Abstract
Elevated [CO2] enhances light interception and carboxylation efficiency in plants. The combined effects of [CO2] and photosynthetic photon flux density (PPFD) on stomatal morphology, leaf anatomy, and photosynthetic capacity in tomato seedlings remain unclear. This study subjected tomato seedlings (Solanum lycopersicum Mill. cv. Jingpeng No.1) to two [CO2] (ambient [a[CO2], 400 µmol·mol−1] and enriched [e[CO2], 800 µmol·mol−1]) and three PPFD levels (L; low[Ll: 200 µmol·m−2·s−1], moderate[Lm: 300 µmol·m−2·s−1], and high[Lh: 400 µmol·m−2·s−1]) to assess their interactive impacts. Results showed that e[CO2] and increased PPFD synergistically improved relative growth rate and net assimilation rate while reducing specific leaf area and leaf area ratio. Notably, e[CO2] decreased stomatal aperture (−13.81%) and density (−27.76%), whereas elevated PPFD promoted stomatal morphological adjustments. Additionally, Leaf thickness increased by 72.98% under e[CO2], with Lm and Lh enhancing this by 10.79% and 41.50% compared to Ll. Furthermore, photosynthetic performance under e[CO2] was further evidenced by improved chlorophyll fluorescence parameters (excluding non-photochemical quenching). While both e[CO2] and increased PPFD Photosynthetic performance under e[CO2] was further evidenced by improved chlorophyll fluorescence parameters (excluding non-photochemical quenching). Moreover, e[CO2]-Lh treatment maximized total dry mass and seedling health index. Correlation analysis indicated that synergistic optimization of stomatal traits and leaf structure under a combination of e[CO2] and increased PPFD enhanced light harvesting and CO2 diffusion, thereby promoting carbon assimilation. These findings highlight e[CO2]-Lh as an optimal strategy for tomato seedling growth, providing empirical guidance for precision CO2 fertilization and light management in controlled cultivation.
1. Introduction
Tomato is a globally preeminent horticultural crop [1], driven by its dual nutritional and economic value [2]. Notably, China cultivates 1.16 million hectares of tomato, yielding 70.21 million tons annually (Food and Agriculture Organization of the United Nations [FAO], 2023) [3], highlighting its critical role in global agriculture [4]. Vigorous seedling establishment is essential for optimal vegetative growth, fruit yield, and quality [5]. However, seedling development is highly sensitive to environmental cues [6,7,8,9,10], with photosynthetic photon flux density (PPFD) and CO2 concentration ([CO2]) being key regulators of energy capture and carbon assimilation, respectively [11]. Although numerous studies have examined the individual and combined effects of [CO2] and PPFD on greenhouse vegetables [12,13,14,15,16], their synergistic impacts on leaf anatomical plasticity, stomatal responses, and photochemical efficiency are not well understood.
Atmospheric [CO2] has reached unprecedented modern levels (≈425 ppm in 2024), though geological records show higher concentrations in ancient eras [17]. Projections indicate a potential doubling by 2100, exceptional given its long-term atmospheric stability (comprising ~0.04% of the atmosphere) [18]. Elevated [CO2] (e[CO2]) consistently promotes seedling development, critical for optimizing crop productivity and fruit quality [13,16]. Mechanistically, in C3 plants, this results from e[CO2]-enhanced carboxylation via ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), coupled with reduced photorespiratory losses through ribulose 1,5-bisphosphate (RuBP) regulation, boosting net photosynthesis (Pn) and biomass accumulation [19]. By contrast, C4 and crassulacean acid metabolism (CAM) plants show muted responses due to anatomical/biochemical adaptations that minimize photorespiration regardless of [CO2] [20]. Concomitantly, these physiological improvements in C3 plants drive higher relative growth rate (RGR), linked to sink-source dynamics and root-zone conditions [21]. Additionally, e[CO2] reduces stomatal conductance (gs) and transpiration (Tr), improving water use efficiency [16], while enhancing chlorophyll fluorescence parameters (e.g., maximum PSII photochemical efficiency (Fv/Fm), PSII quantum yield (ΦPSII)) to boost light-use efficiency [22]. Structural modifications, including increased mesophyll thickness, altered stomatal density (SD), and reduced stomatal aperture (SA), often accompany these functional changes [23]. Notably, responses vary by species and context, influenced by developmental stage, root microenvironment, and experimental duration [24]. Given tomato seedlings’ sensitivity to environmental cues, characterizing e[CO2]-driven morphophysiological adaptations is vital for refining precision management in controlled cultivation.
Light—encompassing intensity, spectral quality, and photoperiod—is a fundamental regulator of plant ontogenesis [25,26]. Among these, light intensity not only serves as the energy substrate for carbon fixation but also initiates photoreceptor-mediated signaling pathways governing morphogenesis and stress acclimation [27]. Specifically, plants harness light energy via photosynthesis, generating adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) during light-dependent reactions [26]. However, this process inherently involves solar radiation-induced reactive oxygen species (ROS) generation, particularly under high light, where excessive excitation energy can overwhelm the photosynthetic apparatus and induce oxidative stress [28]. Light influences seedling photosynthetic performance by modulating chloroplast development, anatomical traits, and key Calvin cycle enzymes [16,29]. Optimal light intensity fosters robust growth, enhances light energy conversion, and improves seedling health indices [6]. This threshold varies across C3, C4, and CAM plants due to divergent growth habits [30]. Notably, it promotes leaf structural integrity and hydraulic efficiency, while supraoptimal intensities induce photoinhibition via PSII destabilization [31]. Conversely, light intensity fluctuations drive dynamic stomatal adjustments: high light elevates stomatal index and conductance for maximal CO2 uptake, whereas low light promotes stomatal expansion to enhance light interception [32]. This dual role is evident in gas exchange: increasing light intensity boosts Pn while often reducing gs [12]. Within physiological thresholds (below 450 µmol·m−2·s−1), elevated light intensity improves ΦPSII and Fv/Fm in tomato seedlings, optimizing light energy absorption and carbon assimilation [33]. Additionally, plants also employ photoreceptor-mediated photoprotection mechanisms under excessive light, including signaling-driven heat dissipation pathways [34]. Collectively, this phenotypic plasticity underscores the need for precision light management in greenhouses to balance growth promotion and stress mitigation.
Stomatal dynamics, regulated by interactive [CO2] and light intensity, mediate the balance between carbon assimilation and transpirational water loss in plants [35,36]. Stomata exhibit acute behavioral adjustments and chronic morphological adaptations to environmental fluctuations, including CO2 variations [23,37]. These responses are often coordinated, though species- and genotype-specific differences exist [37]. In C3 plants like Arabidopsis, high light (175 µmol·m−2·s−1) triggers phytochrome-mediated increases in stomatal density [38], while elevated [CO2] suppresses stomatal aperture via anion channel modulation and abscisic acid signaling crosstalk [39]. These opposing effects suggest a complex regulatory network, yet mechanistic insights into their combined impacts on stomatal morphology and photosynthetic performance remain limited. Concurrently, leaf anatomical traits are key phenotypic adaptations to environmental change [29]. Although tomato seedling leaf anatomy is sensitive to environmental cues, comprehensive data on [CO2]-light intensity interactions are scarce. Chlorophyll fluorescence analysis provides a powerful tool for dissecting photosynthetic stress responses, underpinning agricultural management strategies in changing climates [22,40]. Previous studies show that elevated [CO2] and light intensity individually enhance Fv/Fm, indicating improved light capture [32]. However, their interactive effects on photochemical efficiency and stomatal-anatomical correlations require systematic investigation.
The objective of this research is to investigate the synergistic effects of elevated e[CO2] and enhanced light intensity on leaf anatomical plasticity, stomatal morphology, and photosynthetic physiology in tomato seedlings. Specifically, we hypothesize that combined optimization of [CO2] and light environments modulates mesophyll tissue architecture and stomatal patterning, thereby improving carbon assimilation efficiency through enhanced light interception and CO2 diffusion. Integrating morphological, anatomical, and physiological measurements, this study aims to elucidate how multi-factor environmental optimization balances growth promotion and resource use efficiency, providing guidelines for sustainable tomato seedling production in horticulture.
2. Materials and Methods
2.1. Plant Material Preparation
Tomato (Solanum lycopersicum Mill. cv. ‘Jingpeng No. 1’) seeds were surface-disinfected by soaking in 55 °C water for 15 min, followed by a 6–8 h soak in room-temperature water. Seeds were then incubated in a dark incubator (28 °C, 95% RH) for 72 h to promote germination, after which they were sown in 72-cell trays filled with a peat:vermiculite:perlite (3:1:1, v/v/v) seedling substrate. Seedlings were cultivated for 35 days in an illumination incubator (PRX-450C-30, Ningbo, China) under controlled conditions: 25 °C/18 °C day/night temperature, 250 µmol·m−2·s−1 PPFD [41], 70% RH, and a 14-h photoperiod. At the 4-leaf stage, they were transplanted into 20 cm (diameter) × 16 cm (height) plastic pots with commercial compost (Jiahui Co., Yangling, China). Artificial illumination was provided by metal halide lamps (HQI, 400 W; Osram Co., Munich, Germany), with spectral outputs validated using a calibrated spectroradiometer (Figure 1). Pure CO2 gas (99.9% purity; Shaanxi Meiqi Trading Co., Ltd., Yangling, China) was used for enrichment treatments.
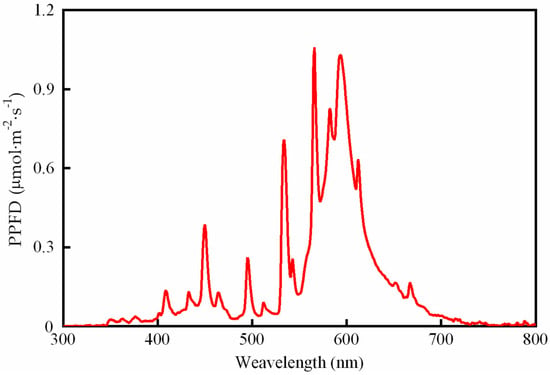
Figure 1.
The spectral distribution of metal halide lamps.
2.2. Experimental Design
The experiment employed a full factorial design with two factors: [CO2] (ambient [a[CO2]: 400 µmol·mol−1] and enriched [e[CO2]: 800 µmol·mol−1]) and light intensity (L; low[Ll: 200 µmol·m−2·s−1], moderate[Lm: 300 µmol·m−2·s−1], and high[Lh: 400 µmol·m−2·s−1]). Notably, prior research [32] showed 300 µmol·m−2·s−1 with red-blue light supplementation optimizes tomato seedling growth, thus defining our moderate light level, with 200 and 400 µmol·m−2·s−1 as the low and high values. Six treatments (a[CO2]-Ll, a[CO2]-Lm, a[CO2]-Lh, e[CO2]-Ll, e[CO2]-Lm, e[CO2]-Lh) were replicated 20 times across two climate chambers (QIUSHI Environment, Zhejiang, China). Environmental parameters were rigorously controlled as follows: (1) Infrared CO2 detectors [16] maintained target concentrations during the 12-h photoperiod, with a[CO2] restored at night. (2) Light levels were calibrated using a quantum sensor (MQ-100, Apogee Instruments, Logan, UT, USA) by adjusting lamp-to-canopy distances. Electric fans ensured uniform [CO2] distribution and temperature stability. (3) Day/night temperatures of 23 °C/18 °C and 65% humidity. Plants were randomized every 48 h to minimize positional bias and irrigated biweekly with half-strength Hoagland solution (pH 6.5, EC 1.3 mS·cm−1), with irrigation volume adjusted according to growth status to ensure adequate nutrient supply. Substrates were covered with aluminum foil to reduce evaporation. The experiment lasted 15 days post-transplant.
2.3. Growth Parameter Quantification
At 0 and 15 days after treatment (DAT), six plants per treatment were destructively sampled. Total leaf area was measured using a Li-3000 leaf area meter (LI-COR, Lincoln, NE, USA), and dry mass was determined via oven-drying (80 °C, 72 h) followed by gravimetric analysis (ME204E balance, Mettler-Toledo, ±0.0001 g, Greifensee, Switzerland). The formulas for calculating RGR, net assimilation rate (NAR), leaf area ratio (LAR), and specific leaf area (SLA) were as follows [42]: RGR = [ln(W2) − ln(W1)]/(t2 − t1); NAR = (W2 − W1)/[(t2 − t1) × (L1 + L2)/2]; LAR = L2/W2; SLA = L2/WL2. Note: W1,2 and L1,2 denote total dry mass and leaf area of the plant at 0 (t1) and 15 DAT (t2), respectively, while WL2 denotes the leaf dry mass at 15 DAT (t2).
2.4. Determination of Stomatal Traits
At 10 DAT, leaf abaxial surfaces were gently cleared of debris using a rubber bulb. From the third fully expanded tomato leaf, 4–6 leaflets were excised and immersed in 4% glutaraldehyde. Primary fixation proceeded in fresh glutaraldehyde: first at 25 °C (≥2 h), then at 4 °C (≥6 h). Samples underwent four 10-min rinses in 0.1 M phosphate buffer (pH 6.8). Ethanol dehydration followed graded concentrations (30% to 90%, 15 min/step) with three final 100% ethanol washes (30 min each). Critical point drying employed isoamyl acetate substitution (1 mL, 10–15 min). Dried specimens were mounted on stubs, gold-sputter coated, and imaged using a JSM-6360LV tungsten SEM (JEOL, Tokyo, Japan). Stomatal density and morphology (length/width/aperture) were determined by analyzing 15 fields per treatment in ImageJ (v1.53, NIH, Baltimore, MD, USA).
2.5. Determination of Leaf Anatomical Structure
At 10 DAT, the third fully expanded apical leaf underwent paraffin sectioning. Six leaf segments were fixed in FAA (50% ethanol, 10% formaldehyde, 5% glacial acetic acid, 35% distilled water) for 24 h. Samples were dehydrated through an ethanol series (70%, 85%, 90%, 95%; 3 h per step), then absolute ethanol (≥16 h). Clearing proceeded in xylene/absolute ethanol (1:1, v/v), followed by pure xylene (4 h total). Tissues were embedded in paraffin (55–60 °C, 24 h) and solidified on a cooling stage. Sections (8–10 µm) cut with a rotary microtome were stained with safranin-fast green and imaged using an Olympus BX51 microscope (OlympusOptical Co., Tokyo, Japan). For each treatment, 4–6 leaves were sectioned, with 15 fields analyzed per section to quantify leaf thickness, palisade mesophyll tissue (PM), spongy mesophyll tissue (SM), and upper/lower epidermis thickness.
2.6. Determination of Gas Exchange and Chlorophyll Fluorescence
From 11 to 12 DAT, diurnal photosynthesis was profiled using two synchronized LI-6400XT systems (Li-COR Inc., Lincoln, NE, USA) measuring gas exchange in third fully-expanded leaves within controlled-environment chambers. LED-illuminated leaf chambers maintained treatment-specific [CO2] and PPFD levels (e.g., 400 µmol·mol−1 CO2 + 200 µmol·m−2·s−1 PPFD for a[CO2]-Ll conditions). Following 60–120 s stabilization to achieve steady-state, photosynthetic parameters (Pn, gs, transpiration rate (Tr)) were recorded at 2-h intervals (09:00–19:00 h) with five biological replicates. This parallel measurement design minimized environmental variability while capturing diurnal physiological responses.
Dark-adapted yields (initial fluorescence, F0; maximum fluorescence, Fm) and light-adapted yields (minimum fluorescence under actinic light, F0′; maximum fluorescence under actinic light, Fm′; steady-state fluorescence, Fs) were recorded. Key photochemical parameters (Fv/Fm, ΦPSII, electron transport rate (ETR), photochemical quenching coefficient (qP), non-photochemical quenching (NPQ), and effective photochemical quantum efficiency (Fv′/Fm′)) were calculated using standard equations: Fv/Fm = (Fm − F0)/Fm; ΦPSII = (Fm′ − Fs)/Fm′; ETR = ΦPSII × PAR × 0.85 × 0.5; qP = (Fm′ − Fs)/(Fm′ − F0′); NPQ = (Fm − Fm′)/Fm′; Fv′/Fm′ = (Fm′ − F0′)/Fm′.
2.7. Determination of Dry Mass Accumulation and Seedling Health Index
After 15 DAT, growth parameters were quantified in four randomly selected plants per treatment. Plant height and stem diameter were measured using a metric ruler and digital caliper (0.01 mm resolution), respectively. Whole plants were rinsed with deionized water, surface-dried, and oven-dried at 65 °C (48 h) to constant mass. Total dry mass (TDM) was determined using an analytical balance (ME203, Mettler-Toledo; ±0.001 g, Greifensee, Switzerland). Thereafter, seedling health index (SHI) was calculated as: SHI = (stem diameter × total dry mass)/plant height, providing an integrated assessment of seedling vigor.
2.8. Statistical Analysis
Statistical analyses employed SAS 9.4 (SAS Institute, Cary, NC, USA) for two-way ANOVA (excluding diurnal gas exchange data), evaluating [CO2] and light intensity main effects and interactions. Data met ANOVA assumptions (normality, homoscedasticity) verified through SAS procedures. Tukey’s HSD tests (p < 0.05) determined treatment differences. Pearson correlations among morphological, anatomical, stomatal, photosynthetic, and SHI parameters were computed in Origin 2024 (OriginLab Corp., Northampton, MA, USA). Results expressed as mean ± SE. Figures generated with Origin 2024 and GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Growth Traits
RGR, NAR, SLA, and LAR of tomato seedlings exhibited strong correlations (p < 0.001) with [CO2] and light intensity (Figure 2), with significant CO2 × light interactions for RGR and NAR (p < 0.05). Elevated [CO2] increased RGR by 7.49–23.10% and NAR by 14.06–28.44% across light gradients (low to high), while reducing SLA (10.97–16.74%) and LAR (11.75–18.42%) (Figure 2A–D). Independently, higher light intensities enhanced RGR (19.59–40.11%) and NAR (24.46–61.74%) but decreased SLA and LAR versus low light. The e[CO2]-Lh treatment produced maximal growth metrics, demonstrating distinct synergistic interaction.
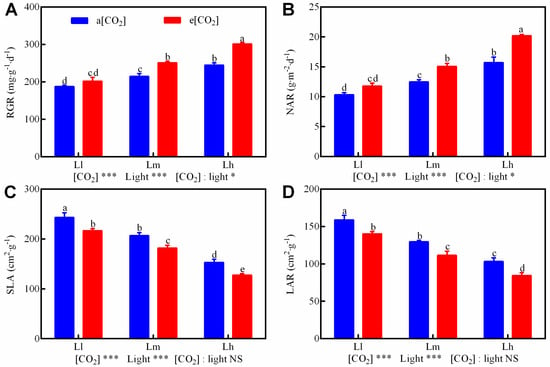
Figure 2.
Growth parameters of tomato seedling under a factorial design of two CO2 levels (ambient [aCO2], 400 μmol·mol−1 and elevated [eCO2], 800 μmol·mol−1) and three PPFD treatments (200 [Ll], 300 [Lm], and 400 [Lh] μmol·m−2·s−1) in growth chambers. Measured parameters included: RGR (A), NAR (B), SLA (C), and LAR (D). Data: mean ± SE (n = 6). Lowercase letters indicate significant differences among treatments (Tukey’s HSD, p < 0.05). NS-not significant, * p < 0.05, *** p < 0.001 (two-way ANOVA).
3.2. Stomatal Traits
All stomatal traits responded significantly to light and [CO2] (p < 0.05; Table 1/Figure 3). Significant CO2 × light interactions occurred for SA (p < 0.05) and SD (p < 0.01). Elevated [CO2] increased stomatal length (SL) by 13.61–44.14% and width (SW) by 27.30–65.90% versus ambient counterparts under moderate-high light, while reducing SA (4.39–10.78%) and SD (23.66–35.39%) across all light levels. Within e[CO2] treatments, SL and SW showed light-responsive increases. Under ambient [CO2], moderate-high light boosted SA (6.63–9.68%) and SD (45.98–66.31%), peaking at a[CO2]-Lh conditions.

Table 1.
Stomatal morphological responses of tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. Data: mean ± SE (n = 15). Lowercase letters indicate significant differences among treatments (Tukey’s HSD, p < 0.05). NS-not significant, * p < 0.05, ** p < 0.01, *** p < 0.001 (two-way ANOVA).
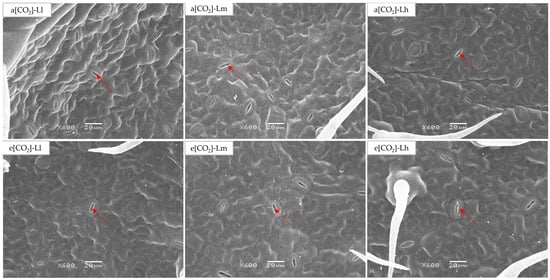
Figure 3.
Stomatal structure in leaves of tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. The red arrows indicate the stoma.
3.3. Leaf Anatomical Structure
Leaf anatomical parameters responded significantly to [CO2], light intensity, and their interaction (p < 0.05; Table 2/Figure 4). Elevated [CO2] augmented palisade mesophyll (PM, +71. 07%), spongy mesophyll (SM, +86.94%), and epidermal thickness (upper, UE + 41.11%; lower, LE + 42.79%), collectively increasing total leaf thickness (LT) by 75.65% versus ambient [CO2]. The ratio of PM to SM (PM/SM) responded non-uniformly to e[CO2], increasing at low/high light (+1.92–10.71%) but decreasing at moderate light (−41.82%). Across both CO2 regimes, LT, SM, UE, and LE thicknesses increased with light intensity. Under ambient [CO2], PM/SM ratio increased linearly with light intensity, whereas e[CO2] induced nonlinear responses peaking at high light. The e[CO2]-Lh treatment yielded maximal LT, PM, UE, and PM/SM values.

Table 2.
Leaf anatomical characteristics of tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. Data are presented as mean ± SE (n = 15). Lowercase letters indicate significant differences among treatments (Tukey’s HSD, p < 0.05). * p < 0.05, ** p < 0.01 (two-way ANOVA).
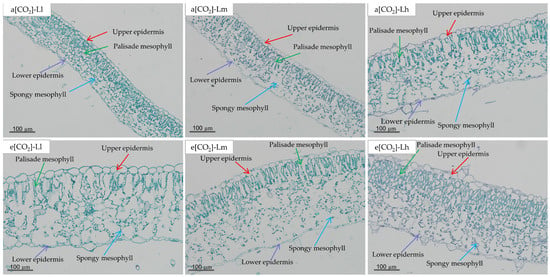
Figure 4.
Leaf anatomical structure of tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers.
3.4. Diurnal Variation in Photosynthesis, Transpiration Rate, and Stomatal Conductance
To characterize the interactive effects of elevated [CO2] and light intensity on plant physiology, diurnal dynamics of Pn, Tr, and gs were systematically analyzed. All treatments exhibited a bimodal diurnal pattern with distinct peaks and troughs (Figure 5). Elevated [CO2] significantly enhanced daytime Pn by 10.06–18.22% (Figure 5A) but reduced Tr and gs by 20.32–25.88% (Figure 5B) and 3.78–18.43% (Figure 5C), respectively. Moderate and high light intensities increased Pn by 20.39–29.37% and 50.95–58.95% compared to low light (Figure 5A), with concurrent Tr enhancements of 15.66–39.97% (moderate) and 30.93–62.38% (high) (Figure 5B). Stomatal conductance followed a similar trend, increasing by 8.55–28.88% and 42.43–81.87% under moderate and high light, respectively (Figure 5C). The highest Pn was observed under e[CO2]-high light (Lh), while a[CO2]-Lh elicited maximal Tr and gs across all conditions.
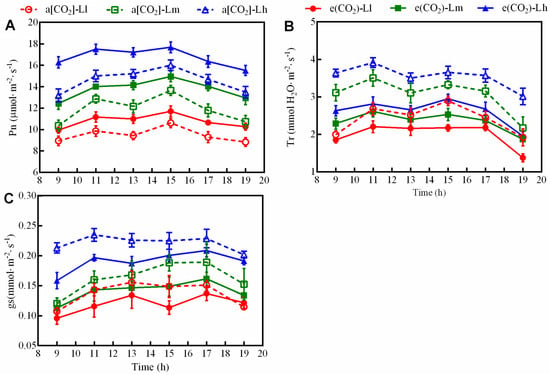
Figure 5.
Diurnal gas exchange patterns—including Pn (A), Tr (B), and gs (C)—were monitored in tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. Data are shown as means ± SE (n = 5).
3.5. Chlorophyll Fluorescence Parameters
Chlorophyll fluorescence parameters showed significant associations with [CO2], light intensity, and their interactions (p < 0.05, 0.01, or 0.001; Table 3). Across [CO2] levels, Fv/Fm, ΦPSII, ETR, qP, and Fv′/Fm′ increased with rising light intensity. Moderate and high light intensities enhanced Fv/Fm by 26.72% and 22.62%, respectively, and ΦPSII by 22.97% and 22.23%, relative to low light. NPQ exhibited a U-shaped pattern, decreasing at moderate light before increasing under high light. Elevated [CO2] further improved photochemical efficiency, boosting Fv/Fm (+11.78%), ΦPSII (+11.41%), ETR (+17.54%), qP (+16.64%), and Fv′/Fm′ (+17.07%), while reducing NPQ by 13.85%. The e[CO2]-Lh treatment maximized all fluorescence parameters except NPQ, highlighting synergistic enhancements in light harvesting and energy conversion under combined high CO2 and light intensity.

Table 3.
Chlorophyll fluorescence parameters were measured in tomato leaves under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. Values are means ± SE (n = 5). Lowercase letters indicate significant differences among treatments (Tukey’s HSD, p < 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001 (two-way ANOVA).
3.6. Total Dry Mass and Seedling Health Index
To evaluate the combined effects of e[CO2] and enhanced light intensity on seedling quality, TDM and SHI were measured (Figure 6). Both parameters were significantly influenced by [CO2], light intensity, and their interactions (p < 0.05–0.001), following parallel response trends. Specifically, e[CO2] increased TDM and SHI by 40.36% (Figure 6A) and 5.95% (Figure 6B) versus ambient [CO2]. Moderate and high light intensities further boosted TDM by 32.20% and 73.29%, and SHI by 31.66% and 64.96%, respectively, relative to low light. The e[CO2]-Lh treatment achieved the highest TDM (6.28 g·plant−1) and SHI (0.79), surpassing all other combinations. These results highlight synergistic benefits of e[CO2] and high light intensity in promoting biomass accumulation and seedling vigor, with e[CO2]-Lh representing an optimal strategy for controlled-environment tomato seedling production.
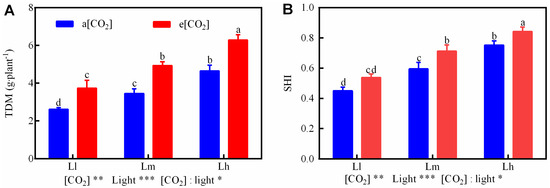
Figure 6.
Totlal dry mass(TDM, (A)) and sedling health index (SHI, (B)) were measured in tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. Data are means ± SE (n = 4). Lowercase letters indicate significant differences among treatments (Tukey’s HSD, p < 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001 (two-way ANOVA).
3.7. Correlations Among Plant Physiological and Morphological Traits
Pearson correlation analysis (Figure 7) revealed a near-perfect positive correlation between RGR and NAR (r = 0.99). Notably, both RGR and NAR were strongly negatively correlated with SLA (r = −0.95 to −0.97) and LAR (r = −0.97), indicating a trade-off between leaf expansion and carbon allocation efficiency. Moreover, PM and SM thicknesses showed strong positive correlations with total leaf thickness (LT; r = 0.85–0.95), supporting their combined role in optimizing light interception. Furthermore, UE thickness positively correlated with RGR (r = 0.82) and Pn (r = 0.98), suggesting that epidermal reinforcement enhances photosynthetic capacity through structural support. Pn positively correlated with stomatal dimensions (length [SL], r = 0.64; width [SW], r = 0.83) and growth metrics (RGR, r = 0.98; NAR, r = 0.98; TDM, r = 0.96; SHI, r = 0.97), thereby indicating that enlarged stomatal apertures facilitate CO2 diffusion and carbon assimilation. Conversely, SD weakly negatively correlated with Pn (r = −0.22), potentially reflecting a trade-off between gas exchange and water conservation under high vapor pressure deficit. In addition, chlorophyll fluorescence parameters showed Fv′/Fm′ strongly correlated with ETR (r = 0.94), while NPQ negatively correlated with SLA and LAR, suggesting enhanced photoprotection in low-SLA leaves. SHI robustly correlated with TDM (r = 0.87), Pn (r = 0.97), RGR (r = 0.91), and NAR (r = 0.92), thus indicating that e[CO2] and increased light intensity promote seedling vigor via enhanced carbon assimilation and biomass accumulation.
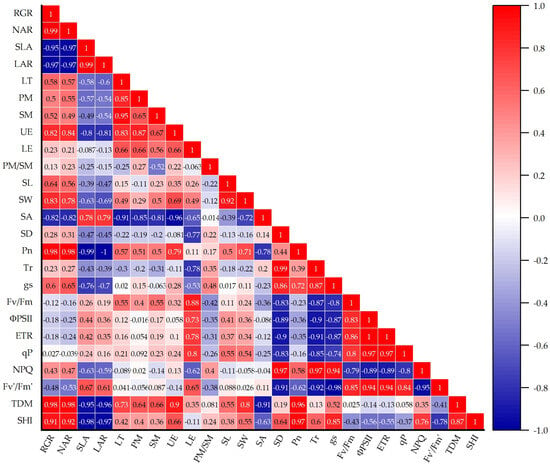
Figure 7.
The Pearson correlation of physiological, anatomical, stomatal, and morphological traits in tomato seedlings under a factorial design of two CO2 levels and three PPFD treatments in growth chambers. Note: Pn, Tr, and gs represent daily average net photosynthesis, transpiration rate, and stomatal conductance, respectively. Color-coded ellipses depict r values, with blue/red shading indicating positive/negative associations and intensity reflecting coefficient magnitude (|r| ≥ 0.7 highlighted in intense colors).
Collectively, these results show that elevated [CO2] and PPFD synergistically enhance growth traits (RGR and NAR) while reducing leaf area parameters (SLA and LAR; Figure 2), thereby facilitating light absorption and organic matter accumulation (Figure 6). Notably, these treatments optimize stomatal morphology (Table 1/Figure 3) and leaf anatomy (Table 2/Figure 4), enhancing light harvesting, CO2 diffusion, and energy utilization. At the physiological level, e[CO2] improves net photosynthesis (Pn; Figure 5) and photochemical parameters (Fv/Fm, ΦPSII, ETR), while decreasing NPQ (Table 3)—findings indicative of enhanced light energy use and photoprotective acclimation under high light. Mechanistically, Pn correlates positively with stomatal dimensions (e.g., SL, SW), leaf anatomical traits (e.g., UE), and growth metrics (RGR, NAR, TDM, SHI; Figure 7). Moreover, SHI shows a robust association with TDM, RGR, and NAR, reinforcing its role as a key vigor indicator. By integrating morphological, anatomical, and photosynthetic data—particularly TDM and SHI—the e[CO2]-Lh treatment emerges as optimal for promoting seedling growth and vigor in this study. These findings validate the study’s hypotheses, providing empirical support for enhanced plant growth under combined CO2 levels and PPFD treatments.
4. Discussion
Recent advances in understanding the interactions between [CO2] and PPFD have substantially influenced plant physiological ecology, attracting considerable research attention [11,12,13,14,15,16]. Leaf phenotypic traits, including anatomical architecture, stomatal density, and leaf area, exhibit high sensitivity to environmental variables such as [CO2] [43] and light intensity [44], thereby affecting carbon assimilation and plant growth [45]. This study explores the short-term effects of e[CO2] and increased light intensity on these traits, employing correlation heatmaps to decipher trait interrelationships. Our results shed light on how tomato seedlings adjust their growth, leaf anatomy, stomatal characteristics, and photosynthetic performance under ambient and elevated [CO2] conditions across a light intensity gradient in controlled environments.
Growth parameters, pivotal for vegetative and reproductive development, are deeply influenced by environmental factors such as light availability [46] and [CO2] [47]. These parameters—including SLA, NAR, and LAR—integrate eco-physiological signals, reflecting carbon allocation and resource use efficiency. RGR, a key vigor indicator, arises from the synergistic interaction between NAR and LAR. Elucidating these relationships enhances our understanding of plant-environment adaptation, providing theoretical grounds for agricultural and ecological research [48]. Notably, elevated [CO2] substantially increased RGR and NAR while reducing SLA and LAR (p < 0.001, Figure 2), consistent with prior studies [47,49]. This suggests e[CO2] enhances carbon assimilation via optimized light interception efficiency rather than increased leaf mass per unit area. Conversely, reduced light intensity decreased NAR but upregulated SLA and LAR (p < 0.001, Figure 2), a phenotypic plasticity strategy to maximize light capture, in line with earlier findings [13,49]. These results highlight plant adaptive strategies through trait modulation and photosynthetic enhancement [50]. RGR and NAR exhibited significant [CO2]-light intensity interactions, driving enhanced dry mass accumulation and seedling robustness (p < 0.05, Figure 7), which correlated with increased net photosynthesis (Figure 5). The greater RGR and NAR increments under high light (Figure 2A,B) likely stem from coupled light/dark reaction enhancements, adaptive leaf structural modifications (Table 1 and Table 2, Figure 3 and Figure 4), alleviated RuBisCO activity limitations (Table 3) [16], and optimized carbon allocation (Figure 2). Collectively, these findings underscore the potential of CO2 fertilization to enhance C3 plant (e.g., tomato) growth via light environment synergies, offering insights for precision agriculture.
Stomata serve as primary conduits for water and gas exchange with the external environment, their behavior and morphology regulated by [CO2] and light intensity, thereby influencing Pn and gs [23,51]. Short-term exposure to e[CO2] and increased light intensity elicits opposing effects on stomatal morphology: e[CO2] induces stomatal closure to conserve water, whereas enhanced light intensity promotes stomatal opening to facilitate CO2 uptake for photosynthesis [52]. Stomatal aperture and density are critical determinants of photosynthetic performance, balancing gas exchange efficiency with water conservation [53]. In this study, e[CO2] significantly reduced stomatal aperture and density (Table 1, Figure 3), minimizing water loss and enhancing water use efficiency, consistent with prior research [16]. Conversely, increased light intensity tended to elevate these parameters, aligning with studies showing moderate light improves Pn, Tr, and gs [32,54]. Notably, e[CO2]-Lh induced a marked reduction in stomatal aperture relative to low/moderate light conditions. This may reflect a potential protective mechanism under combined high CO2 and light stress, involving increased LT and carbon allocation to structural tissues, as evidenced by elevated leaf and plant dry masses in tomato seedlings. A potential confounding factor is heat emission from metal halide lamps, which may have indirectly influenced stomatal dynamics [23,32]. Future studies using LED lighting systems could dissociate light quality/intensity effects from thermal influences, enabling precise characterization of stomatal responses to photic stimuli.
Leaf anatomical structure is dynamically modulated by environmental factors, including light intensity and [CO2] [23,43,44,55], with profound effects on photosynthetic efficiency and drought tolerance via carbon partitioning and water retention mechanisms. Consistent with prior studies [56], e[CO2] substantially enhanced LT, PM, and SM (Table 2, Figure 4), indicating that elevated CO2 optimizes leaf architecture to facilitate CO2 diffusion and dissolution, thereby boosting photosynthetic capacity and biomass accumulation. Concurrently, this structural optimization likely synergized with nutrient sufficiency to enhance carbon assimilation, as elevated CO2 has been shown to upregulate plant nutrient demand [57]. Furthermore, increased light intensity enhanced leaf mass and PM across [CO2] levels, with significant interactions promoting LT. This suggests higher light intensities improve light interception efficiency [58], acting synergistically with e[CO2] to enhance light energy absorption and CO2 fixation [16]. Notably, e[CO2] reduced the PM/SM ratio under moderate light intensity, contrasting with previous reports [57]. This discrepancy likely arises from divergent environmental contexts and the optimal nutrient management in our study, where elevated CO2-treated plants prioritized balancing photosynthetic efficiency with resource utilization [24], reflecting phenotypic resilience and plasticity in response to climate variables—a phenomenon warranting further mechanistic investigation [58].
Gas exchange dynamics (Figure 5) corroborate leaf structural and stomatal observations, indicating that e[CO2] significantly augmented Pn while reducing gs and Tr. These findings validate the structure-function paradigm, whereby e[CO2] enhances photosynthesis through leaf architectural adjustments, including increased thickness and stomatal trait modifications, in line with prior studies [23,45]. Enhanced Pn, gs, and Tr under elevated light intensity align with established mechanisms of light-driven stomatal activation and carbon fixation [31,51,59]. Notably, diurnal fluctuations in photosynthetic parameters reflect dynamic environmental cues and plant water status, modulating the balance between carbon fixation and transpirational water loss [60]. In this study, e[CO2] significantly increased Fv/Fm, ΦPSII, and ETR (Table 3), consistent with earlier reports [40,61]. This suggests elevated CO2 enhances photosynthetic performance by optimizing light energy partitioning—augmenting photochemical efficiency while reducing NPQ—and improving photosynthetic machinery functionality [62].
Plant phenotypic traits—including leaf area, anatomical structure, TDM, and SHI—serve as direct indicators of adaptability to environmental variables [23,32]. In this study, e[CO2], increased light intensity, and their interactions substantially influenced leaf area-related traits (SLA, LAR; Figure 2) and leaf structural parameters (stomatal, mesophyll, and epidermal characteristics; Table 1 and Table 2/Figure 3 and Figure 4). Notably, TDM and SHI showed significant positive correlations with [CO2], light intensity, and their interaction (Figure 6), supporting our prior hypothesis: combined optimization of CO2 and light intensity enhances mesophyll architecture and stomatal patterning, thereby boosting carbon assimilation capacity. Given the rising demands of the vegetable industry for high-quality seedlings [5], scientifically optimized environmental control strategies—such as CO2 fertilization and light regulation—will become pivotal for intensive seedling production systems [16]. While short-term e[CO2] is widely acknowledged to enhance photosynthetic performance and growth, its efficacy may decline across developmental stages due to metabolic reprogramming [16,23]. Thus, future research should explore the dynamic adaptive mechanisms of tomato plants to concurrent [CO2] and light intensity increases, enabling sustainable optimization of greenhouse cultivation protocols.
The correlation matrix (Figure 7) reveals key associations among growth, anatomical, and photosynthetic traits in tomato plants, highlighting adaptive strategies under environmental gradients. In this study, RGR and NAR showed strong negative correlations with SLA and LAR, indicating resource allocation trade-offs in light-limited environments where reduced leaf expansion prioritizes carbon assimilation efficiency [52,63]. Notably, RGR and NAR were positively associated with Pn, TDM, and SHI, consistent with prior findings [64], suggesting that e[CO2] and increased PPFD enhance biomass accumulation by boosting carbon assimilation. Leaf anatomical traits (e.g., UE, LT, SM) correlated positively with Pn, aligning with studies demonstrating structural optimization for carbon assimilation [65]. Pn exhibited a positive correlation with SW but a negative correlation with SA [66], reflecting the interplay between CO2-driven carboxylation efficiency and light-induced photoprotection mechanisms. NPQ negatively correlated with growth traits, likely balancing photoprotection and resource allocation: e[CO2] promoted low-SLA leaf structures, while high light induced NPQ upregulation, collectively limiting leaf expansion (low LAR) [24]. These dynamics align with research on CO2 fertilization and photoprotective acclimation [65], emphasizing the trade-off between light harvesting and carbon gain as a critical acclimation strategy [13].
This study comprehensively analyzed morphological, physiological, anatomical, and photosynthetic responses to e[CO2] and increased PPFD. Importantly, transferring tomato seedlings to differing light intensities triggered stepwise activation of photoprotective pathways and morphological adjustments [41,67,68]. Prior research shows 240 µmol·m−2·s−1 optimizes seedling growth, photosynthetic efficiency, and quality traits, while suboptimal or excessive light reduces chlorophyll content and stomatal conductance [67]. O’Carrigan et al. [68] further documented that prolonged high-light exposure (≥6 weeks) alters guard cell/stomatal morphology, induces stomatal closure, and suppresses photosynthesis. Whether pre-culture enhances seedling resilience to fluctuating light environments remains unaddressed. Moreover, its short-term design limits insights into long-term dynamics (e.g., full growth cycles). Long-term acclimation of stomatal behavior, photosynthetic apparatus, and leaf anatomy—along with yield and quality outcomes—warrants further investigation [24,49]. Future studies should characterize these parameters over extended periods to develop robust tomato production strategies under climate-smart greenhouse management.
5. Conclusions
Elevated [CO2] and increased light intensity significantly enhanced RGR and NAR while reducing SLA and LAR. Concurrently, e[CO2] decreased stomatal aperture and density, whereas increased light intensity modulated stomatal morphology to enhance gas exchange. These treatments collectively optimized leaf anatomical traits, thereby enhancing light interception and CO2 diffusion efficiency. Elevated [CO2] improved photochemical parameters (Fv/Fm, ΦPSII, ETR) while reducing NPQ, indicative of enhanced light energy utilization and potential photoprotective acclimation under high light. Individually and synergistically, e[CO2] and increased PPFD promoted TDM and SHI, with the highest values observed under e[CO2]-Lh. Collectively, these findings, coupled with correlation analysis, demonstrate that e[CO2] and increased light intensity optimize leaf structural and stomatal traits, thereby enhancing light capture, CO2 assimilation, and energy conversion efficiency. Based on these results, the e[CO2]-Lh treatment (800 µmol·mol−1 [CO2] and 400 µmol·m−2·s−1 PPFD) is recommended for tomato seedling production in controlled growth environments to maximize seedling vigor and resource use efficiency.
Author Contributions
Formal Analysis, T.P. and X.Z.; Investigation, T.P. and Y.W.; Data Curation, T.P., G.L. and Y.W.; Writing—Original Draft, T.P., W.D., X.Z., G.L., Y.W. and W.Z.; Writing—Review and Editing, T.P., W.D., W.Z., G.L., B.F., E.B., K.C. and X.Z. Funding acquisition, K.C. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Key Research and Development Program of Ningxia Hui Autonomous Region (2023BCF01022), the Key Laboratory for Crop Production and Smart Agriculture of Yunnan Province (2024ZHNY03), the Key Laboratory of Spectroscopy Sensing, Ministry of Agriculture and Rural Affairs, P.R. China (2024ZJUGP002), and the National Key Laboratory of Wheat Improvement of China (KFKT202507).
Data Availability Statement
The raw data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge the undergraduates and the graduate students in our laboratory for their assistance in data collection and senior colleagues for their valuable guidance in the technical aspects of data analysis.
Conflicts of Interest
The authors declare no conflicts of interest in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| a[CO2] | Ambient CO2 concentration |
| ANOVA | Analysis of variance |
| ATP | Adenosine triphosphate |
| CAM | Crassulacean acid metabolism |
| DAT | Day after treatment |
| e[CO2] | Elevated CO2 concentration |
| ETR | Electron transport rate |
| FAO | Food and Agriculture Organization of the United Nations |
| Fm | Maximum fluorescence |
| Fm′ | Maximum fluorescence under actinic light |
| F0 | Initial fluorescence |
| F0′ | Minimum fluorescence under actinic light |
| Fv/Fm | Maximum PSII photochemical efficiency |
| FS | Steady-state fluorescence |
| Fv′/Fm′ | Effective photochemical quantum efficiency |
| gs | Stomatal conductance |
| LAR | Leaf area ratio |
| LE | Lower epidermis |
| Lh | High light intensity |
| Ll | Low light intensity |
| Lm | Moderate light intensity |
| LT | Leaf thickness |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NAR | Net assimilation rate |
| NPQ | Non-photochemical quenching |
| PM | Palisade mesophyll |
| PM/SM | The ratio of palisade mesophyll to spongy mesophyll |
| Pn | Net photosynthetic rate |
| PPFD | Photosynthetic photon flux density |
| PSII | Photosystem II |
| ΦPSII | Actual PSII quantum yield |
| qP | Photochemical quenching coefficient |
| RGR | Relative growth rate |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| RuBP | Ribulose 1,5-bisphosphate |
| ROS | Reactive oxygen species |
| SA | Stomatal aperture |
| SD | Stomatal density |
| SE | Standard error |
| SHI | Seedling health index |
| SL | Stomatal length |
| SLA | Specific leaf area |
| SM | Spongy mesophyll |
| SW | Stomatal width |
| TDM | Total dry mass per plant |
| Tr | Transpiration rate |
| UE | Upper epidermis |
References
- Xu, X.; Yang, F.; Song, J.; Zhang, R.; Cai, W. Does the daily light integral influence the sowing density of tomato plug seedlings in a controlled environment? Horticulturae 2024, 10, 730. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Yan, H.; Ullah, I.; Zuo, Z.; Li, L.; Yu, J. Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Database 2023. Available online: http://www.fao.org/statistics/en (accessed on 1 March 2025).
- Akhlaq, M.; Zhang, C.; Yan, H.; Ou, M.; Zhang, W.; Liang, S.; Ikram, R.M.A. Response of tomato growth to continuous elevated CO2 concentration under controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 51–59. [Google Scholar] [CrossRef]
- Kumi, F.; Mao, H.; Li, Q.; Luhua, H. Assessment of tomato seedling substrate-root quality using X-ray computed tomography and scanning electron microscopy. Appl. Eng. Agric. 2016, 32, 417–427. [Google Scholar] [CrossRef]
- Song, J.; Zhang, R.; Yang, F.; Wang, J.; Cai, W.; Zhang, Y. Nocturnal LED supplemental lighting improves quality of tomato seedlings by increasing biomass accumulation in a controlled environment. Agronomy 2024, 14, 1888. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, W.; Liu, C.; Xu, C.; Wei, G.; Cui, B.; Hou, J.; Wan, H.; Chen, Y.; Zhang, J.; et al. Effect of CO2 elevation on tomato gas exchange, root morphology and water use efficiency under two N-fertigation levels. Plants 2024, 13, 2373. [Google Scholar] [CrossRef]
- An, W.; Wang, G.; Dou, J.; Zhang, Y.; Zhang, Q.; He, Y.; Tang, Z.; Yu, J. Protective mechanisms of exogenous melatonin on chlorophyll metabolism and photosynthesis in tomato seedlings under heat stress. Front. Plant Sci. 2025, 16, 1519950. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, P.; Fadiji, T.; Li, Z.; Ni, J. Biomechanical response of the above-ground organs in tomato seedling at different age levels under wind-flow disturbance. Sci. Hortic. 2023, 312, 111835. [Google Scholar] [CrossRef]
- Ma, G.; Chen, X.; Liu, Y.; Hu, J.; Han, L.; Mao, H. Effects of compound biochar substrate coupled with water and nitrogen on the growth of cucumber plug seedlings. Agronomy 2022, 12, 2855. [Google Scholar] [CrossRef]
- Pan, T.; Ding, J.; Qin, G.; Wang, Y.; Xi, L.; Yang, J.; Li, J.; Zhang, J.; Zou, Z. Interaction of supplementary light and CO2 enrichment improves growth, photosynthesis, yield, and quality of tomato in autumn through spring greenhouse production. Hort. Sci. 2019, 54, 246–252. [Google Scholar] [CrossRef]
- Amarasinghe, A.A.Y.; Polwaththa, K.P.G.D.M.; Suratissa, D.M. Effects of elevated CO2 and light intensity on growth, yield, and nutritional quality of tomato (Solanum lycopersicum) in controlled environment agriculture systems. Am. J. Life Sci. Innov. 2025, 4, 1–7. [Google Scholar] [CrossRef]
- Esmaili, M.; Aliniaeifard, S.; Mashal, M.; Ghorbanzadeh, P.; Seif, M.; Gavilan, M.U.; Carrillo, F.F.; Lastochkina, O.; Li, T. CO2 enrichment and increasing light intensity till a threshold level, enhance growth and water use efficiency of lettuce plants in controlled environment. Not. Bot. Horti Agrobot. 2020, 48, 2244–2262. [Google Scholar] [CrossRef]
- Koo, J.K.; Hwang, H.S.; Hwang, J.H.; Park, E.W.; Yu, J.; Yun, J.H.; Hwang, S.Y.; Choi, H.E.; Hwang, S.J. Supplemental lighting and CO2 enrichment on the growth, fruit quality, and yield of cucumber. Hortic. Environ. Biotechnol. 2025, 66, 77–85. [Google Scholar] [CrossRef]
- Li, X.; Kang, S.; Li, F.; Zhang, X.; Huo, Z.; Ding, R.; Tong, L.; Du, T.; Li, S. Light supplement and carbon dioxide enrichment affect yield and quality of off-season pepper. Agron. J. 2017, 109, 2107–2118. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Wang, L.; Ding, J.; Cao, Y.; Qin, G.; Yan, L.; Xi, L.; Zhang, J.; Zou, Z. Increased CO2 and light intensity regulate growth and leaf gas exchange in tomato. Physiol. Plant. 2020, 168, 694–708. [Google Scholar] [CrossRef]
- Soukharev, B. Why Humans Would Not Be Able to Stop Global Warming in the Coming Decades Even if There Were No Climate Feedbacks. In Global Warming and Mass Migration, 1st ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2025; Volume 4, pp. 113–173. [Google Scholar] [CrossRef]
- Kumari, S.; Agrawal, M. Growth, yield and quality attributes of a tropical potato variety (Solanum tuberosum L. cv Kufri chandramukhi) under ambient and elevated carbon dioxide and ozone and their interactions. Ecotoxicol. Environ. Saf. 2014, 101, 146–156. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Keeley, J.E.; Rundel, P.W. Evolution of CAM and C4 carbon-concentrating mechanisms. Int. J. Plant Sci. 2003, 164, S55–S77. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef]
- Zhang, C.; Akhlaq, M.; Yan, H.; Ni, Y.; Liang, S.; Zhou, J.; Xue, R.; Li, M.; Adnan, R.M.; Li, J. Chlorophyll fluorescence parameter as a predictor of tomato growth and yield under CO2 enrichment in protective cultivation. Agric. Water Manag. 2023, 284, 108333. [Google Scholar] [CrossRef]
- Liu, B.; LI, M.; Li, Q.; Cui, Q.; Zhang, W.; Ai, X.; Bi, H. Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 2018, 56, 942–952. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Yang, J.; Liang, T.; Liu, L.; Pan, T.; Zou, Z. Stomatal opening and growth in tomato seedlings treated with different proportions of red and blue light. Can. J. Plant Sci. 2019, 99, 688–700. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. Hort. Sci. 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zou, Z.; Zou, Z. Effects of daily light integral on tomato (Solanum lycopersicon L.) grafting and quality in a controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Tan, Y.; Duan, Y.; Chi, Q.; Wang, R.; Yin, Y.; Cui, D.; Li, S.; Wang, A.; Ma, R.; Li, B.; et al. The role of reactive oxygen species in plant response to radiation. Int. J. Mol. Sci. 2023, 24, 3346. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. Bmc Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. J. Plant Growth Regul. 2021, 40, 2191–2207. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Z.; Liu, X.; Tang, C.; Wang, L.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- He, W.; Huang, Z.; Li, J.; Su, W.; Gan, L.; Xu, Z. Effect of different light intensities on the photosynthate distribution in cherry tomato seedlings. J. Hortic. Sci. Biotechnol. 2019, 94, 611–619. [Google Scholar] [CrossRef]
- Khan, I.; Sohail; Zaman, S.; Li, G.; Fu, M. Adaptive responses of plants to light stress: Mechanisms of photoprotection and acclimation. A review. Front. Plant Sci. 2025, 16, 1550125. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xing, D.; Wu, Y.; Wang, W.; Li, M.; Solangi, K. Diurnal variation in transport and use of intracellular leaf water and related photosynthesis in three karst plants. Agronomy 2022, 12, 2758. [Google Scholar] [CrossRef]
- Yan, H.; Acquah, S.; Zhang, C.; Wang, G.; Huang, S.; Zhang, H.; Zhao, B.; Wu, H. Energy partitioning of greenhouse cucumber based on the application of Penman-Monteith and Bulk Transfer models. Agric. Water Manag. 2019, 217, 201–211. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. Phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef]
- Ando, E.; Kinoshita, T. Red light-induced phosphorylation of plasma membrane H+-ATPase in stomatal guard cells. Plant Physiol. 2018, 178, 838–849. [Google Scholar] [CrossRef]
- Akhlaq, M.; Miao, H.; Zhang, C.; Yan, H.; Run, X.; Chauhdary, J.N.; Rehman, M.M.U.; Li, J.; Ren, J. Resilience assessment of tomato crop chlorophyll fluorescence against water stress under elevated CO2 and protective cultivation. Irrig. Drain. 2025, 0, 1–19. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, K.; Zhang, J.; Wang, Z.; Sun, Z.; Zhang, H.; Hu, J. Response analysis of fluorescence parameters of tomato seedlings oriented to vertical light environment adaptation. Plant Sci. 2022, 314, 111118. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Li, J.; Chang, Y.; Du, Q.; Pan, T. Regulation of vapor pressure deficit by greenhouse micro-fog systems improved growth and productivity of tomato via enhancing photosynthesis during summer season. PLoS ONE 2015, 10, e0133919. [Google Scholar] [CrossRef]
- Mishra, A.K.; Gupta, S.; Agrawal, S.B.; Tiwari, S. Role of stomatal and leaf anatomical features in defining plant performance under elevated carbon dioxide and ozone, in the changing climate scenario. Environ. Sci. Pollut. Res. 2025, 32, 2536–2550. [Google Scholar] [CrossRef] [PubMed]
- Janova, J.; Kubasek, J.; Grams, T.E.E.; Zeisler-Diehl, V.; Schreiber, L.; Santrucek, J. Effect of light-induced changes in leaf anatomy on intercellular and cellular components of mesophyll resistance for CO2 in Fagus sylvatica. Plant Biol. 2024, 26, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chang, Z.; Lu, Y.; Tian, Y.; Zhou, H.; Wang, Y.; Liu, L.; Wang, P.; Zheng, Y.; Wu, J. Drought dampens the positive acclimation responses of leaf photosynthesis to elevated [CO2] by altering stomatal traits, leaf anatomy, and Rubisco gene expression in Pyrus. Environ. Exp. Bot. 2023, 211, 105375. [Google Scholar] [CrossRef]
- Li, X.; Schmid, B.; Wang, F.; Wang, T. Net assimilation rate determines the growth rates of 14 species of subtropical forest trees. PLoS ONE 2016, 11, e0150644. [Google Scholar] [CrossRef]
- Oguchi, R.; Ozaki, H.; Hanada, K.; Hikosaka, K. Which plant trait explains the variations in relative growth rate and its response to elevated carbon dioxide concentration among Arabidopsis thaliana ecotypes derived from a variety of habitats? Oecologia 2016, 180, 865–876. [Google Scholar] [CrossRef]
- Shipley, B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: Relationship with daily irradiance. Func. Ecol. 2002, 16, 682–689. [Google Scholar] [CrossRef]
- Centritto, M.; Lee, H.S.J.; Jarvis, P.G. Increased growth in elevated [CO2]: An early, short-term response? Glob. Change Biol. 1999, 5, 623–633. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Hattori, T.; Sonobe, K.; Inanaga, S.; An, P.; Tsuji, W.; Araki, H.; Eneji, A.E.; Morita, S. Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environ. Exp. Bot. 2007, 60, 177–182. [Google Scholar] [CrossRef]
- Driesen, E.; Ende, W.V.D.; Proft, M.D.; Saeys, W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Lawson, T.; von Caemmerer, S.; Baroli, I. Photosynthesis and Stomatal Behaviour. In Progress in Botany, 1st ed.; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 72, pp. 265–304. [Google Scholar] [CrossRef]
- Lee, S.H.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. plantlets. Plant Cell Tissue Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, X.; Feng, C.; Niu, M.; Liu, C.; Wang, H.; Yin, W.; Xia, X. Light and light signals regulate growth and development in woody plants. Forests 2024, 15, 523. [Google Scholar] [CrossRef]
- Wang, N.; Gao, G.; Wang, Y.; Wang, D.; Wang, Z.; Gu, J. Coordinated responses of leaf and absorptive root traits under elevated CO2 concentration in temperate woody and herbaceous species. Environ. Exp. Bot. 2020, 179, 104199. [Google Scholar] [CrossRef]
- De Graaff, M.A.; Van Groenigen, K.J.A.N.; Six, J.; Hungate, B.; Van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Global Change Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Radochová, B.; Tichá, I. Leaf anatomy during leaf development of photoautotrophically in vitro-grown tobacco plants as affected by growth irradiance. Biol. Plant. 2009, 53, 21–27. [Google Scholar] [CrossRef]
- Arantes, M.K.; da Silva Filho, M.P.; Pennacchi, J.P.; das Chagas Mendonça, A.M.; Barbosa, J.P.R.A.D. Phenotypic plasticity of leaf anatomical traits helps to explain gas-exchange response to water shortage in grasses of different photosynthetic types. Theor. Exp. Plant Physiol. 2020, 32, 341–356. [Google Scholar] [CrossRef]
- Matthews, J.S.; Vialet-Chabrand, S.R.M.; Lawson, T. Diurnal variation in gas exchange: The balance between carbon fixation and water loss. Plant Physiol. 2017, 174, 614–623. [Google Scholar] [CrossRef]
- Akhlaq, M.; Zhang, Z.; Yan, H.; Liang, S.; Ni, Y.; Zhou, J.; Xue, R.; Li, J.; Hussain, Z.; Iqbal, S. Exploring adequate CO2 elevation for optimum tomato growth and yield under protected cultivation. J. Plant Physiol. 2023, 289, 154093. [Google Scholar] [CrossRef]
- Kitao, M.; Tobita, H.; Kitaoka, S.; Harayama, H.; Yazaki, K.; Komatsu, M.; Agathokleous, E.; Koike, T. Light energy partitioning under various environmental stresses combined with elevated CO2 in three deciduous broadleaf tree species in Japan. Climate 2019, 7, 79. [Google Scholar] [CrossRef]
- Ma, F.; Na, X.; Xu, T. Drought responses of three closely related Caragana species: Implication for their vicarious distribution. Ecol. Evol. 2016, 6, 2763–2773. [Google Scholar] [CrossRef]
- Borsuk, A.M.; Roddy, A.B.; Theroux-Rancourt, G.; Brodersen, C.R. Structural organization of the spongy mesophyll. New Phytol. 2022, 234, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Marçal, D.M.S.; Avila, R.T.; Quiroga-Rojas, L.F.; de Souza, R.P.B.; Junior, C.C.G.; Ponte, L.R.; Barbosa, M.L.; Oliveira, L.A.; Martins, S.C.V.; Ramalho, J.D.C.; et al. Elevated [CO2] benefits coffee growth and photosynthetic performance regardless of light availability. Plant Physiol. Biochem. 2021, 158, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Dun, Z.; Wang, Y.; Yang, D.; Xiong, D.; Cui, K.; Peng, S.; Huang, J. Effect of stomatal morphology on leaf photosynthetic induction under fluctuating light in rice. Front. Plant Sci. 2022, 12, 754790. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y.; et al. Effects of different light intensity on the growth of tomato seedlings in a plant factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).