Effects of Exogenous Naphthylacetic Acid Application on the Graft Union Healing of Oriental Melon Scion Grafted onto Squash Rootstock and the Qualities of Grafted Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Grafting Methods
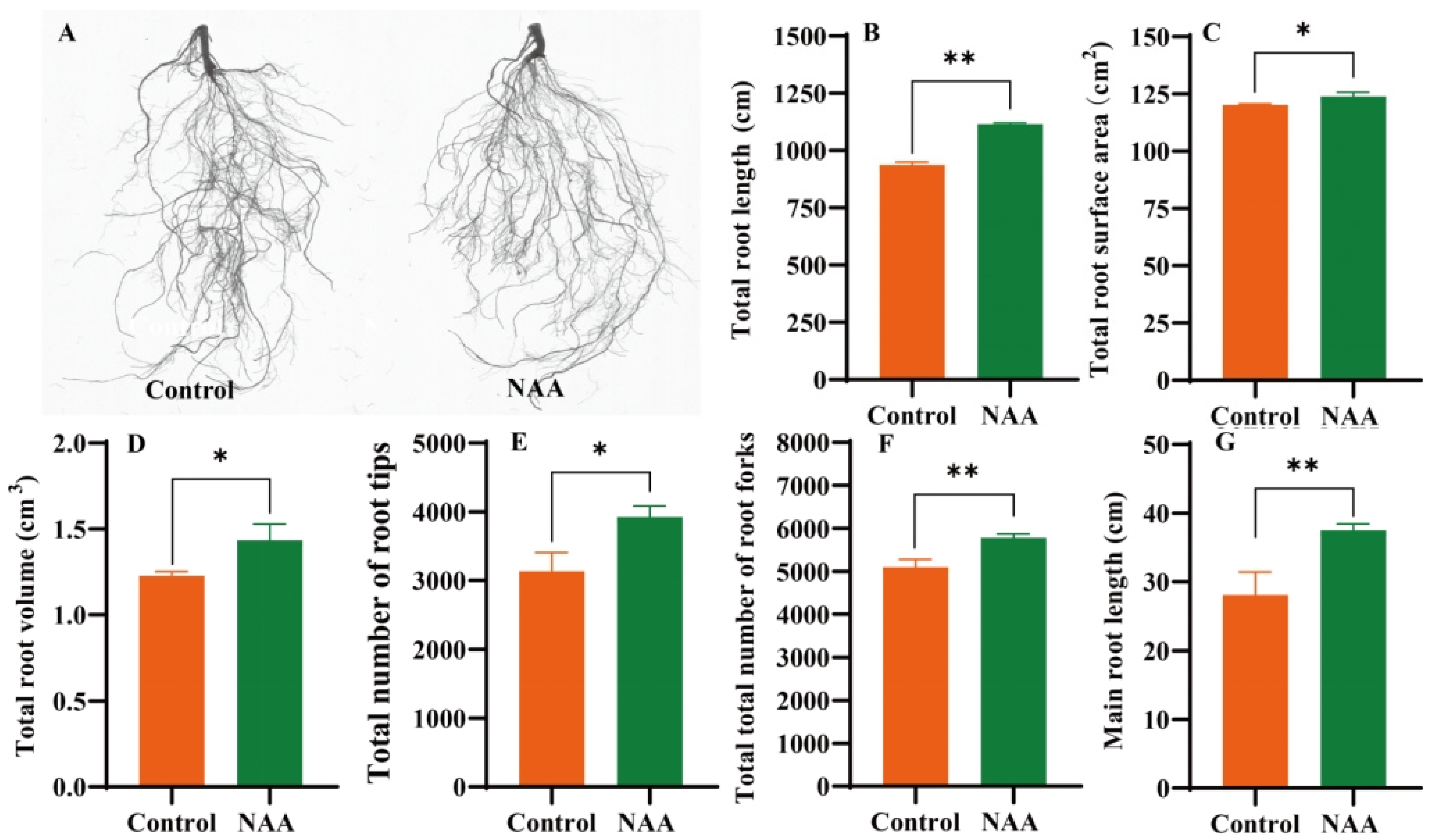
2.2. Acid Fuchsin Absorption Assay and Root Morphology Analysis
2.3. Determination of IAA Content and Enzyme Activities
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results
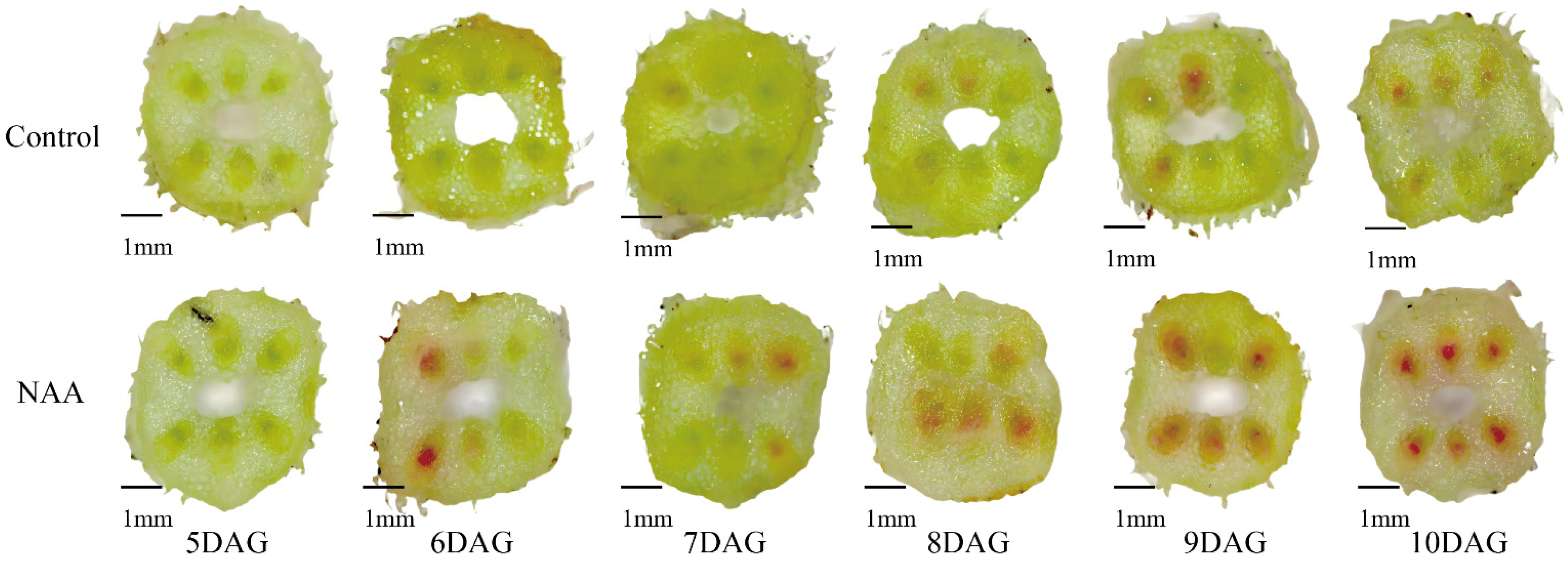
3.1. The Exogenous NAA Treatment Accelerated the Graft Union Healing of the Oriental Melon Scion Grafted onto Squash Rootstock
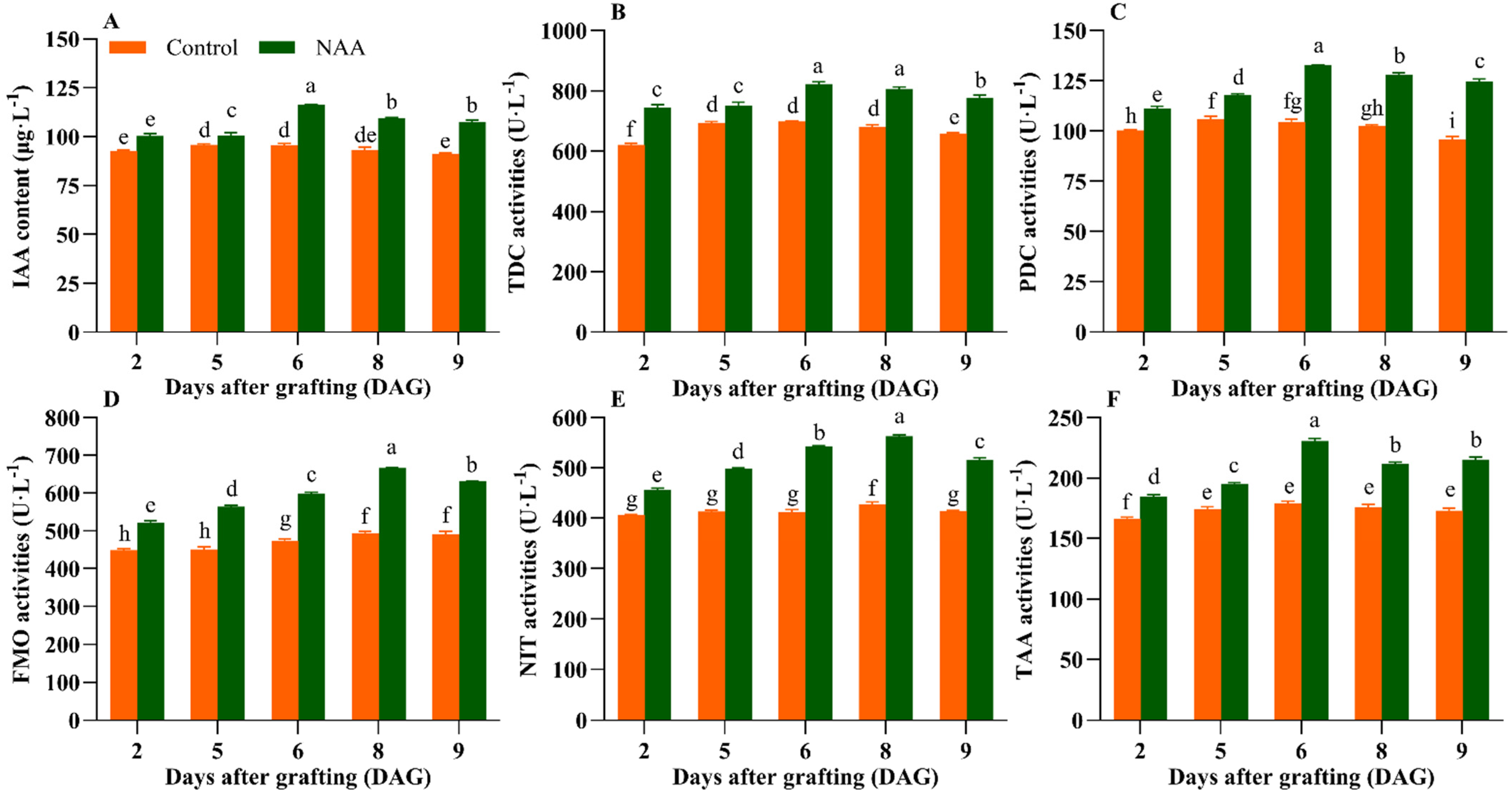
3.2. The Exogenous NAA Treatment Increased the IAA Content and Related Enzyme Activities in the Graft Union Tissues
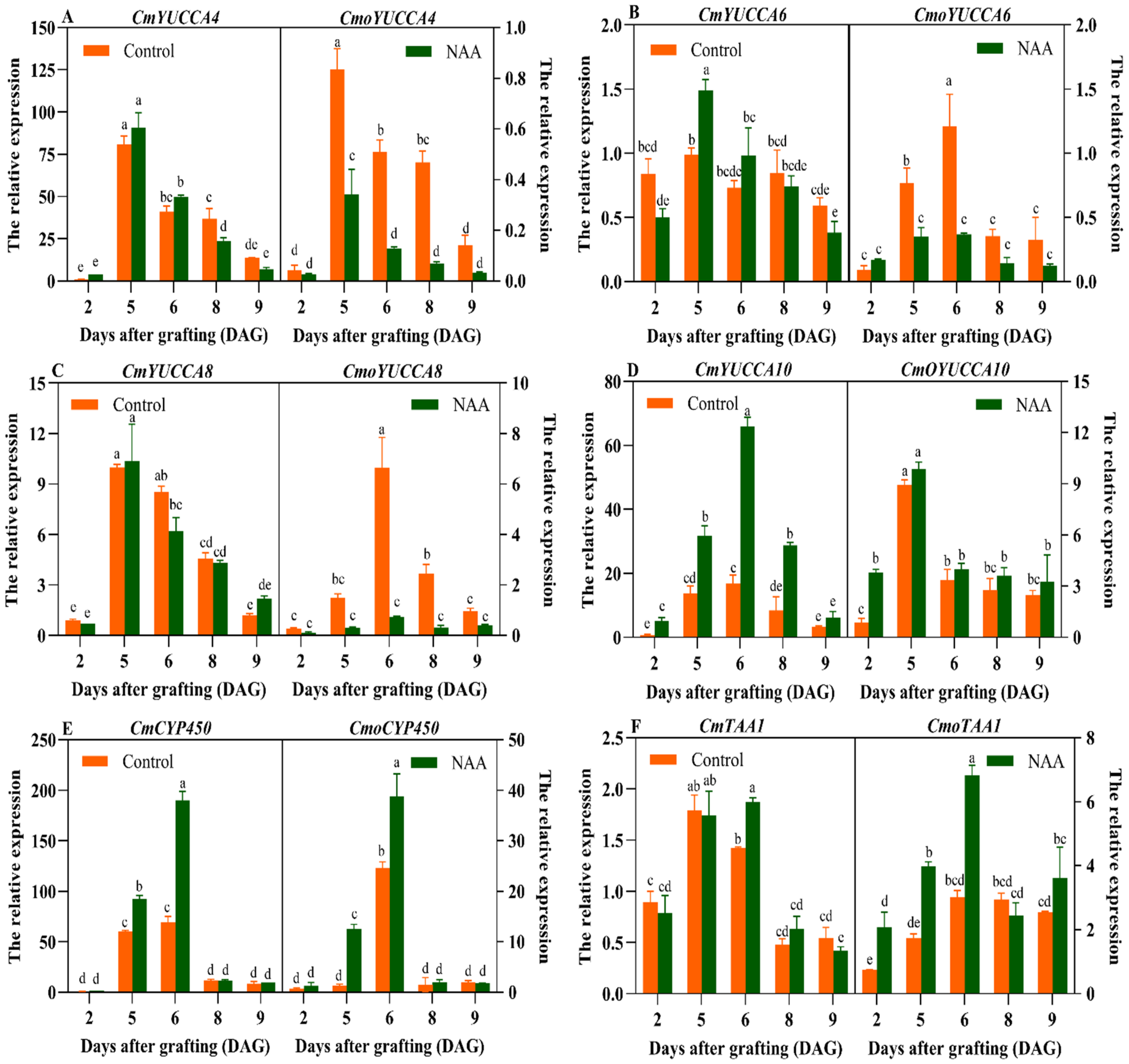
3.3. Analysis of the Key Gene Expression of IAA Biosynthesis During Graft Union Healing Under the Exogenous NAA Treatment
3.4. Expression Profiles of the Genes Related to Graft Union Healing of the Oriental Melon Scion Grafted onto Squash Rootstock by the Exogenous NAA Treatment
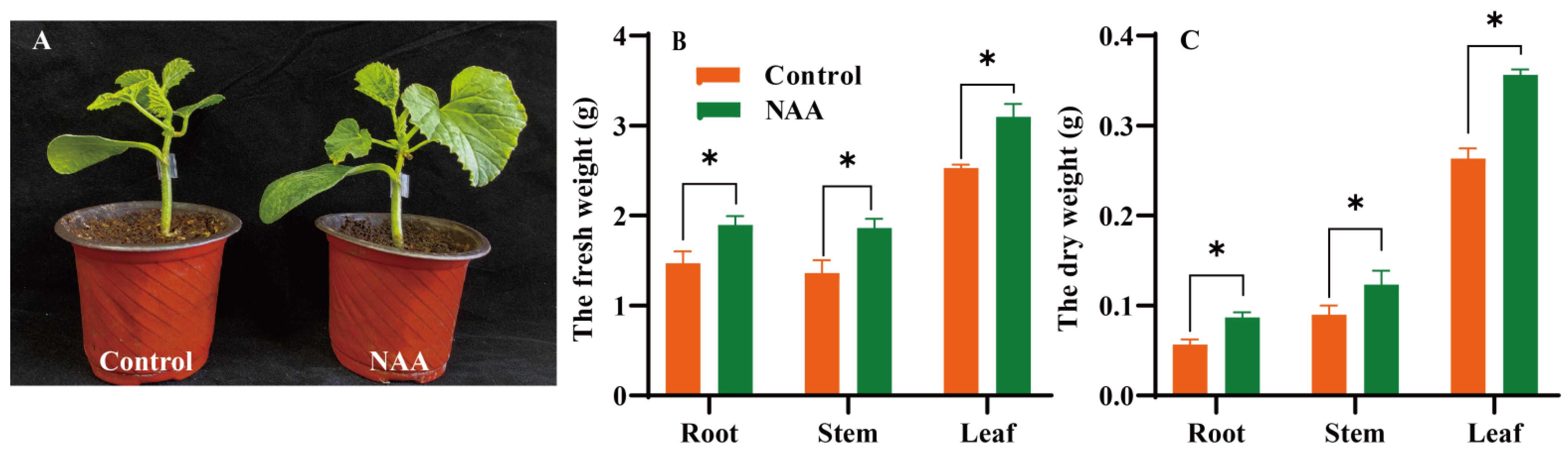
3.5. Effects of the Exogenous NAA Treatment on the Growth Performance of the Grafted Oriental Melon Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jensipershiya, S.; Sendhilvel, V.; Geethalakshmi, V.; Manoranjitham, S.; Malathi, P.; Siva, M. Valourizing agricultural farm waste with bioinoculants for plant growth promotion and disease management. Plant Sci. Today 2025, 12, 1–10. [Google Scholar] [CrossRef]
- Minchev, Z.; Ramírez-Serrano, B.; Dejana, L.; Díaz, A.; Zitlalpopoca-Hernandez, G.; Orine, D.; Saha, H.; Papantoniou, D.; García, J.M.; González-Céspedes, A.; et al. Beneficial soil fungi enhance tomato crop productivity and resistance to the leaf-mining pest Tuta absoluta in agronomic conditions. Agron. Sustain. Dev. 2024, 44, 55. [Google Scholar] [CrossRef]
- Teasdale, J.; Abdul-Baki, A.; Mill, D.; Thorpe, K. Enhanced pest management with cover crop mulches. Acta Hortic. 2004, 638, 135–140. [Google Scholar] [CrossRef]
- Ming, F.; Augstein, F.; Kareem, A.; Melnyk, C. Plant grafting: Molecular mechanisms and applications. Mol. Plant 2023, 17, 75–91. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Tan, J.; Huang, S.; Chen, X.; Dong, H.; Zhang, R.; Wang, Y.; Wang, B.; Xiao, X.; et al. The chromosome-scale reference genome and transcriptome analysis of Solanum torvum provides insights into resistance to root-knot nematodes. Front. Plant Sci. 2023, 14, 1210513. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Msabila, S.; Nordey, T.; Ernest, Z.; Mlowe, N.; Manickam, R.; Ramasamy, S.; Huat, J. Boosting Tomato Resilience in Tanzania: Grafting to Combat Bacterial Wilt and Abiotic Stress. Horticulturae 2024, 10, 338. [Google Scholar] [CrossRef]
- Janaharshini, R.; Velmurugan, M.; Rani, C.; Venkatachalam, S.; Saravanan, P. Vegetables grafting: Green surgical fusion to combat biotic and abiotic stresses. Plant Sci. Today 2024, 11, 1429–1439. [Google Scholar] [CrossRef]
- Bahadur, A.; Singh, P.; Rai, N.; Singh, A.; Singh, A.; Karkute, S.; Behera, T. Grafting in vegetables to improve abiotic stress tolerance, yield and quality. J. Hortic. Sci. Biotechnol. 2024, 99, 385–403. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Robledo-Torres, V.; González-Cortés, A.; Luna-García, L.; Mendoza-Villarreal, R.; Rodríguez, M.; Montejo, N. Histological Variations in Cucumber Grafted Plants and Their Effect on Yield. Agronomy 2024, 14, 1377. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Davis, A.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.; Zhang, X. Grafting Effects on Vegetable Quality. HortScience 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Ashraf, I.; Cipriani, G.; Mori, G. Bridging the Gap: Genetic Insights into Graft Compatibility for Enhanced Kiwifruit Production. Int. J. Mol. Sci. 2025, 26, 2925. [Google Scholar] [CrossRef]
- Loupit, G.; Valls, J.; Prigent, S.; Prodhomme, D.; Spilmont, A.; Hilbert, G.; Franc, C.; Revel, G.; Richard, T.; Ollat, N.; et al. Identifying early metabolite markers of successful graft union formation in grapevine. Hortic. Res. 2022, 9, uhab070. [Google Scholar] [CrossRef] [PubMed]
- Loupit, G.; Cookson, S. Identifying Molecular Markers of Successful Graft Union Formation and Compatibility. Front. Plant Sci. 2020, 11, 610352. [Google Scholar] [CrossRef]
- Rashedy, A.; Hamed, H. Morphological, physio-biochemical and nutritional status as potential markers for grafting compatibility in Kalamata olive cultivar. BMC Plant Biol. 2023, 23, 334. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.; Hassan, G.; Baba, T.; Alyemeni, M.; Alsahli, A.; El-Serehy, H.; Paray, B.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Gautier, A.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.; Delrot, S.; Cookson, S. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2018, 70, 747–755. [Google Scholar] [CrossRef]
- Tandonnet, J.; Cookson, S.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2009, 16, 290–300. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, Á.; Thompson, A.; Dodd, I.; Pérez-Alfocea, F. Unravelling rootstockxscion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef]
- Loupit, G.; Valls, J.; Lorensen, M.; Franc, C.; Revel, G.; Janfelt, C.; Cookson, S. Tissue-specific stilbene accumulation is an early response to wounding/grafting as revealed by using spatial and temporal metabolomics. Plant Cell Environ. 2023, 46, 3871–3886. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Feng, G.; Su, W.; Liu, Z.; Peng, F. Transcriptomic Analysis Provides Insights into Grafting Union Development in Pecan (Carya illinoinensis). Genes 2018, 9, 71. [Google Scholar] [CrossRef]
- Keerthana, P.; Pugalendhi, L.; Priya, R.; Devi, H. Cytological Studies on Graft Union Development with Perennial Chilli Rootstocks. Int. J. Plant Soil Sci. 2021, 33, 86–94. [Google Scholar] [CrossRef]
- Alqardaeai, T.; Alharbi, A.; Alenazi, M.; Al-Omran, A.; Alghamdi, A.; Obadi, A.; Elfeky, A.; Osman, M. Effectiveness of Grafting in Enhancing Salinity Tolerance of Tomato (Solanum lycopersicum L.) Using Novel and Commercial Rootstocks in Soilless Systems. Sustainability 2025, 17, 4333. [Google Scholar] [CrossRef]
- Amerian, M.; Palangi, A.; Gohari, G.; Ntatsi, G. Enhancing salinity tolerance in cucumber through Selenium biofortification and grafting. BMC Plant Biol. 2024, 24, 24. [Google Scholar] [CrossRef]
- Talavera, M.; Sayadi, S.; Chirosa-Ríos, M.; Salmerón, T.; Flor-Peregrín, E.; Verdejo-Lucas, S. Perception of the impact of root-knot nematode-induced diseases in horticultural protected crops of south-eastern Spain. Nematology 2012, 14, 517–527. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, J.; Wu, F.; Yu, H.; Min, J.; Zhao, Y.; Tan, C.; Liu, Y.; Xu, C. Exogenous Melatonin Application Accelerated the Healing Process of Oriental Melon Grafted onto Squash by Promoting Lignin Accumulation. Int. J. Mol. Sci. 2024, 25, 3690. [Google Scholar] [CrossRef]
- Xu, C.; Wu, F.; Guo, J.; Hou, S.; Wu, X.; Xin, Y. Transcriptomic analysis and physiological characteristics of exogenous naphthylacetic acid application to regulate the healing process of oriental melon grafted onto squash. PeerJ. 2022, 10, e13980. [Google Scholar] [CrossRef]
- Asahina, M.; Iwai, H.; Kikuchi, A.; Yamaguchi, S.; Kamiya, Y.; Kamada, H.; Satoh, S. Gibberellin Produced in the Cotyledon Is Required for Cell Division during Tissue Reunion in the Cortex of Cut Cucumber and Tomato Hypocotyls. Plant Physiol. 2002, 129, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.; Zhang, Z.; Yan, X.; Chai, J.; Ren, Z.; Zheng, G.; Liu, H. Graft-union development: A delicate process that involves cell–cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef]
- Fukaki, H.; Okushima, Y.; Tasaka, M. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 2007, 256, 111–137. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, X.; Tang, D.; Qi, Q.; Yer, H.; Jiang, X.; Han, Z.; McAvoy, R.; Li, W.; Li, Y. Molecular and physiological characterization of the effects of auxin-enriched rootstock on grafting. Hortic. Res. 2021, 8, 74. [Google Scholar] [CrossRef]
- Matsuoka, K.; Sugawara, E.; Aoki RTakuma, K.; Terao-Morita, M.; Satoh, S.; Asahina, M. Differential cellular control by cotyledon-derived hytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant Cell Physiol. 2016, 57, 2620–2631. [Google Scholar] [CrossRef]
- Asahina, M.; Azuma, K.; Pitaksaringkarn, W.; Yamazaki, T.; Mitsuda, N.; Ohme-Takagi, M.; Yamaguchi, S.; Kamiya, Y.; Okada, K.; Nishimura, T.; et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16128–16132. [Google Scholar] [CrossRef]
- Iwase, A.; Kondo, Y.; Laohavisit, A.; Takebayashi, A.; Ikeuchi, M.; Matsuoka, K.; Shirasu, K.; Fukuda, H.; Sugimoto, K. WIND transcription factors orchestrate wound-induced callus formation, vascular reconnection and defense response in Arabidopsis. New Phytol. 2021, 232, 734–752. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Ito, T.; Tanaka, H.; Favero, D.; Kawamura, A.; Sakamoto, S.; Wakazaki, M.; Tameshige, T.; Fujii, H.; et al. Wound-inducible WUSCHEL-RELATED HOMEOBOX 13 is required for callus growth and organ reconnection. Plant Physiol. 2021, 188, 425–441. [Google Scholar] [CrossRef]
- Rymen, B.; Kawamura, A.; Lambolez, A.; Inagaki, S.; Takebayashi, A.; Iwase, A.; Sakamoto, Y.; Sako, K.; Favero, D.S.; Ikeuchi, M.; et al. Histone acetylation orchestrates wound-induced transcriptional activation and cellular reprogramming in Arabidopsis. Commun. Biol. 2019, 2, 404. [Google Scholar] [CrossRef]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2022, 34, 535–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Matsuoka, K.; Kareem, A.; Robert, M.; Roszak, P.; Blob, B.; Bisht, A.; Veylder, L.D.; Voiniciuc, C.; Asahina, M.; et al. Cell-wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana. Curr. Biol. 2022, 32, 1883–1894. [Google Scholar] [CrossRef]
- Tan, T.T.; Endo, H.; Sano, R.; Kurata, T.; Yamaguchi, M.; Ohtani, M.; Demura, T. Transcription Factors VND1-VND3 Contribute to Cotyledon Xylem Vessel Formation. Plant Physiol. 2018, 176, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Tetsuro, M.; Hiroo, F.; Taku, D. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef]
- Davis, A.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.; Lee, S.; Hun, Y.; Sun, Z.; Miguel, A.; King, S.; et al. Cucurbit Grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, Y.; Wu, X.; Xin, Y.; Guo, J.; Wu, F.; Yu, H.; Sun, Z.; Xu, C. Scion-to-Rootstock Mobile Transcription Factor CmHY5 Positively Modulates the Nitrate Uptake Capacity of Melon Scion Grafted on Squash Rootstock. Int. J. Mol. Sci. 2022, 24, 162. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, S.; Min, J.; Zhao, Y.; Yu, H.; Irfan, M.; Xu, C. Transcriptomic analysis provides an insight into the function of CmGH9B3, a key gene of β-1,4-glucanase, during the graft union healing of oriental melon scion grafted onto squash rootstock. Biotechnol. J. 2024, 19, 2400006. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, M.; Qi, L.; Song, J.; Li, Q.; Wang, R. Differential expression analysis of genes related to graft union healing in Pyrus ussuriensis Maxim by cDNA-AFLP. Sci. Hortic. 2017, 225, 700–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Wang, G.; Hou, M.; Zhai, M.; Hu, L.; Xuan, J.; Mo, Z. Genome-Wide Identification and Expression Profiling of the Response Regulator (RR) Gene Family in Pecan Reveals Its Possible Association with Callus Formation during Grafting. Forests 2024, 15, 473. [Google Scholar] [CrossRef]
- Song, W.; Song, Y.; Liu, X.; Zhang, X.; Xin, R.; Duan, S.; Guan, S.; Sun, X. Improvement of Culture Conditions and Plant Growth Regulators for In Vitro Callus Induction and Plant Regeneration in Paeonia lactiflora Pall. Plants 2023, 12, 3968. [Google Scholar] [CrossRef] [PubMed]
- Sheldrake, A.; Northcote, D. The Production of Auxin by Tobacco Internode Tissues. New Phytol. 1968, 67, 1–13. [Google Scholar] [CrossRef]
- Wetmore, R.; Rier, J. Experimental Induction of Vascular Tissues in Callus of Angiosperms. Am. J. Bot. 1963, 50, 418–430. [Google Scholar] [CrossRef]
- Liang, H.; Liu, J.; Shi, X.; Ge, M.; Zhu, J.; Wang, D.; Zhou, M. An Integrated Analysis of Anatomical and Sugar Contents Identifies How Night Temperatures Regulate the Healing Process of Oriental Melon Grafted onto Pumpkin. Plants 2024, 13, 1506. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Shi, A.; Li, Y.; He, C.; Yan, Y.; Wang, J.; Sun, M.; Yu, X. Genome-wide analysis of the AINTEGUMENTA-like (AIL) transcription factor gene family in pumpkin (Cucurbita moschata Duch.) and CmoANT1.2 response in graft union healing. Plant Physiol. Biochem. 2021, 162, 706–715. [Google Scholar] [CrossRef]
- Thokchom, A.; Singh, R.; Begane, N.; Mathukmi, K.; Sabastian, K. Influence of Grafting Height and Scion Length on Healing of Graft Union and Growth Characteristics of Citrus reticulata cv. Nagpur Mandarin Grafted on Rough Lemon Rootstocks. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2066–2074. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Guan, L.; Li, Z.; Wang, H.; Luo, J. Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar. Life 2023, 13, 1896. [Google Scholar] [CrossRef]
- Ao, M.; Zhu, Y.; He, S.; Li, D.; Li, P.; Li, J.; Cao, Y. Preparation and characterization of 1-naphthylacetic acid–silica conjugated nanospheres for enhancement of controlled-release performance. Nanotechnology 2012, 24, 035601. [Google Scholar] [CrossRef]
- Shirai, T.; Hagimori, M. Studies in Establishment of Transplant Production Method of Sweet Pepper (Capsicum annuum L.) by Grafting Shoots Harvested from Mother Plants: Effects of Healing Conditions of Grafts on the Rate and Quality of Successful Union. J. Jpn. Soc. Hortic. Sci. 2004, 73, 380–385. [Google Scholar] [CrossRef]
- Wei, H.; Muneer, S.; Manivannan, A.; Liu, Y.; Park, J.; Jeong, B. Slight vapor deficit accelerates graft union healing of tomato plug seedling. Acta Physiol. Plant. 2018, 40, 147. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, J.; Miao, X.; Jiang, H.; Zhang, X.; Xu, C. Effects of Exogenous Naphthylacetic Acid Application on the Graft Union Healing of Oriental Melon Scion Grafted onto Squash Rootstock and the Qualities of Grafted Seedlings. Horticulturae 2025, 11, 765. https://doi.org/10.3390/horticulturae11070765
Wu H, Liu J, Miao X, Jiang H, Zhang X, Xu C. Effects of Exogenous Naphthylacetic Acid Application on the Graft Union Healing of Oriental Melon Scion Grafted onto Squash Rootstock and the Qualities of Grafted Seedlings. Horticulturae. 2025; 11(7):765. https://doi.org/10.3390/horticulturae11070765
Chicago/Turabian StyleWu, Hongxi, Jingwei Liu, Xinzhuo Miao, Hao Jiang, Xindi Zhang, and Chuanqiang Xu. 2025. "Effects of Exogenous Naphthylacetic Acid Application on the Graft Union Healing of Oriental Melon Scion Grafted onto Squash Rootstock and the Qualities of Grafted Seedlings" Horticulturae 11, no. 7: 765. https://doi.org/10.3390/horticulturae11070765
APA StyleWu, H., Liu, J., Miao, X., Jiang, H., Zhang, X., & Xu, C. (2025). Effects of Exogenous Naphthylacetic Acid Application on the Graft Union Healing of Oriental Melon Scion Grafted onto Squash Rootstock and the Qualities of Grafted Seedlings. Horticulturae, 11(7), 765. https://doi.org/10.3390/horticulturae11070765





