Harnessing a Microbial Consortium and Compost to Control Grapevine Pathogens: A Sustainable Viticulture Strategy for Disease Suppression and Quality Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Detection of Fungal and Fungal-like Pathogens
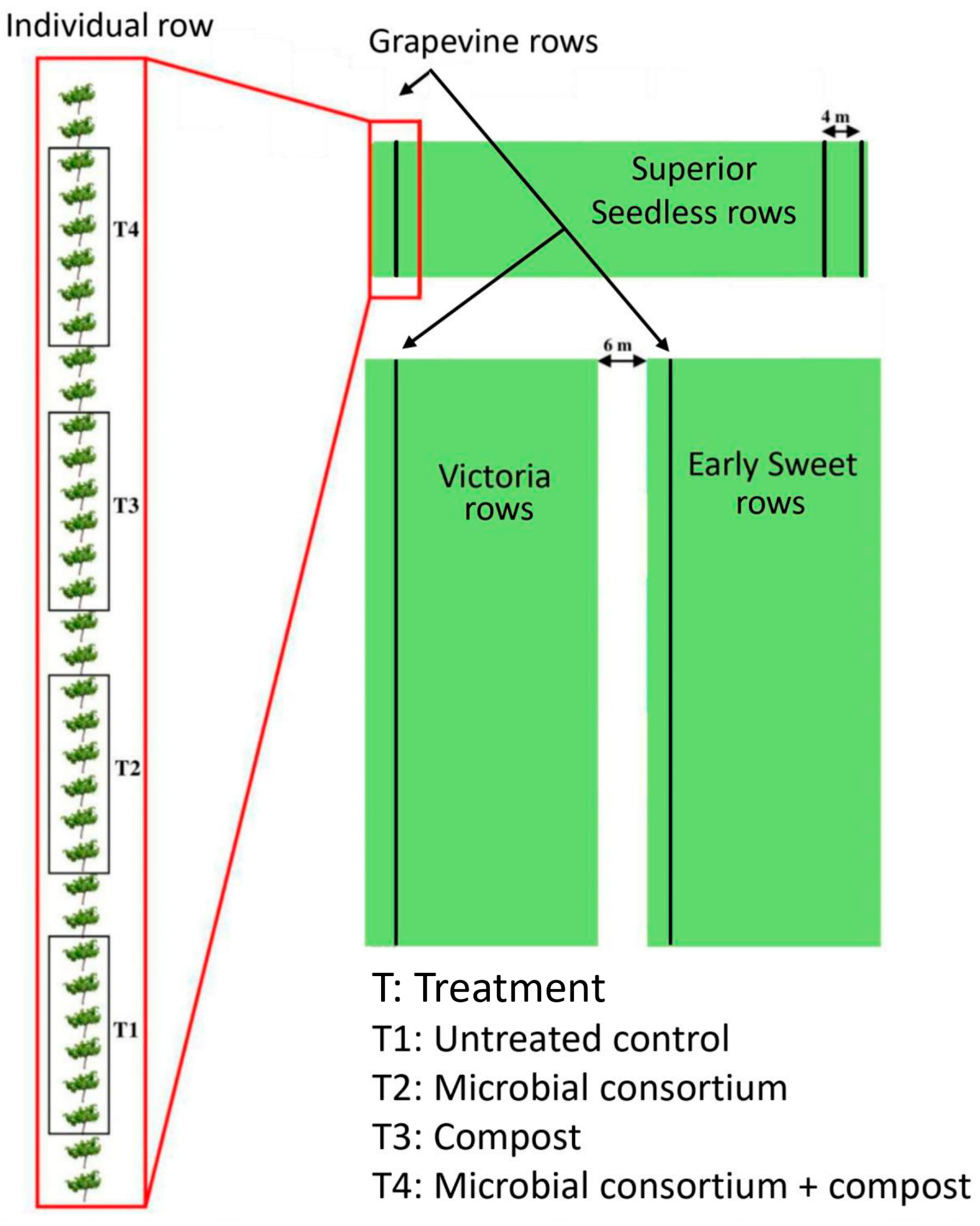
2.3. Experimental Design
2.4. Biocontrol Strains and Compost Preparation for Field Applications
2.5. Soil Sampling After Treatment
2.6. Soil Physicochemical and Microbial Analyses
2.7. Disease Severity and Incidence Assessments
2.8. Physiological Parameters
2.8.1. Chlorophyll Content
2.8.2. Biochemical and Antioxidant Analyses
2.9. Fruit Quality Analysis
2.10. Mineral Analysis of Leaves
2.11. Statistical Analysis
3. Results
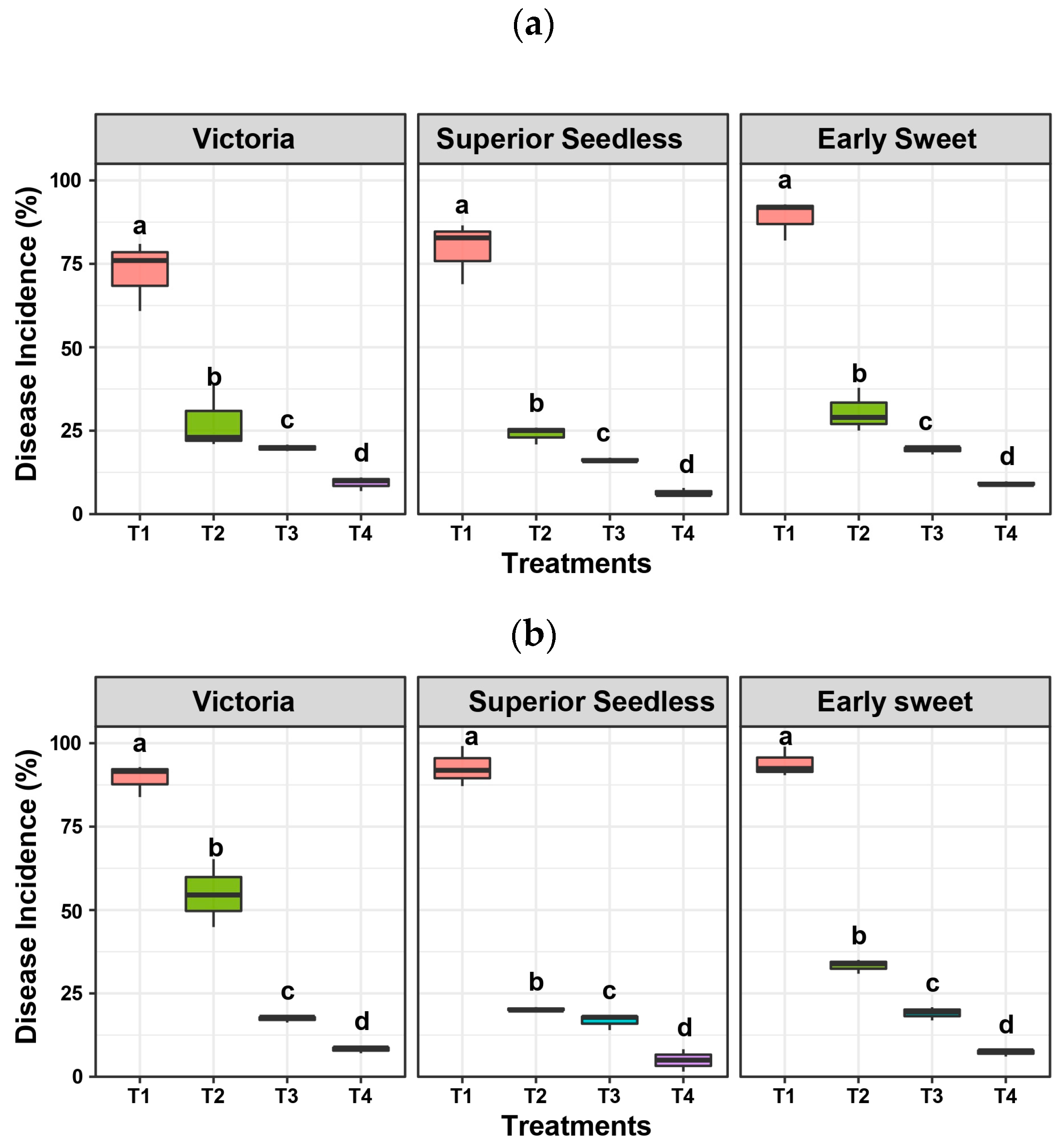
3.1. Antifungal Activity of the Microbial Consortium and Compost Against Fungal Diseases
3.2. Effects of the Microbial Consortium and Compost on Plant Growth Promotion
3.3. Impacts of the Microbial Consortium and Compost on the Grapevine Plant Defense System
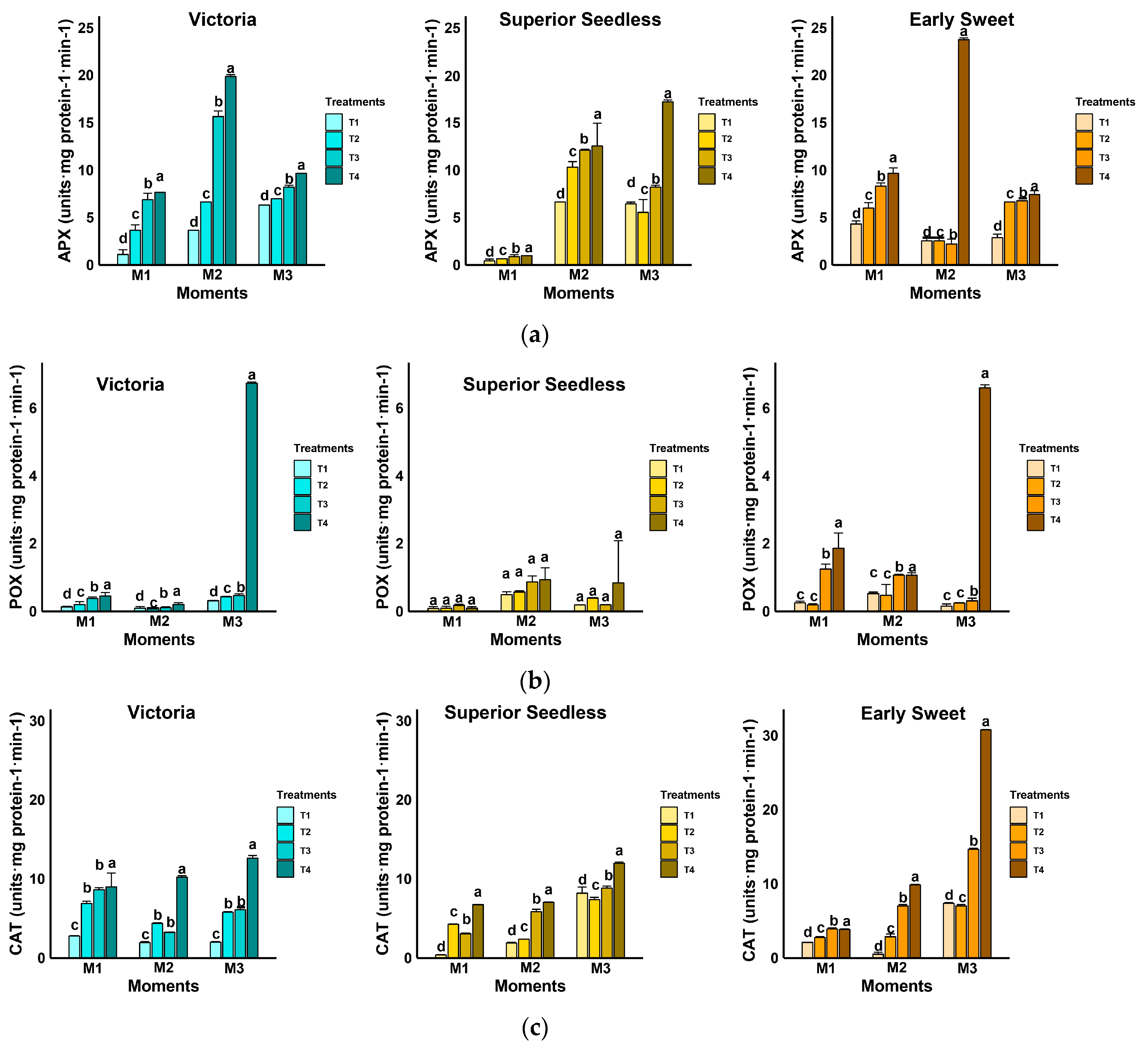
3.3.1. Antioxidant Enzymatic Activities in Grapevine Leaves
3.3.2. Stress Markers and Defense-Related Compounds in Leaves
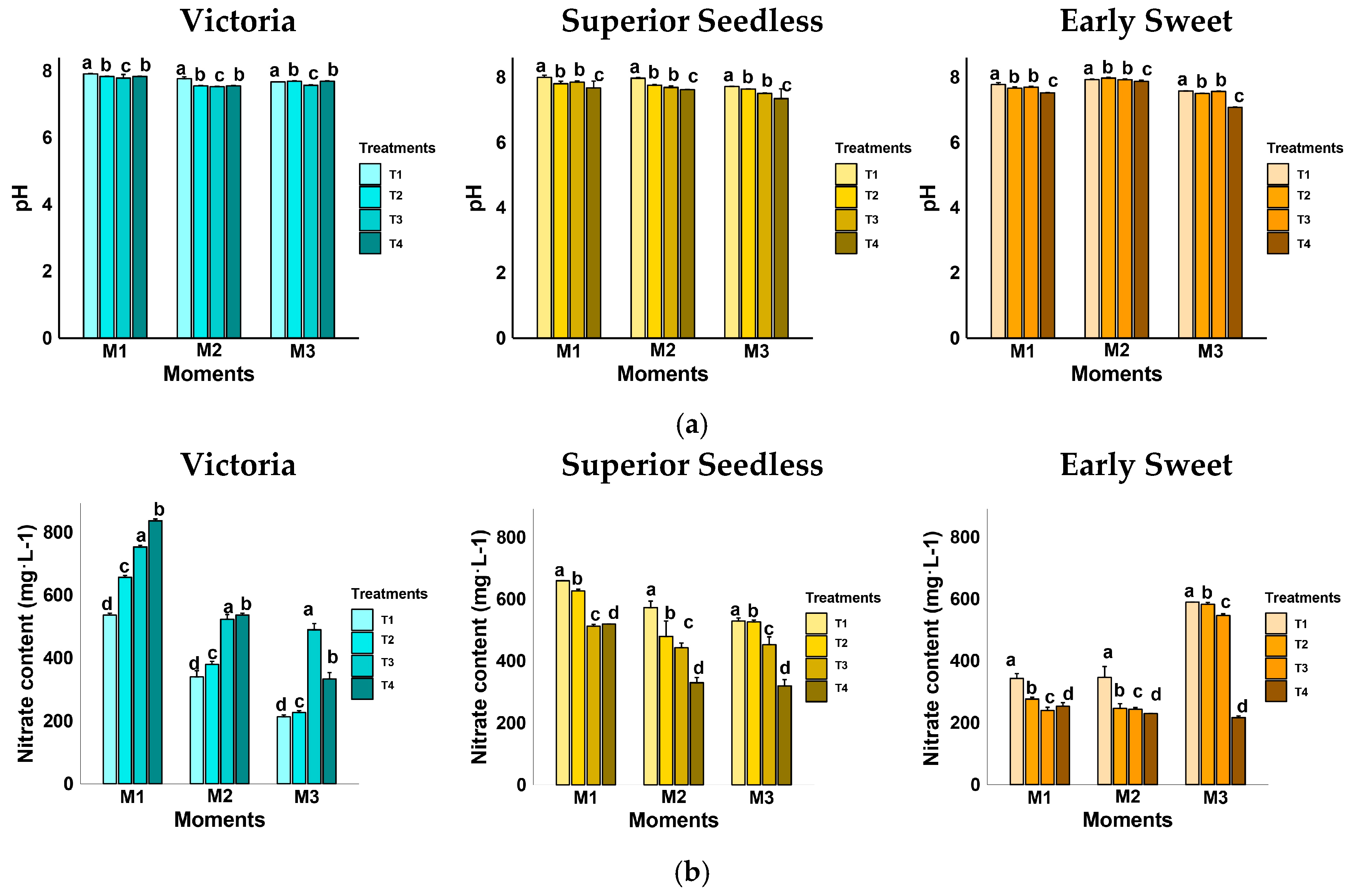
3.4. Soil Physicochemical Responses
3.5. Soil Microbial Abundance
3.6. Antioxidant Enzyme Activities and Stress Markers in Grapevine Fruits
3.7. Mineral Composition of Grapevine Fruits
3.8. Antifungal Activity Against Grapevine Pathogens
3.9. Fruit Quality Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kenfaoui, J.; El Hamss, H.; El Modafar, C.; Wahbi, S.; Ait Barka, E.; Ibriz, M.; Filali-Maltouf, A.; Benkirane, R. Unlocking the potential of rhizobacteria in Moroccan vineyard soils: Biocontrol of grapevine trunk diseases and plant growth promotion. Biol. Control 2023, 186, 105306. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). OIV Statistical Report on World Vitiviniculture. Available online: https://www.oiv.int (accessed on 2 June 2025).
- Gai, Y.; Wang, H. Plant disease: A growing threat to global food security. Agronomy 2024, 14, 1615. [Google Scholar] [CrossRef]
- Cheung, N.; Gill, M.; Wise, C.; Grewal, P.S.; Myers, K.; Leathers, T.D. The destructive fungal pathogen Botrytis cinerea: Insights from genes studied with mutant analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Váczy, K.Z.; Kun, F.; Tóth, E.; Hoffmann, B.; Gere, A.; Kallai, M.; Mocsai, R.; Bényei, F. Botrytis cinerea causes different plant responses in grape (Vitis vinifera) berries during noble and grey rot: Diverse metabolism versus simple defence. Front. Plant Sci. 2024, 15, 1433161. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, H.; Hu, X.; Liu, L.; Wang, M. Occurrence regularity and epidemic factors of Botrytis cinerea in greenhouse in Hubei province. Plant Prot. 2019, 45, 164–169. [Google Scholar]
- Qiu, W.; Feechan, A.; Dry, I. Current understanding of grapevine defense mechanisms against the biotrophic fungus (Erysiphe necator), the causal agent of powdery mildew disease. Hortic. Res. 2015, 2, 15020. [Google Scholar] [CrossRef] [PubMed]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Mu, B.; Teng, Z.; Tang, R.; Lu, M.; Chen, J.; Xu, X.; Wen, Y.Q. An effector of Erysiphe necator translocates to chloroplasts and plasma membrane to suppress host immunity in grapevine. Hortic. Res. 2023, 10, uhad163. [Google Scholar] [CrossRef]
- Ramya Sree, M.; Singh, S.K.; Prakash, J.; Kumar, C.; Kumar, A.; Mishra, G.P.; Sevanthi, A.M.; Sreekanth, H.; Amala, E. Powdery mildew pathogen Erysiphe necator induced physiological and biochemical alterations in leaf tissue of grapevines (Vitis spp.). Physiol. Mol. Plant Pathol. 2024, 133, 102386. [Google Scholar] [CrossRef]
- Yin, L.; An, Y.; Qu, J.; Li, X.; Zhang, Y.; Dry, I.; Wu, H.; Lu, J. Genome sequence of Plasmopara viticola and insight into the pathogenic mechanism. Sci. Rep. 2017, 7, 46553. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Liviz, C.D.A.M.; Maciel, G.M.; Pinheiro, D.F.; Lima, N.F.; Ribeiro, I.S.; Haminiuk, C.W.I. Pesticide residues in grapes and wine: An overview on detection, health risks, and regulatory challenges. Food Res. Int. 2025, 203, 115771. [Google Scholar] [CrossRef]
- Mesguida, O.; Haidar, R.; Yacoub, A.; Berthon, J.; Guyoneaud, R.; Attard, E.; Rey, P. Microbial biological control of fungi associated with grapevine trunk diseases: A review of strain diversity, modes of action, and advantages and limits of current strategies. J. Fungi 2023, 9, 638. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Wannassi, T.; El-Maradny, Y. Fungal endophytes: Insight into evolution, classification, and ecological functions in plants. Microb. Biosyst. 2025, 10, 51–67. [Google Scholar] [CrossRef]
- Soltero-Rios, M.A.; Medina-Orozco, A.Y.; Valenzuela-Yerena, M.A.; Andrade-Robles, N.M.; Mendoza-Flores, A.A.; Morelia-Jiménez, J.A.; Ochoa-Castañeda, M.Y.; Robles-Hernández, A.; González-Estrada, R.R. Microbial consortia: An approach in plant growth promotion and plant diseases management. In Microbial Biocontrol Techniques; Kumar, A., Solanki, M.K., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2024; Volume 54. [Google Scholar] [CrossRef]
- Suthin Raj, T.; Vignesh, S.; Nishanthi, P.; Hane Graff, K.; Ann Suji, H. Induction of defence enzymes activities in grape plant treated by seaweed algae against Plasmopara viticola and Uncinula necator causing downy and powdery mildews of grapes. Nov. Res. Microbiol. J. 2018, 2, 122–137. [Google Scholar]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef]
- Ziedan, E.H.E.; El-Mohamedy, R.S.R. Application of Pseudomonas fluorescens for Controlling Root-Rot Disease of Grapevine. Res. J. Agric. Biol. Sci. 2008, 4, 346–353. [Google Scholar]
- Yendyo, S.; GC, R.; Pandey, B.R. Evaluation of Trichoderma spp., Pseudomonas fluorescens and Bacillus subtilis for Biological Control of Ralstonia Wilt of Tomato. F1000Research 2018, 6, 2028. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.F.; Issa, G.J.; Smernik, R.; Wilkinson, K. Chemical Composition of Composted Grape Marc. Water Sci. Technol. 2009, 60, 1265–1271. [Google Scholar] [CrossRef]
- Barakat, R.; Al-Masri, M. Trichoderma harzianum in Combination with Sheep Manure Enhances Soil Suppressiveness of Fusarium Wilt of Tomato. Phytopathol. Mediterr. 2010, 48, 385–395. [Google Scholar]
- Edy, N.; Arsih, D.W.; Panggeso, J.; Anshary, A.; Yunus, M.; Lakani, I.; Rosmini, R. Double Action Plant Growth Promotion Microorganisms in Suppressing Fusarium Wilt Disease and Increase Tomato Production. IOP Conf. Ser. Earth Environ. Sci. 2023, 1253, 012024. [Google Scholar] [CrossRef]
- Bandara, A.Y.; Kang, S. Trichoderma Application Methods Differentially Affect the Tomato Growth, Rhizomicrobiome, and Rhizosphere Soil Suppressiveness against Fusarium oxysporum. Front. Microbiol. 2024, 15, 1366690. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Rhouma, A.; Hajlaoui, H.; Hajlaoui, F.; Rebouh, N.Y. Understanding the Influence of Applying Two Culture Filtrates to Control Gray Mold Disease (Botrytis cinerea) in Tomato. Agronomy 2023, 13, 1774. [Google Scholar] [CrossRef]
- Köppen, W. Das Geographische System der Klimate; Gebrüder Borntraeger: Berlin, Germany, 1936; pp. 1–44. [Google Scholar]
- Zhou, Y.J.; Zhang, J.; Wang, X.D.; Yang, L.; Jiang, D.H.; Li, G.Q.; Hsiang, T.; Zhuang, W.Y. Morphological and Phylogenetic Identification of Botrytis sinoviticola, a Novel Cryptic Species Causing Gray Mold Disease of Table Grapes (Vitis vinifera) in China. Mycologia 2014, 106, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Blancard, D.; Lecomte, P.; Levis, C. Phenotypic Differences between vacuma and transposa Subpopulations of Botrytis cinerea. Eur. J. Plant Pathol. 2003, 109, 479–488. [Google Scholar] [CrossRef]
- Tanovic, B.; Delibasic, G.; Milivojevic, J.; Nikolic, M. Characterization of Botrytis cinerea Isolates from Small Fruits and Grapevine in Serbia. Arch. Biol. Sci. 2009, 61, 419–429. [Google Scholar] [CrossRef]
- Tanovic, B.; Hrustic, J.; Mihajlovic, M.; Grahovac, M.; Delibasic, G. Botrytis cinerea in Raspberry in Serbia I: Morphological and Molecular Characterization. J. Pestic. Phytomed. 2014, 29, 237–247. [Google Scholar] [CrossRef]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of the Erysiphales (Powdery Mildews); CBS Biodiversity Series; CBS: Utrecht, The Netherlands, 2012; Volume 11, pp. 1–707. [Google Scholar]
- Darsaraei, H.; Khodaparast, S.A.; Mousanejad, S.; Asgari, B.; Aliabadi, F.; Sajedi, S. A Taxonomic Revision of Erysiphe sect. Uncinula (Erysiphaceae, Helotiales) in Iran. Mycol. Iran. 2021, 8, 1–15. [Google Scholar]
- Karthick, M.; Kamalakannan, A.; Malathi, V.G.; Paranidharan, V.; Sivakumar, U.; Kavino, M.; Gowrisri, N. Morphological Characterization of Plasmopara viticola, the Inciting Agent of Grapes Downy Mildew. J. Pharmacogn. Phytochem. 2019, 8, 209–212. [Google Scholar]
- Wong, C.T.; Falcone, M.; Rich, G.; Stubler, C.; Malama, B.; Lazcano, C.; Decock, C. Short-Term Effects of Increasing Compost Application Rates on Soil C and Greenhouse Gas (N2O and CO2) Emissions in a California Central Coast Vineyard. Front. Environ. Sci. 2023, 11, 1123510. [Google Scholar] [CrossRef]
- Wilson, S.G.; Lambert, J.J.; Dahlgren, R. Compost Application to Degraded Vineyard Soils: Effect on Soil Chemistry, Fertility, and Vine Performance. Am. J. Enol. Vitic. 2021, 72, 85–93. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Walkley, A.; Black, A. Étude de la Méthode DEGT JAREFF pour le Dosage de la Matière Organique, Modification Apportée au Dosage de l’Acide Chromique. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Mouria, B.; Ouazzani-Touhami, A.; Douira, A. Isolement et Identification de la Mycoflore du Compost des Déchets Urbains Solides. Nature Technol. 2013, 9, 13. [Google Scholar]
- Nakano, Y.; Azada, K. Purification of Ascorbate Peroxidase in Spinach Chloroplasts: Its Inactivation in Ascorbate-Depleted Medium and Reactivation by Monodehydroascorbate Radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Velazhahan, R.; Vidhyasekaran, P. Role of phenolic compounds, peroxidase and polyphenol-oxidase in resistance of groundnut to rust. Acta Phytopathol. Entomol. Hung. 1994, 29, 23–29. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimétrie des Phénols Totaux avec des Réactifs Phosphomolybdic-Phosphotungstiques. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Haraguchi, H.; Saito, T.; Okamura, N.; Yagi, A. Inhibition of Lipid Peroxidation and Superoxide Generation by Diterpenoids from Rosmarinus officinalis. Planta Med. 1995, 61, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Minchev, Z.; Kostenko, O.; Soler, R.; Pozo, M.J. Microbial Consortia for Effective Biocontrol of Root and Foliar Diseases in Tomato. Front. Plant Sci. 2021, 12, 756368. [Google Scholar] [CrossRef]
- Fournier, P.; Pellan, L.; Jaswa, A.; Cambon, M.C.; Chataigner, A.; Bonnard, O.; Raynal, M.; Debord, C.; Poeydebat, C.; Labarthe, S.; et al. Revealing Microbial Consortia That Interfere with Grapevine Downy Mildew through Microbiome Epidemiology. Environ. Microbiome 2025, 20, 37. [Google Scholar] [CrossRef]
- Maachia, B.; Rafik, E.; Chérif, M.; Nandal, P.; Mohapatra, T.; Bernard, P. Biological Control of the Grapevine Diseases ‘Grey Mold’ and ‘Powdery Mildew’ by Bacillus B27 and B29 Strains. Indian J. Exp. Biol. 2015, 53, 109–115. [Google Scholar]
- Fernandez-Ortuno, D.; Pérez-García, A.; Chamorro, M.; de la Peña, E.; de Vicente, A.; Torés, J.A. Resistance to the SDHI Fungicides Boscalid, Fluopyram, Fluxapyroxad, and Penthiopyrad in Botrytis cinerea from Commercial Strawberry Fields in Spain. Plant Dis. 2017, 101, 1306–1313. [Google Scholar] [CrossRef]
- Perazzolli, M.; Roatti, B.; Bozza, E.; Pertot, I. Trichoderma harzianum T39 Induces Resistance against Downy Mildew by Priming for Defense without Costs for Grapevine. Biol. Control 2011, 58, 74–82. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the Characteristics of Organic Soil Amendments That Suppress Soilborne Plant Diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam, M.; Barka, E.A.; et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 1625. [Google Scholar] [CrossRef] [PubMed]
- Ouhaddou, R.; Ikan, C.; Soussani, F.E.; Errouh, F.; Boutasknit, A.; Rodrigez, J.C.; Duponnois, R.; Meddich, A. Investigation of the Impact of Dual Inoculations of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria on Drought Tolerance of Maize Grown in a Compost-Amended Field under Mediterranean Conditions. Front. Microbiol. 2024, 15, 1432637. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a Leaf Chlorophyll Content Index to Improve the Prediction of Above-Ground Biomass and Productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef]
- Lafontaine, P.J.; Benhamou, N. Chitosan Treatment: An Emerging Strategy for Enhancing Resistance of Greenhouse Tomato Plants to Infection by Fusarium oxysporum f. sp. radicis-lycopersici. Biocontrol Sci. Technol. 1996, 6, 111–124. [Google Scholar] [CrossRef]
- Ebel, J.; Cosio, E.G. Elicitors of Plant Defense Responses. Int. Rev. Cytol. 1994, 148, 1–36. [Google Scholar]
- Wei, G.; Kloepper, J.W.; Tuzun, S. Induced Systemic Resistance to Cucumber Diseases and Increased Plant Growth by Plant Growth-Promoting Rhizobacteria under Field Conditions. Phytopathology 1996, 86, 221–224. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-Evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Sharifi, M.; Hajiaghaei-Kamrani, M. Biochar–Compost Mixture and Cover Crop Effects on Soil Carbon and Nitrogen Dynamics, Yield, and Fruit Quality in an Irrigated Vineyard. Can. J. Soil Sci. 2023, 103, 200–212. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, H.; Hu, J.; Li, H.; Zhao, Z.; Wu, Y.; Li, J.; Zhou, Y.; Yang, K.; Yang, H. Trichoderma harzianum Inoculation Promotes Sweet Sorghum Growth in the Saline Soil by Modulating Rhizosphere Available Nutrients and Bacterial Community. Front. Plant Sci. 2023, 14, 1258131. [Google Scholar] [CrossRef]
- Srivastava, S.N.; Singh, V.; Awasthi, S.K. Trichoderma-Induced Improvement in Growth, Yield and Quality of Sugarcane. Sugar Tech 2006, 8, 166–169. [Google Scholar] [CrossRef]
- Wong, C.K.F.; Zulperi, D.; Saidi, N.B.; Vadamalai, G. A Consortium of Pseudomonas aeruginosa and Trichoderma harzianum for Improving Growth and Induced Biochemical Changes in Fusarium Wilt-Infected Bananas. Trop. Life Sci. Res. 2021, 32, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Bai, R.; Yu, Q.; Bao, Y.; Yang, W. The Effect of Nitrogen Reduction and Applying Bio-Organic Fertilisers on Soil Nutrients and Apple Fruit Quality and Yield. Agronomy 2024, 14, 345. [Google Scholar] [CrossRef]
- Okba, S.K.; Abo Ogiela, H.M.; Mehesen, A.; Mikhael, G.B.; Alam-Eldein, S.M.; Tubeileh, A.M. Influence of Compost and Biological Fertilization with Reducing the Rates of Mineral Fertilizers on Vegetative Growth, Nutritional Status, Yield and Fruit Quality of ‘Anna’ Apples. Agronomy 2025, 15, 662. [Google Scholar] [CrossRef]





| Cultivars | Treatments | M1 (30 Days) | M2 (60 Days) | M3 (90 Days) |
|---|---|---|---|---|
| Victoria | T1 | 19.5 ± 3.20 c | 31.7 ± 0.361 c | 31.6 ± 1.70 c |
| T2 | 23.0 ± 0.723 c | 32.1 ± 1.54 c | 39.9 ± 2.56 b | |
| T3 | 23.4 ± 1.25 b | 36.0 ± 3.38 b | 39.5 ± 4.21 b | |
| T4 | 35.1± 1.74 a | 47.6 ± 1.72 a | 45.4 ± 0.416 a | |
| p-value | <0.01 | <0.01 | <0.01 | |
| Superior Seedless | T1 | 22.4 ± 2.89 c | 33.9 ± 0.900 b | 36.6 ± 2.95 b |
| T2 | 24.1 ± 0.755 c | 35.2 ± 6.28 b | 34.6 ± 4.80 b | |
| T3 | 26.4 ± 0.458 b | 38.6 ± 0.458 b | 46.3 ± 3.50 a | |
| T4 | 34.9 ± 0.954 a | 45.4 ± 4.59 a | 44.7 ± 3.44 a | |
| p-value | <0.01 | <0.01 | <0.01 | |
| Early Sweet | T1 | 33.0 ± 0.153 c | 37.4 ± 2.14 c | 37.4 ± 2.48 c |
| T2 | 41.7 ± 1.01 b | 38.1 ± 0.265 c | 40.3 ± 1.47 c | |
| T3 | 42.0 ± 0.737 b | 41.7 ± 0.802 b | 42.4 ± 2.80 b | |
| T4 | 43.7 ± 1.32 q | 46.1 ± 0.379 a | 48.6 ± 1.90 a | |
| p-value | <0.01 | <0.01 | <0.01 |
| Cultivars | Treatments | APX (Units·mg Protein−1·min−1) | POX (Units·mg Protein−1·min−1) | CAT (Units·mg Protein−1·min−1) | MDA (µmol/g) | TPC (mg/g) | TP (mg/g) |
|---|---|---|---|---|---|---|---|
| Victoria | T1 | 3.51 ± 0.81 d | 1.48 ± 0.27 b | 17.50 ± 1.74 c | 1.03 ± 0.95 b | 0.47 ± 0.01 d | 1.09 ± 0.51 c |
| T2 | 9.40 ± 0.59 c | 1.55 ± 0.45 b | 19.52 ± 1.52 bc | 2.71 ± 0.27 a | 0.98 ± 0.57 c | 8.06 ± 0.92 b | |
| T3 | 9.83 ± 0.66 b | 1.78 ± 0.59 a | 23 ± 0.97 ab | 0.39 ± 0.02 c | 1.83 ± 0.48 a | 13.41 ± 0.87 a | |
| T4 | 9.92 ± 0.48 a | 1.91 ± 0.63 a | 24.64 ± 1.22 a | 0.65 ± 0.08 c | 1.15 ± 0.92 b | 13.64 ± 1.23 a | |
| p-value | <0.01 | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Superior Seedless | T1 | 3.80 ± 0.44 d | 2.21 ± 0.21 b | 4.22 ± 0.91 d | 10.19 ± 0.45 a | 1.07 ± 0.33 c | 10.31 ± 1.08 b |
| T2 | 7.52 ± 0.34 c | 2.24 ± 0.13 b | 4.86 ± 0.67 c | 7.57 ± 0.69 b | 0.42 ± 0.64 d | 11.86 ± 0.97 a | |
| T3 | 8.16 ± 0.17 b | 2.30 ± 0.44 b | 6.10 ± 0.58 b | 2.82 ± 0.34 c | 1.71 ± 0.28 b | 10.54 ± 0.84 b | |
| T4 | 9.51 ± 0.75 a | 2.49 ± 0.98 a | 7.06 ± 0.04 a | 0.47 ± 0.05 d | 2.50 ± 0.49 a | 12.40 ± 0.66 a | |
| p-value | <0.01 | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Early Sweet | T1 | 1.09 ± 0.18 d | 2.05 ± 0.18 a | 5.06 ± 0.19 c | 4.24 ± 0.08 a | 0.92 ± 0.15 d | 0.85 ± 0.02 c |
| T2 | 4.99 ± 0.29 c | 2.16 ± 0.65 a | 5.64 ± 0.36 b | 2.47 ± 0.23 b | 1.13 ± 0.69 c | 7.36 ± 0.31 b | |
| T3 | 7.81 ± 0.96 b | 2.32 ± 0.32 a | 6.14 ± 0.48 b | 0.99 ± 0.19 c | 1.43 ± 0.09 b | 11.01 ± 0.45 a | |
| T4 | 15.38 ± 0.33 a | 2.42 ± 0.46 a | 6.98 ± 0.53 a | 0.97 ± 0.24 c | 1.80 ± 0.37 a | 11.16 ± 0.75 a | |
| p-value | <0.01 | ≥0.05 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cultivars | Treatments | Fe (µmol/g) | Mg (µmol/g) | Cl (µmol/g) | Ca (µmol/g) | Zn (µmol/g) | P (µmol/g) |
|---|---|---|---|---|---|---|---|
| Victoria | T1 | 2.42 | 183.75 | 919.53 | 5.57 | 0.33 | 0.48 |
| T2 | 1.67 | 73.4 | 699.52 | 2.23 | 0.24 | 0.47 | |
| T3 | 2.01 | 68.87 | 801.06 | 2.09 | 0.15 | 0.57 | |
| T4 | 2.31 | 141.37 | 648.75 | 4.29 | 0.2 | 0.87 | |
| Superior Seedless | T1 | 2.09 | 28.22 | 603.62 | 0.86 | 0.38 | 0.61 |
| T2 | 2.65 | 16.05 | 614.9 | 0.49 | 0.52 | 0.62 | |
| T3 | 2.31 | 75.79 | 535.92 | 2.3 | 0.76 | 0.65 | |
| T4 | 2.44 | 76.94 | 660.03 | 2.33 | 0.81 | 0.79 | |
| Early Sweet | T1 | 1.27 | 144.99 | 784.14 | 4.4 | 0.04 | 0.5 |
| T2 | 1.75 | 16.13 | 693.88 | 0.49 | 0.06 | 0.52 | |
| T3 | 1.87 | 34.89 | 880.04 | 1.06 | 0.12 | 0.56 | |
| T4 | 1.84 | 4.2 | 547.2 | 0.13 | 0.08 | 0.64 |
| Cultivars | Treatments | pH | EC (mS cm−1) | Sugar Content (Brix) | Nitrate Content (mg/kg)) | WC (%) | Fruits Caliber Size (mm) |
|---|---|---|---|---|---|---|---|
| Victoria | T1 | 3.54 ± 0.51 d | 3.44 ± 0.70 | 12.67 ± 0.53 b | 100 ± 3.05 a | 59.50 ± 0.64 | 16.95 ± 0.81 b |
| T2 | 3.80± 0.68 b | 3.71 ± 0.24 | 12.70 ± 0.84 b | 94.67 ± 2.67 b | 51.17 ± 0.57 | 19.03 ± 0.75 ab | |
| T3 | 3.71 ± 0.77 c | 3.41 ± 0.71 | 14.33 ± 0.99 a | 94 ± 1.94 b | 58.83 ± 0.92 | 19.29 ± 0.49 ab | |
| T4 | 3.82 ± 0.92 a | 3.26 ± 0.37 | 14.80 ± 0.68 a | 77 ± 1.88 c | 72.17 ± 0.38 | 21.20 ± 0.93 a | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | ≥0.05 | <0.05 | |
| Superior Seedless | T1 | 3.85 ± 0.36 d | 4.33 ± 0.09 | 13.10 ± 0.42 c | 87 ± 1.28 b | 27.83 ± 0.45 c | 17.55 ± 1.02 b |
| T2 | 3.91 ± 0.75 c | 4.16 ± 0.65 | 13.60 ± 0.37 b | 98.33 ± 1.36 a | 38.83 ± 0.69 bc | 18.37 ± 0.36 ab | |
| T3 | 3.94 ± 0.16 b | 4.50 ± 0.44 | 14.07 ± 0.91 a | 81.67 ± 1.45 c | 57.67 ± 0.88 a | 20.24 ± 0.69 ab | |
| T4 | 3.99 ± 0.22 a | 3.99 ± 0.18 | 14.40 ± 0.64 a | 83.33 ± 1.89 c | 46.83 ± 0.32 ab | 20.55 ± 0.55 a | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.05 | <0.05 | |
| Early Sweet | T1 | 3.73 ± 0.69 d | 4.26 ± 0.74 a | 11.37 ± 0.86 c | 97 ± 1.69 a | 68.50 ± 0.22 b | 15.76 ± 0.23 a |
| T2 | 3.87 ± 0.82 c | 4.01 ± 0.98 a | 12.30 ± 0.75 b | 65.67 ± 2.09 b | 79.17 ± 0.75 a | 15.88 ± 1.14 a | |
| T3 | 3.91 ± 0.55 b | 3.92 ± 0.80 a | 12.33 ± 0.67 ab | 86.67 ± 1.08 b | 79.33 ± 0.64 a | 17.03 ± 0.42 a | |
| T4 | 3.96 ± 0.45 a | 3.34 ± 0.62 a | 12.53 ± 0.52 a | 84 ± 0.98 b | 75.33 ± 0.80 ab | 17.81 ± 0.89 a | |
| p-value | <0.01 | ≥0.05 | <0.01 | <0.01 | <0.05 | ≥0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajji-Hedfi, L.; Wannassi, T.; Abdel-Azeem, A.M. Harnessing a Microbial Consortium and Compost to Control Grapevine Pathogens: A Sustainable Viticulture Strategy for Disease Suppression and Quality Enhancement. Horticulturae 2025, 11, 769. https://doi.org/10.3390/horticulturae11070769
Hajji-Hedfi L, Wannassi T, Abdel-Azeem AM. Harnessing a Microbial Consortium and Compost to Control Grapevine Pathogens: A Sustainable Viticulture Strategy for Disease Suppression and Quality Enhancement. Horticulturae. 2025; 11(7):769. https://doi.org/10.3390/horticulturae11070769
Chicago/Turabian StyleHajji-Hedfi, Lobna, Takwa Wannassi, and Ahmed M. Abdel-Azeem. 2025. "Harnessing a Microbial Consortium and Compost to Control Grapevine Pathogens: A Sustainable Viticulture Strategy for Disease Suppression and Quality Enhancement" Horticulturae 11, no. 7: 769. https://doi.org/10.3390/horticulturae11070769
APA StyleHajji-Hedfi, L., Wannassi, T., & Abdel-Azeem, A. M. (2025). Harnessing a Microbial Consortium and Compost to Control Grapevine Pathogens: A Sustainable Viticulture Strategy for Disease Suppression and Quality Enhancement. Horticulturae, 11(7), 769. https://doi.org/10.3390/horticulturae11070769










