The Impact of Light Quality on the Growth and Quality of Celery
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
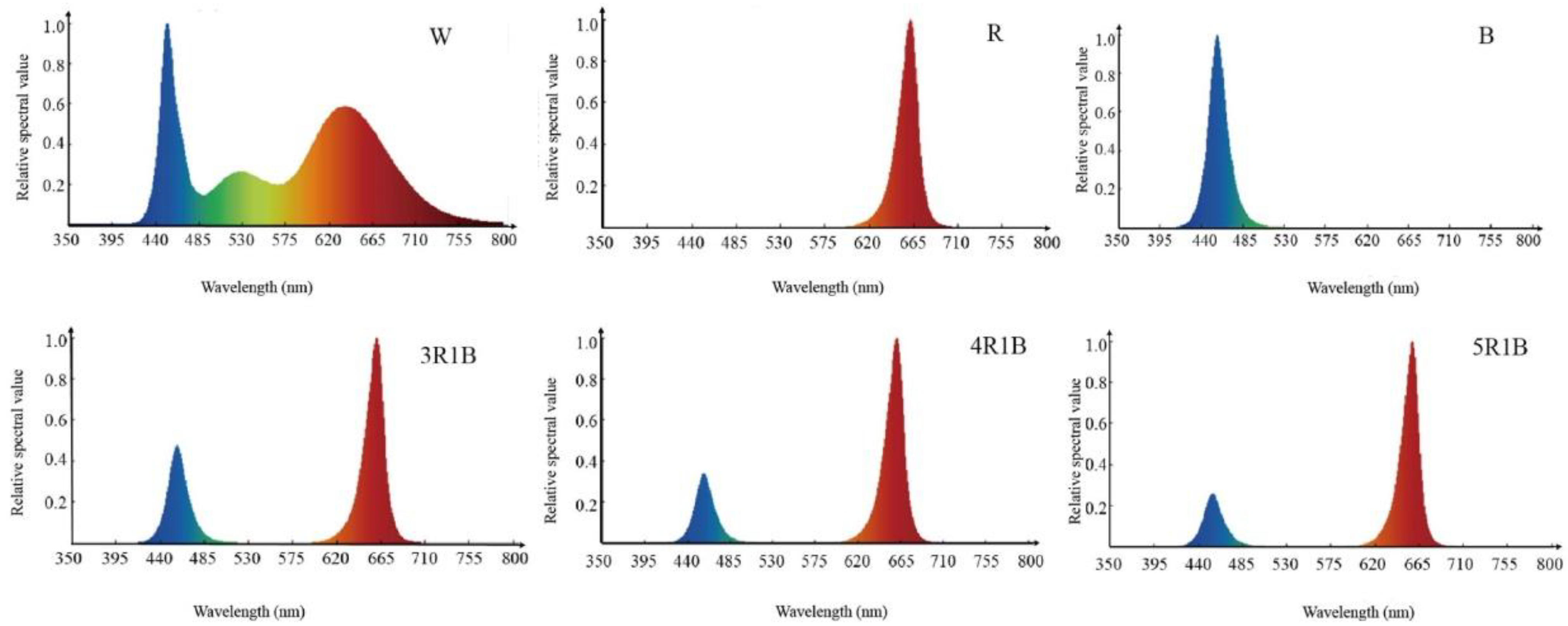
2.2. Experimental Design
2.3. Determination of Biomass
2.4. Determination of Photosynthetic Parameters
2.5. Determination of Quality Indicators
2.6. Determination of Mineral Elements
2.7. Determination of Enzyme Activities Associated with Major Flavonoid Compounds
2.8. Determination of Expression Analysis of Key Flavonoid-Related Genes
2.9. Statistical Analysis
3. Results and Analysis
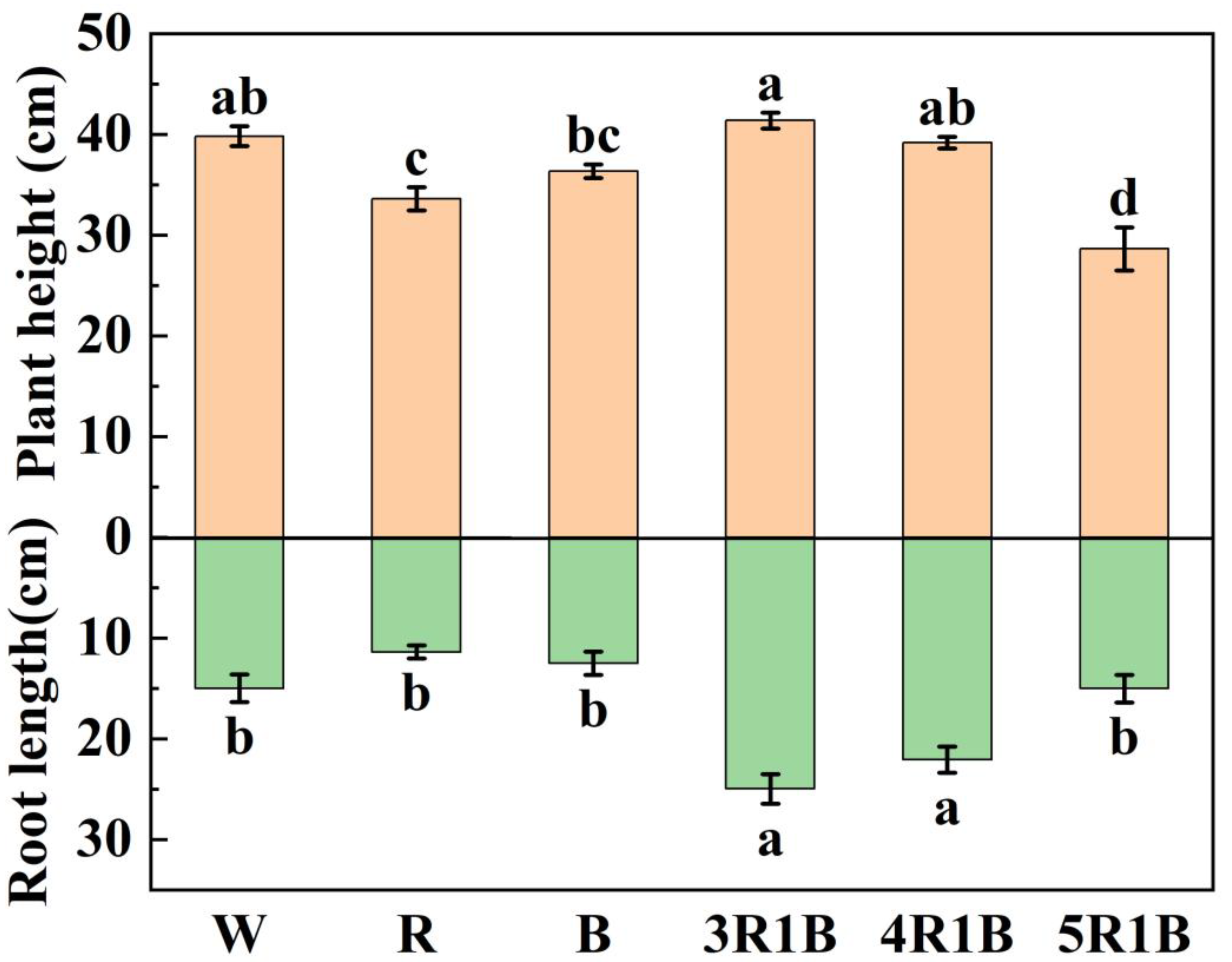
3.1. Effects of Different Light Quality Treatments on Biological Parameters of Celery
3.2. Effects of Different Light Quality Treatments on Photosynthetic and Fluorescence Characteristics of Celery
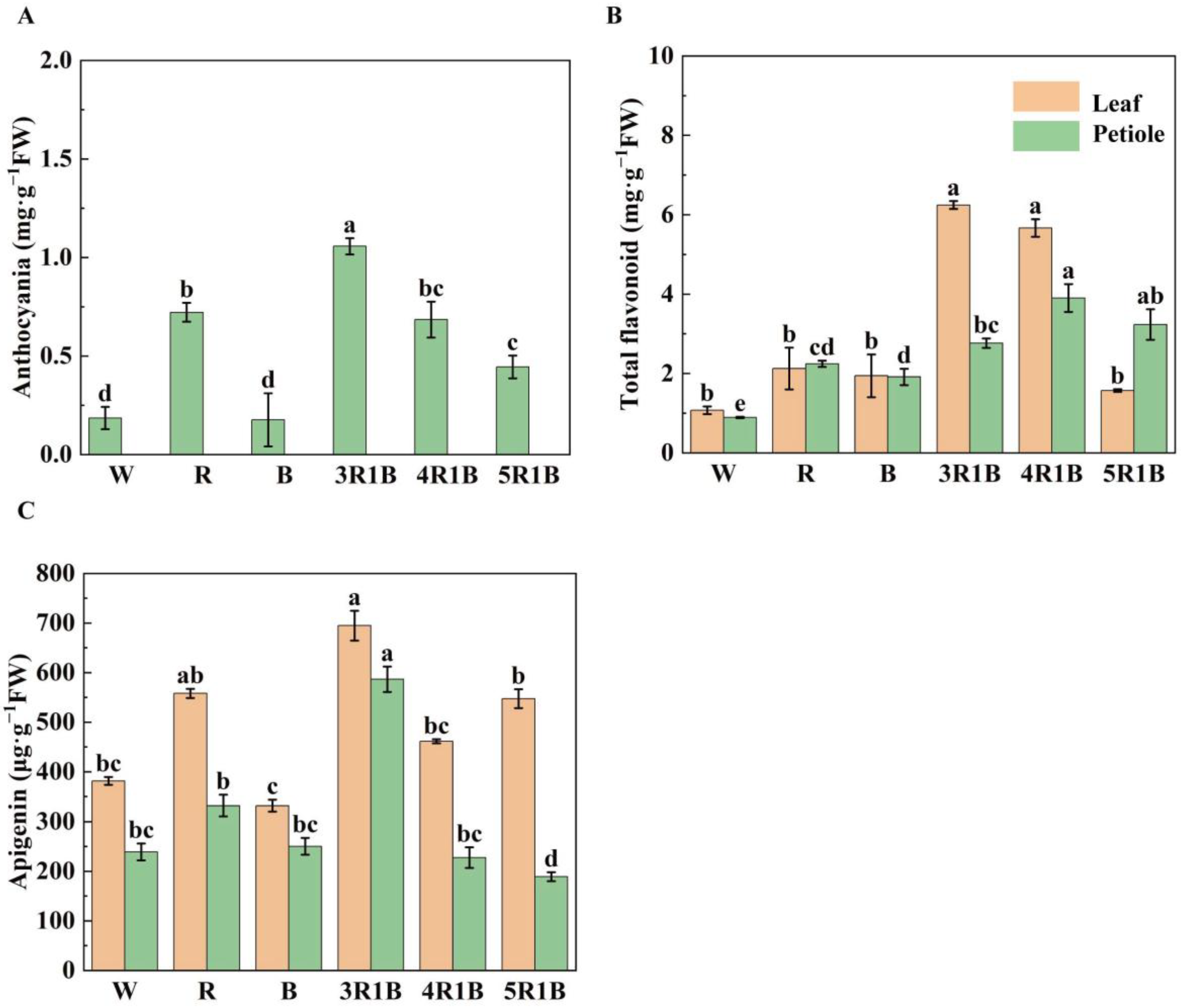
3.3. Effects of Different Light Quality Treatments on Quality of Celery
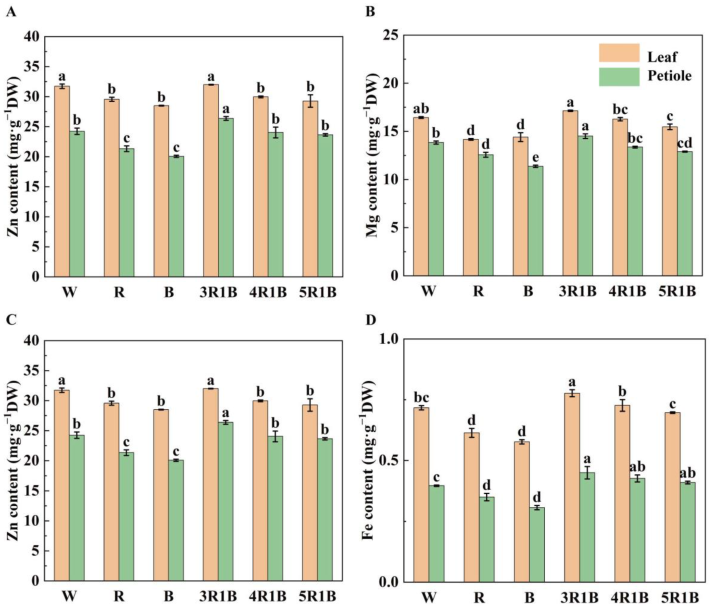
3.4. Effects of Different Light Quality Treatments on the Content of Mineral Elements in Celery
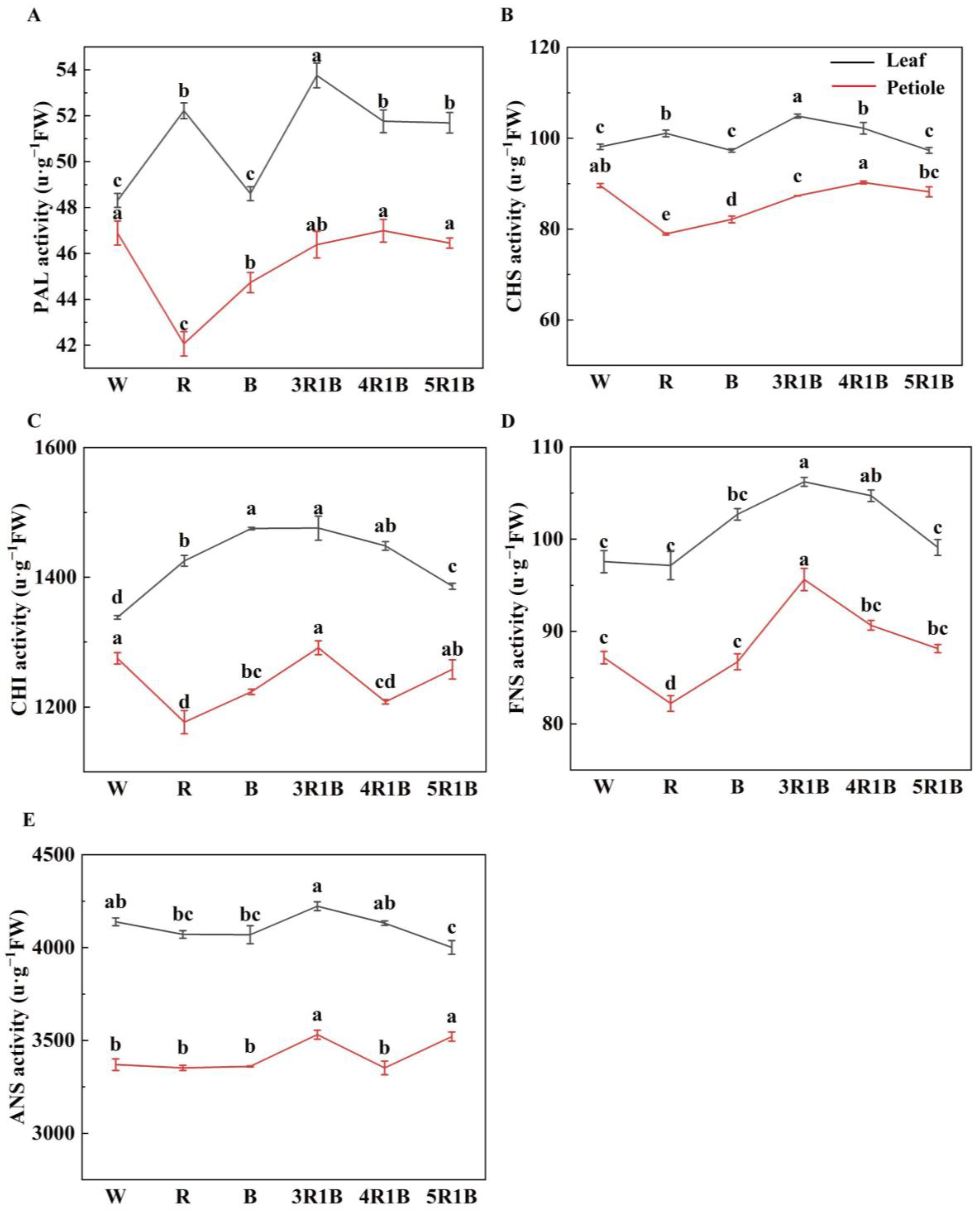
3.5. Effects of Light Quality on Enzyme Activity Related to Flavonoid Synthesis of Celery
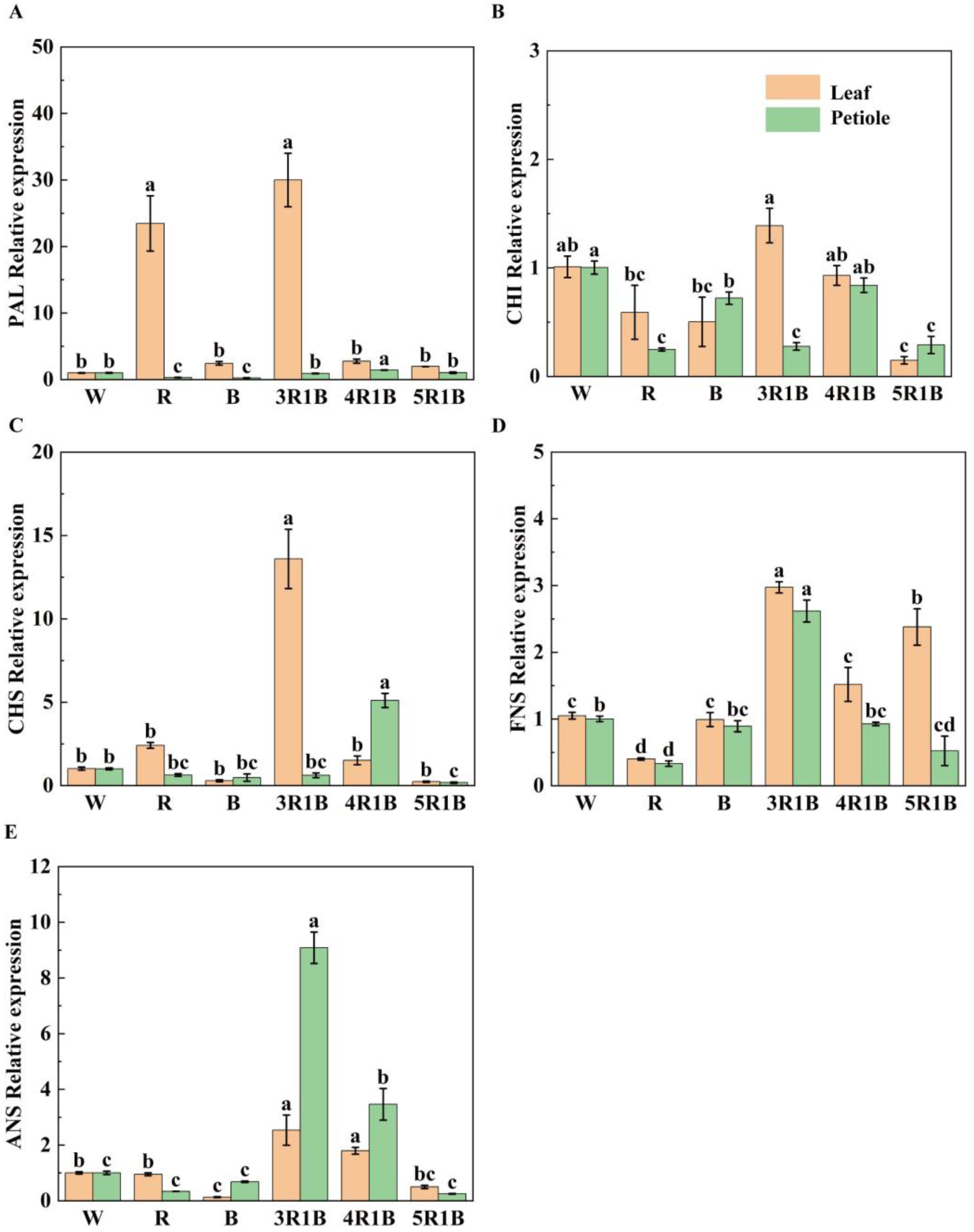
3.6. Effects of Light Quality on Genes Related to Celery Flavonoid Synthesis
3.7. Correlation Analysis of Celery Leaves and Petioles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi. J. Photochem. Photobiol. B-Biol. 2019, 193, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Song, K.H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Si, C.C.; Lin, Y.; Luo, S.M.; Yu, Y.H.; Liu, R.Q.; Naz, M.; Dai, Z.C. Effects of LED Light Quality Combinations on Growth and Leaf Colour of Tissue Culture-Generated Plantlets in Sedum rubrotinctum. Hortic. Sci. Technol. 2024, 42, 53–67. [Google Scholar] [CrossRef]
- Yan, Z.; Li, X.; Li, Z.; Qi, Y.; Song, J.; Cheng, F.; Dou, H.; Yang, Y. Partially Substituting Photosynthetic Photon Flux Density with Far-red Photons Differentially Alters Biomass Accumulation and Photochemical Efficiency of Greenhouse Lettuce. HortScience 2024, 59, 1691–1699. [Google Scholar] [CrossRef]
- Stamford, J.D.; Stevens, J.; Mullineaux, P.M.; Lawson, T. LED Lighting: A Grower’s Guide to Light Spectra. HortScience 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patane, C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef]
- Li, W.L.; Liang, Z.L. Effect of different LED light sources on growth characteristics of cucumber seedlings. Mod. Hortic. 2017, 3, 15–18. (In Chinese) [Google Scholar]
- Zhang, X.; Zhang, M.; Xu, B.; Mujumdar, A.S.; Guo, Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 106–126. [Google Scholar] [CrossRef]
- Kong, L.; Wen, Y.; Jiao, X.; Liu, X.; Xu, Z. Interactive regulation of light quality and temperature on cherry tomato growth and photosynthesis. Environ. Exp. Bot. 2021, 182, 104326. [Google Scholar] [CrossRef]
- Kinoshita, T.; Shimazaki, K. Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999, 18, 5548–5558. [Google Scholar] [CrossRef] [PubMed]
- D’Egidio, S.; Galieni, A.; Stagnari, F.; Pagnani, G.; Pisante, M. Yield, Quality and Physiological Traits of Red Beet Under Different Magnesium Nutrition and Light Intensity Levels. Agronomy 2019, 9, 379. [Google Scholar] [CrossRef]
- Braidot, E.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Tubaro, F.; Wahlby, U.; Coan, M.; Vianello, A.; Zancain, M. Low-intensity light cycles improve the quality of lamb’s lettuce during storage at low temperature. Postharvest Biol. Technol. 2014, 90, 15–23. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Song, W.; Wang, L.; Guo, W.; Xue, X. Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue LED irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Pan, Q.; Khan, A.; Bai, X.; Ali, M.; Yang, W.; Zhang, L.; Li, B. The effects of short term blue light treatment on promoting nutrition value in Chinese cabbage. Food Chem. 2013, 412, 135542. [Google Scholar] [CrossRef]
- Jain, K.; Desai, N.; Sharma, K.; Marwal, A. Development and screening of byproduct for its secondary metabolites, antioxidant and anti-diabetic potential from anthracnose-infected fruits of pomegranate: A sustainable approach. 3 Biotech 2021, 11, 74. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Du, M.Z.; Wei, Y.; Wang, C.T.; Shen, L.Q. A review on the structure–activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018, 42, 0145–8884. [Google Scholar] [CrossRef]
- Danila, C.; Tamara, Y.; Forbes, H.; Lucia, R.; José, M.; Alvarez, S.; Maria, D.N.H.; Xiao, J.B.; José, L.; Quiles, M.B.; et al. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar]
- Alokam, S. Red/Far-Red Light-Mediated Stem Elongation Response and Regulation of Anthocyanin Biosynthesis in Alpine and Prairie Ecotypes of Stellaria longipe. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2000. [Google Scholar]
- Liu, C.; Wan, H.; Yang, Y.; Ye, Q.; Zhou, G.; Wang, X.; Ahammed, G.J.; Cheng, Y. Post-Harvest LED Light Irradiation Affects Firmness, Bioactive Substances, and Amino Acid Compositions in Chili Pepper. Foods 2022, 11, 2712. [Google Scholar] [CrossRef]
- Fu, B.; Ji, X.; Zhao, M.; He, F.; Wang, X.; Wang, Y.; Liu, P.; Niu, L. The influence of light quality on the accumulation of flavonoids in tobacc leaves. J. Photochem. Photobiol. B-Biol. 2016, 162, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, X.; Li, C.; Chu, Q.; Cheng, S.; Su, L.; Shao, D.; Guo, X.; He, Z.; Zhou, X. Effect of light intensity on celery growth and flavonoid synthesis. Front. Plant Sci. 2024, 14, 1326218. [Google Scholar] [CrossRef] [PubMed]
- Yusni, Y.; Zufry, H.; Meutia, F.; Sucipto, K.W. The effects of celery leaf treatment on blood glucose and insulin levels in elderly pre-diabetics. Saudi Med. J. 2018, 39, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, H.; Zhang, B.; Chen, M.; Liu, Y.; Sui, Y. Quantitative Perturbation Analysis of Plant Factory LED Heat Dissipation on Crop Microclimate. Horticulturae 2023, 9, 660. [Google Scholar] [CrossRef]
- Sun, J.; Tan, X.; Liu, B.; Battino, M.; Meng, X.; Zhang, F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biol. Technol. 2024, 215, 113006. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.J.J.W.; Sons, I. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1-F4.3.8. [Google Scholar] [CrossRef]
- Shabbir, A.; Mao, H.; Ullah, I.; Buttar, N.A.; Ajmal, M.; Lakhiar, I.A. Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato. Agronomy 2020, 10, 1685. [Google Scholar] [CrossRef]
- Nejad, M.S.; Niroomand, A. Carbohydrate content and its roles in alternate bearing in olive. Pak. J. Biol. Sci. 2007, 10, 2744–2747. [Google Scholar]
- Blakesley, R.W.; Boezi, J.A. A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal. Biochem. 1977, 82, 580–582. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Communications in soil science and plant analysis rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Chen, G.; Mo, L.; Li, S.; Zhou, W.; Wang, H.; Liu, J.; Yang, C. Separation and determination of reduced vitamin C in polymerized hemoglobin-based oxygen carriers of the human placenta. Artif. Cells Nanomed. Biotechnol. 2015, 43, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Antia, B.S.; Akpan, E.J.; Okon, P.A.; Umoren, I.U. Nutritive and Anti-Nutritive Evaluation of Sweet Potatoes (Ipomoea batatas) Leaves. Pak. J. Nutr. 2006, 5, 166–168. [Google Scholar] [CrossRef]
- Ghadage, D.M.; Kshirsagar, P.R.; Pai, S.R.; Chavan, J.J. Extraction efficiency, phytochemical profiles and antioxidative properties of different parts of Saptarangi (Salacia chinensis L.)—An important underutilized plant. Biochem. Biophys. Rep. 2017, 12, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Moldovan, B.; David, L.; Donca, R.; Chisbora, C. Degradation kinetics of anthocyanins from crude ethanolic extract from sour cherries. Stud. Univ. Babes-Bolyai Chem. 2011, 56, 189–194. [Google Scholar]
- Sandoval, L.; Ettiene, G.; Perez-Perez, E.; Fuenmayor, M.; Raga, J.; Silva, N. HPLC determination of flavonoids in fruits of soursop from different plants. Rev. Fac. Agron. Univ. Zulia 2014, 31, 785–800. [Google Scholar]
- GB/T 23375-2009; Determination of Copper, Iron, Zinc, Calcium, Magnesium And Phosphorus in Vegetables And Their Products. National Standard of the People’s Republic of China: Beijing, China, 2009.
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous Irradiation with Alternating Red and Blue Light Enhances Plant Growth While Keeping Nutritional Quality in Lettuce. HortScience 2018, 53, 1804. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W. Effects of light quality, light intensity, and photoperiod on growth and yield of cherry radish grown under red plus blue LEDs. Hortic. Environ. Biotechnol. 2018, 59, 511–518. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of Spectral Quality of Monochromatic LED Lights on the Growth of Artichoke Seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Zhang, F.; Gu, Y.; Tian, W.; Tian, W.; Tong, Y.; Li, J. The combination of red and blue light increases the biomass and steroidal saponin contents of Paris polyphylla var. yunnanensis. Ind. Crops Prod. 2023, 194, 116311. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Chu, Q.; Qin, Y.; Li, C.; Cheng, S.; Su, L.; He, Z.; Zhou, X.; Shao, D.; Guo, X. Effects of Different Photoperiods on the Growth and Nutritional Characteristics of Two Celery Cultivars in Plant Factory. Agronomy 2023, 13, 3039. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kolton, A.; Dlugosz-Grochowska, O.; Knop, E. Nitrate content in Valerianella locust L. plants is affected by supplemental LED lighting. Sci. Hortic. 2016, 211, 179–186. [Google Scholar] [CrossRef]
- Samuoliene, G.; Sirtautas, R.; Brazaityte, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef]
- Kolton, A.; Dlugosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Park, S.-A.; Park, B.-J.; Lee, Y.; Oh, M.-M. Growth and Antioxidant Phenolic Compounds in Cherry Tomato Seedlings Grown under Monochromatic Light-emitting Diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Diatloff, E. Roles and functions of plant mineral nutrients. Methods Mol. Biol. 2013, 953, 1–21. [Google Scholar]
- Gao, S.; Kong, Y.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion. Food Res. Int. 2022, 156, 111329. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Eichholz-Duendar, I.; Hassenberg, K.; Herppich, W.B. Impact of light quality (white, red, blue light and UV-C irradiation) on changes in anthocyanin content and dynamics of PAL and POD activities in apical and basal spear sections of white asparagus after harvest. Postharvest Biol. Technol. 2020, 161, 111069. [Google Scholar] [CrossRef]
- Sng, B.J.R.; Mun, B.; Mohanty, B.; Kim, M.; Phua, Z.W.; Yang, H.; Lee, D.Y.; Jang, I.C. Combination of red and blue light induces anthocyanin and other secondary metabolite biosynthesis pathways in an age-dependent manner in Batavia lettuce. Plant Sci. 2021, 310, 110977. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; He, L.; Xu, S.; Wan, Y.; Wang, H.; Wang, Y.; Yu, L.; Zhu, W. Expression Analysis, Functional Marker Development and Verification of AgFNSI in Celery. Sci. Rep. 2020, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photochem. Photobiol. B-Biol. 2018, 179, 66–73. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| AgACTIN | CTTCCTGCCATATATGATTGG | GCCAGCACCTCGATCTTCATG |
| AgPAL | TGATGCAGGGGAAGCCTGAATTTA | TGAGGACCAAGCCACTGAGGAGAT |
| AgCHS | GGGCCTTACCTTCCATCTTCTTAA | GGTCGCTCGCATTTTTTCTTCCTT |
| AgCHI | CACTTGTCATTCCTTCTTCCTTGC | AACTGGTCTGCCGTCTTGCCCTTC |
| AgFNS | AAGGCGGCTTTACTATCTCCACTC | CACCTAGCACCATTAACTTCTCAC |
| AgANS | CTCTTTCCTCCTCGTACCTTTGCT | CTTCGGTGTTCTTATGTTCCCCTG |
| Treatment | Shoot FW (g) | Shoot DW (g) | Root FW (g) | Root DW (g) | Stem Thickness (mm) |
|---|---|---|---|---|---|
| W | 26.12 ± 0.582 b | 2.08 ± 0.073 b | 7.95 ± 0.367 b | 0.96 ± 0.205 bc | 12.49 ± 0.465 ab |
| R | 22.64 ± 0.711 c | 1.82 ± 0.010 bc | 7.16 ± 1.252 b | 0.91 ± 0.165 bc | 10.51 ± 1.462 b |
| B | 17.21 ± 0.655 d | 1.45 ± 0.063 d | 3.98 ± 0.967 c | 0.54 ± 0.033 c | 11.01 ± 0.950 ab |
| 3R1B | 32.71 ± 1.155 a | 3.19 ± 0.138 a | 11.42 ± 0.357 a | 1.62 ± 0.040 a | 13.88 ± 0.122 a |
| 4R1B | 24.36 ± 1.566 bc | 2.05 ± 0.085 b | 8.18 ± 0.105 b | 0.61 ± 0.188 c | 11.30 ± 0.966 ab |
| 5R1B | 23.95 ± 0.680 c | 1.64 ± 0.255 bc | 9.45 ± 0.429 ab | 1.15 ± 0.021 b | 10.42 ± 0.398 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Chu, Q.; Liu, K.; Lu, Y.; Cheng, S.; Pan, T.; Zhou, X.; He, Z. The Impact of Light Quality on the Growth and Quality of Celery. Horticulturae 2025, 11, 774. https://doi.org/10.3390/horticulturae11070774
Tang L, Chu Q, Liu K, Lu Y, Cheng S, Pan T, Zhou X, He Z. The Impact of Light Quality on the Growth and Quality of Celery. Horticulturae. 2025; 11(7):774. https://doi.org/10.3390/horticulturae11070774
Chicago/Turabian StyleTang, Li, Qianwen Chu, Kaiyue Liu, Yingyi Lu, Shaobo Cheng, Tonghua Pan, Xiaoting Zhou, and Zhongqun He. 2025. "The Impact of Light Quality on the Growth and Quality of Celery" Horticulturae 11, no. 7: 774. https://doi.org/10.3390/horticulturae11070774
APA StyleTang, L., Chu, Q., Liu, K., Lu, Y., Cheng, S., Pan, T., Zhou, X., & He, Z. (2025). The Impact of Light Quality on the Growth and Quality of Celery. Horticulturae, 11(7), 774. https://doi.org/10.3390/horticulturae11070774






