Comparative Evaluation of Appearance and Nutritional Qualities of 57 Tomato (Solanum lycopersicum L.) Accessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Determination of Appearance Qualities
2.4. Determination of Nutritional Qualities
2.5. Statistical Analysis
3. Results
3.1. Quality Traits of Different Tomato Accessions
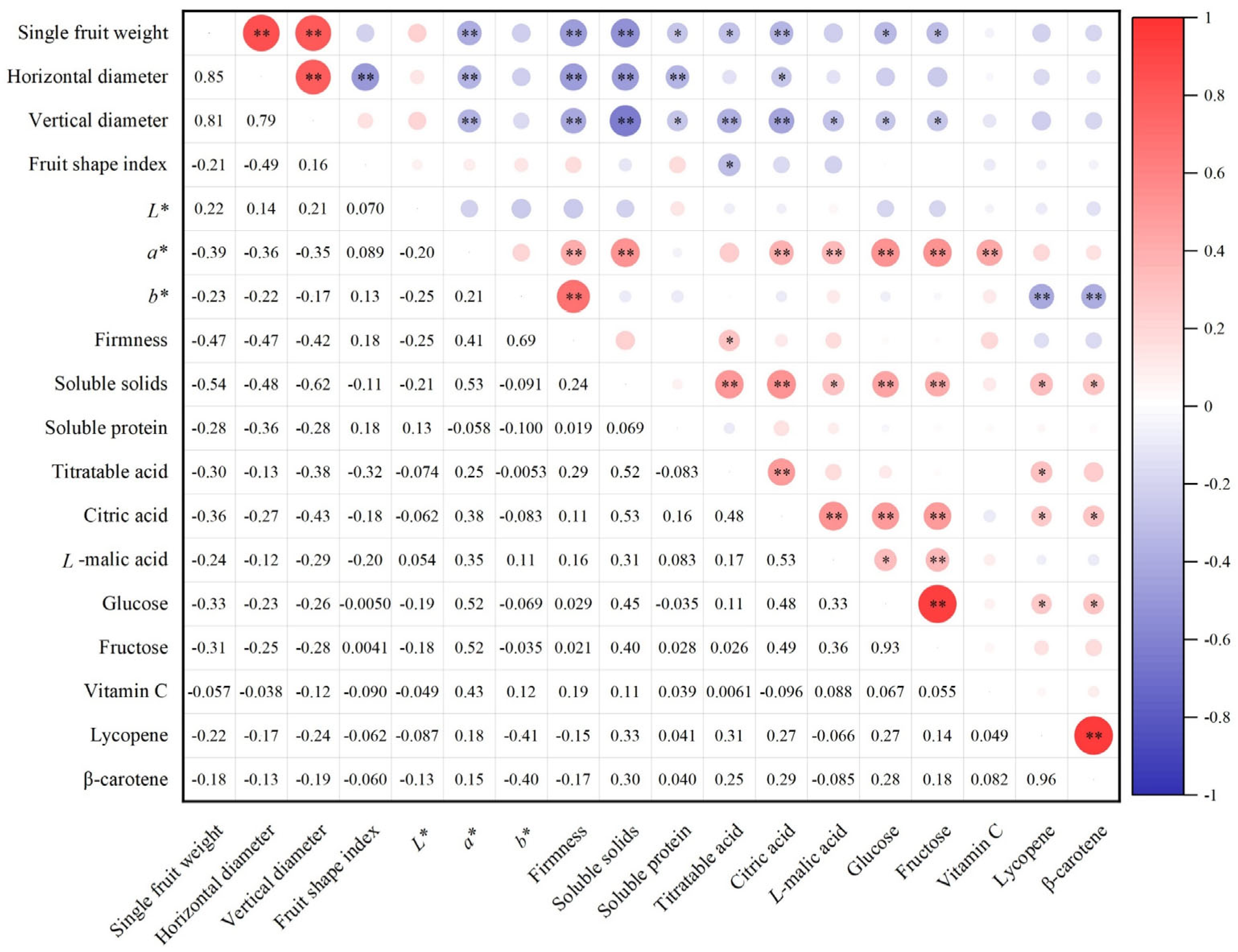
3.2. Correlation Analysis
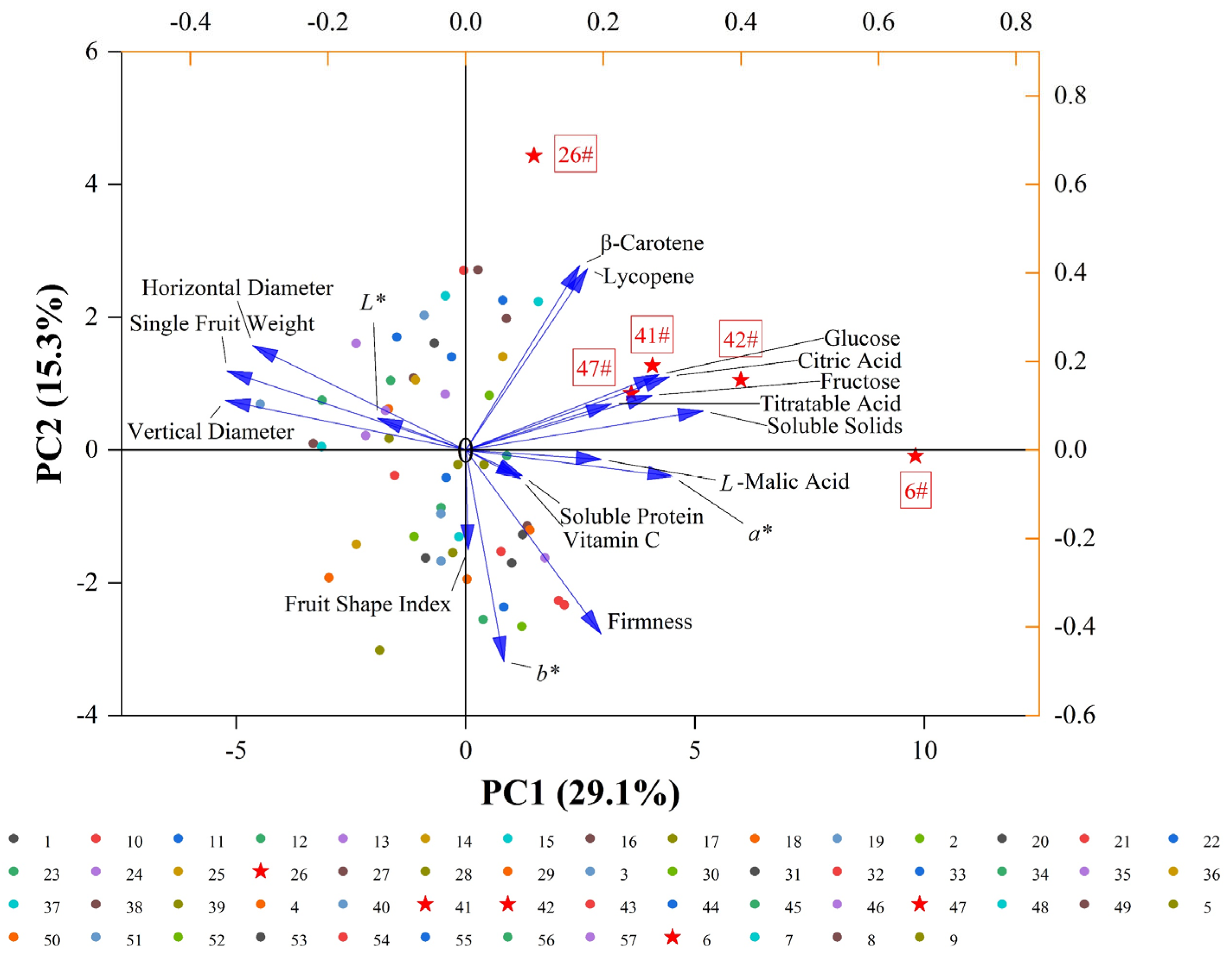
3.3. PCA of Tomato Quality Traits
3.4. Comprehensive Evaluation of Tomato Quality
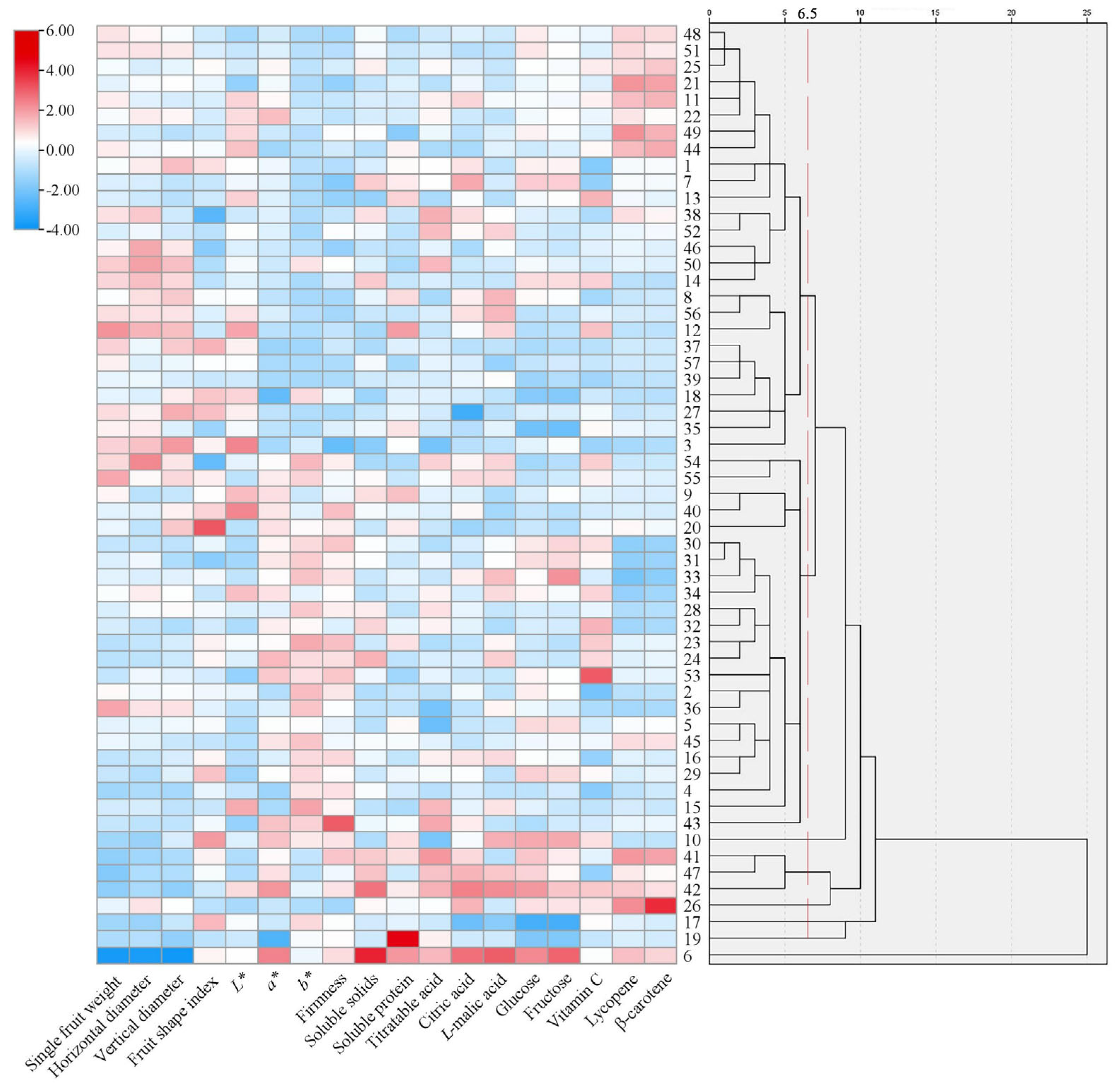
3.5. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, W.; Jiang, P.; Liu, Y.; Miao, X.; Liu, A. Distinct Changes of Taste Quality and Metabolite Profile in Different Tomato Varieties Revealed by LC-MS Metabolomics. Food Chem. 2024, 442, 138456. [Google Scholar] [CrossRef]
- Brainer, V.-L.; Yendry, C.-M.; Laura, B.-P.; Marianela, G.-M.; Jimmy, V.-P.; Gerardo, R.; Pablo, J.-B.; Victor, Á.-V. Phytonutraceutical Evaluation of Five Varieties of Tomato (Solanum lycopersicum) during Ripening and Processing. LWT 2022, 164, 113592. [Google Scholar]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simoes, S. Lycopene in Human Health. LWT Food Sci. Technol. 2020, 127, 109323. [Google Scholar] [CrossRef]
- Khalid, S.; Hassan, S.A.; Javaid, H.; Zahid, M.; Naeem, M.; Bhat, Z.F.; Abdi, G.; Aadil, R.M. Factors Responsible for Spoilage, Drawbacks of Conventional Packaging, and Advanced Packaging Systems for Tomatoes. J. Agric. Food Res. 2024, 15, 100962. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, S.; Yang, X.; Hou, F.; Fan, L.; Wang, W.; Xu, E.; Cheng, H.; Guo, M.; Liu, D. Evaluation of Extraction Technologies of Lycopene: Hindrance of Extraction, Effects on Isomerization and Comparative Analysis-A Review. Trends Food Sci. Technol. 2021, 115, 285–296. [Google Scholar] [CrossRef]
- Wu, X.; Yu, L.; Pehrsson, P.R. Are Processed Tomato Products as Nutritious as Fresh Tomatoes? Scoping Review on the Effects of Industrial Processing on Nutrients and Bioactive Compounds in Tomatoes. Adv. Nutr. 2022, 13, 138–151. [Google Scholar] [CrossRef]
- Zhao, L.; Maimaitiyiming, R.; Hong, J.; Wang, L.; Mu, Y.; Liu, B.; Zhang, H.; Chen, K.; Aihaiti, A. Optimization of Tomato (Solanum lycopersicum L.) Juice Fermentation Process and Analysis of Its Metabolites during Fermentation. Front. Nutr. 2024, 11, 1344117. [Google Scholar] [CrossRef]
- Dan, Y.; Yousef, G.; Campbell, F.N.; Phelps, D.W.; Burnett, C.; Kekkonen, A.; Shockley, A.; Lila, M.A. Development, and Genetic and Metabolic Characterization of New Tomato Mutants with Enhanced and Deficient Carotenoid Content. J. Hortic. Sci. Biotechnol. 2017, 92, 475–483. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, L.; Wang, Y.; Zhou, R.; Song, L.; Xu, L.; Cui, X.; Li, R.; Yu, W.; Zhao, T. Genetic Diversity of 324 Cultivated Tomato Germplasm Resources Using Agronomic Traits and InDel Markers. Euphytica 2019, 215, 69. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, M.F.R., Jr.; Lin, T.; Taylor, M.; Zhang, B.; Ikeda, H.; Liu, Z.; Fisher, J.; Zemach, I.; et al. A Chemical Genetic Roadmap to Improved Tomato Flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The Genetics of Fruit Flavour Preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Cheng, G.; Chang, P.; Shen, Y.; Wu, L.; El-Sappah, A.H.; Zhang, F.; Liang, Y. Comparing the Flavor Characteristics of 71 Tomato (Solanum lycopersicum) Accessions in Central Shaanxi. Front. Plant Sci. 2020, 11, 586834. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Wu, Y.; Tang, Z.; Zhang, D.; Lyu, J.; Khan, K.S.; Xiao, X.; Yu, J. Evaluation of Nutritional Composition, Biochemical, and Quality Attributes of Different Varieties of Tomato (Solanum lycopersicum L.). J. Food Compos. Anal. 2024, 132, 106384. [Google Scholar] [CrossRef]
- Panthee, D.R.; Labate, J.A.; McGrath, M.T.; Breksa, A.P., III; Robertson, L.D. Genotype and Environmental Interaction for Fruit Quality Traits in Vintage Tomato Varieties. Euphytica 2013, 193, 169–182. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, X.; Wang, C.; Ma, N.; Xie, J.; Zhang, J. Fruit Quality Analysis and Flavor Comprehensive Evaluation of Cherry Tomatoes of Different Colors. Foods 2024, 13, 1898. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Physicomechanical, Phytochemical, Volatile Compounds and Free Radical Scavenging Properties of Eight Pomegranate Cultivars and Classification by Principal Component and Cluster Analyses. Br. Food J. 2014, 116, 544–567. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, X.; Bi, J.; Jiao, Y.; Wu, X.; Ruan, W. Characterization of Chinese White-Flesh Peach Cultivars Based on Principle Component and Cluster Analysis. J. Food Sci. Technol. 2017, 54, 3818–3826. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Yin, J.; Wu, M.; Zhou, W.; Yang, X.; Zhang, R.; He, J. Color and Nutritional Analysis of Ten Different Purple Sweet Potato Varieties Cultivated in China via Principal Component Analysis and Cluster Analysis. Foods 2024, 13, 904. [Google Scholar] [CrossRef]
- Brewer, M.T.; Lang, L.; Fujimura, K.; Dujmovic, N.; Gray, S.; van der Knaap, E. Development of a Controlled Vocabulary and Software Application to Analyses Fruit Shape Variation in Tomato and Other Plant Species. Plant Physiol. 2006, 141, 15–25. [Google Scholar] [CrossRef]
- Huang, J.; Gao, W.; Qiao, H.; Wang, J. Condition Optimization in Extracting Lycopene from Fresh Tomato. Feed Res. 2019, 42, 86–90. (In Chinese) [Google Scholar]
- Figas, M.R.; Prohens, J.; Raigon, M.D.; Fita, A.; Garcia-Martinez, M.D.; Casanova, C.; Borras, D.; Plazas, M.; Andujar, I.; Soler, S. Characterization of Composition Traits Related to Organoleptic and Functional Quality for the Differentiation, Selection and Enhancement of Local Varieties of Tomato from Different Cultivar Groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef]
- Luo, J.; Liang, G.; Luo, F.; Tang, Y.; Yang, Y.; Gao, J. Quality Analysis and Evaluation of Fleshy Stems and Leaves of 10 Lettuce Varieties. Sci. Technol. Food Ind. 2024, 45, 336–344. (In Chinese) [Google Scholar]
- Liu, X.-H.; Song, H.-Z.; Zhang, G.-M.; Duan, W.-X.; Zhang, R.-H.; Li, Y.-R. Phenotypic Variation and Genetic Diversity in the Collections of Erianthus arundinaceus (Retz.) Jesw. Sugar Tech 2017, 19, 359–367. [Google Scholar] [CrossRef]
- Adhikari, P.; McNellie, J.; Panthee, D.R. Detection of Quantitative Trait Loci (QTL) Associated with the Fruit Morphology of Tomato. Genes 2020, 11, 1117. [Google Scholar] [CrossRef]
- de Souza, L.M.; Melo, P.C.T.; Luders, R.R.; Melo, A.M.T. Correlations between Yield and Fruit Quality Characteristics of Fresh Market Tomatoes. Hortic. Bras. 2012, 30, 627–631. [Google Scholar] [CrossRef]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; Cruz-Izquierdo, S.; García-Zavala, J.J.; Chávez-Servia, J.L.; Hernández-Leal, E.; Bonilla-Barrientos, O. Fruit Size QTLs Affect in a Major Proportion the Yield in Tomato. Chil. J. Agric. Res. 2015, 75, 402–409. [Google Scholar] [CrossRef]
- Nadia, B.; Mathilde, C.; Béatrice, B.; David, T.; Michel, G. Identification of Growth Processes Involved in QTLs for Tomato Fruit Size and Composition. J. Exp. Bot. 2009, 60, 237–248. [Google Scholar]
- Zhang, R.; Tao, J.; Song, L.; Zhang, J.; Liu, H.; Zhu, W.; Zhao, T.; Xiong, A. Identification of Genes of Molecular Marker TGS0892 on Chromosome 6 and Its Mechanism of Soluble Solids Metabolism in Tomato. Horticulturae 2023, 9, 52. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Gálvez, L.; Quezada, C.; Navarro, R. Principal Component Analysis as an Exploration Tool for Kinetic Modeling of Food Quality: A Case Study of a Dried Apple Cluster Snack. J. Food Eng. 2013, 119, 229–235. [Google Scholar] [CrossRef]
- Santos, S.A.; Almeida, B.M.; Mata, C.U.M.F.; Oliveira, R.C.J.; Cotrim, S.B.; Galvão, N.C.; Venâncio, A.E.R. An Introductory Review on the Application of Principal Component Analysis in the Data Exploration of the Chemical Analysis of Food Samples. Food Sci. Biotechnol. 2024, 33, 1323–1336. [Google Scholar]
- Piombino, P.; Sinesio, F.; Moneta, E.; Cammareri, M.; Genovese, A.; Lisanti, M.T.; Mogno, M.R.; Peparaio, M.; Termolino, P.; Moio, L.; et al. Investigating Physicochemical, Volatile and Sensory Parameters Playing a Positive or a Negative Role on Tomato Liking. Food Res. Int. 2013, 50, 409–419. [Google Scholar] [CrossRef]
- Azzi, L.; Deluche, C.; Gevaudant, F.; Frangne, N.; Delmas, F.; Hernould, M.; Chevalier, C. Fruit Growth-Related Genes in Tomato. J. Exp. Bot. 2015, 66, 1075–1086. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Q.; Zhao, Y.; Wan, H.; Wang, R.; Zhang, X.; Cheng, Y. Quantitative Extraction and Evaluation of Tomato Fruit Phenotypes Based on Image Recognition. Front. Plant Sci. 2022, 13, 859290. [Google Scholar] [CrossRef]
- Shi, Y.; Dai, Z.; Chen, K. Transcription Factors Involved in Tomato Fruit Quality Improvement. Food Qual. Saf. 2024, 8, fyad050. [Google Scholar] [CrossRef]
- Baldina, S.; Picarella, M.E.; Troise, A.D.; Pucci, A.; Ruggieri, V.; Ferracane, R.; Barone, A.; Fogliano, V.; Mazzucato, A. Metabolite Profiling of Italian Tomato Landraces with Different Fruit Types. Front. Plant Sci. 2016, 7, 664. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors Affecting the Postharvest Soluble Solids and Sugar Content of Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J. A Comprehensive Evaluation of Tomato Fruit Quality and Identification of Volatile Compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef]
- Galiana-Balaguer, L.; Ibanez, G.; Cebolla-Cornejo, J.; Rosello, S. Evaluation of Germplasm in Solanum Section Lycopersicon for Tomato Taste Improvement. Turk. J. Agric. For. 2018, 42, 309–321. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Yang, X.; Zhang, Q.; Shi, R.; Li, J. Online Detection of Lycopene Content in the Two Cultivars of Tomatoes by Multi-Point Full Transmission Vis-NIR Spectroscopy. Postharvest Biol. Technol. 2024, 211, 112813. [Google Scholar] [CrossRef]
- Gorecka, D.; Wawrzyniak, A.; Jedrusek-Golinska, A.; Dziedzic, K.; Hamulka, J.; Kowalczewski, P.L.; Walkowiak, J. Lycopene in Tomatoes and Tomato Products. Open Chem. 2020, 18, 752–756. [Google Scholar] [CrossRef]
- Tufail, T.; Ul Ain, H.B.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Meng, F.; Li, Y.; Li, S.; Chen, H.; Shao, Z.; Jian, Y.; Mao, Y.; Wang, Q.; Liu, L. Carotenoid Biofortification in Tomato Products along Whole Agro-Food Chain from Field to Fork. Trends Food Sci. Technol. 2022, 124, 296–308. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tieman, D.M.; Sims, C.A.; Odabasi, A.Z.; Clark, D.G.; Klee, H.J. Carotenoid Content Impacts Flavor Acceptability in Tomato (Solanum lycopersicum). J. Sci. Food Agric. 2010, 90, 2233–2240. [Google Scholar] [CrossRef]
| Indicator | Min | Max | Mean | SD | CV |
|---|---|---|---|---|---|
| Single fruit weight (g) | 87.86 | 260.67 | 197.31 | 29.39 | 14.90 |
| Horizontal diameter (mm) | 55.6 | 85.7 | 73.9 | 5.0 | 6.79 |
| Vertical diameter (mm) | 45.2 | 65.9 | 58.7 | 3.5 | 6.00 |
| Fruit shape index | 0.71 | 0.90 | 0.80 | 0.03 | 4.17 |
| L* value | 40.44 | 46.71 | 42.87 | 1.62 | 3.77 |
| a* value | 18.20 | 28.49 | 23.63 | 2.02 | 8.53 |
| b* value | 18.36 | 34.57 | 24.93 | 5.16 | 20.71 |
| Firmness (g) | 334.16 | 757.24 | 516.14 | 78.94 | 15.29 |
| Soluble solids (%) | 4.2 | 6.8 | 5.0 | 0.5 | 9.23 |
| Soluble protein (mg 100 g−1) | 1.26 | 13.43 | 4.52 | 1.94 | 42.92 |
| Titratable acid (mg g−1) | 4.50 | 7.03 | 5.80 | 0.59 | 10.13 |
| Citric acid (mg g−1) | 7.57 | 33.80 | 21.14 | 4.61 | 21.80 |
| L-malic acid (mg g−1) | 6.06 | 21.91 | 11.70 | 3.37 | 28.79 |
| Glucose (mg g−1) | 10.81 | 17.41 | 14.47 | 1.28 | 8.82 |
| Fructose (mg g−1) | 10.88 | 21.20 | 16.01 | 1.77 | 11.08 |
| Vitamin C (mg 100 g−1) | 13.83 | 28.51 | 19.64 | 2.87 | 14.63 |
| Lycopene (mg 100 g−1) | 4.90 | 15.62 | 9.89 | 2.53 | 25.60 |
| β-carotene (mg 100 g−1) | 2.44 | 9.50 | 4.55 | 1.28 | 28.18 |
| Indicator | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| Single fruit weight | −0.793 | 0.297 | 0.337 | −0.015 | 0.078 | 0.079 |
| Horizontal diameter | −0.708 | 0.392 | 0.495 | −0.187 | 0.056 | 0.041 |
| Vertical diameter | −0.799 | 0.186 | 0.235 | 0.207 | 0.199 | −0.011 |
| Fruit shape index | 0.008 | −0.373 | −0.454 | 0.581 | 0.203 | −0.071 |
| L* value | −0.293 | 0.119 | −0.090 | 0.169 | −0.501 | 0.427 |
| a* value | 0.689 | −0.099 | 0.315 | 0.102 | 0.326 | 0.274 |
| b* value | 0.127 | −0.791 | 0.217 | −0.158 | 0.189 | −0.111 |
| Firmness | 0.450 | −0.688 | 0.022 | −0.279 | 0.169 | −0.005 |
| Soluble solids | 0.789 | 0.147 | 0.016 | −0.164 | −0.065 | −0.012 |
| Soluble protein | 0.189 | −0.097 | −0.455 | 0.270 | −0.446 | 0.333 |
| Titratable acid | 0.484 | 0.173 | 0.046 | −0.652 | −0.153 | −0.081 |
| Citric acid | 0.677 | 0.275 | 0.219 | −0.028 | −0.382 | −0.108 |
| L-malic acid | 0.451 | −0.035 | 0.488 | 0.046 | −0.446 | 0.186 |
| Glucose | 0.643 | 0.283 | 0.377 | 0.472 | 0.148 | −0.139 |
| Fructose | 0.619 | 0.204 | 0.413 | 0.541 | 0.073 | −0.129 |
| Vitamin C | 0.183 | −0.107 | 0.157 | −0.097 | 0.417 | 0.807 |
| Lycopene | 0.405 | 0.679 | −0.416 | −0.130 | 0.287 | 0.044 |
| β-carotene | 0.379 | 0.691 | −0.387 | −0.093 | 0.328 | 0.038 |
| Eigenvalue | 5.240 | 2.748 | 1.930 | 1.636 | 1.473 | 1.138 |
| Variance contribution rate (%) | 29.109 | 15.269 | 10.722 | 9.088 | 8.186 | 6.321 |
| Cumulative variance contribution Rate (%) | 29.109 | 44.378 | 55.100 | 64.188 | 72.374 | 78.696 |
| Accession | PC Score | Comprehensive Score (Z) | Rankings | |||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | |||
| 6# | 9.803 | −0.088 | −0.754 | 1.282 | −2.270 | 0.790 | 2.740 | 1 |
| 42# | 5.997 | 1.054 | 1.068 | −0.128 | −1.126 | 1.639 | 2.021 | 2 |
| 41# | 4.071 | 1.270 | −2.062 | −0.641 | 0.873 | −0.710 | 1.126 | 3 |
| 26# | 1.492 | 4.434 | −0.768 | −0.394 | 1.495 | −0.023 | 1.114 | 4 |
| 47# | 3.608 | 0.853 | −0.030 | −0.202 | −0.863 | −1.766 | 0.977 | 5 |
| 10# | 2.149 | −2.333 | 0.733 | 3.743 | 0.359 | 0.759 | 0.766 | 6 |
| 7# | 1.585 | 2.234 | −0.012 | 0.945 | −1.711 | −1.342 | 0.662 | 7 |
| 11# | 0.811 | 2.259 | −0.274 | −0.271 | 0.100 | 1.007 | 0.599 | 8 |
| 53# | 1.241 | −1.272 | 1.259 | −0.813 | 3.123 | 1.391 | 0.572 | 9 |
| 25# | 0.812 | 1.406 | −0.646 | −0.032 | 1.403 | 0.276 | 0.511 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Luo, J.; Tang, Y.; Li, Z.; Yang, L.; Gao, J. Comparative Evaluation of Appearance and Nutritional Qualities of 57 Tomato (Solanum lycopersicum L.) Accessions. Horticulturae 2025, 11, 796. https://doi.org/10.3390/horticulturae11070796
Yang Y, Luo J, Tang Y, Li Z, Yang L, Gao J. Comparative Evaluation of Appearance and Nutritional Qualities of 57 Tomato (Solanum lycopersicum L.) Accessions. Horticulturae. 2025; 11(7):796. https://doi.org/10.3390/horticulturae11070796
Chicago/Turabian StyleYang, Yiwen, Jinghong Luo, Yueming Tang, Zhi Li, Liang Yang, and Jia Gao. 2025. "Comparative Evaluation of Appearance and Nutritional Qualities of 57 Tomato (Solanum lycopersicum L.) Accessions" Horticulturae 11, no. 7: 796. https://doi.org/10.3390/horticulturae11070796
APA StyleYang, Y., Luo, J., Tang, Y., Li, Z., Yang, L., & Gao, J. (2025). Comparative Evaluation of Appearance and Nutritional Qualities of 57 Tomato (Solanum lycopersicum L.) Accessions. Horticulturae, 11(7), 796. https://doi.org/10.3390/horticulturae11070796





