The Effect of Additional Night and Pre-Harvest Blue and Red LEDs and White Lighting During the Day on the Morphophysiological and Biochemical Traits of Basil Varieties (Ocimum basilicum L.) Under Hydroponic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
2.2. Light Conditions
- The light of white LEDs has a predominance of the green part of the spectrum. Photosynthetic photon flux density (PPFD) is ~139.9 µmol m−2·s−1 (Table 1). The color temperature of the white LEDs is 4000 K.
- The light of white and blue LEDs. Photosynthetic photon flux density of the blue LED (PPFD) is ~50 µmol m−2·s−1, with a peak wavelength of 450 nm.
- The light of white and red LEDs. Photosynthetic photon flux density of the red LED (PPFD) is ~50 µmol m−2·s−1, with a peak wavelength of 660 nm.
- Additional night exposure
- Additional pre-harvest irradiation
2.3. Biometric Indicators
2.4. The Content of Chlorophyll and Carotenoids in the Leaves
2.5. The Content of Macronutrients and Nitrates in the Leaves
2.6. The Content of Essential Oil in the Leaves
2.7. The Content of Quercetin and Rosmarinic Acid in the Leaves
2.8. Statistical Data Analysis
3. Results
3.1. The Effect of Nighttime Spectral Irradiation on the Economically Valuable Traits of Basil Plants
3.1.1. Morphometric Parameters of Plants
3.1.2. The Content of Photosynthetic Pigments and Macronutrients in Leaves
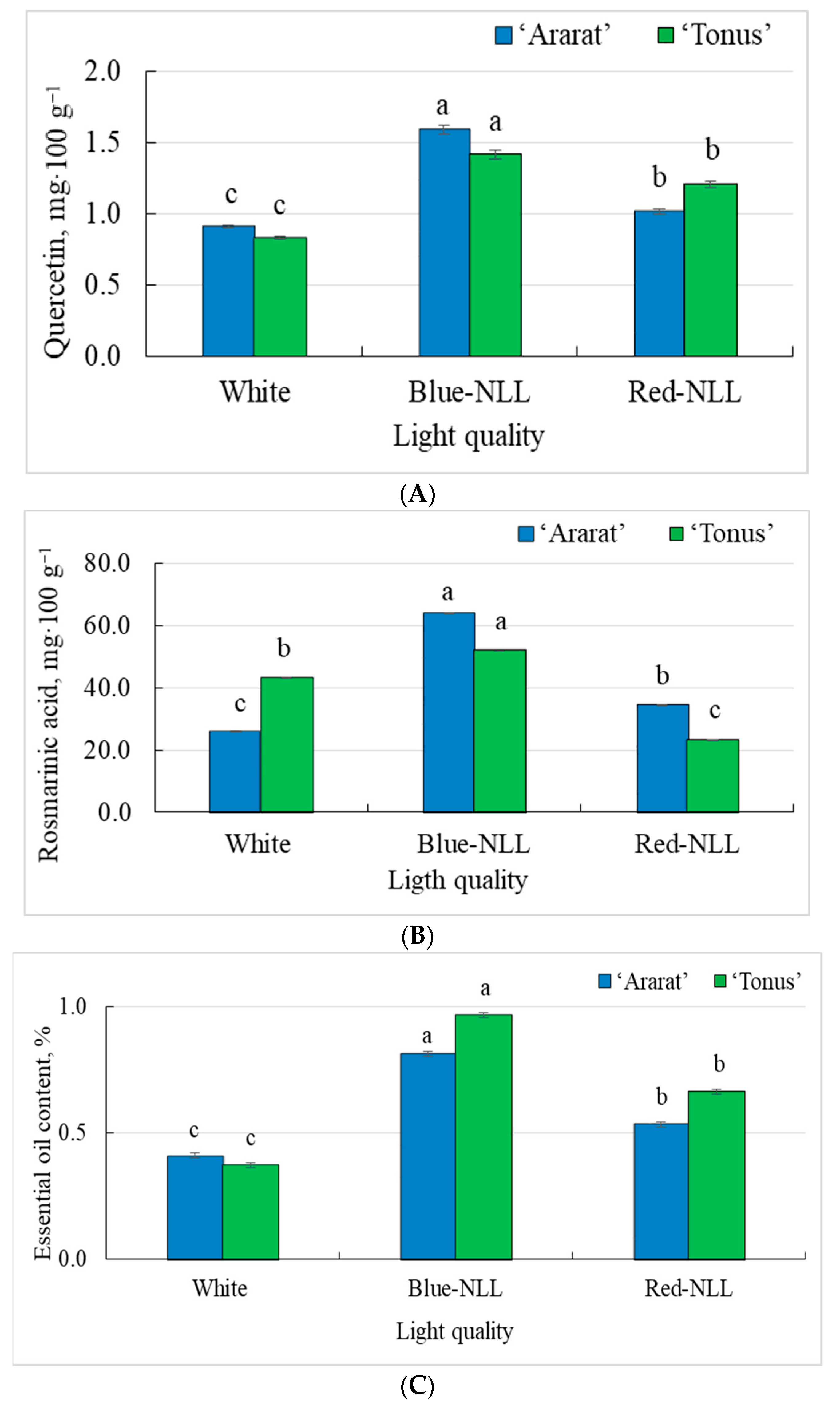
3.1.3. The Content of Quercetin, Rosmarinic Acid, and Essential Oil in the Vegetative Mass of Plants
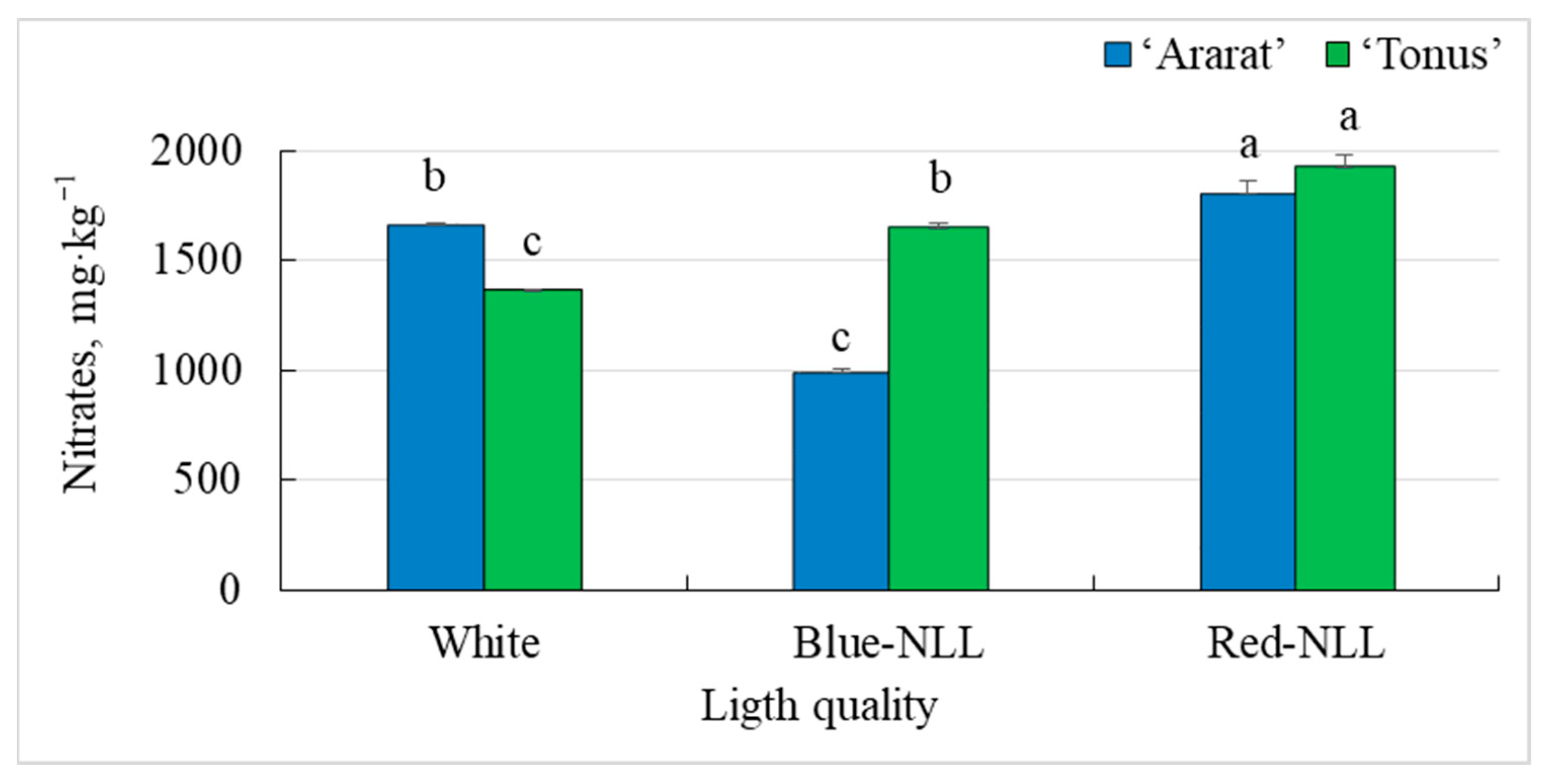
3.1.4. Nitrate Content in the Vegetative Part of Plants
3.2. The Effect of Pre-Harvest Irradiation on the Economically Valuable Characteristics of Basil Plants
3.2.1. Morphological Parameters of Plants
3.2.2. The Content of Photosynthetic Pigments and Macronutrients in Leaves
3.2.3. The Content of Quercetin, Rosmarinic Acid, and Essential Oil in the Vegetative Mass of Plants
3.2.4. Nitrate Content in the Vegetative Part of Plants
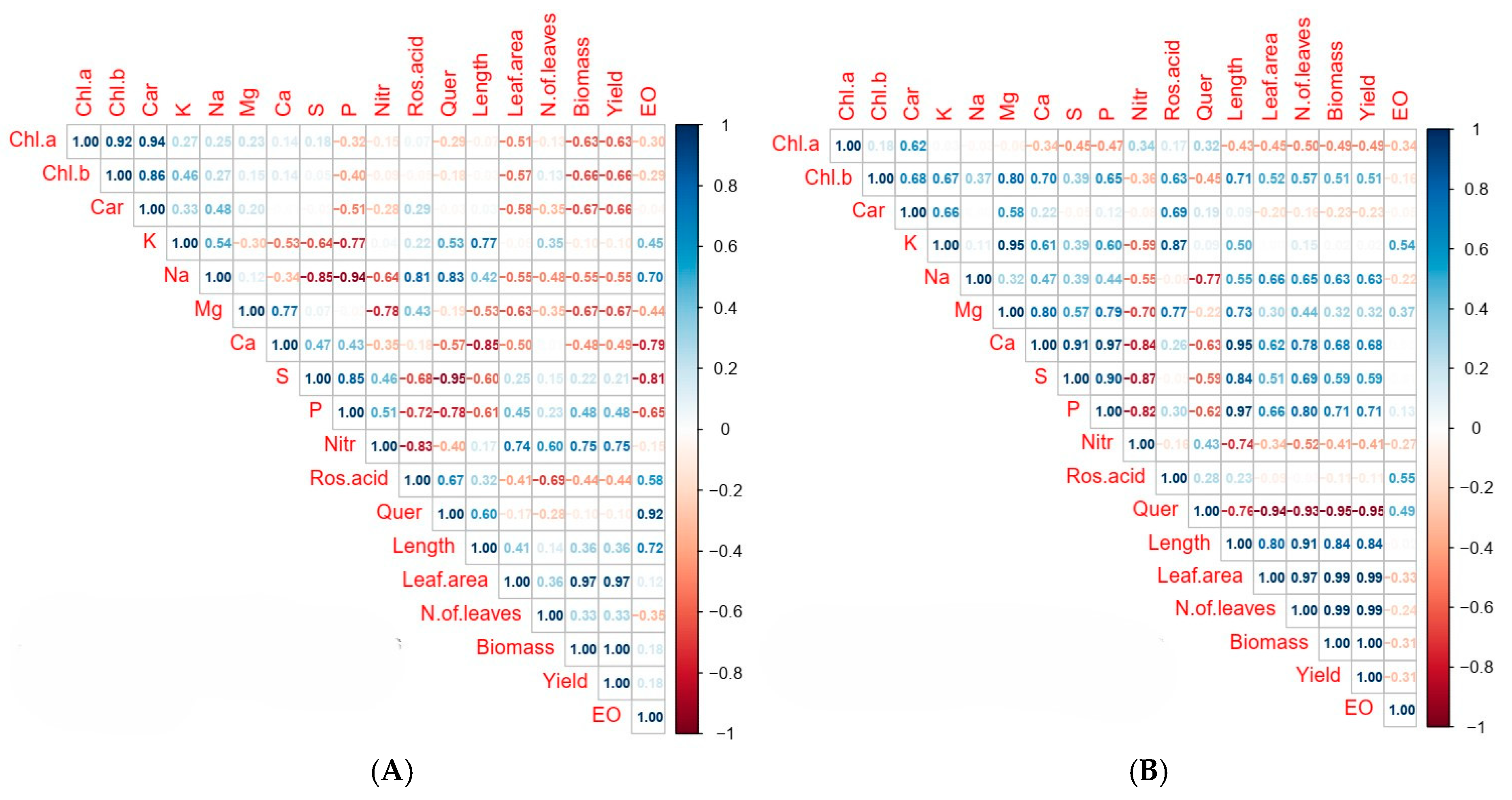
3.3. Correlation Analysis Among Morphophysiological and Biochemical Traits Under LED Lighting Treatments
LED Lighting
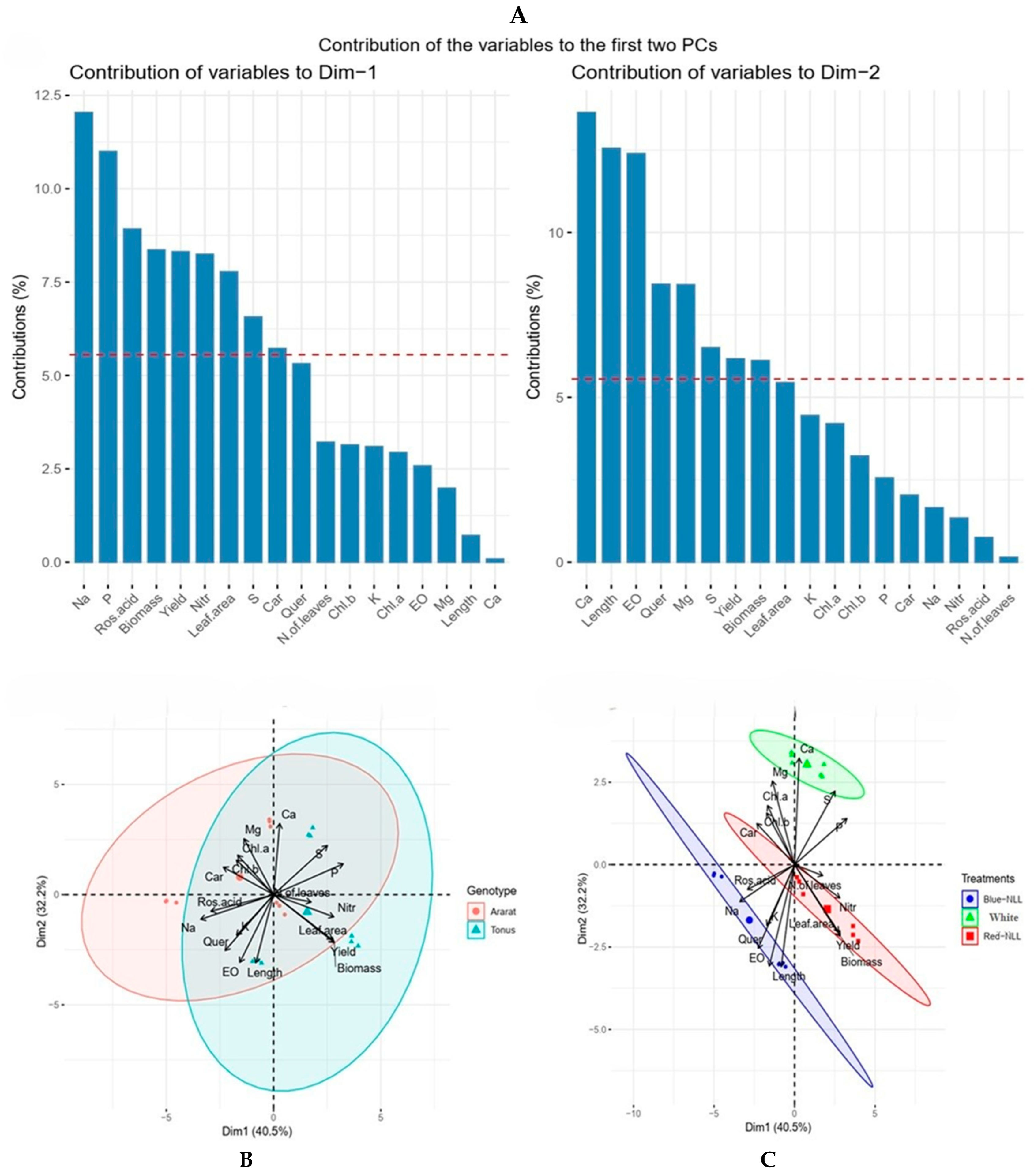
3.4. PCA—Biplot Analysis for Morphophysiological and Biochemical Traits Under LED Lighting at Night Treatments
3.5. PCA—Biplot Analysis for Morphophysiological and Biochemical Traits Under Pre-Harvest LED Lighting Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ivanitskikh, A.; Tarakanov, I.G. Effect of light spectral composition on the essential oils accumulation in basil plants grown with artificial lighting. Probl. Biol. Med. Pharm. Chem. 2022, 25, 3–9. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Pauletti, G.F. Evaluation of extraction yield and chemical composition of the essential oil of five commercial varieties of basil (Ocimum basilicum L.). Rev. Interdiscip. Ciênc. Apl. 2021, 6, 44–50. [Google Scholar] [CrossRef]
- Date, M.B.; Rivero, W.C.; Tan, J.; Specca, D.; Simon, J.E.; Salvi, D.A.; Karwe, M.V. Growth of Hydroponic Sweet Basil (O. basilicum L.) Using Plasma-Activated Nutrient Solution (PANS). Agriculture 2023, 13, 443. [Google Scholar] [CrossRef]
- Degtereva, M.; Levin, Y.; Gubina, A.; Degterev, A.; Lamkin, I.; Konoplev, G.; Tarasov, S.; Whaley, A.; Whaley, A.; Suloev, I.; et al. Influence of the Spectral Composition of Illuminating Light Sources on Biometric and Phytochemical Characteristics of Ocimum basilicum L. Photonics 2023, 10, 1369. [Google Scholar] [CrossRef]
- Mason-D’Croz, D.; Bogard, J.R.; Sulser, T.B.; Cenacchi, N.; Dunston, S.; Herrero, M.; Wiebe, K. Gaps between fruit and vegetable production, demand, and recommended consumption at global and national levels: An integrated modelling study. Lancet Planet. Health 2019, 3, e318–e329. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, K.; Singh, A.K.; Kumar, R.; Singh, S. Co-cultivation of sweet basil (Ocimum basilicum L.) with food crops for efficient resource utilization and increasing the farm profits. Ind. Crops Prod. 2024, 214, 118495. [Google Scholar] [CrossRef]
- Rawat, R.; Negi, K.S.; Mehta, P.S.; Tiwari, V.; Verma, S.K.; Bisht, I.S. Study of six varieties of sweet basil (Ocimum basilicum L.) and their morphological variations. J. Non-Timb. Forest Prod. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Knaus, U.; Pribbernow, M.; Xu, L.; Appelbaum, S.; Palm, H.W. Basil (Ocimum basilicum) Cultivation in Decoupled Aquaponics with Three Hydro-Components (Grow Pipes, Raft, Gravel) and African Catfish (Clarias gariepinus) Production in Northern Germany. Sustainability 2020, 12, 8745. [Google Scholar] [CrossRef]
- Teliban, G.-C.; Stoleru, V.; Burducea, M.; Lobiuc, A.; Munteanu, N.; Popa, L.-D.; Caruso, G. Biochemical, Physiological and Yield Characteristics of Red Basil as Affected by Cultivar and Fertilization. Agriculture 2020, 10, 48. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Hydroponic greenhouse basil production: Comparing systems and cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef]
- Wierdak, R.; Kocka, A.; Kowalski, R.; Borowski, B. Changes in the chemical composition of the essential oil of sweet basil (Ocimum basilicum L.) depending on the plant growth stage. Chemija 2012, 23, 216–222. [Google Scholar]
- Avgoustaki, D.D. Optimization of Photoperiod and Quality Assessment of Basil Plants Grown in a Small-Scale Indoor Cultivation System for Reduction of Energy Demand. Energies 2019, 12, 3980. [Google Scholar] [CrossRef]
- Rahman, M.M.; Vasiliev, M.; Alameh, K. LED Illumination Spectrum Manipulation for Increasing the Yield of Sweet Basil (Ocimum basilicum L.). Plants 2021, 10, 344. [Google Scholar] [CrossRef]
- Litvin, A.G.; Currey, C.J.; Wilson, L.A. Effects of supplemental light source on basil. dill. and parsley growth. morphology. aroma and flavor. J. Am. Soc. Hortic. Sci. 2020, 145, 18–29. [Google Scholar] [CrossRef]
- Oehler, M.A.; Kelly, N.; Fonseca, J.M.; Evensen, E.; Park, E.; Gu, G.; Teng, Z.; Luo, Y. Influence of Supplementary Blue and Far-Red Light on the Morphology and Texture of Ocimum basilicum L. Grown in Controlled Environments. Horticulturae 2025, 11, 287. [Google Scholar] [CrossRef]
- Hammock, H.A.; Sams, C.E. Variation in supplemental lighting quality influences key aroma volatiles in hydroponically grown ‘Italian Large Leaf’ basil. Front. Plant Sci. 2023, 14, 1184664. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. LED light sources improved the essential oil components and antioxidant activity of two genotypes of lemon balm (Melissa ofcinalis L.). Bot. Stud. 2021, 62, 9. [Google Scholar] [CrossRef]
- Maran, B.; Silvestre, W.P.; Pauletti, G.F. Preliminary study on the effect of artificial lighting on the production of basil, mustard, and red cabbage seedlings. AgriEngineering 2024, 6, 1043–1054. [Google Scholar] [CrossRef]
- Semenova, N.A.; Smirnov, A.A.; Ivanitskikh, A.S.; Izmailov, A.Y.; Dorokhov, A.S.; Proshkin, Y.A.; Yanykin, D.V.; Sarimov, R.R.; Gudkov, S.V.; Chilingaryan, N.O. Impact of Ultraviolet Radiation on the Pigment Content and Essential Oil Accumulation in Sweet Basil (Ocimum basilicum L.). Appl. Sci. 2022, 12, 7190. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light quality dependent changes in morphology. antioxidant capacity and volatile production in sweet basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Hsu, M.-H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT 2022, 153, 112210. [Google Scholar] [CrossRef]
- Varga, F.; Carovic-Stanko, K.; Ristic, M.; Grdisa, M.; Liber, Z.; Satovic, Z. Morphological and biochemical intraspecific characterization of Ocimum basilicum L. Ind. Crops Prod. 2017, 109, 611–618. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Ozdemir, F.A.; Kołodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest preservation method–Convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef]
- Toncer, O.; Karaman, S.; Diraz, E.; Tansi, S. Essential oil composition of Ocimum basilicum L. at different phenological stages in semi-arid environmental conditions. Fersenius Environ. Bull. 2017, 8, 5441–5446. [Google Scholar]
- Al-Kateb, H.; Mottram, D. The relationship between growth stages and aroma composition of lemon basil Ocimum citriodorum. Vis. Food Chem. 2014, 152, 440–446. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.; Wright, C. Variation in the Essential Oils in Different Leaves of Basil (Ocimum basilicum L.) at Day Time. Open Hortic. J. 2009, 2, 13–16. [Google Scholar] [CrossRef]
- Brindisi, L.J.; Simon, J.E. Preharvest and postharvest techniques that optimize the shelf life of fresh basil (Ocimum basilicum L.): A review. Front. Plant Sci. 2023, 14, 1237577. [Google Scholar] [CrossRef]
- Knyazeva, I.V.; Panfilova, O.; Vershinina, O.; Smirnov, A.A.; Dorokhov, A.S.; Kahramanoğlu, I. The Effect of Nighttime LED Lighting on Tomato Growth, Yield, and Nutrient Content of Fruits. Horticulturae 2024, 10, 1259. [Google Scholar] [CrossRef]
- Panfilova, O.; Ryago, N.; Ondrasek, G.; Knyazeva, I.V.; Kahramanoğlu, I.; Vershinina, O.; Tsoy, M.; Izmailov, A.Y.; Dorokhov, A.S. Optimizing Microclonal Propagation of Red Currant Cultivars: The Role of Nutrient Media, Sterilizers, and LED Lighting in Plant Adaptation. Horticulturae 2025, 11, 149. [Google Scholar] [CrossRef]
- Stepanova, N.Y. Evaluation of basil cultivars suitability for drying and freezing. Sci. Support Dev. Agro Ind. Complex Import. Cond. 2020, 1, 115–118. [Google Scholar]
- Hosseini, A.; Mehrjerdi, M.Z.; Aliniaeifard, S. Alteration of bioactive compounds in two varieties of basil (Ocimum basilicum) grown under different light spectra. J. Essent. Oil Bear. Plants 2018, 21, 913–923. [Google Scholar] [CrossRef]
- Gavrilenko, V.F.; Ladygina, M.E.; Khandobina, L.M. Large Workshop on Plant Physiology, Photosynthesis, Respiration; Higher School: Moscow, Russia, 1975; pp. 133–134. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- De Carvalho, L.M.; Raabe, A.; Martini, M.; Santanna, C.S.; Da Silveira, G.D.; Do Nascimento, P.C.; Bohrer, D. Contactless Conductivity Detection of 14 Inorganic Cations in Mineral and Phytotherapeutic Formulations after Capillary Electrophoretic Separation. Electroanalysis 2011, 23, 2574–2581. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A. Protein Isolate from Basil Seeds (Ocimum basilicum L.): Physicochemical and Functional Characterisation. Food Chem. Adv. 2023, 3, 100424. [Google Scholar] [CrossRef]
- Burbott, A.; Loomis, W. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967, 42, 20–28. [Google Scholar] [CrossRef]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.N.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.R. Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 5 May 2025).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses (Version 1.0.7). 2020. Available online: https://cran.r-project.org/package=factoextra (accessed on 5 May 2025).
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Shaidullin, A.K.; Makarov, P.N. Productivity and nitrate content in green crop products (Ocimum basilicum L., Eruca sativa Mill.) under light culture conditions. In Proceedings of the III All-Russian Scientific and Practical Conference, Surgut, Russia, 11–12 November 2020. [Google Scholar]
- Nelson, J.A.; Bugbee, B. Economic Analysis of Greenhouse Lighting: Light Emitting Diodes vs. High Intensity Discharge Fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef]
- Lee, S.H.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. plantlets. Plant Cell Tissue Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Casson, S.A.; Hetherington, A.M. Phytochrome B Is required for light-mediated systemic control of stomatal development. Curr. Biol. 2014, 24, 1216–1221. [Google Scholar] [CrossRef]
- Beatrice, P.; Saviano, G.; Reguzzoni, M.; Divino, F.; Fantasma, F.; Chiatante, D.; Montagnoli, A. Light spectra of biophilic LED-sourced system modify essential oils composition and plant morphology of Mentha piperita L. and Ocimum basilicum L. Front. Plant Sci. 2023, 14, 1093883. [Google Scholar] [CrossRef]
- Branca, F.; Treccarichi, S.; Ruberto, G.; Renda, A.; Argento, S. Comprehensive Morphometric and Biochemical Characterization of Seven Basil (Ocimum basilicum L.) Genotypes: Focus on Light Use Efficiency. Agronomy 2024, 14, 224. [Google Scholar] [CrossRef]
- Larsen, D.H.; Woltering, E.J.; Nicole, C.C.S.; Marcelis, L.F.M. Response of basil growth and morphology to light intensity and spectrum in a vertical farm. Front. Plant Sci. 2020, 11, 597906. [Google Scholar] [CrossRef]
- Sale, A.I.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Pre-Harvest Supplemental LED Treatments Led to Improved Postharvest Quality of Sweet Basil Leaves. J. Photochem. Photobiol. B Biol. 2023, 248, 112788. [Google Scholar] [CrossRef]
- Jensen, N.B.; Clausen, M.R.; Kjaer, K.H. Spectral quality of supplemental LED grow light permanently alters stomatal functioning and chilling tolerance in basil (Ocimum basilicum L.). Sci. Hortic. 2018, 227, 38–47. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Emerson, R.; Chalmers, R.; Cederstrand, C. Some Factors Influencing The Long-Wave Limit of Photosynthesis. Proc. Natl. Acad. Sci. USA 1957, 43, 133–143. [Google Scholar] [CrossRef]
- De Keyser, E.; Dhooghe, E.; Christiaens, A.; Van Labeke, M.-C.; Van Huylenbroeck, J. LED light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Iwai, M.; Ohta, M.; Tsuchiya, H.; Suzuki, T. Enhanced accumulation of caffeic acid, rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV-A irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Aldarkazali, M.; Rihan, H.Z.; Carne, D.; Fuller, M.P. The Growth and Development of Sweet Basil (Ocimum basilicum) and Bush Basil (Ocimum minimum) Grown under Three Light Regimes in a Controlled Environment. Agronomy 2019, 9, 743. [Google Scholar] [CrossRef]
- Amaki, W.; Yamazaki, N.; Ichimura, M.; Watanabe, H. Effects of light quality on the growth and essential oil content in sweet basil. Acta Hortic. 2011, 907, 91–94. [Google Scholar] [CrossRef]
- Chu, H.T.T.; Vu, T.N.; Dinh, T.T.T.; Do, P.T.; Chu, H.H.; Tien, T.Q.; Tong, Q.C.; Nguyen, M.H.; Ha, Q.T.; Setzer, W.N. Effects of Supplemental Light Spectra on the Composition, Production and Antimicrobial Activity of Ocimum basilicum L. Essential Oil. Molecules 2022, 27, 5599. [Google Scholar] [CrossRef]
- Mirgos, M.; Geszprych, A.; Przybył, J.L.; Niedzińska, M.; Sujkowska-Rybkowska, M.; Gajc-Wolska, J.; Kowalczyk, K. Effect of Lighting Type on the Nitrates Concentration, Selective Bioactive Compounds and Yield of Sweet Basil (Ocimum basilicum L.) in Hydroponic Production. Agronomy 2025, 15, 966. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.C. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]







| Variant | Photon Flux, µmol Photons m−2·s−1 | Percentage Composition of Light (B:G:R) | |||
|---|---|---|---|---|---|
| Blue (B) | Green (G) | Red (R) | PPFD | ||
| White LED | 23.9 ± 1.3 | 63.2 ± 3.2 | 52.9 ± 3.0 | 139.9 ± 7.5 | 17:45:38 |
| Additional night exposure | |||||
| Blue-NLL | 49.7 ± 1.9 | 0.1 ± 0.08 | 0.2 ± 0.1 | 50 ± 2.1 | 100:0:0 |
| Red-NLL | 0.1 ± 0.01 | 0.1 ± 0.01 | 49.9 ± 1.8 | 50 ± 1.8 | 0:0:100 |
| Additional pre-harvest irradiation | |||||
| Blue-P-hLL | 85.6 ± 3.8 | 57.4 ± 3.1 | 47.7 ± 1.3 | 190.7 ± 8.2 | 45:30:25 |
| Red-P-hLL | 29.1 ± 1.5 | 67.1 ± 2.2 | 94.0 ± 4.1 | 190.2 ± 7.8 | 15:35:50 |
| Cultivar | Experiment Variant | Content, mg·g−1 Raw Weight of Plants | |||
|---|---|---|---|---|---|
| Chl a | Chl b | Chl (a + b) | Car | ||
| ‘Ararat’ | White | 1.93 ± 0.12 a | 0.88 ± 0.02 a | 2.81 ± 0.14 a | 0.58 ± 0.03 a |
| Blue-NLL | 1.59 ± 0.07 b | 0.71 ± 0.01 c | 2.30 ± 0.07 b | 0.51 ± 0.04 b | |
| Red-NLL | 1.84 ± 0.10 a | 0.83 ± 0.02 b | 2.68 ± 0.09 a | 0.53 ± 0.02 ab | |
| ‘Tonus’ | White | 1.50 ± 0.05 a | 0.53 ± 0.02 b | 2.03 ± 0.05 a | 0.42 ± 0.02 b |
| Blue-NLL | 1.54 ± 0.09 a | 0.58 ± 0.02 a | 2.12 ± 0.09 a | 0.51 ± 0.03 a | |
| Red-NLL | 1.08 ± 0.06 b | 0.45 ± 0.02 c | 1.53 ± 0.07 b | 0.29 ± 0.02 c | |
| Cultivar | Experiment Variant | Content, mg·100 g−1 Raw Weight of Plants | |||||
|---|---|---|---|---|---|---|---|
| K | Na | Mg | Ca | S | P | ||
| ‘Ararat’ | White | 380.40 ± 8.99 b | 6.96 ± 0.15 b | 31.59 ± 0.82 b | 53.17 ± 1.22 a | 11.97 ± 0.25 a | 49.39 ± 0.31 a |
| Blue-NLL | 432.23 ± 10.78 a | 11.02 ± 0.56 a | 35.75 ± 0.69 a | 49.21 ± 1.36 b | 8.76 ± 0.25 c | 34.14 ± 0.80 c | |
| Red-NLL | 453.60 ± 12.26 a | 6.84 ± 0.22 b | 29.74 ± 1.15 c | 44.76 ± 0.92 c | 11.06 ± 0.10 b | 43.20 ± 0.68 b | |
| ‘Tonus’ | White | 331.47 ± 9.37 b | 5.39 ± 0.22 b | 37.53 ± 1.56 a | 56.03 ± 2.70 a | 12.25 ± 0.24 a | 55.41 ± 0.96 a |
| Blue-NLL | 402.08 ± 3.07 a | 9.03 ± 0.52 a | 26.82 ± 0.78 b | 38.22 ± 0.93 c | 10.25 ± 0.38 b | 42.35 ± 0.27 c | |
| Red-NLL | 389.16 ± 12.51 a | 5.48 ± 0.15 b | 26.74 ± 1.04 b | 46.54 ± 2.57 b | 10.78 ± 0.13 b | 53.08 ± 0.67 b | |
| Cultivar | Experiment Variant | Content, mg·g−1 Raw Weight of Plants | |||
|---|---|---|---|---|---|
| Chl a | Chl b | Chl (a + b) | Car | ||
| ‘Ararat’ | White | 1.78 ± 0.08 a | 0.62 ± 0.03 a | 2.40 ± 0.09 a | 0.51 ± 0.02 a |
| Blue-P-hLL | 1.48 ± 0.07 b | 0.48 ± 0.02 c | 1.97 ± 0.08 c | 0.38 ± 0.02 c | |
| Red-P-hLL | 1.69 ± 0.07 a | 0.53 ± 0.02 b | 2.22 ± 0.08 b | 0.44 ± 0.02 b | |
| ‘Tonus’ | White | 1.54 ± 0.06 ab | 0.67 ± 0.03 b | 2.21 ± 0.07 b | 0.42 ± 0.02 b |
| Blue-P-hLL | 1.63 ± 0.04 a | 0.78 ± 0.03 a | 2.41 ± 0.07 a | 0.55 ± 0.02 a | |
| Red-P-hLL | 1.48 ± 0.04 b | 0.60 ± 0.02 c | 2.08 ± 0.05 c | 0.40 ± 0.02 b | |
| Cultivar | Experiment Variant | Content, mg·100 g−1 Raw Weight of Plants | |||||
|---|---|---|---|---|---|---|---|
| K | Na | Mg | Ca | S | P | ||
| ‘Ararat’ | White | 467.47 ± 9.12 b | 9.65 ± 0.40 b | 29.47 ± 1.06 b | 48.86 ± 1.36 b | 6.10 ± 0.31 c | 48.33 ± 1.28 b |
| Blue-P-hLL | 503.60 ± 6.91 a | 8.69 ± 0.45 c | 31.35 ± 1.12 b | 42.66 ± 1.81 c | 7.22 ± 0.30 b | 54.00 ± 1.28 a | |
| Red-P-hLL | 485.58 ± 14.67 a | 15.86 ± 0.58 a | 33.76 ± 1.11 a | 53.79 ± 1.87 a | 9.59 ± 0.28 a | 52.60 ± 1.38 a | |
| ‘Tonus’ | White | 438.11 ± 14.73 c | 15.80 ± 0.41 a | 39.38 ± 0.78 c | 85.68 ± 2.89 b | 12.24 ± 0.23 c | 67.58 ± 2.35 b |
| Blue-P-hLL | 832.28 ± 22.24 a | 14.01 ± 0.38 b | 86.12 ± 3.32 a | 125.25 ± 2.68 a | 18.90 ± 0.64 b | 80.93 ± 1.63 a | |
| Red-P-hLL | 523.43 ± 8.38 b | 13.58 ± 0.25 b | 48.19 ± 1.20 b | 126.62 ± 3.71 a | 28.52 ± 1.10 a | 80.56 ± 1.01 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knyazeva, I.V.; Panfilova, O.; Vershinina, O.; Kahramanoğlu, I.; Smirnov, A.A.; Titenkov, A. The Effect of Additional Night and Pre-Harvest Blue and Red LEDs and White Lighting During the Day on the Morphophysiological and Biochemical Traits of Basil Varieties (Ocimum basilicum L.) Under Hydroponic Conditions. Horticulturae 2025, 11, 784. https://doi.org/10.3390/horticulturae11070784
Knyazeva IV, Panfilova O, Vershinina O, Kahramanoğlu I, Smirnov AA, Titenkov A. The Effect of Additional Night and Pre-Harvest Blue and Red LEDs and White Lighting During the Day on the Morphophysiological and Biochemical Traits of Basil Varieties (Ocimum basilicum L.) Under Hydroponic Conditions. Horticulturae. 2025; 11(7):784. https://doi.org/10.3390/horticulturae11070784
Chicago/Turabian StyleKnyazeva, Inna V., Olga Panfilova, Oksana Vershinina, Ibrahim Kahramanoğlu, Alexander A. Smirnov, and Andrey Titenkov. 2025. "The Effect of Additional Night and Pre-Harvest Blue and Red LEDs and White Lighting During the Day on the Morphophysiological and Biochemical Traits of Basil Varieties (Ocimum basilicum L.) Under Hydroponic Conditions" Horticulturae 11, no. 7: 784. https://doi.org/10.3390/horticulturae11070784
APA StyleKnyazeva, I. V., Panfilova, O., Vershinina, O., Kahramanoğlu, I., Smirnov, A. A., & Titenkov, A. (2025). The Effect of Additional Night and Pre-Harvest Blue and Red LEDs and White Lighting During the Day on the Morphophysiological and Biochemical Traits of Basil Varieties (Ocimum basilicum L.) Under Hydroponic Conditions. Horticulturae, 11(7), 784. https://doi.org/10.3390/horticulturae11070784








