organ and a higher mass accumulator than fruit [55,154], results in most of the Ca2+ being deposited in the leaf. Fruit sap uptake can be facilitated by either reducing leaf transpiration or enhancing stomatal closure, leading to better Ca2+ uptake and thus minimizing BER [25,154,155,157]. Ca2+ content does not decrease in the leaves by being phloem-immobile; instead, it may increase due to dehydration during senescence [158,159].
6.3. Calcium Translocation Within the Fruit
Xylem sap Ca2+ enters the fruit through the peduncle and is distributed within the fruit based mainly on the xylem network. Being phloem-immobile, Ca2+ accumulation within the fruit depends on fruit transpiration. Fruit transpiration rate is lower than that of leaves, resulting in a low Ca2+ supply to the fruit. However, phloem may contribute to the transport of Ca2+ as it contains higher Ca2+ in the pedicel, and xylem functionality is lost at maturity [160]. High N causes fruit expansion, leading to reduced Ca2+ availability to fruit through dilution [34,161], resulting in BER. Though high [K+] and [Mg2+] may replace plasma membrane Ca2+, they cannot substitute the function of Ca2+ in the membrane [162,163], which may also lead to loss of membrane permeability and make the fruit susceptible to Ca2+ deficiency disorders. Compared to the total fruit Ca2+, the relative Ca2+ contents, such as the ratios N/Ca2+, K+/Ca2+, Mg2+/Ca2+, (K+ + Mg2+)/Ca2+, are better predictors of Ca2+ deficiency disorders such as BER [164,165,166]. Fruits can also regulate Ca2+ translocation by altering aquaporin activity and cell wall properties [153].
7. Calcium Compartmentalization Within the Cell
Partitioning of Ca2+ within cellular compartments is the final step of Ca2+ regulation. Fruit sensitivity to Ca2+ deficiency disorders is triggered by modifying cellular Ca2+-partitioning [42,167]. Ca2+ compartmentalization is regulated by the capacity of binding Ca2+ to the cell wall and the presence of Ca2+ channels, ATPases, and exchangers in the membranes of organelles [168]. The cellular plasma membrane is located between the apoplast and cytosol. Apoplastic Ca2+ includes water-soluble plasma membrane and cell wall Ca2+ [55]. Water-soluble Ca2+ stabilizes the plasma membrane by binding phosphate and carboxylate from phospholipids and proteins, respectively, and keeps it functional [50,69]. A certain threshold of water-soluble Ca2+ is always maintained in the apoplast to avoid membrane damage and leakiness [69,169], and replacement of Ca2+ with other ions can damage the membrane [64,170]. Cell wall Ca2+ binds with the pectin matrix to obtain the rigidity of the cell wall. Newly synthesized pectic polysaccharides are highly methyl-esterified. Removal of a methyl group by pectin methyl esterases (PMEs) creates a carboxylate group with which Ca2+ binds strongly [60,171].
The Ca2+ concentration of organelles varies greatly, and the cell maintains a certain Ca2+ threshold level for its function. The vacuole is the biggest store of Ca2+, maintaining 1–10 mM Ca2+ [21,172]. Other Ca2+ storage sites are the ER (1–5 mM) [173], chloroplast (0.1–10 µM) [174,175], mitochondria (0.2–1.2 µM) [174,175], Golgi apparatus (0.7 µM), nucleus (0.1–0.2 µM) [176], and cytosol (100–200 nM) [84] (Figure 1).
8. Calcium Deficiency Disorders
Ca2+ deficiency leads to physiological disorders like BER in tomatoes, peppers, watermelon, eggplant, squash, and apples [177,178,179] (Figure 4). It weakens cell walls, leading to disease and pest susceptibility and cell death [180,181]. Tip burn is another relevant physiological disorder. It is characterized by necrosis of rapidly growing young leaves in cabbage, Chinese cabbage, Brussels sprouts, lettuce, chervil, chicory, escarole, onion, fennel, and potatoes [24,26,182,183,184,185] (Table 1). Other disorders include bitter pit—the development of brown/black depressed spots on the blossom end of fruit—in apples [21,138,164]; blackheart—collapsing of young leaf tissue that turns black, usually at the center (heart) of the plant—in celery [130,186]; brown heart—necrosis of the tip of young leaves that covers the entire leaf successively—in leafy vegetables [21]; empty pod—poor or no development of seed kernel resulting in an empty pod/shell—in peanut [21]; and fruit cracking—splitting of skin or cuticle—in apple, tomatoes, and cherry [21] (Table 1).

Figure 4.
Blossom-end rot (BER) in tomato and bell pepper fruits. Blossom-end rot affects the distal end of the fruit and occurs during the first few weeks of fruit growth. BER is considered a calcium deficiency disorder that other environmental conditions can exacerbate. In advanced stages, dry, sunken, black/brown symptoms appear that can cover the entire blossom part of the fruit. Upper panel: BER in tomato; middle panel: development of BER in bell pepper while on the plant; and bottom panel: different stages of development of BER symptoms in bell pepper (from left to right: very low, low, moderate, high, and very high BER). Photos are from the first author’s experiments conducted in Athens, GA, USA, from December 2015 to April 2018.

Table 1.
Calcium deficiency disorders of crops.
Table 1.
Calcium deficiency disorders of crops.
| Deficiency Symptoms | Crops | Description | Reference |
|---|---|---|---|
| Blossom-end rot | Bell pepper, tomato, watermelon, eggplant, squash | Blossom-end rot in fruit and vegetables develops dry, brown/black, sunken spots, leading to rotting that may cover most of the fruit. | [21,23,25,177,187,188] |
| Blackheart | Celery | Young leaf tissue collapses and turns black, usually at the center (heart) of celery. | [132,186] |
| Bitter pit | Apple | Development of brown/black depressed spots on the fruits. | [21,138,164] |
| Empty pod | Peanut | Poor or no development of the seed kernel, resulting in the empty pod/shell of the peanut. | [21] |
| Tip burn | Cabbage, Chinese cabbage, other cabbages | The tip of rapidly growing young leaves becomes necrotic. | [24,26,183] |
| Brussels sprouts, lettuce | Necrosis of the tip of rapidly growing young leaves. | [24,184,185] | |
| Chervil | The tip of rapidly growing young leaves becomes necrotic. | [24,182] | |
| Chicory, escarole, onion, fennel, potatoes | Necrosis of the tip of rapidly growing young leaves. | [24] | |
| Brown heart | Leafy vegetables | Necrosis of the tip of young leaves that covers the entire leaf successively. | [21] |
| Fruit cracking | Tomato, cherry, apple | Splitting of skin or cuticle. | [21] |
In addition to deficiency, Ca2+ toxicity is reported in crop plants, such as gold spot/yellowish flecks—tiny flecks that develop around the calyx and shoulder of fruit—in tomato [189], and Ca2+ toxicity halts germination and growth of vegetables [24].
8.1. Genesis of Blossom-End Rot Development
Although Ca2+ is the most abundant mineral (3.64%) in the Earth’s crust, it may not be available to plants [190]. Among the three forms (bound, exchangeable, and soluble) of soil Ca2+, soluble Ca2+ is readily available; availability of exchangeable Ca2+ depends on pH, and bound Ca2+ remains unavailable. Soil solution Ca2+ depends on the weathering of parent rock material, the mineralization of primary minerals and soil organic matter, soil pH, fertilization, and diffusion along the gradient. In acidic soil (pH < 5.0), Ca2+ can be leached out with rainwater, and in alkaline soil (pH > 7.5), it can be precipitated with phosphorus, resulting in less availability. Ca2+ availability also depends on the presence of other cations, salinity, root growth, anoxia, and root zone temperature [191]. Ca2+ can be supplied to the plants by applying Ca2+ in the soil and on leaf and fruit surfaces.
Cations decrease, while anions increase, Ca2+ uptake by the plants. The presence of cations such as K+, manganese (Mn2+), magnesium (Mg2+), ammonium (NH4+), Al3+, and Na+ antagonize [25,35,192], and anions such as nitrate (NO3−) and phosphate (PO43−) synergize Ca2+ uptake by the plant root system. Although soil contains about 10 times more Ca2+ than K+, the uptake of Ca2+ is lower than that of K+ [69], which might be due to the higher valency of Ca2+ [11]. NH4+ competes with Ca2+ to be taken up by plants. Moreover, high N fertilization promotes shoot growth, which diverts absorbed Ca2+ to the leaf instead of the fruit because of the higher leaf transpiration rate than the fruit [55,193]. At high soil calcium availability, fruit Ca2+ deficiency disorder may not appear. However, at low calcium availability, BER can appear due to depletion of apoplastic Ca2+ content. BER may also occur at high calcium availability due to improper Ca2+ compartmentalization through disruptions to calcium transport and signaling pathways (Figure 5).
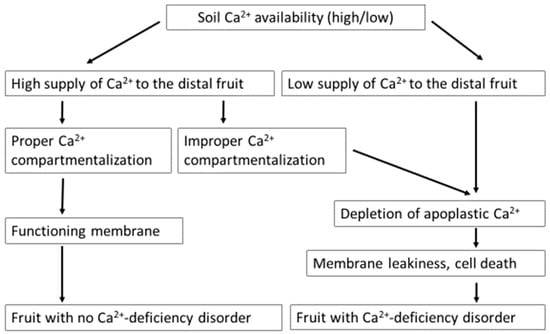
Figure 5.
Calcium availability affects the development of the calcium deficiency disorder blossom-end rot (BER). At high soil calcium availability, fruit Ca2+ deficiency disorder may not appear. However, at low calcium availability, the deficiency disorder appears through depletion of apoplastic calcium and subsequent membrane leakiness. However, BER may occur at high calcium availability due to improper calcium compartmentalization.
8.2. Incidence of BER Based on Variety, Season, and Truss
The incidence of BER may vary from variety to variety, as reported from cultivated peppers and tomatoes. Ca2+ deficiency, differential fruit growth rate, and variation in xylem development are considered the basis of this variability [194]. Ca2+-efficient cultivars absorbed Ca2+ more efficiently than Ca2+-inefficient cultivars when the availability of Ca2+ is low on the substrate. Thus, Ca2+-efficient cultivars could be selected against BER, but the difficulty is that they yield poorly. However, no significant difference in BER susceptibility has been found between efficient and inefficient varieties [194]. Tomato varieties ‘Calypso’ and ‘Spectra’ showed higher incidences of BER than ‘Counter’ [195]; ‘Petomech II’ higher than ‘IPA-L’ [196]; ‘Celebrity’ higher than ‘Rutgers,’ ‘Mountain Pride’, and ‘Mountain Spring’ [197]; STEP 158 (breeding line) higher than ‘Rutgers’ and ‘Doublerich’ [198], and ‘Boludo’ higher than ‘Daniela’ [199]. Elongated tomato varieties are more susceptible to BER [200]. BER never occurs in small-fruit and wild tomato cultivars [55]. Lack or excess of minerals may cause deficiency disorders [201,202]. A low fruit apoplastic calcium, which results in leaky cell membranes, leads to BER development [203]. BER occurs in tomatoes if the calcium concentrations of the lateral ends of green fruits are <0.2 µmol g−1 [204]. The fruit growth rate of the BER-susceptible tomato accession (Solanum lycopersicum lycopersicum) is higher than the BER-resistant one (Solanum lycopersicum cerasiforme); however, it is not clear whether this may contribute to BER development [191]. Phytohormones may also affect BER incidence. Foliar spray of ABA reduces BER incidence by increasing calcium availability [39,204], and gibberellins increase it through increasing oxidative stresses in plants [205]. ABA increases, and GA decreases calcium accumulation [148]. Thus, applying ABA or GA inhibitors (paclobutrazol and prohexadione-Ca2+) may decrease BER in peppers [148]. Transport and homeostasis of calcium ions are crucial for preventing BER in peppers [148].
Pepper varieties with larger final fruit sizes and faster growth rates, such as ‘Marconi’ and ‘J27’, had higher sensitivity to BER than ‘Jerid’, which produces a smaller final fruit size [206]. No incidence of BER has been reported from wild-type tomatoes (small fruit size). This observation indicates that BER might be associated with larger fruits under favorable growth conditions that influence rapid fruit growth. ‘Marmande’ tomatoes had a higher BER incidence than cherry, cocktail, or round tomatoes [207]. Pygmy fruits, having no rapid growth phase, usually do not develop BER [208]. BER-affected tomatoes ripen earlier and are smaller than healthy fruit [209].
The onset of BER can vary based on trusses and seasons. Frequent incidence of BER was observed with the first truss [37], followed by a subsequent decrease [32,210,211], or increase [211,212]. Basal fruits of a truss had more severe BER than the others [29]. Based on seasons, BER incidence can increase or decrease from the first to the upper trusses [156,195].
9. Control of BER
BER, a critical physiological disorder for several vegetables, has been studied for over a century, although the mechanism is unclear. Most researchers agree that this condition is a Ca2+-deficiency disorder, and supplying sufficient Ca2+ to the fruit may prevent the symptom development. However, the Ca2+ route from soil to the target organ, i.e., fruit, is not straightforward. Many factors act on the journey of Ca2+ from the soil to the fruit. Moreover, it is not the effect of a single factor, rather a combined effect of one or more factors [25], such as low soluble soil Ca2+, high Mg2+, NH4+, and K+ concentrations, high salinity, inconsistent soil moisture (high, low, or fluctuating), rapid fruit growth rate, poor xylem network toward the blossom end of the fruit, high temperature, and high or low transpiration of the target organ [27,69,210,213,214,215,216,217]. Cultural management approaches should favor Ca2+ translocation to the fruit to control BER. However, the transport of Ca2+ to the fruit may not ensure BER control, as cellular Ca2+ partitioning is the final and most crucial control level for this disorder [168]. Reports show severe BER incidence in the distal part of the fruit despite high Ca2+-concentration [215]. Moreover, no BER symptoms occurred when the Ca2+ supply was low in the case of slow-growing plants [216]. The relatively high Ca2+ concentration in the BER-affected fruit might be explained through abnormal cellular partitioning, such as transport of abundant Ca2+ to the vacuoles, which may cause Ca2+ deficiency in other subcellular organelles and may develop BER. Moreover, an unanticipated change in cellular Ca2+ concentration response to environmental stimuli or hormonal effects may cause localized Ca2+ deficiency, leading to BER development [168].
BER occurs due to abiotic stresses, high temperature, drought, salinity, water logging, higher transpiration, production of ROS, and low availability of ascorbic acid [200,218]. Control of BER is complicated due to the involvement of many changing and unpredictable abiotic factors, and proper management can only reduce the incidence rate [37]. Spraying calcium on the fruit during the developmental stage reduces BER incidence [55]. Spraying should be started at the early stage of the fruit and, of course, before the onset of BER, and it needs to be continued for the entire development stage. However, spraying may not be effective [200]. Balanced fertilizer and avoiding vigorous foliage growth may help reduce BER [200]. Shade net may also reduce BER incidence [219,220,221,222,223]. However, the incidence of BER in tomatoes is positively correlated with fruit plant−1, K content, root relative water content, firmness, vitamin C, titratable acidity, and peroxidase, and negatively correlated with calcium content [40]. A high calcium concentration (e.g., 20 mmol·L−1) decreases growth [plant height, diameter, biomass production (leaf, stem, root, and total)], physiological attributes [photosynthesis, stomatal conductance, transpiration, and chlorophyll content (chlorophyll a; chlorophyll b)], enzyme levels (superoxide dismutase, catalase, and peroxidase), and water use efficiency in poplar seedlings [50]. Therefore, correctly understanding calcium nutrition helps reduce crop cultivation costs, minimize environmental pollution, and boost crop production [224].
Cultural and physiological crop management that ensures Ca2+ transportation to the fruit and appropriate cellular distribution may reduce the incidence of BER. The selection of BER-resistant and Ca2+-efficient cultivars may also help to reduce BER incidence. Controlling or skipping calcium deficiency disorders may include modifications of watering, light, temperature, transpiration, and application of mulching and growth regulators. Using resistant varieties and customization of planting and harvesting (early planting and harvesting) may help skip calcium deficiency disorders [24]. Understanding the molecular mechanisms of BER may help in better managing the disorder [225]. With the combination of appropriate management practices and BER-resistant cultivars, the incidence of this disorder may be eliminated in the future [55].
10. Climate Change and Calcium Availability
Climate change, characterized by temperature extremes, prolonged drought, elevated atmospheric CO2 levels, and episodes of heavy precipitation (Figure 6), poses significant challenges to crop productivity and quality. Understanding calcium’s role in enhancing crop resilience to such environmental stressors is increasingly vital [226]. Elevated temperatures increase leaf transpiration relative to fruit transpiration, thereby limiting calcium transport to fruit tissues and promoting the onset of blossom-end rot (BER) [223]. Additionally, high air and root-zone temperatures impair calcium uptake and exacerbate deficiency-related disorders [227]. Elevated temperatures also enhance the incidence of plant diseases and pest infestations, further restricting nutrient availability.
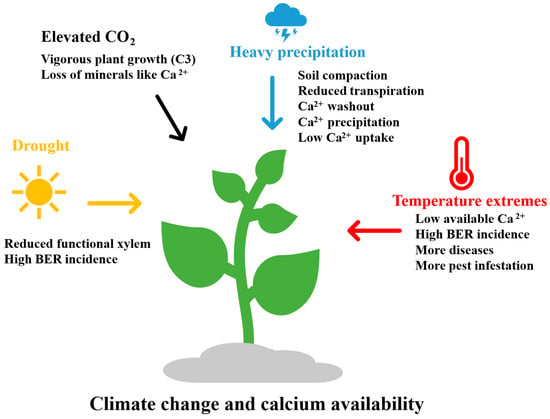
Figure 6.
A diagram illustrating how climate change affects calcium availability in plants.
Drought stress limits xylem functionality, diminishing calcium translocation and contributing to BER development [228]. Since calcium is primarily transported via the xylem in the transpiration stream, water scarcity significantly impairs calcium delivery to actively growing tissues such as fruits, as observed in tomatoes and bell peppers [220,222].
Conversely, excessive precipitation can compact soil, reduce transpiration, leach soluble calcium, and induce calcium precipitation with phosphates—factors that collectively lower calcium bioavailability and increase the prevalence of disorders like BER [229]. Waterlogged or compacted soils, damaged root systems (caused by pathogens or insects), and inconsistent soil moisture regimes all further impede calcium uptake. Elevated atmospheric CO2 can enhance the growth of C3 plants, yet this often coincides with reduced mineral concentrations in plant tissues [230]. Enhanced vegetative growth may dilute essential minerals, including calcium, magnesium, iron, and zinc. It is estimated that elevated CO2 levels can reduce mineral content in food crops by 5% to 15% [231].
11. Recent Advances and Future Directions
Calcium (Ca2+) plays a central role in plant physiology, acting both as a structural nutrient critical for cell wall integrity and membrane stabilization and as a versatile secondary messenger in a wide range of physiological processes [50]. Recent progress in plant science has introduced promising approaches to enhance calcium delivery, engineer calcium transporter genes, and monitor intracellular calcium dynamics with high spatial and temporal resolution. Key emerging areas include: (1) nanotechnology-based calcium delivery systems, (2) CRISPR-mediated modification of calcium transporter genes, and (3) advanced imaging and biosensor tools for real-time calcium monitoring.
11.1. Nanotechnology-Based Calcium Delivery Systems
Conventional calcium fertilization methods often face limitations, such as low solubility, leaching losses, and inefficient uptake, particularly in calcareous soils or under abiotic stress conditions. Nanotechnology offers a novel solution by enabling the formulation of nanoscale calcium carriers that enhance solubility, mobility, and targeted delivery to plant tissues [224]. Calcium-based nanoparticles (Ca-NPs), including calcium carbonate, calcium phosphate, and hydroxyapatite forms, have demonstrated improved foliar and root uptake due to their high surface area and controlled-release properties. For example, foliar application of calcium phosphate nanoparticles has been shown to enhance calcium mobility and mitigate blossom-end rot in tomato by improving calcium allocation to distal fruit tissues [232]. Nanoencapsulation—embedding calcium salts in polymeric or lipid nanocarriers—further improves nutrient bioavailability and ensures sustained release. These carriers protect calcium ions from premature precipitation or interaction with soil constituents, thereby enhancing availability in the rhizosphere or phyllosphere [233]. In addition to nutrient delivery, Ca-NPs may influence stress signaling by modulating calcium-dependent cascades, thus simultaneously correcting deficiencies and enhancing stress resilience [49,232]. Calcium transients, whether transient, sustained, or oscillatory, serve as signals that are decoded into physiological responses [81]. Despite promising results, the agricultural application of calcium phosphate nanomaterials remains in its infancy. Critical gaps persist in understanding the physiological and molecular effects, cost-effectiveness, and scalability of these approaches. Further research is needed to elucidate plant responses to these formulations and to tailor nanoparticle design for crop-specific needs [234]. For instance, nano-calcium carbonate at 200 mg L−1 significantly improved wheat seedling growth, enhanced photosynthetic pigments, antioxidant enzyme activity, Rubisco function, and stomatal conductance [235].
11.2. CRISPR-Mediated Gene Manipulation of Calcium Transporters
Calcium homeostasis in plants is orchestrated by a group of transporters, including Ca2+-ATPases, Ca2+/H+ exchangers, and cyclic nucleotide-gated channels (CNGCs) [236]. Genome editing via CRISPR-Cas9 has emerged as a tool for functional dissection and targeted manipulation of these transporters. For instance, the CRISPR-based knockout of the vacuolar Ca2+/H+ antiporter gene CAX1 in Arabidopsis altered cytosolic calcium levels and increased sensitivity to abiotic stress, underscoring its regulatory role in calcium partitioning and stress responses [50]. Furthermore, CRISPR-dCas9 systems enable precise transcriptional regulation without altering genomic sequences, allowing fine-tuned modulation of calcium influx via CNGC genes [237]. These strategies not only improve calcium-use efficiency but also provide a broader understanding of the regulatory networks underlying calcium signaling. Calcium transients in the cytosol ([Ca2+]cyt) are triggered by diverse stimuli, including light, touch, hormones, pathogens, and abiotic stresses like salinity and drought. These transients result from a dynamic interplay of calcium influx through channels and efflux via pumps, primarily mediated by Ca2+-ATPases and H+/Ca2+ antiporters. Calcium-binding proteins and calcium-dependent protein kinases decode these signals, regulating gene expression and conferring stress tolerance [238]. The use of CRISPR tools is accelerating insights into calcium-regulated stress responses. For example, their application in dissecting plant adaptation to drought and salinity has proven effective, particularly when combined with genomic-assisted breeding strategies [239]. Additionally, CRISPR systems have expanded into phytometabolic editing, targeting regulatory mechanisms such as upstream open reading frames (uORFs), post-translational modifications, and protein–protein interactions [240]. However, regulatory uncertainty remains a barrier to the widespread adoption of CRISPR-edited crops [241].
11.3. Imaging and Biosensors for Calcium Dynamics
Deciphering calcium signaling requires real-time monitoring of transient and spatially specific cytosolic Ca2+ changes [242]. Genetically encoded calcium indicators (GECIs) have enabled in vivo imaging of calcium dynamics with high resolution. These biosensors comprise calcium-binding domains fused to fluorescent proteins, which emit signals upon binding to calcium. Each physiological process—ranging from growth and differentiation to stress and defense—exhibits unique “calcium signatures” defined by amplitude, frequency, and duration. Understanding how transporter activity shapes these signatures remains a significant research challenge [242]. Recent biosensor advancements include red-shifted indicators for deep-tissue imaging, multiplex systems for co-detection of additional analytes, and microfluidic integration for real-time environmental monitoring. Beyond imaging, calcium acts as a signaling nexus across various physiological processes, including cell wall maintenance and development, enzyme regulation, and programmed cell death. GECI-based imaging has revealed refined subcellular dynamics and is now supported by artificial intelligence tools for analyzing complex datasets, as well as miniaturized systems for in-field applications [243]. Improvements such as calibration-free sensors, quantum dot incorporation, and bio-orthogonal activation will further advance live-cell calcium imaging. In Arabidopsis, GECIs have been utilized to monitor calcium responses to various stimuli, including mechanical stress, gravity, cold, osmotic changes, reactive oxygen species, and hormones [244].
11.4. Future Directions
Key areas requiring further investigation include: (1) limited understanding of long-distance calcium transport mechanisms [153]; (2) incomplete characterization of calcium transport and intracellular compartmentalization [20]; (3) poor resolution of how calcium signatures encode specific responses [245]; and (4) the complex interactions between calcium signaling and hormonal or nutritional networks [238]. To bridge these gaps, interdisciplinary research integrating molecular biology, systems imaging, nanotechnology, and agronomic studies is essential. Such integration is crucial for developing crops with optimized calcium nutrition and enhanced stress resilience.
12. Conclusions
Calcium is an essential element for plant growth, development, and productivity. While most soils worldwide are not typically deficient in Ca2+, calcium-deficiency disorders in crops are prevalent and lead to substantial yield losses in several horticultural crops. Among the Ca2+-deficiency disorders, BER is widely prevalent worldwide. The complex route of Ca2+ from soil to the appropriate cellular compartments, such as the cytosol, depends on multiple factors, e.g., soil (moisture availability, the competition of Ca2+ with other cations, pH, anoxia, and salinity), plant [genotypes, growth habit (dwarf, tall), xylem network, root and shoot growth, and yield], and environment [temperature (air and root zone), relative humidity, vapor pressure deficit, and transpiration, which renders it difficult to control BER. Moreover, the cellular Ca2+ compartmentalization, particularly in the vacuoles, depletes cytosolic Ca2+ levels and may disintegrate the plasma membrane, leading to BER development despite high Ca2+ content in the blossom end of the fruit. Therefore, possible ways of minimizing and controlling BER include (i) an integrated approach that ameliorates soil, plant, and environmental factors toward supplying sufficient Ca2+ into the cells; (ii) appropriate cultural and physiological management of crops; (iii) a favorable environment; and (iv) BER-resistant and Ca2+-efficient cultivars. Other approaches, such as agronomic, physiological, breeding, and molecular methods, may also contribute to minimizing BER. Although calcium is well studied, the molecular identity and regulation of many calcium channels, pumps, and sensors remain uncharacterized. The relation between calcium as a nutrient and secondary messenger is also poorly understood. Therefore, future investigations should explore how calcium signatures encode specific responses and how the complex interactions between calcium signaling and nutritional or hormonal networks interplay.
Author Contributions
M.Y.K.: Conceptualization; writing—original draft, review and editing; J.C.D.-P.: writing—review and editing, supervision, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This review is part of the first author’s Ph.D. program, supported by the United States Agency for International Development as part of the Feed the Future initiative under the CGIAR Fund, award number BFS-G-11-00002, and the predecessor fund, the Food Security and Crisis Mitigation II grant, award number EEM-G-00-04-00013.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Keiser, J.R.; Mullen, R.E. Calcium and relative humidity effects on soybean seed nutrition and seed quality. Crop Sci. 1993, 33, 1345–1349. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Wimmer, R. Tansley Review No. 104 Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Zartdinova, R.; Nikitin, A. Calcium in the life cycle of legume root nodules. Indian J. Microbiol. 2023, 63, 410–420. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology Online Fifth Edition [WWW Document]. 2011. Available online: http://5e.plantphys.net (accessed on 31 May 2025).
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients A2-Marschner, Petra. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Saito, S.; Uozumi, N. Calcium-regulated phosphorylation systems controlling uptake and balance of plant nutrients. Front. Plant Sci. 2020, 11, 44. [Google Scholar] [CrossRef]
- Kim, M.C.; Chung, W.S.; Yun, D.J.; Cho, M.J. Calcium and calmodulin-mediated regulation of gene expression in plants. Mol. Plant 2009, 2, 13–21. [Google Scholar] [CrossRef]
- Feng, D.; Wang, X.; Gao, J.; Zhang, C.; Liu, H.; Liu, P.; Sun, X. Exogenous calcium: Its mechanisms and research advances involved in plant stress tolerance. Front. Plant Sci. 2023, 14, 1143963. [Google Scholar] [CrossRef]
- Hu, W.; Liu, J.; Liu, T.; Zhu, C.; Wu, F.; Jiang, C.; Wu, Q.; Chen, L.; Lu, H.; Shen, G.; et al. Exogenous calcium regulates the growth and development of Pinus massoniana detecting by physiological, proteomic, and calcium-related gene expression analysis. Plant Physiol. Biochem. 2023, 196, 1122–1136. [Google Scholar] [CrossRef]
- Hu, W.; Tian, S.B.; Di, Q.; Duan, S.H.; Dai, K. Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum Linn.) under drought stress. Photosynthetica 2018, 56, 1204–1211. [Google Scholar] [CrossRef]
- Sun, X.; Pan, B.; Wang, Y.; Xu, W.; Zhang, S. Exogenous calcium improved resistance to Botryosphaeria dothidea by increasing autophagy activity and salicylic acid level in pear. Mol. Plant-Microbe Interact. 2020, 33, 1150–1160. [Google Scholar] [CrossRef]
- Marschner, H. 8-Functions of Mineral Nutrients: Macronutrients. In Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Kulik, L.V.; Epel, B.; Lubitz, W.; Messinger, J. Electronic structure of the Mn4O x Ca cluster in the S0 and S2 States of the oxygen-evolving complex of photosystem II based on pulse 55Mn-ENDOR and EPR spectroscopy. J. Am. Chem. Soc. 2007, 129, 13421–13435. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Hetherington, A.M. Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 1998, 3, 32–36. [Google Scholar] [CrossRef]
- Simon, E.W. The symptoms of calcium deficiency in plants. New Phytol. 1978, 80, 1–15. [Google Scholar] [CrossRef]
- Alonso, M.T.; Torres-Vidal, P.; Calvo, B.; Rodriguez, C.; Delrio-Lorenzo, A.; Rojo-Ruiz, J.; Garcia-Sancho, J.; Patel, S. Use of aequorin-based indicators for monitoring Ca2+ in acidic organelles. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2023, 1870, 119481. [Google Scholar] [CrossRef]
- Vaughan, M.K. Arginine vasotocin and vertebrate reproduction. In The Pineal Gland; CRC Press: Boca Raton, FL, USA, 2020; pp. 125–163. [Google Scholar]
- Lecourieux, D.; Mazars, C.; Pauly, N.; Ranjeva, R.; Pugin, A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 2002, 14, 2627–2641. [Google Scholar] [CrossRef]
- Carafoli, E. Calcium–a universal carrier of biological signals: Delivered on 3 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005, 272, 1073–1089. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Morard, P.; Lacoste, L.; Silvestre, J. Effects of calcium deficiency on nutrient concentration of xylem sap of excised tomato plants. J. Plant Nutr. 2000, 23, 1051–1062. [Google Scholar] [CrossRef]
- Díaz-P’erez, J.C.; Hook, J.E. Plastic-mulched bell pepper (Capsicum annuum L.) plant growth and fruit yield and quality as influenced by irrigation rate and calcium fertilization. HortScience 2017, 52, 774–781. [Google Scholar] [CrossRef]
- Olle, M.; Bender, I. Causes and control of calcium deficiency disorders in vegetables: A review. J. Hortic. Sci. Biotechnol. 2009, 84, 577–584. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J. Blossom-end rot: A calcium deficiency. J. Plant Nutr. 2004, 27, 123–139. [Google Scholar] [CrossRef]
- Aloni, B.; Pashkar, T.; Libel, R. The possible involvement of gibberellins and calcium in tipburn of Chinese cabbage: Study of intact plants and detached leaves. Plant Growth Regul. 1986, 4, 3–11. [Google Scholar] [CrossRef]
- Geraldson, C.M. The use of calcium for control of blossom-end rot of Tomatoes. Proc. Fla. State Hortic. Soc. 1956, 68, 197–202. [Google Scholar]
- Adams, P.; El-Gizawy, A.M. Effect of calcium stress on the calcium status of tomatoes grown in NFT. Acta Hortic. 1988, 222, 15–22. [Google Scholar] [CrossRef]
- Banuelos, G.S.; Offermann, G.P.; Seim, E.C. High relative humidity promotes blossom-end rot on growing tomato fruit. HortScience 1985, 20, 894–895. [Google Scholar] [CrossRef]
- Barke, R.E. Absorption and translocation of calcium foliar sprays in relation to the incidence of blossom-end rot in tomatoes. Qld. J. Agric. Anim. Sci. 1968, 25, 179–197. [Google Scholar]
- Cho, I.H.; Lee, E.H.; Kim, T.Y.; Woo, Y.H.; Kwon, Y.S. Effects of high humidity on occurrence of tomato blossom-end rot. J. Korean Soc. Hortic. Sci. 1998, 39, 47–249. [Google Scholar]
- El-Gizawy, A.M.; Adams, P. Effect of temporary calcium stress on the calcium status of tomato fruit and leaves. Symp. Nutr. Grow. Tech. Plant Substrates 1985, 178, 37–44. [Google Scholar] [CrossRef]
- Gutteridge, C.G.; Bradfield, E.G. Root pressure stops blossom-end rot. Grower 1983, 100, 25–26. [Google Scholar]
- Saure, M.C. Blossom-end rot of tomato (Lycopersicon esculentum Mill.)—A calcium-or a stress-related disorder? Sci. Hortic. 2001, 90, 193–208. [Google Scholar] [CrossRef]
- Spurr, A.R. Anatomical aspects of blossom-end rot in the tomato with special reference to calcium nutrition. Hilgardia 1959, 28, 269–295. [Google Scholar] [CrossRef]
- Wada, T.; Ikeda, H.; Ikeda, M.; Furukawa, H. Effects of foliar application of calcium solutions on the incidence of blossom-end rot of tomato fruit. J. Jpn. Soc. Hortic. Sci. 1996, 65, 553–558. [Google Scholar] [CrossRef]
- Westerhout, J. Relation of fruit development to the incidence of blossom-end rot of tomatoes. Neth. J. Agric. Sci. 1962, 10, 223–234. [Google Scholar] [CrossRef]
- Wui, M.; Takano, T. Effect of Temperature and Concentration of Nutrient Solution during the Stage of the Fruit Development on the Incidence of Blossom-End Rot in Fruits of Tomato, Lycopersicon esculentum L. Environ. Control Biol. 1995, 33, 7–14. [Google Scholar] [CrossRef]
- De Freitas, S.T.; McElrone, A.J.; Shackel, K.A.; Mitcham, E.J. Calcium partitioning and allocation and blossom-end rot development in tomato plants in response to whole-plant and fruit-specific abscisic acid treatments. J. Exp. Bot. 2014, 65, 235–247. [Google Scholar] [CrossRef]
- Abdelkader, M.; Elkhawaga, F.A.; Suliman, A.A.; Puchkov, M.; Kuranova, K.N.; Mahmoud, M.H.; Abdelkader, M.F. Understanding the Regular Biological Mechanism of Susceptibility of Tomato Plants to Low Incidences of Blossom-End Rot. Horticulturae 2024, 10, 648. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Díaz-Pérez, J.C.; Doyle, J.W.; Berenguer, E.I.; van der Knaap, E.; Nambeesan, S.U. The Effect of Calcium Application and Irrigation on Development of Blossom-end Rot in Tomato. In Proceedings of the 2017 ASHS Annual Conference, Waikoloa, HI, USA, 19–22 September 2017. [Google Scholar]
- De Freitas, S.T.; Padda, M.; Wu, Q.; Park, S.; Mitcham, E.J. Dynamic alternations in cellular and molecular components during blossom-end rot development in tomatoes expressing sCAX1, a constitutively active Ca2+/H+ antiporter from Arabidopsis. Plant Physiol. 2011, 156, 844–855. [Google Scholar] [CrossRef]
- Gao, H.; Wu, X.; Zorrilla, C.; Vega, S.E.; Palta, J.P. Fractionating of calcium in tuber and leaf tissues explains the calcium deficiency symptoms in potato plant overexpressing CAX1. Front. Plant Sci. 2020, 10, 1793. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.K.; Pike, L.M.; Smith, R.H.; Hirschi, K.D. Increased calcium in carrots by expression of an Arabidopsis H+/Ca 2+ transporter. Mol. Breed. 2004, 14, 275–282. [Google Scholar] [CrossRef]
- Wu, Q.; Shigaki, T.; Han, J.S.; Kim, C.K.; Hirschi, K.D.; Park, S. Ectopic expression of a maize calreticulin mitigates calcium deficiency-like disorders in sCAX1-expressing tobacco and tomato. Plant Mol. Biol. 2012, 80, 609–619. [Google Scholar] [CrossRef]
- Zorrilla, C.; Schabow, J.E.; Chernov, V.; Palta, J.P. CAX1 vacuolar antiporter overexpression in potato results in calcium deficiency in leaves and tubers by sequestering calcium as calcium oxalate. Crop Sci. 2019, 59, 176–189. [Google Scholar] [CrossRef]
- Wyatt, S.E.; Tsou, P.L.; Robertson, D. Expression of the high capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res. 2002, 11, 1–10. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, H. Identification of the Causative Genes of Calcium Deficiency Disorders in Horticulture Crops: A Systematic Review. Agriculture 2021, 11, 906. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Ren, C.; Zhou, Y.; Zhu, W.; Zhang, S.; Liu, L. Calcium regulates growth and nutrient absorption in poplar seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef]
- Kitano, M.; Araki, T.; Yoshida, S.; Eguchi, H. Dependence of calcium uptake on water absorption and respiration in roots of tomato plants (Lycopersicon esculentum Mill.). Biotronics Environ. Control Environ. Biol. 1999, 28, 121–130. Available online: https://www.researchgate.net/publication/237406446 (accessed on 2 July 2025).
- Hewitt, E.J. The essential nutrient elements: Requirements and interactions. In Plant Physiology; Academic Press: Cambridge, MA, USA, 1963; pp. 137–360. [Google Scholar]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef]
- Ho, L.C.; White, P.J. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley & Sons: New York, NY, USA, 1972. [Google Scholar]
- Fuller, G.M.; Ellison, J.J.; McGill, M.; Sordahl, L.A.; Brinkley, B.R. Studies on the inhibitory role of calcium in the regulation of microtubule assembly in vitro and in vivo. In Microtubules and Microtubule Inhibitors; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1975; pp. 379–390. [Google Scholar]
- Ninkuu, V.; Yan, J.; Fu, Z.; Yang, T.; Ziemah, J.; Ullrich, M.S.; Kuhnert, N.; Zeng, H. Lignin and its pathway-associated phytoalexins modulate plant defense against fungi. J. Fungi 2022, 9, 52. [Google Scholar] [CrossRef]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef]
- Wehr, J.B.; Menzies, N.W.; Blamey, F.P.C. Inhibition of cell-wall autolysis and pectin degradation by cations. Plant Physiol. Biochem. 2004, 42, 485–492. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, A.; Plasencia, J.; Monribot-Villanueva, J.L.; Rodríguez-Haas, B.; Ruíz-May, E.; Guerrero-Analco, J.A.; Sánchez-Rangel, D. Virulence factors of the genus Fusarium with targets in plants. Microbiol. Res. 2023, 277, 127506. [Google Scholar] [CrossRef]
- Ralet, M.C.; Dronnet, V.; Buchholt, H.C.; Thibault, J.F. Enzymatically and chemically de-esterified lime pectins: Characterisation, polyelectrolyte behaviour and calcium binding properties. Carbohydr. Res. 2001, 336, 117–125. [Google Scholar] [CrossRef]
- Goulao, L.F.; Santos, J.; de Sousa, I.; Oliveira, C.M. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biol. Technol. 2007, 43, 307–318. [Google Scholar] [CrossRef]
- Massiot, P.; Baron, A.; Drilleau, J.F. Characterisation and enzymatic hydrolysis of cell-wall polysaccharides from different tissue zones of apple. Carbohydr. Polym. 1994, 25, 145–154. [Google Scholar] [CrossRef]
- Wallace, A.; Frolich, E.; Lunt, O.R. Calcium requirements of higher plants. Nature 1966, 209, 634. [Google Scholar] [CrossRef]
- Lahaye, P.A.; Epstein, E. Calcium and salt toleration by bean plants. Physiol. Plant. 1971, 25, 213–218. [Google Scholar] [CrossRef]
- Asher, C.J.; Edwards, D.G. Modern solution culture techniques. In Inorganic Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 1983; pp. 94–119. [Google Scholar] [CrossRef]
- Cramer, G.R. Sodium-calcium interactions under salinity stress. In Salinity: Environment-Plants-Molecules; Springer: Dordrecht, The Netherlands, 2002; pp. 205–227. [Google Scholar]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Kirkby, E.A.; Pilbeam, D.J. Calcium as a plant nutrient. Plant Cell Environ. 1984, 7, 397–405. [Google Scholar] [CrossRef]
- Lovegrove, A.; Hooley, R. Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci. 2000, 5, 102–110. [Google Scholar] [CrossRef]
- Bush, D.S.; Cornejo, M.J.; Huang, C.N.; Jones, R.L. Ca2+-stimulated secretion of α-amylase during development in barley aleurone protoplasts. Plant Physiol. 1986, 82, 566–574. [Google Scholar] [CrossRef]
- Luan, S.; Wang, C. Calcium signaling mechanisms across kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef]
- Naz, M.; Afzal, M.R.; Raza, M.A.; Pandey, S.; Qi, S.; Dai, Z.; Du, D. Calcium (Ca2+) signaling in plants: A plant stress perspective. South Afr. J. Bot. 2024, 169, 464–485. [Google Scholar] [CrossRef]
- Bu, Y.; Fu, W.; Chen, J.; Takano, T.; Liu, S. Description of AtCAX4 in response to abiotic stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 856. [Google Scholar] [CrossRef]
- Pirayesh, N.; Giridhar, M.; Khedher, A.B.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Shankar, A.; Nalini Chandran, A.K.; Sharma, M.; Jung, K.H.; Suprasanna, P.; Pandey, G.K. Emerging concepts of potassium homeostasis in plants. J. Exp. Bot. 2020, 71, 608–619. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, C.; Li, M.; Zhangzhong, L.; Song, P.; Li, Y. Interaction and adaptation of phosphorus fertilizer and calcium ion in drip irrigation systems: The perspective of emitter clogging. Agric. Water Manag. 2023, 282, 108269. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Narwal, P.; Kumari, P.; Kisku, A.V.; Gahlot, P.; Mittal, N.; Kumar, D. Calcium signaling in coordinating plant development, circadian oscillations and environmental stress responses in plants. Environ. Exp. Bot. 2022, 201, 104935. [Google Scholar] [CrossRef]
- Ghosh, S.; Bheri, M.; Pandey, G.K. Delineating calcium signaling machinery in plants: Tapping the potential through functional genomics. Curr. Genom. 2021, 22, 404–439. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Lim, D.; Carafoli, E. Calcium and signal transduction. Biochem. Mol. Biol. Educ. 2008, 36, 175–180. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Paliyath, G.; Thompson, J.E. Calcium-and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987, 83, 63–68. [Google Scholar] [CrossRef]
- Rudd, J.J.; Franklin-Tong, V.E. Calcium signaling in plants. Cell. Mol. Life Sci. 1999, 55, 214–232. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- Putney, J.W., Jr. Calcium signaling: Up, down, up, down.... What’s the point? Science 1998, 279, 191–192. [Google Scholar] [CrossRef]
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and calcineurin B–like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14 (Suppl. S1), S389–S400. [Google Scholar] [CrossRef]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14 (Suppl. S1), S401–S417. [Google Scholar] [CrossRef]
- Bouché, N.; Scharlat, A.; Snedden, W.; Bouchez, D.; Fromm, H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J. Biol. Chem. 2002, 277, 21851–21861. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef]
- Laohavisit, A.; Davies, J.M. Multifunctional annexins. Plant Sci. 2009, 177, 532–539. [Google Scholar] [CrossRef]
- Miedema, H.; Bothwell, J.H.; Brownlee, C.; Davies, J.M. Calcium uptake by plant cells–channels and pumps acting in concert. Trends Plant Sci. 2001, 6, 514–519. [Google Scholar] [CrossRef]
- White, P.J. Calcium channels in higher plants. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1465, 171–189. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Demidchik, V.; Nichols, C.; Davies, J.M. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1564, 299–309. [Google Scholar] [CrossRef]
- White, P.J. Depolarization-activated calcium channels shape the calcium signatures induced by low-temperature stress. New Phytol. 2009, 183, 6–8. Available online: http://www.jstor.org/stable/40302000 (accessed on 2 July 2025). [CrossRef]
- Miedema, H.; Demidchik, V.; Véry, A.A.; Bothwell, J.H.; Brownlee, C.; Davies, J.M. Two voltage-dependent calcium channels co-exist in the apical plasma membrane of Arabidopsis thaliana root hairs. New Phytol. 2008, 179, 378–385. [Google Scholar] [CrossRef]
- Amtmann, A.; Blatt, M.R. Regulation of macronutrient transport. New Phytol. 2009, 181, 35. [Google Scholar] [CrossRef]
- Kim, M.D.; Kim, Y.H.; Kwon, S.Y.; Yun, D.J.; Kwak, S.S.; Lee, H.S. Enhanced tolerance to methyl viologen-induced oxidative stress and high temperature in transgenic potato plants overexpressing the CuZnSOD, APX and NDPK2 genes. Physiol. Plant. 2010, 140, 153–162. [Google Scholar] [CrossRef]
- Moran, N. Osmoregulation of leaf motor cells. FEBS Lett. 2007, 581, 2337–2347. [Google Scholar] [CrossRef]
- Harper, J.F. Dissecting calcium oscillators in plant cells. Trends Plant Sci. 2001, 6, 395–397. [Google Scholar] [CrossRef]
- Klüsener, B.; Boheim, G.; Liss, H.; Engelberth, J.; Weiler, E.W. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 1995, 14, 2708–2714. [Google Scholar] [CrossRef]
- Hirschi, K.D. Vacuolar H+/Ca2+ transport: Who’s directing the traffic? Trends Plant Sci. 2001, 6, 100–104. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, F.; Hong, B.; Young, J.C.; Sussman, M.R.; Harper, J.F.; Sze, H. An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol. 2002, 130, 128–137. [Google Scholar] [CrossRef]
- Blatt, M.R. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 221–241. [Google Scholar] [CrossRef]
- Ritchie, S.M.; Swanson, S.J.; Gilroy, S. From common signalling components to cell-specific responses: Insights from the cereal aleurone. Physiol. Plant. 2002, 115, 342–351. [Google Scholar] [CrossRef]
- Evans, D.E.; Williams, L.E. P-type calcium ATPases in higher plants–biochemical, molecular and functional properties. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1998, 1376, 1–25. [Google Scholar] [CrossRef]
- Huang, L.; Berkelman, T.; Franklin, A.E.; Hoffman, N.E. Characterization of a gene encoding a Ca (2+)-ATPase-like protein in the plastid envelope. Proc. Natl. Acad. Sci. USA 1993, 90, 10066–10070. [Google Scholar] [CrossRef]
- Harper, J.F.; Hong, B.; Hwang, I.; Guo, H.Q.; Stoddard, R.; Huang, J.F.; Palmgren, M.G.; Sze, H. A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J. Biol. Chem. 1998, 273, 1099–1106. [Google Scholar] [CrossRef]
- Bonza, M.C.; Morandini, P.; Luoni, L.; Geisler, M.; Palmgren, M.G.; De Michelis, M.I. At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol. 2000, 123, 1495–1506. [Google Scholar] [CrossRef]
- George, L.; Romanowsky, S.M.; Harper, J.F.; Sharrock, R.A. The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol. 2008, 146, 716. [Google Scholar] [CrossRef]
- Schiøtt, M.; Romanowsky, S.M.; Bækgaard, L.; Jakobsen, M.K.; Palmgren, M.G.; Harper, J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA 2004, 101, 9502–9507. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Frangne, N.; Gomes, E.; Martinoia, E.; Palmgren, M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, B.G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [CrossRef]
- Liang, F.; Cunningham, K.W.; Harper, J.F.; Sze, H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 8579–8584. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.F.; Doherty, M.L.; López-Marqués, R.L.; Weimar, T.; Dupree, P.; Palmgren, M.G.; Pittman, J.K.; Williams, L.E. ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol. 2008, 146, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chanroj, S.; Wu, Z.; Romanowsky, S.M.; Harper, J.F.; Sze, H. A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol. 2008, 147, 1675–1689. [Google Scholar] [CrossRef]
- Cheng, N.H.; Pittman, J.K.; Shigaki, T.; Lachmansingh, J.; LeClere, S.; Lahner, B.; Salt, D.E.; Hirschi, K.D. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005, 138, 2048–2060. [Google Scholar] [CrossRef]
- Hirschi, K.D.; Korenkov, V.D.; Wilganowski, N.L.; Wagner, G.J. Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 2000, 124, 125–134. [Google Scholar] [CrossRef]
- Luo, G.Z.; Wang, H.W.; Huang, J.; Tian, A.G.; Wang, Y.J.; Zhang, J.S.; Chen, S.Y. A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol. Biol. 2005, 59, 809–820. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. Calcium at the cell wall-cytoplast interface. J. Integr. Plant Biol. 2010, 52, 147–160. [Google Scholar] [CrossRef]
- De Kreij, C. Interactive effects of air humidity, calcium and phosphate on blossom-end rot, leaf deformation, production and nutrient contents of tomato. J. Plant Nutr. 1996, 19, 361–377. [Google Scholar] [CrossRef]
- Clarkson, D.T. Roots and the delivery of solutes to the xylem. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 1993, 341, 5–17. [Google Scholar] [CrossRef]
- Moore, C.A.; Bowen, H.C.; Scrase-Field, S.; Knight, M.R.; White, P.J. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J. 2002, 30, 457–465. [Google Scholar] [CrossRef]
- Park, S.; Waterland, N.L. Evaluation of calcium application methods on delaying plant wilting under water deficit in bedding plants. Agronomy 2021, 11, 1383. [Google Scholar] [CrossRef]
- Santos, E.; Montanha, G.S.; Agostinho, L.F.; Polezi, S.; Marques, J.P.R.; de Carvalho, H.W.P. Foliar calcium absorption by tomato plants: Comparing the effects of calcium sources and adjuvant usage. Plants 2023, 12, 2587. [Google Scholar] [CrossRef]
- Haleema, B.; Shah, S.T.; Basit, A.; Hikal, W.M.; Arif, M.; Khan, W.; Said-Al Ahl, H.A.; Fhatuwani, M. Comparative Effects of Calcium, Boron, and Zinc Inhibiting Physiological Disorders, Improving Yield and Quality of Solanum lycopersicum. Biology 2024, 13, 766. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Gao, X.; Tie, J.; Wu, Y.; Tang, Z.; Hu, L.; Yu, J. Exogenous brassinosteroids alleviate calcium deficiency-induced tip-burn by maintaining cell wall structural stability and higher photosynthesis in mini Chinese Cabbage. Front. Plant Sci. 2022, 13, 999051. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, J.; Zhang, W.; Gao, X.; Wang, X.; Chen, W.; Dawuda, M.M.; Li, W.; Hu, L. Quality Response of Two Mini Chinese Cabbage Cultivars to Different Calcium Levels. Foods 2025, 14, 872. [Google Scholar] [CrossRef]
- Geraldson, C.M. Studies on control of blackheart of celery. Proc. Fla. State Hortic. Soc. 1952, 65, 171–172. [Google Scholar]
- Vergara, C.; Araujo, K.E.C.; Santos, A.P.; de Oliveira, F.F.; de Souza Silva, G.; de Oliveira Miranda, N.; de Souza Barreto, E.; da Silva, I.K.; de Medeiros, J.F. Use of crushed eggshell to control tomato blossom-end rot. Res. Soc. Dev. 2024, 13, e2213545667. [Google Scholar] [CrossRef]
- Geraldson, C.M. The control of blackheart of celery. Proc. Am. Soc. Hortic. Sci. 1954, 63, 353–358. [Google Scholar]
- Kleemann, M. Effects of salinity, nutrients and spraying with cacl2 solution on the development of calcium deficiency in chervil (Anthriscus cerefolium (L.) Hoffm.) and curled parsley (Petroselinum crispum (Mill.) nym. Convar. Crispum). In Integrated View of Fruit and Vegetable Quality; CRC Press: Boca Raton, FL, USA, 2000; pp. 41–53. [Google Scholar]
- Schmitz-Eiberger, M.; Haefs, R.; Noga, G. Calcium deficiency-influence on the antioxidative defense system in tomato plants. J. Plant Physiol. 2002, 159, 733–742. [Google Scholar] [CrossRef]
- Parađiković, N.; Lončarić, Z.; Bertić, B.; Vukadinović, V. Influence of Ca-foliar application on yield and quality of sweet pepper in glasshouse conditions. Poljoprivreda 2004, 10, 24–27. Available online: https://www.researchgate.net/publication/47727972 (accessed on 31 May 2025).
- Raven, J.A. H+ and Ca2+ in phloem and symplast: Relation of relative immobility of the ions to the cytoplasmic nature of the transport paths. New Phytol. 1977, 79, 465–480. [Google Scholar] [CrossRef]
- Kohl, W. Die calciumverteilung in apfeln und ihre veranderung wahrend des wachstums. Die Gartenbauwissenschaft 1966, 31, 513–547. [Google Scholar]
- Perring, M.A.; Jackson, C.H. The mineral composition of apples. Calcium concentration and bitter pit in relation to mean mass per apple. J. Sci. Food Agric. 1975, 26, 1493–1502. [Google Scholar] [CrossRef]
- Bangerth, F. Calcium-related physiological disorders of plants. Annu. Rev. Phytopathol. 1979, 17, 97–122. [Google Scholar] [CrossRef]
- Chamel, A.R. Permeability characteristics of isolated ‘Golden Delicious’ apple fruit cuticles with regard to calcium. J. Am. Soc. Hortic. Sci. 1989, 114, 804–809. [Google Scholar] [CrossRef]
- Glenn, G.M.; Poovaiah, B.W. Cuticular permeability to calcium compounds in ‘Golden Delicious’ apple fruit. J. Am. Soc. Hortic. Sci. 1985, 110, 192–195. [Google Scholar] [CrossRef]
- Schlegel, T.K.; Schönherr, J. Penetration of calcium chloride into apple fruits as affected by stage of fruit development. Acta Hortic. 2002, 594, 527–533. [Google Scholar] [CrossRef]
- Wojcik, P. Effect of calcium chloride sprays at different water volumes on “Szampion” apple calcium concentration. J. Plant Nutr. 2001, 24, 639–650. [Google Scholar] [CrossRef]
- Sarijan, A.; Limbongan, A.A.; Ekowati, N.Y.; Kusumah, R. Calcium nitrate application on tomatoes to increase blossom-end rot disease resistance. IOP Conf. Ser. Earth Environ. Sci. 2025, 1471, 012025. [Google Scholar] [CrossRef]
- Assaha, D.V.; Petang, L.Y. Bone meal enhances the growth and yield of the tomato cultivar Cobra F1 by increasing fruit Ca content and alleviating blossom-end rot. Asian J. Agric. 2024, 8, 1–25. [Google Scholar] [CrossRef]
- Madani, B.; Mirshekari, A.; Sofo, A.; Tengku Muda Mohamed, M. Preharvest calcium applications improve postharvest quality of papaya fruits (Carica papaya L. cv. Eksotika II). J. Plant Nutr. 2016, 39, 1483–1492. [Google Scholar] [CrossRef]
- Parsa, Z.; Roozbehi, S.; Hosseinifarahi, M.; Radi, M.; Amiri, S. Integration of pomegranate peel extract (PPE) with calcium sulphate (CaSO4): A friendly treatment for extending shelf-life and maintaining postharvest quality of sweet cherry fruit. J. Food Process. Preserv. 2021, 45, e15089. [Google Scholar] [CrossRef]
- Zhai, J.; Gao, Y.; Zhang, X.W.; Han, L.J.; Bi, H.A.; Li, Q.M.; Ai, X. Effects of silicon and calcium on photosynthesis, yield and quality of cucumber in solar-greenhouse. Acta Hortic. Sin. 2019, 46, 701–713. [Google Scholar] [CrossRef]
- Ahn, J.; Park, M.; Kim, J.; Huq, E.; Kim, J.; Kim, D.H. Physiological and transcriptomic analyses of healthy and blossom-end-rot (BER)-defected fruit of chili pepper (Capsicum annuum. L.). Hortic. Environ. Biotechnol. 2024, 65, 971–980. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Watkins, C.B. Bitter pit in apple fruit. Hortic. Rev. 1989, 11, 289–355. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Watkins, C.B. Cation distribution and balance in apple fruit in relation to calcium treatments for bitter pit. Sci. Hortic. 1983, 19, 301–310. [Google Scholar] [CrossRef]
- Huang, Z.; Takemoto, T.; Omwange, K.A.; Orsino, M.; Konagaya, K.; Kondo, N. Early detection of Blossom-End Rot in green peppers using fluorescence and normal color images in visible region. Food Control 2025, 172, 111156. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, S.T.; Shackel, K.A.; Mitcham, E.J. Abscisic acid triggers whole-plant and fruit-specific mechanisms to increase fruit calcium uptake and prevent blossom end rot development in tomato fruit. J. Exp. Bot. 2011, 62, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.C. Environmental effects on the diurnal accumulation of 45Ca by young fruit and leaves of tomato plants. Ann. Bot. 1989, 63, 281–288. [Google Scholar] [CrossRef]
- Ho, L.C.; Belda, R.; Brown, M.; Andrews, J.; Adams, P. Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J. Exp. Bot. 1993, 44, 509–518. [Google Scholar] [CrossRef]
- Tadesse, T.; Nichols, M.A.; Hewett, E.W.; Fisher, K.J. Relative humidity around the fruit influences the mineral composition and incidence of blossom-end rot in sweet pepper fruit. J. Hortic. Sci. Biotechnol. 2001, 76, 9–16. [Google Scholar] [CrossRef]
- Fischer, A.M. Nutrient remobilization during leaf senescence. Annu. Rev. Senescence Process. Plants 2007, 26, 87–107. [Google Scholar]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; ISBN 9780123849052. [Google Scholar]
- Song, W.; Yi, J.; Kurniadinata, O.F.; Wang, H.; Huang, X. Linking fruit Ca uptake capacity to fruit growth and pedicel anatomy, a cross-species study. Front. Plant Sci. 2018, 9, 575. [Google Scholar] [CrossRef]
- Bar-Tal, A.; Aloni, B.; Karni, L.; Oserovitz, J.; Hazan, A.; Itach, M.; Gantz, S.; Avidan, A.; Posalski, I.; Tratkovski, N.; et al. Nitrogen nutrition of greenhouse pepper. I. Effects of nitrogen concentration and NO3: NH4 ratio on yield, fruit shape, and the incidence of blossom-end rot in relation to plant mineral composition. HortScience 2001, 36, 1244–1251. [Google Scholar] [CrossRef]
- Schönherr, J.; Bukovac, M.J. Ion exchange properties of isolated tomato fruit cuticular membrane: Exchange capacity, nature of fixed charges and cation selectivity. Planta 1973, 109, 73–93. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Nir, S.; Ben-Hayyim, G.; Kafkafi, U. Quantitative competition of calcium with sodium or magnesium for sorption sites on plasma membrane vesicles of melon (Cucumis melo L.) root cells. J. Membr. Biol. 1994, 138, 55–63. [Google Scholar] [CrossRef]
- De Freitas, S.T.; do Amarante, C.V.; Labavitch, J.M.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 57, 6–13. [Google Scholar] [CrossRef]
- Dris, R.; Niskanen, R.; Fallahi, E. Nitrogen and calcium nutrition and fruit quality of commercial apple cultivars grown in Finland. J. Plant Nutr. 1998, 21, 2389–2402. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kvikliene, N. Effect of calcium foliar application on some fruit quality characteristics of ‘Sinap Orlovskij’apple. Agron. Res. 2006, 4, 31–36. [Google Scholar]
- Park, S.; Cheng, N.H.; Pittman, J.K.; Yoo, K.S.; Park, J.; Smith, R.H.; Hirschi, K.D. Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol. 2005, 139, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, S.T.; Mitcham, E.I. 3 factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar] [CrossRef]
- Picchioni, G.A.; Watada, A.E.; Conway, W.S.; Whitaker, B.D.; Sams, C.E. Postharvest calcium infiltration delays membrane lipid catabolism in apple fruit. J. Agric. Food Chem. 1998, 46, 2452–2457. [Google Scholar] [CrossRef]
- Lund, Z.F. The effect of calcium and its relation to several cations in soybean root growth. Soil Sci. Soc. Am. J. 1970, 34, 456–459. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Bush, D.S. Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Biol. 1995, 46, 95–122. [Google Scholar] [CrossRef]
- Kendall, J.M.; Dormer, R.L.; Campbell, A.K. Targeting aequorin to the endoplasmic reticulum of living cells. Biochem. Biophys. Res. Commun. 1992, 189, 1008–1016. [Google Scholar] [CrossRef]
- Johnson, C.H.; Knight, M.R.; Kondo, T.; Masson, P.; Sedbrook, J.; Haley, A.; Trewavas, A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 1995, 269, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.C.; Knight, M.R. Mitochondrial and cytosolic calcium dynamics are differentially regulated in plants. Plant Physiol. 2003, 133, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Pauly, N.; Knight, M.R.; Thuleau, P.; Graziana, A.; Muto, S.; Ranjeva, R.; Mazars, C. The nucleus together with the cytosol generates patterns of specific cellular calcium signatures in tobacco suspension culture cells. Cell Calcium 2001, 30, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Voyle, G. Blossom End Rot Causes and Cures in Garden Vegetables; Michigan State University: Extension, MI, USA, 2021. [Google Scholar]
- de Freitas, S.T.; Amarante, C.D.; Mitcham, E.J. Calcium deficiency disorders in plants. In Postharvest Ripening Physiology of Crops; CRC Press: Boca Raton, FL, USA, 2016; pp. 477–502. ISBN 9781498703819. [Google Scholar]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, X.; Li, W.; Hussain, J.; Qi, G.; Liu, S. Calcium signaling in plant programmed cell death. Cells 2021, 10, 1089. [Google Scholar] [CrossRef]
- Ojha, R.K.; Jha, S.K. Role of mineral nutrition in management of plant diseases. In Farmers’ Prosperity Through Improved Agricultural Technologies; Singh, H.K., Solankey, S.S., Roy, M.K., Eds.; Jaya Publishing House: Delhi, India, 2021; pp. 241–261. Available online: https://www.researchgate.net/publication/348448043_ (accessed on 2 July 2025).
- Kleemann, M. Development of calcium deficiency symptoms in chervil (Antriscus cerefolium (L.) Hoffm.) and curled parsley (Petroselinum crispum (Mill.) Nym. convar crispum). Zesz. Probl. Postępów Nauk. Rol. 1999, 468, 335–348. [Google Scholar]
- Aloni, B. Enhancement of leaf tip burn by restricting root growth in Chinese cabbage plants. J. Hortic. Sci. 1986, 61, 509–513. [Google Scholar] [CrossRef]
- Collier, G.F.; Tibbitts, T.W. Tipburn of lettuce. Hortic. Rev. 1982, 4, 49–65. [Google Scholar] [CrossRef]
- Cox, E.F.; McKee, J.M.T.; Dearman, A.S. The effect of growth rate on tipburn occurrence in lettuce. J. Hortic. Sci. 1976, 51, 297–309. [Google Scholar] [CrossRef]
- Bible, B.B.; Stiehl, B. Effect of atmospheric modification on the incidence of blackheart and the cation content of celery. Sci. Hortic. 1986, 28, 19–28. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Martinelli, F.; Feng, B.; Reitz, N.F.; Mitcham, E.J. Transcriptome approach to understand the potential mechanisms inhibiting or triggering blossom-end rot development in tomato fruit in response to plant growth regulators. J. Plant Growth Regul. 2018, 37, 183–198. [Google Scholar] [CrossRef]
- Adams, P.; Holder, R. Effects of humidity, Ca and salinity on the accumulation of dry matter and Ca by the leaves and fruit of tomato (Lycopersicon esculentum). J. Hortic. Sci. 1992, 67, 137–142. [Google Scholar] [CrossRef]
- De Kreij, C.; Janse, J.; Van Goor, B.J.; Van Doesburg, J.D.J. The incidence of calcium oxalate crystals in fruit walls of tomato (Lycopersicon esculentum Mill.) as affected by humidity, phosphate and calcium supply. J. Hortic. Sci. 1992, 67, 45–50. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1987; 687p, ISBN 978-1-4020-0008-9/978-9-40-101009-2. [Google Scholar]
- Kabir, M.Y. Abiotic Factors Affecting Plant Physiology and Fruit Yield and Physiological Disorders in Bell Pepper and Tomato. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2019; 219p. [Google Scholar]
- Horst, W.J. Aluminium tolerance and calcium efficiency of cowpea genotypes. J. Plant Nutr. 1987, 10, 1121–1129. [Google Scholar] [CrossRef]
- Ho, L.C.; Hand, D.J.; Fussell, M. Improvement of tomato fruit quality by calcium nutrition. Int. Symp. Grow. Media Hydroponics 1997, 481, 463–468. [Google Scholar] [CrossRef]
- Ho, L.C.; Adams, P.; Li, X.Z.; Shen, H.; Andrews, J.; Xu, Z.H. Responses of Ca-efficient and Ca-inefficient tomato cultivars to salinity in plant growth, calcium accumulation and blossom-end rot. J. Hortic. Sci. 1995, 70, 909–918. [Google Scholar] [CrossRef]
- Adams, P.; Ho, L.C. The susceptibility of modern tomato cultivars to blossom-end rot in relation to salinity. J. Hortic. Sci. 1992, 67, 827–839. [Google Scholar] [CrossRef]
- Cardoso, M.O.; Queiróz, M.D.; Souza, R.F. Incidence of blossom-end rot in five tomato cultivars grown in soil with three calcium levels. Hortic. Bras. 1995, 13, 172–175. [Google Scholar]
- Sperry, W.J.; Davis, J.M.; Sanders, D.C. Soil moisture and cultivar influence cracking, blossom-end rot, zippers, and yield of staked fresh-market tomatoes. HortTechnology 1996, 6, 21–24. [Google Scholar] [CrossRef]
- Maynard, D.N.; Barham, W.S.; McCombs, C.L. The effect of calcium nutrition of tomatoes as related to the incidence and severity of blossom-end rot. Proc. Amer. Soc. Hort. Sci 1957, 69, 318–322. [Google Scholar]
- Magán, J.J.; Gallardo, M.; Thompson, R.B.; Lorenzo, P. Effects of salinity on fruit yield and quality of tomato grown in soil-less culture in greenhouses in Mediterranean climatic conditions. Agric. Water Manag. 2008, 95, 1041–1055. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, L.; Debnath, B.; Shukla, M.; Kumar, S.; Rana, M.; Meshram, S. Blossom end-rot (BER): An abiotic devastating disorder of tomato. Clim. Change Environ. Sustain. 2022, 10, 15–22. [Google Scholar] [CrossRef]
- Kumar, V.; Darvhankar, M.; Srivastava, S. Evaluation of Trichoderma species and fungicides on nutritional quality and yield of tomato. Pl. Cell Biotechnol. Mol. Biol 2019, 20, 331–338. [Google Scholar]
- Das, K.A.; Bhosale, R.K.; Chavan, S.S.; Rana, M.; Srivastava, S. Effect of physiological factors and different growth media on Alternaria solani under in vitro condition and eco-friendly management of early blight of tomato (Solanum lycopersicum L.). Ann Biol 2023, 39, 66–72. [Google Scholar]
- Zhou, L.; Fu, Y.; Yang, Z. A genome-wide functional characterization of Arabidopsis regulatory calcium sensors in pollen tubes. J. Integr. Plant Biol. 2009, 51, 751–761. [Google Scholar] [CrossRef]
- Yoshida, Y.; Irie, N.; Vinh, T.D.; Ooyama, M.; Tanaka, Y.; Yasuba, K.I.; Goto, T. Incidence of blossom-end rot in relation to the water-soluble calcium concentration in tomato fruits as affected by calcium nutrition and cropping season. J. Jpn. Soc. Hortic. Sci. 2014, 83, 282–289. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid increases carotenoid and chlorophyll concentrations in leaves and fruit of two tomato genotypes. J. Am. Soc. Hortic. Sci. 2014, 139, 261–266. [Google Scholar] [CrossRef]
- R’him, T.; Jebari, H. Blossom-end rot in relation to morphological parameters and calcium content in fruits of four pepper varieties (Capsicum annuum L.). Biotechnol. Agron. Société Environ. 2008, 12, 361–366. [Google Scholar]
- Grasselly, D.; Rosso, L.; Holgard, S.; Cottet, V.; Navez, B.; Jost, M.; Berier, A. Soilless culture of tomato: Effect of salinity of the nutrient solution. Infos-Ctifl 2008, 239, 41–45. [Google Scholar]
- Robbins, W.R. Relation of nutrient salt concentration to growth of the tomato and to the incidence of blossom-end rot of the fruit. Plant Physiol. 1937, 12, 21. [Google Scholar] [CrossRef]
- Dekock, P.C.; Hall, A.; Inkson, R.H.; Alan Robertson, R. Blossom-end rot in tomatoes. J. Sci. Food Agric. 1979, 30, 508–514. [Google Scholar] [CrossRef]
- Adams, P.; Ho, L.C. Effects of environment on the uptake and distribution of calcium in tomato and on the incidence of blossom-end rot. Plant Soil 1993, 154, 127–132. [Google Scholar] [CrossRef]
- Pill, W.G.; Lambeth, V.N. Effects of soil water regime and nitrogen form on blossom-end rot, yield, water relations, and elemental composition of tomato. J. Am. Soc. Hortic. Sci. 1981, 105, 730–734. [Google Scholar] [CrossRef]
- Paiva, E.A.S.; Sampaio, R.A.; Martinez, H.E.P. Composition and quality of tomato fruit cultivated in nutrient solutions containing different calcium concentrations. J. Plant Nutr. 1998, 21, 2653–2661. [Google Scholar] [CrossRef]
- Van Goor, B.J. Influence of restricted water supply on blossom-end rot and ionic composition of tomatoes grown in nutrient solution. Commun. Soil Sci. Plant Anal. 1974, 5, 13–24. [Google Scholar] [CrossRef]
- Franco, J.A.; Perez-Saura, P.J.; Ferná Ndez, J.A.; Parra, M.; Garcia, A.L. Effect of two irrigation rates on yield, incidence of blossom-end rot, mineral content and free amino acid levels in tomato cultivated under drip irrigation using saline water. J. Hortic. Sci. Biotechnol. 1999, 74, 430–435. [Google Scholar] [CrossRef]
- Franco, J.; Bañón, S.; Madrid, R. Effects of a protein hydrolysate applied by fertigation on the effectiveness of calcium as a corrector of blossom-end rot in tomato cultivated under saline conditions. Sci. Hortic. 1994, 57, 283–292. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Ho, L.C. Blossom-end rot in relation to growth rate and calcium content in fruits of sweet pepper (Capsicum annuum L.). J. Exp. Bot. 1999, 50, 357–363. [Google Scholar] [CrossRef]
- Chiu, T.F.; Bould, C. Effects of shortage of calcium and other cations on 45Ca mobility, growth and nutritional disorders of tomato plants (Lycopersicon esculentum). J. Sci. Food Agric. 1976, 27, 969–977. [Google Scholar] [CrossRef]
- Saure, M.C. Why calcium deficiency is not the cause of blossom-end rot in tomato and pepper fruit—A reappraisal. Sci. Hortic. 2014, 174, 151–154. [Google Scholar] [CrossRef]
- Gent, M.P. Effect of degree and duration of shade on quality of greenhouse tomato. HortScience 2007, 42, 514–520. [Google Scholar] [CrossRef]
- Díaz-Perez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Fruit yield, quality, and postharvest attributes, and incidence of phytophthora blight (caused by Phytophthora capsici Leon.). HortScience 2014, 49, 891–900. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Díaz-Pérez, J.C.; Nambeesan, S.U. Effect of shade levels on plant growth, physiology, and fruit yield in bell pepper (Capsicum annuum L.). In Proceedings of the XI International Symposium on Protected Cultivation in Mild Winter Climates and I International Symposium on Nettings and 1268, Tenerife, Canary Islands, Spain, 27 January 2020; pp. 311–318. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Nambeesan, S.U.; Bautista, J.; Díaz-Pérez, J.C. Plant water status, plant growth, and fruit yield in bell pepper (Capsicum annum L.) under shade nets. Sci. Hortic. 2022, 303, 111241. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Nambeesan, S.U.; Díaz-Pérez, J.C. Shade nets improve vegetable performance. Sci. Hortic. 2024, 334, 113326. [Google Scholar] [CrossRef]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A.; et al. Role of calcium nutrition in plant Physiology: Advances in research and insights into acidic soil conditions—A comprehensive review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef]
- Topcu, Y.; Nambeesan, S.U.; van der Knaap, E. Blossom-end rot: A century-old problem in tomato (Solanum lycopersicum L.) and other vegetables. Mol. Hortic. 2022, 2, 1. [Google Scholar] [CrossRef]
- Rezvi, H.U.A.; Tahjib-Ul-Arif, M.; Azim, M.A.; Tumpa, T.A.; Tipu, M.M.H.; Najnine, F.; Dawood, M.F.; Skalicky, M.; Brestič, M. Rice and food security: Climate change implications and the future prospects for nutritional security. Food Energy Secur. 2023, 12, e430. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J.; Alligood, M.R. Blossom-end rot incidence of tomato as affected by irrigation quantity, calcium source, and reduced potassium. HortScience 2004, 39, 1110–1115. [Google Scholar] [CrossRef]
- Lens, F.; Gleason, S.M.; Bortolami, G.; Brodersen, C.; Delzon, S.; Jansen, S. Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytol. 2022, 236, 2019–2036. [Google Scholar] [CrossRef]
- Oishy, M.N.; Shemonty, N.A.; Fatema, S.I.; Mahbub, S.; Mim, E.L.; Raisa, M.B.H.; Anik, A.H. Unravelling the effects of climate change on the soil-plant-atmosphere interactions: A critical review. Soil Environ. Health 2025, 3, 100130. [Google Scholar] [CrossRef]
- Taub, D. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat. Educ. Knowl. 2010, 3, 21. [Google Scholar]
- Ebi, K.L.; Loladze, I. Elevated atmospheric CO2 concentrations and climate change will affect our food’s quality and quantity. Lancet Planet. Health 2019, 3, e283–e284. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Rehman, H.U.; Cheema, S.A. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2021, 780, 146467. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized calcium phosphates as novel macronutrient nano-fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, S.; Li, Y.; Shi, Y. Effect of nano-calcium carbonate on morphology, antioxidant enzyme activity and photosynthetic parameters of wheat (Triticum aestivum L.) seedlings. Chem. Biol. Technol. Agric. 2023, 10, 31. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W., III; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Shelake, R.M.; Kadam, U.S.; Kumar, R.; Pramanik, D.; Singh, A.K.; Kim, J.Y. Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: Targets, tools, challenges, and perspectives. Plant Commun. 2022, 3, 100417. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Zeinali, M.; Naghavi, M.R. CRISPR-based metabolic editing: Next-generation metabolic engineering in plants. Gene 2020, 759, 144993. [Google Scholar] [CrossRef] [PubMed]
- Choudry, M.W.; Riaz, R.; Nawaz, P.; Ashraf, M.; Ijaz, B.; Bakhsh, A. CRISPR-Cas9 mediated understanding of plants’ abiotic stress-responsive genes to combat changing climatic patterns. Funct. Integr. Genom. 2024, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Navazio, L.; Szabo, I. The contribution of organelles to plant intracellular calcium signalling. J. Exp. Bot. 2018, 69, 4175–4193. [Google Scholar] [CrossRef]
- Ghosh, S.; Dahiya, M.; Kumar, A.; Bheri, M.; Pandey, G.K. Calcium imaging: A technique to monitor calcium dynamics in biological systems. Physiol. Mol. Biol. Plants 2023, 29, 1777–1811. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Long, Y.; Schmitz-Thom, I.; Wang, X.P.; Zhang, C.; Li, H.; Steinhorst, L.; Manishankar, P.; Ren, X.L.; Offenborn, J.N.; et al. Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol. 2017, 213, 739–750. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).