Abstract
Chinese kale sprouts are widely cultivated and consumed due to their elevated nutritional worth and ease of cultivation in controlled environments. This study focused on the effects of several light types (white, red, and blue) both by themselves and in conjunction with calcium chloride (CaCl2) on the physiological development and nutritional value of Chinese kale sprouts. Analysis proved that under differing light treatments, white light resulted in the highest pigment content, and red light markedly increased plant height, fresh mass, and soluble sugar accumulation. Additionally, blue light significantly enhanced the antioxidant capacity. When light quality and calcium chloride were applied together, fresh mass increased by 9.50%, 9.85%, and 12.47% (compared with single light treatments), and significantly elevated the indolic glucosinolate content. Besides, red light combined with calcium chloride also enhanced the antioxidant capacity (ferric reducing antioxidant power (FRAP) content) compared with red light alone, increasing by 14.31%. This research determined that red light in conjunction with calcium chloride obtained the highest yield of sprouts, and it can also offer new strategies for increasing the antioxidant capacity, while the blue light in conjunction with calcium chloride can provide more ways for accumulating glucosinolates. In conclusion, incorporating light quality and calcium chloride treatments in Chinese kale sprout cultivation is an effective approach for increasing yield and quality.
1. Introduction
Chinese kale (Brassica oleracea var. alboglabra), an annual herbaceous plant of the genus Brassica of the Cruciferae family, is a distinctive vegetable in China, rich in nutrients and bioactive substances, such as glucosinolates and phenolic compounds [1]. These compounds are associated with the prevention of chronic diseases and cancer [2]. Moreover, Chinese kale seeds are readily accessible, and the sprouts exhibit a short growth cycle, are not limited by time or space, do not require fertilizer, only need to be watered, and can be harvested in 7–10 d. Notably, the glucosinolate content in sprouts is much higher than that in other edible parts (e.g., leaves and stems), making them highly appealing to consumers [1].
Light is a critical environmental component that affects plant growth and development stages. It is essential for controlling metabolic pathways, morphogenesis, and physiological processes [3]. Different light qualities exert specific effects on plant development [4]. In recent years, red and blue light have attracted a lot of attention as vital energy sources for photosynthetic carbon assimilation [5]. According to earlier research, in terms of growth, red light promotes the height of tomato seedlings, whereas blue light has been shown to be inhibitory [6]. Similarly, red light enhances fresh mass accumulation in water spinach, while blue light restricts stem elongation [7]. In terms of nutritional quality, red light (660 nm) promotes the accumulation of soluble sugars in wheat and lentil sprouts [8], whereas blue light increases the ascorbic acid content and antioxidant activity in Chinese cabbage seedlings [9]. In particular for Chinese kale sprouts, it has been reported that red light encourages the formation of glucosinolates, whereas Chinese kale sprouts grown in blue light (470 nm) have a significantly greater total phenolic level and a strong antioxidant possibility [10]. These results emphasize the potential of light quality for sprout culture optimization by demonstrating the distinct effects it has on plant morphology and nutritional quality. So, by exploring their specific effects, we can regulate the growth and development of sprouts and improve nutritional benefits.
In cellular signaling, calcium serves as a secondary messenger and is involved in plant metabolic processes [11]. It is also involved in multiple pathways of plant responses to environmental stimuli and regulates photosynthesis; it affects gas exchange related to photosynthesis by regulating stomatal movement and also directly or indirectly regulates several photosynthetic proteins [12]. A variety of Ca2+-related proteins located in the chloroplast outer membrane may directly link the cytoplasmic signal with the chloroplast signal [12]. By modifying primary metabolism, substance transport, and signal transduction, calcium chloride has been shown to increase soybean sprout yield and healthy qualities [13]. Additionally, calcium also promotes the accumulation of secondary metabolites, e.g., calcium chloride increases the antioxidant content in broccoli sprouts [14]. It has been shown that applying a 10 mM solution of calcium chloride pre-harvest enhances broccoli microgreens’ yield and markedly increases their glucosinolate content [15]. However, when exogenous calcium is applied in excess, it may cause ionic toxicity and inhibit growth in plants [16]. High calcium treatments also inhibit carbon assimilation processes [17].
Some studies have shown that Ca2+ signaling in the light signaling pathways regulate plant growth by modulating brassinosteroid biosynthesis in response to changes in ambient light and temperature, and light-induced Ca2+ entry into the cytoplasm from the extracellular space will promote photomorphogenesis [18], showing that light and Ca2+ signaling have some interaction. The influence of calcium chloride and light quality treatments on cruciferous sprout development and nutritional quality is still understudied. By examining the effects of various light characteristics both alone and in conjunction with calcium chloride treatments, this study seeks to provide a theoretical scientific foundation for the agricultural production and quality enhancement of Chinese kale sprouts.
2. Materials and Methods
2.1. Plant Materials and Treatments
The Chinese kale variety identified as ‘Sijicutiao’ (produced by Guangzhou Hongye Seed Technology Co., ltd., Guangzhou, China) was used as the material in this experiment. Four grams of seeds were soaked in a centrifuge tube containing 40 mL of 55 °C distilled water for 10 min and then placed in a thermostatic water bath at 37 °C for 3 h. Subsequently, seedlings were evenly spaced in a hydroponic plastic seedling tray (22.5 cm × 14.4 cm × 2.5 cm). After two days of dark culture in adjustable light incubators (Jiangnan Co. Ltd., Ningbo, China), they were exposed to white (400–700 nm, 6500 K), red (660 nm), and blue light (445 nm) in light incubators. The experiment was divided into six treatments: white light (W), white light + 10 mM calcium chloride solution (WC), red light (R), red light + 10 mM calcium chloride solution (RC), blue light (B), and blue light + 10 mM calcium chloride solution (BC) (Table 1). The incubation conditions comprised 80 µmol·m−2·s−1 of light intensity, 25 °C daytime and 23 °C nighttime temperatures, a photoperiod of 16 h of light and 8 h of darkness, and 75% humidity. Photographs were taken at 0, 2, 4, 6, 8, and 10 days after sowing. Distilled water or 10 mM calcium chloride solution was applied once in the morning and once in the evening at 3, 4, 5, 6, 7, 8, and 9 days after sowing. On the 10th day, the sprouts were harvested and sampled by cutting them off the roots, weighing the fresh weight of the aboveground section, freezing in liquid nitrogen, and preserving in a −80 °C refrigerator. They were freeze-dried and analyzed for nutritional indicators.

Table 1.
Names of experimental treatments and their abbreviations.
2.2. Growth Parameters
A total of 30 Chinese kale sprouts were selected at random from each treatment, with 10 plants per replication. A ruler was used to measure the plant height of sprouts. The length and width of a single cotyledon were measured, and the single cotyledon was approximated as a rectangle to calculate the leaf area, with the area formula length × width. The area of the single cotyledon was doubled to represent the total leaf area. The above-ground weight (fresh weight of the above-ground part after root removal) was weighed using an electronic balance. Each treatment was reproduced three times.
2.3. Chlorophylls and Carotenoids Content
The pigment content was analyzed by high performance liquid chromatography (HPLC). The lyophilized powder (50 mg) was weighed and extracted with 10 mL of acetone. The samples were sonicated for 20 min, and centrifuged at 4000× g at room temperature for 5 min. The supernatant was filtered through 0.22 μm nylon syringe filters and analyzed by high-performance liquid chromatography (HPLC). HPLC analysis of chlorophylls and carotenoids was carried out using an Agilent 1260 instrument with a variable wavelength detector (VWD) (Agilent Technologies, Inc., Palo Alto, CA, USA). Samples (10 μL) were separated at 30 °C on a Waters C18 column using isopropanol and 80% acetonitrile–water at a flow rate of 0.5 mL min−1. Absorbance was detected at 448 and 428 nm [19].
2.4. Soluble Sugar Content
Freeze-dried sample powder (50 mg) was extracted in 10 mL of distilled water for 20 min at 90 °C, following which the homogenates were centrifuged at 4000× g for 5 min. A combination of 1 mL of sample extract, 0.5 mL anthrone-ethyl acetate reagent, and 5 mL concentrated sulfuric acid was homogenized, boiled for 5 min, and then cooled rapidly using ice water. The absorbance of the reaction mixtures was measured at 630 nm using a spectrophotometer, and the soluble sugar content was determined using a standard curve of sucrose [19].
2.5. Soluble Protein Content
Freeze-dried sample powder (50 mg) was soaked in 10 mL of distilled water. The solution was stirred for 30 s using a vortex mixer (DLAB Scientific co., ltd., Shanghai, China), after which it was allowed to settle for 30 min. The solution was then centrifuged for 5 min at 4000× g, and 1 mL was transferred to a polypropylene tube. Subsequently, Coomassie brilliant blue G-250 was combined with 1 mL of supernatant. The absorbance was measured at 595 nm within 20 min of the reaction. Soluble proteins in the samples were calculated based on a standard curve of bovine serum albumin [20].
2.6. Ascorbic Acid Content
Freeze-dried sample powder (50 mg) was extracted with 5 mL of 1.0% (w/v) oxalic acid and subsequently centrifuged 5 min at 4000× g. Each sample was filtered through a 0.45 mm cellulose acetate filter. HPLC analysis of ascorbic acid was carried out using a Waters instrument with a Model 2996 PDA detector (Waters Inc., Milford, CT, USA). Sample (20 µL) were separated at room temperature on a Waters Spherisorb C18 column (250 × 4.6 mm id; 5 µm particle size), using a solvent of 0.1% oxalic acid at a flow rate of 1.0 mL min−1. The amount of ascorbic acid was calculated from absorbance values at 243 nm, using authentic ascorbic acid as a standard. The results were expressed as mg g−1 dry weight [19].
2.7. Flavonoids Content
Freeze-dried sample powder (40 mg) was extracted in 50% ethanol and incubated at room temperature for 24 h in the dark. The suspension was then centrifuged at 4000× g for 5 min at room temperature. A 1.2 mL aliquot of the supernatant was mixed with 60 mL 2% aluminum trichloride, the same volume (60 mL) of 1 mol L−1 potassium acetate, and 1.680 mL distilled water. Absorption was read at 415 nm after 40 min. The flavonoid content was determined using a standard calibration curve with quercetin in 50% ethanol as a reference standard and expressed as mg of quercetin equivalence per g dry weight [19]
2.8. Total Phenolics Content
Total phenolics were extracted with 50% ethanol and incubated at room temperature for 24 h in the dark. The suspension was centrifuged at 4000× g for 5 min at room temperature. The supernatant (300 mL) was mixed with 1.5 mL 0.2 mol L−1 Folin–Ciocalteu reagent in a polypropylene tube; after 3 min, 1.2 mL saturated sodium carbonate was added to each polypropylene tube. The mixtures were allowed to stand for 20 min at room temperature, and the absorbance was measured at 760 nm [19].
2.9. Ferric Reducing Antioxidant Power (FRAP)
To extract lyophilized powder (40 mg), 50% ethanol was used. After completing centrifugation, the extracted samples (300 μL) were combined with 2.7 mL of FRAP working solution and incubated at 37 °C. After 10 min of incubation at 37 degrees Celsius, the absorbance was measured at 593 nm and computed the value [19].
2.10. Glucosinolates Content
Lyophilized powder (100 mg) was heated in 5 mL of water for 10 min, resulting in a liquid layer that was transferred to a DEAE-Sephadex A-25 column (GE Healthcare, Piscataway, NJ, USA). Glucosinolates were converted into desulfated equivalents using aryl sulfatase. Following this treatment, the desulfated glucosinolates were isolated and evaluated by HPLC [19].
2.11. Statistical Analysis
The DPS data processing system version 9.01 was used for statistical analysis. Two-factors analysis of variance was performed on the data (Supplementary Table S1). Graphs were created using OriginPro 2024 (Origin Lab Corporation, Northampton, MA, USA). Principal component analysis (PCA) was performed using SIMCA 14.1 Demo software (Umetrics, Malmö, Sweden).
3. Results
3.1. Sprout Growth
Regarding growth appearance, Chinese kale sprouts treated with R greatly outgrew those treated with W in terms of height, while those treated with B significantly decreased in height. The R treatment plant height was 1.25 times greater than the W treatment. The R treatment had the smallest leaf area, 26.42% smaller than the W treatment. Chinese kale sprouts showed no significant change in plant height following the combined application concerning light quality and calcium chloride; nevertheless, the leaf area rose significantly, with WC, RC, and BC treatments being 1.26, 1.28, and 1.24 times greater than W, R, and B treatments, respectively (Figure 1A–C).
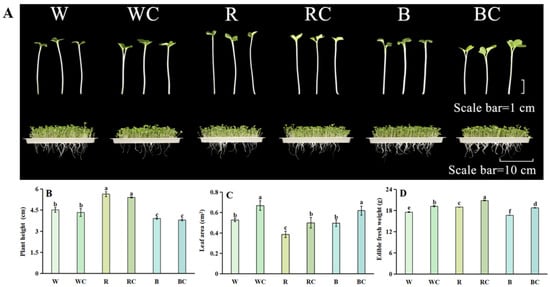
Figure 1.
Chinese kale sprouts’ growth morphology and corresponding physiological indicators following different treatments. (A) Growth morphology of Chinese kale sprouts under white light (W), white light + 10 mM calcium chloride solution (WC); red light (R), red light + 10 mM calcium chloride solution (RC), blue light (B), blue light + 10 mM calcium chloride solution (BC) treatments; (B) plant height of Chinese kale sprouts; (C) leaf area of Chinese kale sprouts; (D) fresh weight of Chinese kale sprouts. According to the LSD test, different letters in the figures indicate significant differences between treatments (p < 0.05).
The fresh weight of Chinese kale sprouts was greatly increased by the R treatment, 1.08 times higher than the W treatment, while it was significantly decreased by the B treatment, 5.07% lower than the W treatment. After undergoing both light quality and calcium chloride treatments, with respect to the W, R, and B treatments, the WC, RC, and BC treatments’ fresh weight performed 1.10, 1.10, and 1.12 times higher, respectively (Figure 1D).
3.2. Chlorophylls and Carotenoids
Chinese kale sprouts were found to contain four carotenoids and two chlorophylls (Figure 2A–H). The W treatment had the highest total chlorophyll content under several light quality treatments, second with the B treatment and the R treatment (Figure 2C). When light quality and calcium chloride together were used, the total chlorophyll quantity of the WC, RC, and BC treatments significantly decreased by 9.37%, 16.51%, and 23.86%, respectively. Among them, the chlorophyll a and chlorophyll b contents, as well as the total chlorophyll content, all followed the same trend (Figure 2A–C), indicating that the W treatment was higher than the R and B treatments and that the total chlorophyll content greatly decreased after the combined light quality and calcium chloride treatment.
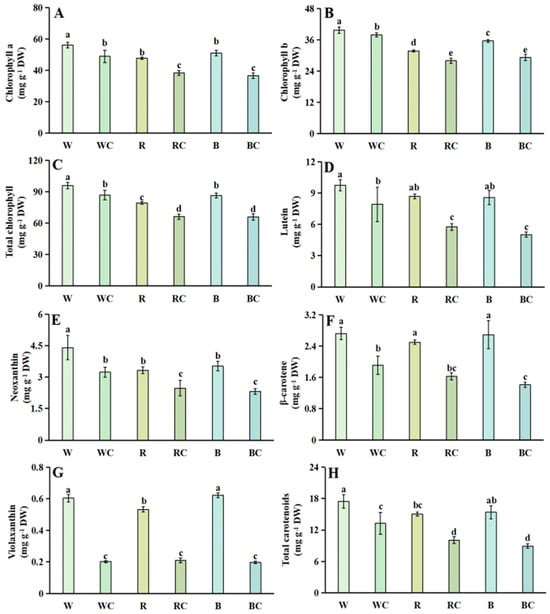
Figure 2.
Chlorophyll and carotenoid accumulation in Chinese kale sprouts following different treatments. (A) chlorophyll a; (B) chlorophyll b; (C) total chlorophyll; (D) lutein; (E) neoxanthin; (F) β-carotene; (G) violaxanthin; (H) total carotenoids. According to the LSD test, different letters in the figures indicate significant differences between treatments (p < 0.05).
Among the four carotenoids, lutein was the most prevalent, followed by neoxanthin and β-carotene. The least prominent carotenoid was violaxanthin (Figure 2D–G). The total carotenoids of the B treatment did not significantly differ from the content of the W treatment, and the R treatment had the smallest amounts of total carotenoids under diverse light quality treatments and was 14.02% lower than the W treatment. After calcium chloride and light quality treatment, the total carotenoids of the WC, RC, and BC treatments were markedly lower at 24.10%, 33.02%, and 41.94%, respectively (Figure 2H). Among them, the changes in lutein and β-carotene contents were not significantly different between light qualities, and the contents were significantly reduced after the combined treatment of light qualities and calcium chloride (Figure 2D–F). The neoxanthin concentration of Chinese kale sprouts was dramatically decreased by both R and B treatments across all three light quality treatments; however, there was no apparent distinction comparing the two R and B treatments. In comparison to the W, R, and B treatments, the WC, RC, and BC treatments decreased the neoxanthin concentration by 26.59%, 25.60%, and 34.38%, respectively, following the simultaneous application of light quality and calcium chloride (Figure 2E). Regarding the violaxanthin content, during different lighting situations, the violaxanthin content was lowest in the R treatment, but there was no discernible difference between the two treatments of W and B. Additionally, there was no noticeable distinction between the WC, RC, and BC treatments following the combined application of calcium chloride and light quality (Figure 2G).
3.3. Soluble Sugars and Soluble Proteins
Regarding soluble sugars, the R treatment had the highest soluble sugar content under several light quality treatments, next by the B treatment and the W treatment. The R treatment additionally generated a 1.11-fold increase in content when compared to the W treatment. After light quality and calcium chloride were interconnected, the soluble sugar content of the WC and RC treatments dropped considerably by 10.23% and 9.21%, respectively in comparison to the W and R treatments, while the BC treatment’s content did not change significantly (Figure 3A).
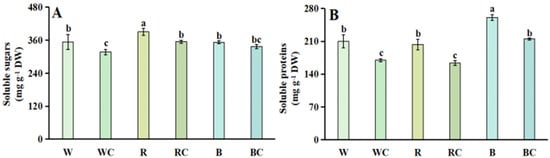
Figure 3.
The amount of soluble sugar and soluble protein in Chinese kale sprouts following different treatments. (A) soluble sugar; (B) soluble protein. According to the LSD test, different letters in the figures indicate significant differences between treatments (p < 0.05).
Regarding soluble protein, under several light quality treatments, the B treatment had the maximum soluble protein amount, preceded by the W treatment and the R treatment. Contrasting the B treatment to the W treatment, there was a notable 1.24-fold rise. The soluble protein content of WC, RC, and BC treatments was considerably reduced by 19.26%, 19.51%, and 17.53%, respectively, in comparison to W, R, and B treatments with the simultaneous treatment of light quality and calcium chloride (Figure 3B).
3.4. Antioxidant Content and Antioxidant Capacity
Regarding ascorbic acid, under diverse light quality treatments, the ascorbic acid level was significantly elevated under B treatment (1.08 times higher than under W treatment) and much lower under R treatment (19.68% lower than under W). The ascorbic acid content of the RC treatment substantially rose after the light quality and calcium chloride treatment combination to 1.09 times higher than that of the R treatment. In contrast, the ascorbic acid content of the WC and BC treatments significantly decreased to 8.12% and 14.09% lower than that of the W and B treatments, respectively (Figure 4A).
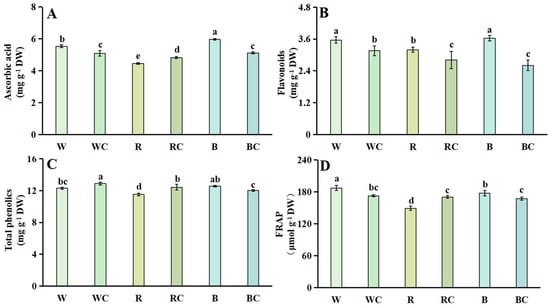
Figure 4.
Antioxidant content and antioxidant capacity of Chinese kale sprouts following different treatments. (A) ascorbic acid; (B) Flavonoids; (C) total phenolics; (D) FRAP. According to the LSD test, different letters in the figures indicate significant differences between treatments (p < 0.05).
The greatest levels of flavonoids and total phenolics were found in treatments B and R that had varying light quality. When light quality and calcium chloride were used together, the flavonoid content significantly decreased, while the total phenolic content significantly increased in WC and RC treatments and reduced in BC treatments. These treatments were 1.05 and 1.08 times higher than W and R treatments, respectively (Figure 4B,C). The greatest FRAP level was detected in the W treatment, followed by the B treatment, and the least in the R treatment. When calcium chloride and light quality were coupled, the considerable content increased significantly in the RC treatment—which was 1.14 times higher than the R treatment—but decreased in the WC and BC treatments (Figure 4D).
3.5. Glucosinolates
Five aliphatic and four indolic glucosinolates were identified in Chinese kale sprouts (Figure 5A–I).
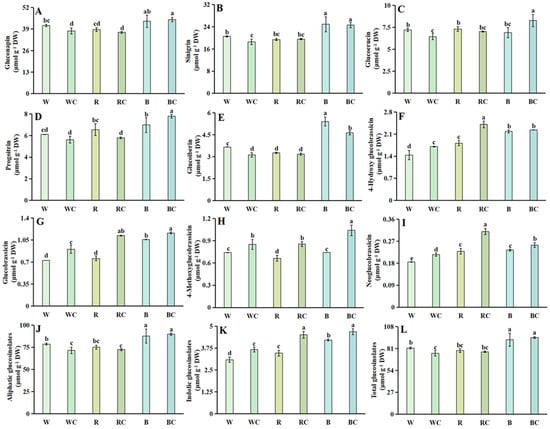
Figure 5.
Glucosinolate content in Chinese kale sprouts following different treatments. (A) gluconapin; (B) sinigrin; (C) glucoerucin; (D) progoitrin; (E) glucoiberin; (F) 4-hydroxy glucobrassicin; (G) glucobrassicin; (H) 4-methoxyglucobrassicin; (I) neoglucobrassicin; (J) aliphatic glucosinolates; (K) indolic glucosinolates; (L) total glucosinolates. According to the LSD test, different letters in the figures indicate significant differences between treatments (p < 0.05).
Gluconapin, sinigrin, glucoerucin, progoitrin, and glucoiberin were among the aliphatic glucosinolates (Figure 5A–E). The R treatment extensively decreased the glucoiberin content by 10.93%, but the B treatment greatly boosted sinigrin, progoitrin, and glucoiberin contents by 1.20, 1.14, and 1.48 times, respectively, in comparison to the W treatment (Figure 5B–D). RC treatment significantly decreased progoitrin; BC treatment significantly decreased the glucoiberin content; and the BC treatment significantly increased glucoerucin and progoitrin, which were 1.20-fold and 1.12-fold higher than those in the B treatment, respectively. After the incorporated treatments, the WC treatment extensively diminished gluconapin, sinigrin, glucoerucin, and glucoiberin contents by 8.05%, 9.74%, 10.69%, and 14.75%, respectively. (Figure 5A–E). B treatment boosted the generation of aliphatic glucosinolate content, which was substantially increased by 1.12 times, whereas the R treatment had no discernible effect on the total aliphatic glucosinolate content when compared to the W treatment. The total aliphatic glucosinolate content was significantly reduced by 9.03 times in WC treatment compared to W treatment following the combined treatments, while the total aliphatic glucosinolates under RC and BC treatments did not significantly change (Figure 5J).
Indolic glucosinolates included 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin (Figure 5F–I). Under various light quality treatments, R treatment significantly inhibited 4-methoxyglucobrassicin contents while significantly promoting the accumulation of 4-hydroxyglucobrassicin and neoglucobrassicin contents, which were 1.26 and 1.28 times higher than those of the W treatment, respectively. The four indolic glucosinolates were encouraged to accumulate by the B treatment, and the amounts of 4-hydroxyglucobrassicin, glucobrassicin, and neoglucobrassicin increased considerably, increasing by 1.51 times, 1.45 times, and 1.28 times, respectively, compared to the W treatment. All four indolic glucosinolates significantly increased following the combination treatment of calcium chloride and light quality (Figure 5F–I). In terms of total indolic glucosinolate content, in comparison to the W treatment, the R and B treatments both markedly increased the generation of total indolic glucosinolate content by 1.12 and 1.37 times, respectively. The accrual of total indolic glucosinolate content was further facilitated by the combined light quality and calcium chloride treatments; the WC, RC, and BC treatments were 1.19, 1.30, and 1.11 times greater than the W, R, and B treatments, respectively (Figure 5K).
Regarding the total glucosinolate content, under differing light conditions, the total glucosinolate content of R treatment was not substantially affected, while B treatment significantly raised the total glucosinolate content, making it 1.13 times more than that of W treatment. The WC treatment showed a major decrease in the total glucosinolate content after the combination treatment of light quality and calcium chloride, which was 7.97% lower than the W treatment. In contrast, the RC and BC treatments did not show any significant modification in total glucosinolate content (Figure 5L).
3.6. PCA
The first component (PC1) and the second component (PC2) explained 44.4% and 30.7% of the variation in the data, respectively (Figure 6A). PC1 distinguished between calcium chloride and non-calcium chloride treatments under red and blue light, and PC2 distinguished between calcium chloride and non-calcium chloride treatments under white light. From the results of the loading plot (Figure 6B), the first and second quadrants contained the majority of the indicators pertaining to the quality and development of Chinese kale sprouts. The findings indicated that while the BC treatment group had a greater effect on the glucosinolate content, the W treatment group influenced the pigment content better. The B treatment had a better impact on soluble protein and antioxidants, and the RC treatment group had the biggest impact on leaf area and fresh weight. The fourth quadrant showed the distribution of soluble sugar and plant height, suggesting that the WC and R treatment groups had a greater impact on these two parameters.
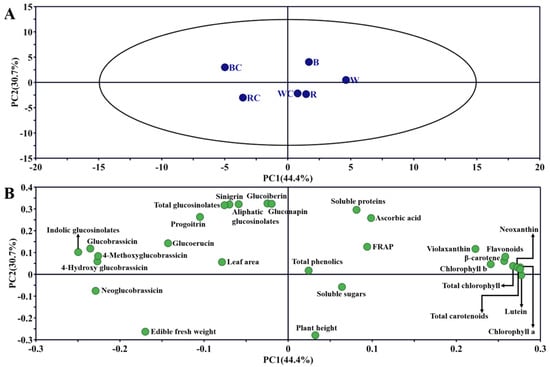
Figure 6.
Principal component analysis in Chinese kale sprouts following different treatments. (A) PCA score plot; (B) loading plot.
3.7. Correlation Analysis
There was a negative correlation between leaf area and soluble sugar (−0.98), a negative correlation between fresh weight and soluble protein (−0.89), and a positive correlation between aliphatic glucosinolates (0.82). Flavonoids were positively correlated with chlorophyll a (0.94), total chlorophyll (0.90), β-carotene (0.95), and total carotenoids (0.94), and indolic glucosinolates were negatively correlated with total chlorophyll (−0.83) and total carotenoids (−0.86). Additionally, there was a strong correlation (0.93) between total carotenoids and total chlorophyll. Sinigrin (0.98), gluconapin (0.97), and total aliphatic glucosinolates (1.00) all showed positive correlations with total glucosinolates (Figure 7, Supplementary Table S2).
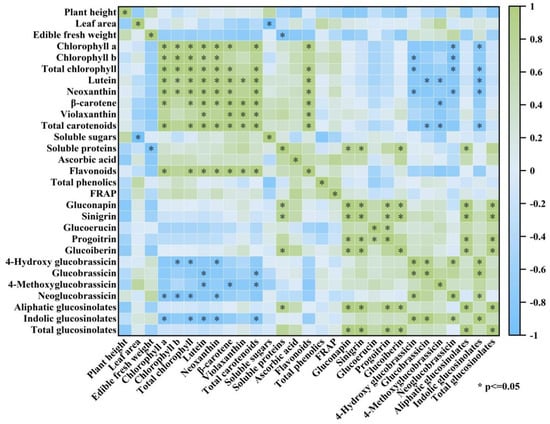
Figure 7.
Correlation shows the relationships between Chinese kale sprouts’ antioxidant capacity and nutritional components.
3.8. Analysis of Variance
The results of variance analysis showed that the effect of calcium chloride on chlorophyll a, chlorophyll b, total chlorophylls, lutein, neoxanthin, β-carotene, total carotenoids, gluconapin, sinigrin, glucoiberin, 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin, aliphatic glucosinolates, indolic glucosinolates, and total glucosinolates in Chinese kale sprouts was greater than that of light quality. The interaction between light quality and calcium chloride had greater effects on plant height, leaf area, edible fresh weight, neoxanthin, β-carotene, violaxanthin, total carotenoids, soluble sugar, soluble protein, ascorbic acid, flavonoids, gluconapin, glucoiberin, 4-methoxyglucobrassicin, aliphatic glucosinolates, and total glucosinolates than a single factor (Supplementary Table S3).
4. Discussion
Because of its high nutrient content, Chinese kale sprouts, a novel vegetable, have gained more and more popularity among consumers [1]. Furthermore, a number of physical and chemical parameters control the nutritional value of Chinese kale sprouts [21,22]. Thus, we examined how the development and nutritional value were affected by different light qualities alone (W, R, and B) and in conjunction with calcium chloride treatments (WC, RC, and BC).
4.1. The Effect of Different Light Qualities Alone and in Conjunction with Calcium Chloride on the Development of Chinese Kale Sprouts
The growth status of sprouts can be reflected in plant height and fresh weight. The appearance morphology and growth potential of sprouts differed significantly under different light environments, which was the result of their different adaptations to the regulation of the light environment [10]. The results of this study demonstrated that red light significantly boosted plant height and fresh weight, whereas blue light greatly reduced the plant height and fresh weight and impeded the growth of Chinese kale sprouts. This is comparable to Artés-Hernández et al.’s findings [23]. Specifically, red light may promote plant growth and development by increasing the soluble sugar in plants, which is consistent with the observation of green leaf lettuce growth under red light [24]. The inhibition of Chinese kale sprouts growth under blue light may be caused by changes in phytohormone levels in plants. It has been demonstrated that blue light inhibits the growth of Arabidopsis thaliana by decreasing the level of IAA in plants [25]. Calcium, as an essential plant nutrient and cellular signal, is closely related to various physiological and developmental processes in plants. Pre-harvest exogenous calcium treatment significantly enhanced soybean sprout yield by enhancing the hypocotyl and radical length of the soybean sprout [26] and promoted peanut seedling growth and development by stimulating the soluble sugar content accumulation [27]. The results of our investigation showed that the simultaneous treatment of calcium chloride and light quality substantially boosted the fresh weight and leaf area of Chinese kale sprouts. This may be because calcium is involved in growth-related signaling, which allocates carbon from the plant to leaf growth and increases photon capture by enlarging the leaf area [28]. Leaf area is an important consideration for determining plant development [29], and there is a near-linear relationship between leaf mass and plant biomass during the trophic stage [30]. Thus, when leaf area increases, leaf mass rises, and plant biomass increases.
Plant photosynthesis, growth, and development depend on chlorophyll and carotenoids. Alteration of these pigment contents have an impact on plants’ ability to photosynthesize, which is a crucial marker of plant development. Plants’ ability to photosynthesize may be hampered when quantities of carotenoids and chlorophyll drop [31]. In the present study, it was found that the chlorophyll and carotenoids exhibited the highest levels under white light compared to different light quality treatments. This phenomenon was also verified in mustard sprouts [32]. Moreover, chlorophyll and carotenoids synthesized by mung bean sprouts under white light also showed significantly higher contents than those synthesized under red and blue light [33]. Following the combination of calcium chloride and light quality treatment, Chinese kale sprouts’ levels of carotenoids and chlorophyll dropped. This may be because light drives Ca2+ to enter chloroplasts, which controls enzymatic activities inside the chloroplasts, in addition to influencing the cytoplasmic concentration of free Ca2+ [17]. Calcium receptors (CAS) located on the chloroplast-like vesicle membranes are closely linked to the cytoplasmic calcium concentration ([Ca2+] cyt) [34]. Thus, the decrease in the chlorophyll concentration may be due to the high concentration of Ca2+, inhibiting the relevant processes within the chloroplasts and resulting in a decrease in chlorophyll synthesis.
4.2. The Effect of Different Light Qualities Alone and in Conjunction with Calcium Chloride on the Antioxidant Capacity of Chinese Kale Sprouts
Plants are rich in compounds called antioxidants, and the synergistic action of many antioxidants demonstrates the plants’ antioxidant capacity. Blue light exhibited the strongest antioxidant capacity under diverse light quality treatments, according to the current study’s analysis of Chinese kale sprouts’ diverse antioxidant components. This outcome is consistent with mustard sprouts growing under blue light [32]. Previous research efforts have also demonstrated a strong correlation between blue light and the improvement of the antioxidant capacity. According to an earlier study, pea seedlings’ phenolic component quantity rose when exposed to blue light [35]. Specifically, blue light enhances plants’ antioxidant capacity by obstructing negative regulators of ascorbic acid production, thus boosting ascorbic acid synthesis [36]. When light quality and calcium chloride treatment were combined, the antioxidant capacity of the composite combination of red light and calcium chloride was substantially enhanced. This phenomenon might be attributed to a complicated interplay between the impact of Ca2+ and red light. It has been experimentally proven that red light influences the calcium concentration ([Ca2+] cyt) in the cytoplasm and causes phytochromes in plants to engage in the corresponding signal transduction, which therefore governs plants development [37].
4.3. The Effect of Different Light Qualities Alone and in Conjunction with Calcium Chloride on the Glucosinolate Content of Chinese Kale Sprouts
As the main secondary metabolites in cruciferous crops [38], glucosinolates have important biological functions and are closely related to the resistance, nutritional quality, and flavor of cruciferous vegetables, and a number of elements influence the content. According to prior research, blue light may dramatically raise the amount of glucosinolates in pakchoi and exhibit a dosage effect [39]. Blue light increases the glucosinolate concentration of broccoli sprouts, but red light decreases the level of aliphatic glucosinolates [40], indicating that the glucosinolate content is impacted by light quality. In this investigation, we discovered that the concentration of glucosinolates in Chinese kale sprouts was altered under different light quality treatments. Blue light enhanced the glucosinolate content of Chinese kale sprouts, and this finding was also corroborated in broccoli sprouts [41]. Chinese kale sprouts’ total glucosinolate concentration varied non-significantly following combination treatment with calcium chloride and light quality. This is different from the results of calcium chloride treated broccoli microgreens, probably due to differences between varieties, which have different ranges of acceptance of external Ca2+ concentrations. In addition, the indolic glucosinolate content in Chinese kale sprouts increased significantly after the compound treatment. It has been shown that indolic glucosinolates have a defense function, and they indicate the defense status through their accumulation [42]. When high concentrations of calcium (Ca2+) outside the plant cause calcium concentrations ([Ca2+] cyt) in the cytoplasm to exceed threshold values, it will cause phytotoxicity, growth inhibition, and defense responses [43]. According to our findings, the increase in indolic glucosinolates in Chinese kale sprouts brought about by the combined treatments could be attributed to the high concentration of calcium ions in Chinese kale sprouts, which causes the plant to develop a defense state in which calcium ion homeostasis in its body is disrupted, causing the plant to shift from growth to defense.
5. Conclusions
This study demonstrated that white and blue light primarily affected the physiological and metabolic processes of Chinese kale sprouts, with the highest pigments in Chinese kale sprouts during white light treatment and boosted the antioxidant capacity during blue light treatment. Red light had a positive effect on Chinese kale sprout growth, increasing the plant height and fresh mass and encouraging the building up of the soluble sugar content. The combined treatment further enhanced the leaf area and fresh weight and promoted the formation of indolic glucosinolates. Among them, the red light combined with calcium chloride had the highest yield of sprouts, which was 1.28 times greater than that under red light alone, and greatly boosted the content of antioxidants, such as ascorbic acid, total phenolics, and FRAP level, which was 1.14 times higher than that under red light alone. The blue light and calcium chloride composite treatment had the highest content of aliphatic, indolic, and total glucosinolates, and the total glucosinolates reached 94.69 μmol g−1 DW. Therefore, red light and calcium chloride composite treatments was the most effective way for increasing yield, and they also boosted the antioxidant capacity. Blue light and calcium chloride composite treatments effectively promoted the accumulation of mustard oleoresin aspect content in Chinese kale sprouts. Distinct combinations of light quality and calcium chloride had differing effects on Chinese kale sprout growth and nutritional quality, and the use in different composite combinations may be targeted to increase yield and quality, offering more alternatives for Chinese kale sprout production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11070780/s1, Table S1: Physiological parameters in Chinese kale sprouts with light quality and combined with calcium chloride treatments; Table S2: Correlation of the nutritional components and antioxidant capacity in Chinese kale sprouts; Table S3: Estimation of growth index, health-promoting compounds and antioxidant capacity variance component ratio of Chinese kale sprouts.
Author Contributions
Conceptualization, B.S. and C.C.; investigation, H.L. (Hongxia Li) and W.C.; data curation, X.Y., K.L., Y.T. and Z.H.; writing—original draft preparation, H.L. (Hongxia Li) and W.C.; writing—review and editing, Y.T., Z.H., H.L. (Huanxiu Li), F.Z., C.C. and B.S.; funding acquisition, F.Z., C.C. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32372732, 32372683), Key Laboratory of Storage of Agricultural Products, Ministry of Agriculture and Rural Affairs (kt202410), and Tianjin Science and Technology Program (24ZYCGSN00950).
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, B.; Liu, N.; Zhao, Y.T.; Yan, H.Z.; Wang, Q.M. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Chang, J.Q.; Wang, M.Y.; Jian, Y.; Zhang, F.; Zhu, J.; Wang, Q.M.; Sun, B. Health-promoting phytochemicals and antioxidant capacity in different organs from six varieties of Chinese kale. Sci. Rep. 2019, 9, 20344. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Rehman, M.; Ullah, S.; Bao, Y.N.; Wang, B.; Peng, D.X.; Liu, L.J. Light-emitting diodes: Whether an efficient source of light for indoor plants. Environ. Sci. Pollut. Res. 2017, 24, 24743–24752. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Xu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, Y.; Xin, G.F.; Wei, M.; Yang, Q.C.; Mi, Q.H. Effects of red and blue light quality on nitrogen levels, activities and gene expression of key enzymes involved in nitrogen metabolism from leaves of tomato seedlings. Acta Hortic. Sin. 2017, 44, 768–776. [Google Scholar]
- Kitayama, M.; Nguyen, D.T.P.; Lu, N.; Takagaki, M. Effect of light quality on physiological disorder, growth, and secondary metabolite content of water spinach (Ipomoea aquatica Forsk) cultivated in a closed-type plant production system. Hortic. Sci. Technol. 2019, 37, 206–218. [Google Scholar] [CrossRef]
- Abdul, M.; Amana, K.; Wajahat, K.; Dilsat, B.K.; Muhammad, M.A.; Muhammad, J.; Shafiq, U.R.; Baber, A.; Alevcan, K.; Sana, W. Effect of light emitting diodes (LEDs) on growth, mineral composition, and nutritional value of wheat & lentil sprouts. Phyton-Int. J. Exp. Bot. 2024, 93, 1117–1128. [Google Scholar]
- Chang, H.K.; Eun, K.Y.; Muthusamy, M.; Jin, A.K.; Mi-Jeong, J.; Soo, I.L. Blue LED light irradiation enhances L-ascorbic acid content while reducing reactive oxygen species accumulation in Chinese cabbage seedlings. Sci. Hortic. 2020, 261, 108924. [Google Scholar]
- Qian, H.M.; Liu, T.Y.; Deng, M.D.; Miao, H.Y.; Cai, C.X.; Shen, W.S.; Wang, Q.M. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Gao, Q.Y.; Xiong, T.T.; Li, X.P.; Chen, W.X.; Zhu, X.Y. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wan, S.; Li, X. The significance of calcium in photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Yang, R.Q.; Zhou, Y.L.; Gu, Z.X. A comparative transcriptome and proteomics analysis reveals the positive effect of supplementary Ca2+ on soybean sprout yield and nutritional qualities. J. Proteom. 2016, 143, 161–172. [Google Scholar] [CrossRef]
- Yang, R.Q.; Hui, Q.R.; Gu, Z.X.; Zhou, Y.L.; Guo, L.P.; Shen, C.; Zhang, W.H. Effects of CaCl2 on the metabolism of glucosinolates and the formation of isothiocyanates as well as the antioxidant capacity of broccoli sprouts. J. Funct. Foods 2016, 24, 156–163. [Google Scholar] [CrossRef]
- Sun, J.H.; Kou, L.P.; Geng, P.; Huang, H.L.; Yang, T.B.; Luo, Y.G.; Chen, P. Metabolomic assessment reveals an elevated level of glucosinolate content in CaCl2 treated broccoli microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Georg, K.; Michael, M.; Joseph, A.M.H.; Erwin, L. Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol. 1988, 86, 423–428. [Google Scholar]
- Zhu, C.G.; Hu, Z.J.; Hu, C.Y.; Ma, H.X.; Zhou, J.; Xia, X.J.; Shi, K.; Foyer, C.H.; Yu, J.Q.; Zhou, Y.H. SlCPK27 cross-links SlHY5 and SlPIF4 in brassinosteroid-dependent photo- and thermo-morphogenesis in tomato. Proc. Natl. Acad. Sci. USA 2024, 121, e2403040121. [Google Scholar] [CrossRef]
- Sun, B.; Di, H.M.; Zhang, J.Q.; Xia, P.X.; Huang, W.L.; Jian, Y.; Zhang, C.L.; Zhang, F. Effect of light on sensory quality, health-promoting phytochemicals and antioxidant capacity in post-harvest baby mustard. Food Chem. 2021, 339, 128057. [Google Scholar] [CrossRef]
- Sun, B.; Tian, Y.X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef]
- Zhou, A.L.; Tang, J.Y.; Wu, S.; Di, H.M.; Ma, J.; Escalona, V.H.; Yu, X.N.; Liang, K.H.; Huang, Z.; Tang, Y.; et al. Low concentration of sodium chloride combined with melatonin improved the nutritional quality of Chinese kale sprouts. LWT-Food Sci. Technol. 2025, 225, 117903. [Google Scholar] [CrossRef]
- Zhou, A.L.; Zhang, Y.T.; Li, L.; Di, H.M.; Bian, J.L.; Ma, J.; Escalona, V.H.; Hong, H.J.; Li, H.X.; Tang, Y.; et al. Effects of combined treatment of light quality and melatonin on health-promoting compounds in Chinese kale sprouts. LWT-Food Sci. Technol. 2024, 199, 116137. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and visible spectrum LED lighting as abiotic elicitors of bioactive compounds in sprouts, microgreens, and baby leaves—A comprehensive review including their mode of action. Foods 2022, 11, 265. [Google Scholar] [CrossRef]
- Akvilė, V.; Aušra, B.; Viktorija, V.K.; Jurga, M.; Julė, J.; Algirdas, N.; Kristina, L.; Giedrė, S. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2020, 310, 125799. [Google Scholar]
- Xu, F.; He, S.B.; Zhang, J.Y.; Mao, Z.L.; Wang, W.X.; Li, T.; Hua, J.; Du, S.S.; Xu, P.B.; Li, L.; et al. Photoactivated CRY1 and phyB interact directly with AUX/IAA proteins to inhibit auxin signaling in Arabidopsis. Mol. Plant 2018, 11, 523–541. [Google Scholar] [CrossRef]
- Adhikari, A.; Sapkota, M.; Savidya, R.N.; Tosin, A.T.; Adam, M.; Alam, M.N.; Kwon, E.-H.; Kang, S.-M.; Shaffique, S.; Lee, I.-J. Calcium enhances the effectiveness of melatonin in improving nutritional properties of soybean sprouts and germination under salt and cadmium stress. Int. J. Mol. Sci. 2025, 26, 878. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, X.; Wang, R.; Hao, F.; Zhang, H.; Zhang, Y.; Lin, G. Exogenous calcium alleviates oxidative stress caused by salt stress in peanut seedling roots by regulating the antioxidant enzyme system and flavonoid biosynthesis. Antioxidants 2024, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Charlotte, M.M.G.; Eric, J.W.V.; Kate, R.S.O.; Laurentius, A.C.J.V.; Ronald, P. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar]
- Park, Y.J.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhou, X.; Huang, H.T.; Wang, C.; Xiao, X.X.; Wen, J.; Wu, J.M.; Zhou, S.; Víctor, R.D.; Lucas, G.R.; et al. ORANGE proteins mediate adaptation to high light and resistance to Pseudomonas syringae in tomato by regulating chlorophylls and carotenoids accumulation. Int. J. Biol. Macromol. 2025, 306, 141379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Tang, J.Y.; Cheng, W.J.; Yao, X.W.; Escalona, V.H.; Qian, G.P.; Ma, J.; Yu, X.N.; Li, H.X.; Huang, Z.; et al. Combination of light quality and melatonin regulates the quality in mustard sprouts. Food Chem. 2024, 23, 101560. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Xiang, N.; Chen, H.L.; Zhao, Y.H.; Wang, L.X.; Cheng, X.Z.; Guo, X.B. The modulation of light quality on carotenoid and tocochromanol biosynthesis in mung bean (Vigna radiata) sprouts. Food Chem.-Mol. Sci. 2023, 6, 100170. [Google Scholar] [CrossRef]
- Weinl, S.; Held, K.; Schlücking, K.; Steinhorst, L.; Kuhlgert, S.; Hippler, M.; Kudla, J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol. 2008, 179, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Chen, Y.Y.; Hu, T.T.; Zhang, S.J.; Zhang, Y.H.; Zhao, T.Y.; Yu, H.E.; Kang, Y.F. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Céline, B.; Kentaro, M.; Paul, D.; Guillaume, D.; Tim, B.; Jean-Philippe, M.; Joana, J.; Stéphanie, G.; Cédric, C.; Mickael, M.; et al. Blue light promotes ascorbate synthesis by deactivating the PAS/LOV photoreceptor that inhibits GDP-L-galactose phosphorylase. Plant Cell 2023, 35, 2615–2634. [Google Scholar]
- Zhao, Y.; Shi, H.; Pan, Y.; Lyu, M.H.; Yang, Z.X.; Kou, X.X.; Deng, X.W.; Zhong, S.W. Sensory circuitry controls cytosolic calcium-mediated phytochrome B phototransduction. Cell 2023, 186, 1230–1243. [Google Scholar] [CrossRef]
- Bansal, S.; Lakra, N.; Mishra, S.; Ahlawat, Y.K. Unraveling the potential of glucosinolates for nutritional enhancement and stress tolerance in Brassica crops. Veg. Res. 2024, 4, e015. [Google Scholar] [CrossRef]
- Mao, P.P.; Li, Q.M.; Li, Y.M.; Xu, Y.L.; Yang, Q.C.; Bian, Z.H.; Wang, S.; He, L.M.; Xu, Z.G.; Zheng, Y.J.; et al. The beneficial functions of blue light supplementary on the biosynthesis of glucosinolates in pakchoi (Brassica rapa L. ssp. chinensis) under greenhouse conditions. Environ. Exp. Bot. 2022, 197, 104834. [Google Scholar]
- Köksal, D.; Gölge, S.; Gamze, Ç.S. Effect of LED lights on the growth, nutritional quality and glucosinolate content of broccoli, cabbage and radish microgreens. Food Chem. 2023, 401, 134088. [Google Scholar]
- Xue, A.H.; Liang, W.J.; Wen, S.D.; Gao, Y.Y.; Huang, X.Y.; Tong, Y.Z.; Hao, Y.B.; Luo, L.P. Metabolomic analysis based on EESI-MS indicate blue LED light promotes aliphatic-glucosinolates biosynthesis in broccoli sprouts. J. Food Compos. Anal. 2021, 97, 103777. [Google Scholar] [CrossRef]
- Agerbirk, N.; De, V.M.; Kim, J.H. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Wang, C.; Tang, R.J.; Kou, S.H.; Xu, X.S.; Lu, Y.; Rauscher, K.; Voelfer, A.; Luan, S. Mechanisms of calcium homeostasis orchestrate plant growth and immunity. Nature 2024, 627, 382–388. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).