Impact of Antibacterial Agents in Horticulture: Risks to Non-Target Organisms and Sustainable Alternatives
Abstract
1. Introduction
2. Types of Antibacterial Agents
2.1. Biological Control Agents
2.2. Chemical Agents
2.3. Nanoparticles (NPs)
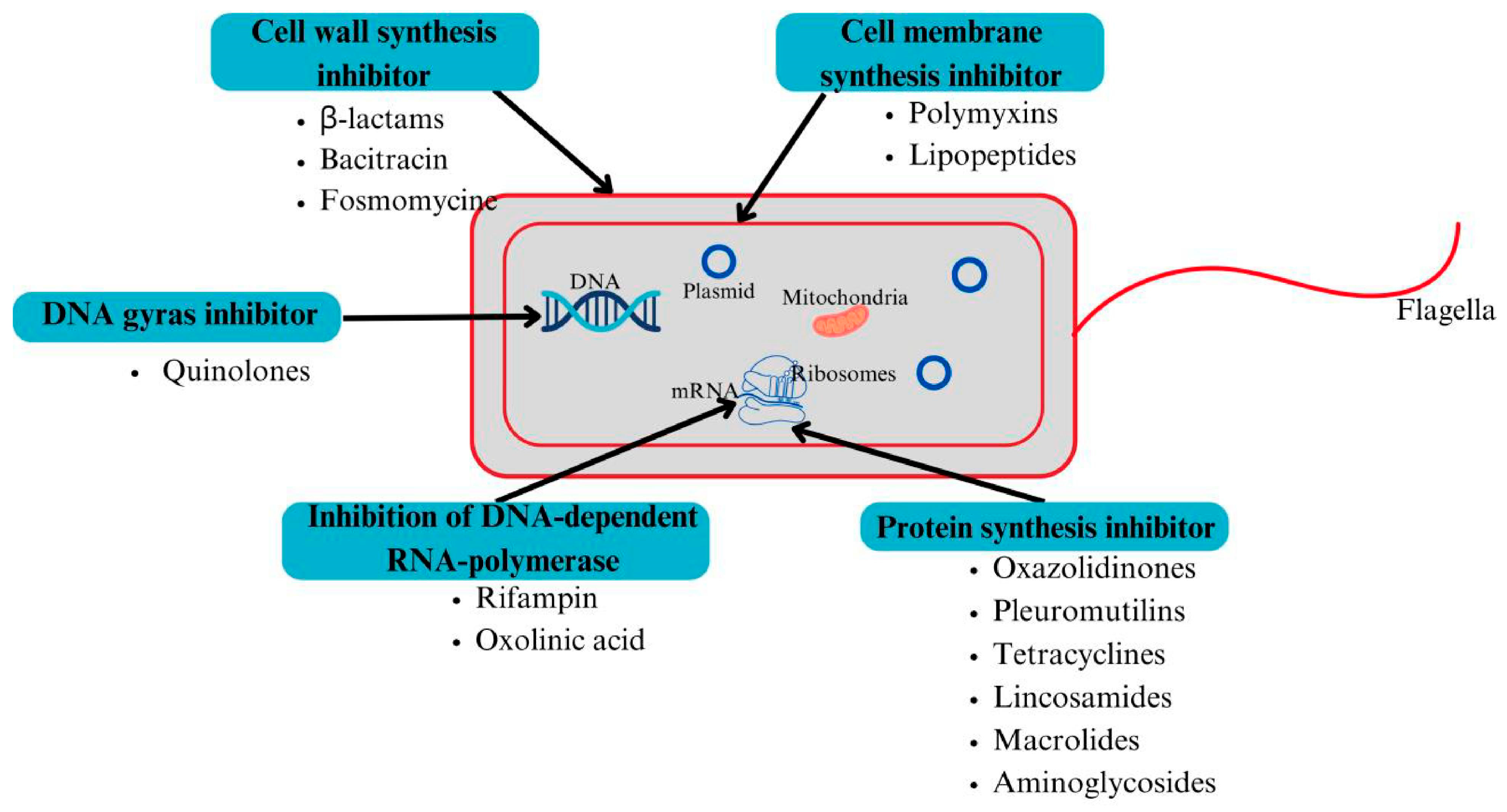
3. Action Mechanisms of Antibacterial Agents
3.1. Disruption of Cell Wall Synthesis
3.2. Inhibition of Protein Synthesis
3.3. Inhibition of Nucleic Acid Synthesis
3.4. Cell Membrane Disruption
3.5. Oxidative Stress-Induced Bacterial Cell Damage
3.6. Induction of Systemic Acquired Resistance (SAR)
4. Effect of Antibacterial Agents on Non-Target Organisms
5. Impact on Water Quality
6. Antibacterial Residues in Agricultural Products and Their Impact on Human Health
7. Ecosystem Influences
8. Current Status
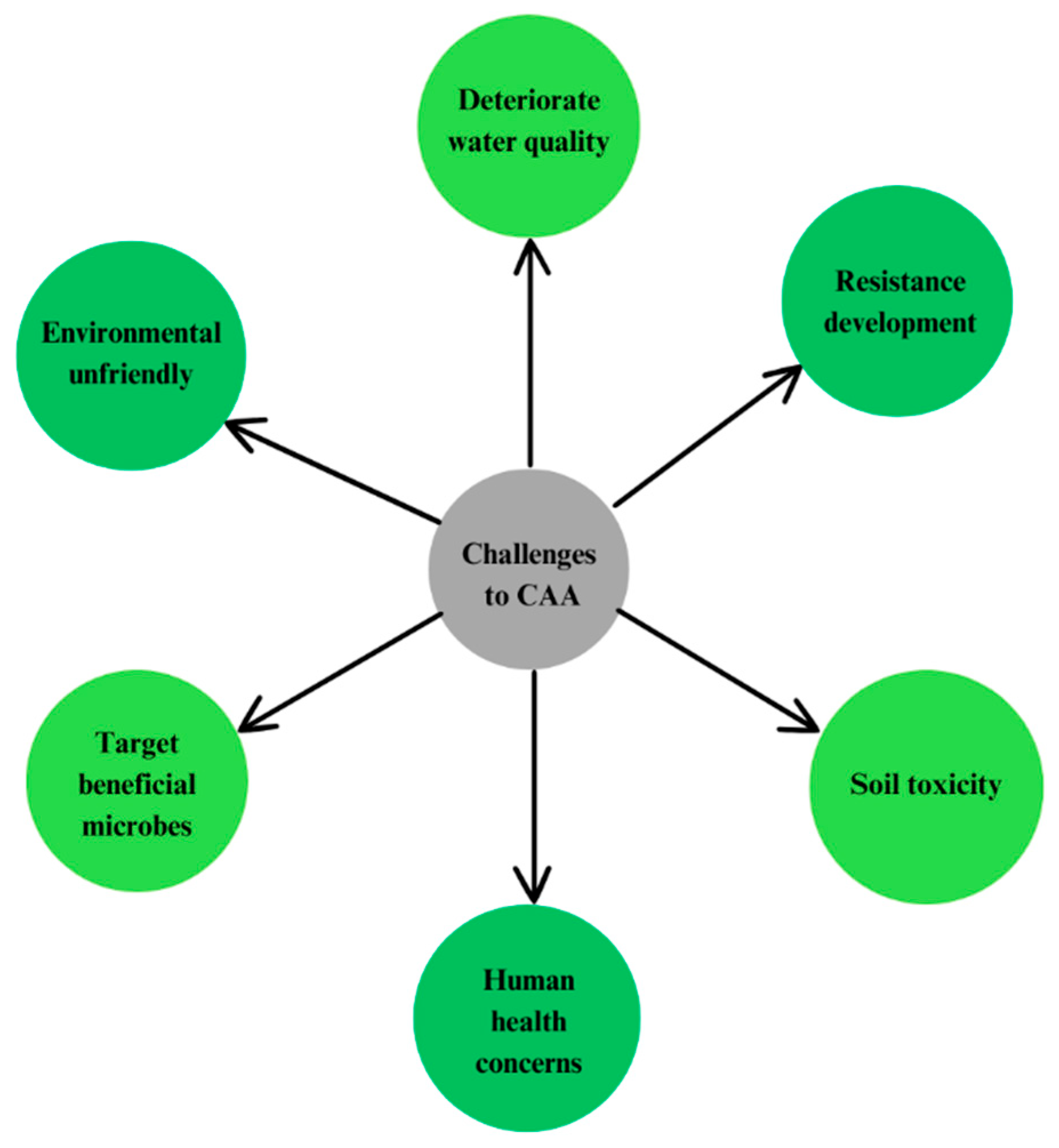
9. Constraints and Challenges in the Use of Conventional Antibacterial Agents
10. Strategies to Use Antibacterial Agents in a Sustainable Way
11. Alternatives to Antibacterial Agents for Bacterial Disease Management
12. Use of Probiotics to Minimize Antibacterial Dependency
13. Biotechnology in Reducing Antibacterial Use
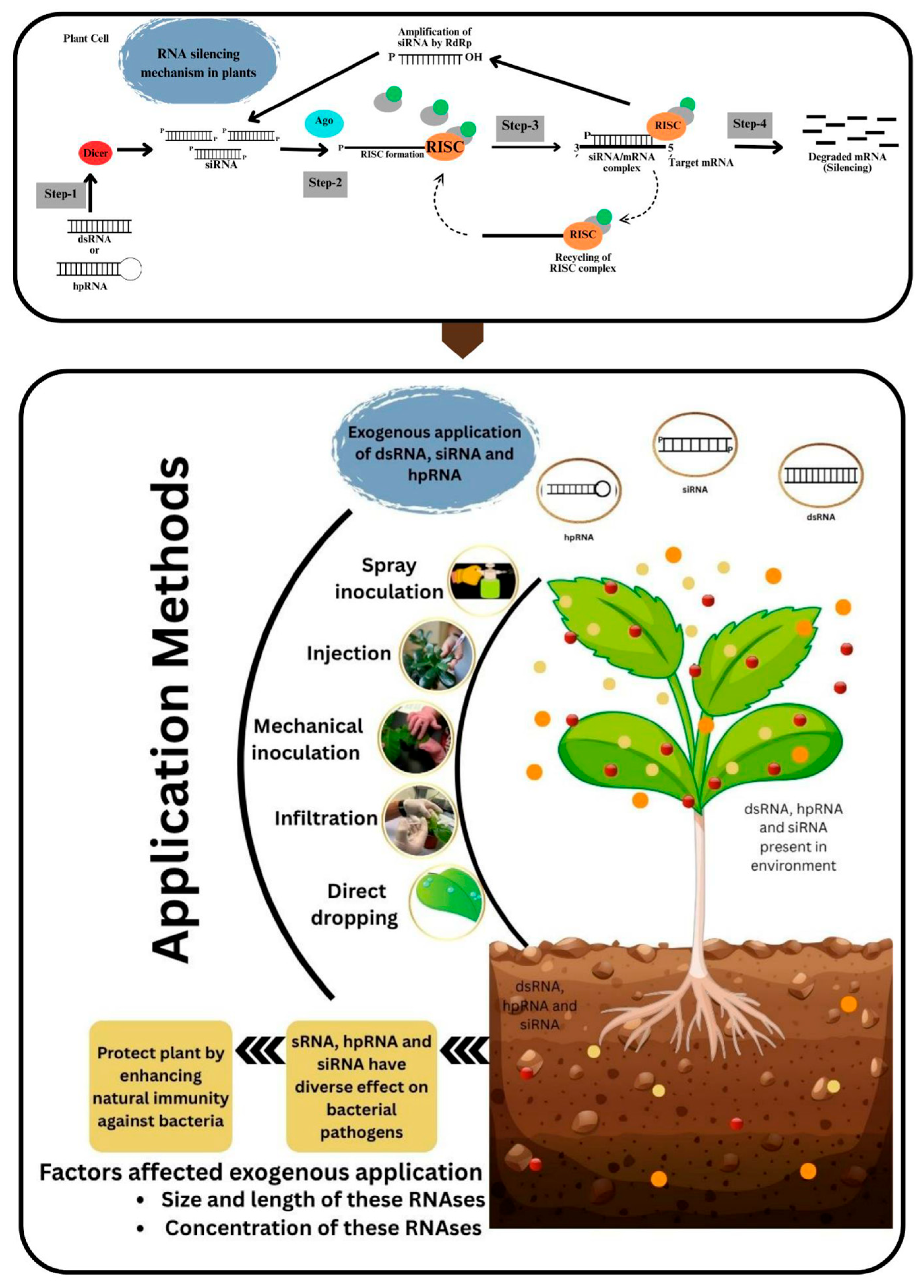
13.1. RNA Interference Technology
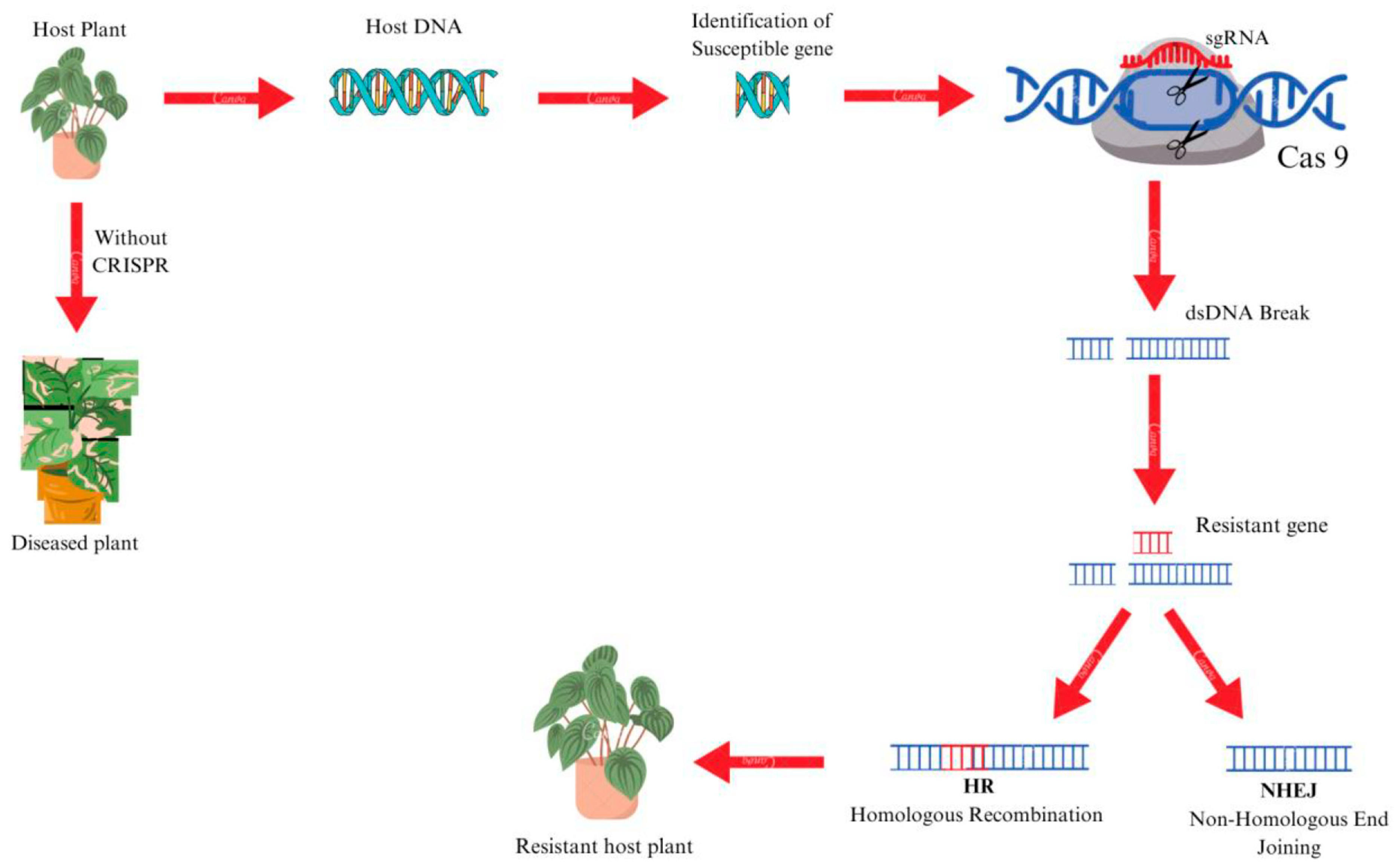
13.2. CRISPR-Cas9
14. Role of Precision Agriculture in Sustainable Application of Antibacterial Agents
15. Future Perspectives
16. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alhshem, H.H.M.; Ghader, M. A Study of Agriculture Value Added Percentage of Gross Domestic Product for Selected Asian Countries. J. Asian Multicult. Res. Soc. Sci. Study 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Elizabath, A.; Babychan, M.; Mathew, A.M.; Syriac, G.M. Application of Nanotechnology in Agriculture. Int. J. Pure Appl. Biosci. 2019, 7, 131–139. [Google Scholar] [CrossRef]
- Nchuchuwe, F.F.; Adejuwon, K.D. The Challenges of Agriculture and Rural Development in Africa: The Case of Nigeria. Int. J. Acad. Res. Progress. Educ. Dev. 2012, 1, 45–61. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- Viola, I.; Marinelli, A. Life Cycle Assessment and Environmental Sustainability in the Food System. Agric. Agric. Sci. Procedia 2016, 8, 317–323. [Google Scholar] [CrossRef]
- Asfaw, S.; Pallante, G.; Palma, A. Distributional Impacts of Soil Erosion on Agricultural Productivity and Welfare in Malawi. Ecol. Econ. 2020, 177, 106764. [Google Scholar] [CrossRef]
- Lachaud, M.A.; Bravo-Ureta, B.E.; Ludena, C.E. Economic Effects of Climate Change on Agricultural Production and Productivity in Latin America and the Caribbean (LAC). Agric. Econ. 2022, 53, 321–332. [Google Scholar] [CrossRef]
- Moore, D.; Robson, G.D.; Trinci, A.P. 21st Century Guidebook to Fungi; University Press: Cambridge, UK, 2020. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A. New and Emerging Water Pollutants Arising from Agriculture. 2012. Available online: https://pure.york.ac.uk/portal/en/publications/new-and-emerging-water-pollutants-arising-from-agriculture (accessed on 19 April 2025).
- Chen, J.S.; Toth, J.; Kasap, M. Nitrogen-Fixation Genes and Nitrogenase Activity in Clostridium Acetobutylicum and Clostridium Beijerinckii. J. Ind. Microbiol. Biotechnol. 2001, 27, 281–286. [Google Scholar] [CrossRef]
- Rice, L.B.; Bonomo, R.A. Mechanisms of Resistance to Antibacterial Agents. In Manual of Clinical Microbiology; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 1082–1114. ISBN 978-1-68367-411-5. [Google Scholar]
- World Food and Agriculture–Statistical Yearbook 2024|FAO. Available online: https://www.fao.org/family-farming/detail/en/c/1725784/ (accessed on 2 June 2025).
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight Principles of Integrated Pest Management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Muhie, S.H. Novel Approaches and Practices to Sustainable Agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Reganold, J.P.; Papendick, R.I.; Parr, J.F. Sustainable Agriculture. Sci. Am. 1990, 262, 112–121. [Google Scholar] [CrossRef]
- Doula, M.K. Soil: Threats and Protection. Sustainable Agriculture. In Social Responsibility and Science in Innovation Economy; Kawalec, P., Wierzchoslawski, R.P., Eds.; John Paul II Catholic University of Lublin: Lublin, Poland, 2015; pp. 193–272. [Google Scholar]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.; Kang, Y.C.; Lee, J.-K. Quorum Sensing Inhibitors as Antipathogens: Biotechnological Applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a Plant’s Best Friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Schaechter, M. Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Stockwell, V.O.; Johnson, K.B.; Sugar, D.; Loper, J.E. Antibiosis Contributes to Biological Control of Fire Blight by Pantoea Agglomerans Strain Eh252 in Orchards. Phytopathology® 2002, 92, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Mikiciński, A.; Sobiczewski, P.; Puławska, J.; Maciorowski, R. Control of Fire Blight (Erwinia amylovora) by a Novel Strain 49M of Pseudomonas graminis from the Phyllosphere of Apple (Malus spp.). Eur. J. Plant Pathol. 2016, 145, 265–276. [Google Scholar] [CrossRef]
- Drenker, C.; El Mazouar, D.; Bücker, G.; Weißhaupt, S.; Wienke, E.; Koch, E.; Kunz, S.; Reineke, A.; Rondot, Y.; Linkies, A. Characterization of a Disease-Suppressive Isolate of Lysobacter Enzymogenes with Broad Antagonistic Activity against Bacterial, Oomycetal and Fungal Pathogens in Different Crops. Plants 2023, 12, 682. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Khedr, A.A.; Mehiar, F.; El-Kady, E.M.; Baazeem, A.; Shimizu, M. Suppression of Pseudomonas syringae pv. tomato Infection by Rhizosphere Fungi. Pest Manag. Sci. 2021, 77, 4350–4356. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, D.K.; Chaudhary, G.; Rana, V.S.; Sharma, R.K. Potential of Bacillus Amyloliquefaciens for Biocontrol of Bacterial Wilt of Tomato Incited by Ralstonia Solanacearum. J. Plant Pathol. Microbiol. 2016, 7, 1000327. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, D.K.; Sinha, S.; Upadhyay, B.K. Utilization of Plant Growth Promoting Bacillus Subtilis Isolates for the Management of Bacterial Wilt Incidence in Tomato Caused by Ralstonia Solanacearum Race 1 Biovar 3. Indian Phytopath 2012, 65, 18–24. [Google Scholar]
- Yan, L.; Khan, R.A.A. Biological Control of Bacterial Wilt in Tomato through the Metabolites Produced by the Biocontrol Fungus, Trichoderma Harzianum. Egypt. J. Biol. Pest Control 2021, 31, 5. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of Biological Efficacy of Trichoderma asperellum against Tomato Bacterial Wilt Caused by Ralstonia solanacearum. Egypt. J. Biol. Pest Control 2018, 28, 63. [Google Scholar] [CrossRef]
- Vidhyasekaran, P.; Kamala, N.; Ramanathan, A.; Rajappan, K.; Paranidharan, V.; Velazhahan, R. Induction of Systemic Resistance by Pseudomonas fluorescens Pf1 against Xanthomonas oryzae pv. Oryzae in Rice Leaves. Phytoparasitica 2001, 29, 155–166. [Google Scholar] [CrossRef]
- Mishra, S.; Arora, N.K. Management of Black Rot in Cabbage by Rhizospheric Pseudomonas Species and Analysis of 2,4-Diacetylphloroglucinol by qRT-PCR. Biol. Control 2012, 61, 32–39. [Google Scholar] [CrossRef]
- Promnuan, Y.; Promsai, S.; Meelai, S. Antimicrobial Activity of Streptomyces spp. Isolated from Apis Dorsata Combs against Some Phytopathogenic Bacteria. PeerJ 2020, 8, e10512. [Google Scholar] [CrossRef] [PubMed]
- Mácha, H.; Marešová, H.; Juříková, T.; Švecová, M.; Benada, O.; Škríba, A.; Baránek, M.; Novotnỳ, Č.; Palyzová, A. Killing Effect of Bacillus velezensis FZB42 on a Xanthomonas campestris pv. Campestris (Xcc) Strain Newly Isolated from Cabbage Brassica oleracea Convar. Capitata (L.): A Metabolomic Study. Microorganisms 2021, 9, 1410. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ma, Q.; Bian, L.; Liu, X.; Xu, Y.; Zhang, H.; Shao, J.; Liu, Y. Bacillus Velezensis CLA178-Induced Systemic Resistance of Rosa Multiflora against Crown Gall Disease. Front. Microbiol. 2020, 11, 587667. [Google Scholar] [CrossRef]
- Hernández-Huerta, J.; Tamez-Guerra, P.; Gomez-Flores, R.; Delgado-Gardea, M.C.E.; Robles-Hernández, L.; Gonzalez-Franco, A.C.; Infante-Ramirez, R. Pepper Growth Promotion and Biocontrol against Xanthomonas euvesicatoria by Bacillus cereus and Bacillus thuringiensis Formulations. PeerJ 2023, 11, e14633. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.; Abd El-Mageed, T.A.; Negm, S.H. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The New Perspective in Precision Agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sholberg, P.L.; Bedford, K.E.; Haag, P.; Randall, P. Survey of Erwinia amylovora Isolates from British Columbia for Resistance to Bactericides and Virulence on Apple. Can. J. Plant Pathol. 2001, 23, 60–67. [Google Scholar] [CrossRef]
- Iqbal, M.; ul Haq, M.E.; Kamran, M.; Idrees, M.; Nazir, S.; Ullah, I.; Naz, S.; Ali, S.; Iqbal, M.Z. Morpho-Molecular Characterization of Xanthomonas axonopodis pv. citri Associated with Kinnow (Mandarin) and Its Management. Pak. J. Agric. Res. 2021, 34, 8. [Google Scholar] [CrossRef]
- Kant, R. Innovative Methods for Management of Black Rot of Cabbage (Brassica oleracea var. capitata L.). Ph.D. Thesis, UHF, Nauni, India, 2018. [Google Scholar]
- Maguvu, T.E.; Frias, R.J.; Hernandez-Rosas, A.I.; Shipley, E.; Dardani, G.; Nouri, M.T.; Yaghmour, M.A.; Trouillas, F.P. Pathogenicity, Phylogenomic, and Comparative Genomic Study of Pseudomonas syringae Sensu Lato Affecting Sweet Cherry in California. Microbiol. Spectr. 2024, 12, e01324-24. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Liu, C.; Gong, H.; Qi, B.; Zhu, R.; Xia, L.; Li, L.; Liu, S.; Jiang, Q.; et al. Application of Trichloroisocyanuric Acid in Controlling Kiwifruit Bacterial Canker Disease Demonstrates Its Promising Potential as an Eco-Friendly Bactericide. Chem. Biol. Technol. Agric. 2025, 12, 3. [Google Scholar] [CrossRef]
- El-Sisi, A.A. Pathological Studies on Bacterial Canker and Wilt Disease on Mango. Ph.D. Thesis, Faculty of Agricultural, Benha University, Benha, Egypt, 2013; 131p. [Google Scholar]
- Ishikawa, R.; Fujimori, K.; Matsuura, K. Antibacterial Activity of Validamycin A against Pseudomonas solanacearum and Its Efficacy against Tomato Bacterial Wilt. Ann. Phytopathol. Soc. Jpn. 1996, 62, 478–482. [Google Scholar] [CrossRef]
- Khanam, R. Management of Bacterial Wilt of Brinjal by Using Some Chemicals and Bio-Agent. 2020. Available online: http://archive.saulibrary.edu.bd:8080/xmlui/handle/123456789/3549 (accessed on 12 April 2025).
- El-Khair, H.A. Variation and Control of Erwinia carotovora subsp. carotovora Isolates, the Causal Agent of Potato Soft Rot Disease. Ann. Agric. Sci. 2004, 49, 377–388. [Google Scholar]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural Nanotechnologies: What Are the Current Possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticles-a Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Elmer, W.; Ma, C.; White, J. Nanoparticles for Plant Disease Management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Pandiyaraj, V.; Murmu, A.; Pandy, S.K.; Sevanan, M.; Arjunan, S. Metal Nanoparticles and Its Application on Phenolic and Heavy Metal Pollutants. Phys. Sci. Rev. 2023, 8, 2879–2897. [Google Scholar] [CrossRef]
- Amer, M.; Awwad, A. Green Synthesis of Copper Nanoparticles by Citrus Limon Fruits Extract, Characterization and Antibacterial Activity. 2020. Available online: https://ssrn.com/abstract=3693721 (accessed on 5 April 2025).
- Bibi, S.; Raza, M.; Shahbaz, M.; Ajmal, M.; Mehak, A.; Fatima, N.; Abasi, F.; Seelan, J.S.S.; Raja, N.I.; Yongchao, B. Biosynthesized Silver Nanoparticles Enhanced Wheat Resistance to Bipolaris Sorokiniana. Plant Physiol. Biochem. 2023, 203, 108067. [Google Scholar] [CrossRef]
- Hashemi Tameh, M.; Primiceri, E.; Chiriacò, M.S.; Poltronieri, P.; Bahar, M.; Maruccio, G. Pectobacterium atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato. Biosensors 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Dorjee, L.; Gogoi, R.; Kamil, D.; Kumar, R.; Verma, A. Copper Nanoparticles Hold Promise in the Effective Management of Maize Diseases without Impairing Environmental Health. Phytoparasitica 2023, 51, 593–619. [Google Scholar] [CrossRef]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia Solanacearum Species Complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 263, p. 032019. [Google Scholar]
- Khan, R.A.A.; Tang, Y.; Naz, I.; Alam, S.S.; Wang, W.; Ahmad, M.; Najeeb, S.; Rao, C.; Li, Y.; Xie, B.; et al. Management of Ralstonia solanacearum in Tomato Using ZnO Nanoparticles Synthesized Through Matricaria chamomilla. Plant Dis. 2021, 105, 3224–3230. [Google Scholar] [CrossRef]
- El-Sayed, M.E. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Carrasco-Sandoval, J.; Aranda-Bustos, M.; Henríquez-Aedo, K.; López-Rubio, A.; Fabra, M.J. Bioaccessibility of Different Types of Phenolic Compounds Co-Encapsulated in Alginate/Chitosan-Coated Zein Nanoparticles. Lwt 2021, 149, 112024. [Google Scholar] [CrossRef]
- Khare, P.; Talreja, N.; Deva, D.; Sharma, A.; Verma, N. Carbon Nanofibers Containing Metal-Doped Porous Carbon Beads for Environmental Remediation Applications. Chem. Eng. J. 2013, 229, 72–81. [Google Scholar] [CrossRef]
- Maleki, R.; Abdollahi, H.; Piri, S. Variation of Active Iron and Ferritin Content in Pear Cultivars with Different Levels of Pathogen Resistance Following Inoculation with Erwinia amylovora. J. Plant Pathol. 2022, 104, 281–293. [Google Scholar] [CrossRef]
- Bytešníková, Z.; Pečenka, J.; Tekielska, D.; Kiss, T.; Švec, P.; Ridošková, A.; Bezdička, P.; Pekárková, J.; Eichmeier, A.; Pokluda, R.; et al. Reduced Graphene Oxide-Based Nanometal-Composite Containing Copper and Silver Nanoparticles Protect Tomato and Pepper against Xanthomonas euvesicatoria Infection. Chem. Biol. Technol. Agric. 2022, 9, 84. [Google Scholar] [CrossRef]
- Bidyarani, N.; Srivastav, A.K.; Gupta, S.K.; Kumar, U. Synthesis and Physicochemical Characterization of Rhamnolipid-Stabilized Carvacrol-Loaded Zein Nanoparticles for Antimicrobial Application Supported by Molecular Docking. J. Nanoparticle Res. 2020, 22, 307. [Google Scholar] [CrossRef]
- Vinay, J.U.; Iliger, K.S. Green Nanotechnology in Agriculture: Plant Disease Diagnosis to Management. In Innovative Approaches in Diagnosis and Management of Crop Diseases; Apple Academic Press: Burlington, ON, USA, 2021; pp. 67–95. [Google Scholar]
- Ayisigi, M.; Cokislerel, A.; Kucukcobanoglu, Y.; Yalcin, T.; Aktas, L.Y. Green Synthesized Silver Nanoparticles for an Effective Control on Soft Rot Disease Pathogen Pectobacterium Carotovorum and Growth Stimulation in Pepper. Bulg. J. Agric. Sci. 2020, 26, 574–584. [Google Scholar]
- Park, H.J.; Kim, S.H.; Kim, H.J.; Choi, S.H. A New Composition of Nanosized Silica-Silver for Control of Various Plant Diseases. Plant Pathol. J. 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Attia, M.S.; Salem, S.S.; Elakraa, A.A.; Abdel-Maksoud, M.A.; Malik, A.; Kiani, B.H.; Malash, M.N.; El-Sayyad, G.S. Promising Antagonistic Effect of Bimetallic Silver-Selenium Nanoparticles against Ralstonia Solanacearum-Causing Wilt Disease in Eggplant (Solanum melongena L.). Physiol. Mol. Plant Pathol. 2024, 133, 102369. [Google Scholar] [CrossRef]
- Makarovsky, D.; Fadeev, L.; Salam, B.B.; Zelinger, E.; Matan, O.; Inbar, J.; Jurkevitch, E.; Gozin, M.; Burdman, S. Silver Nanoparticles Complexed with Bovine Submaxillary Mucin Possess Strong Antibacterial Activity and Protect against Seedling Infection. Appl. Environ. Microbiol. 2018, 84, e02212-17. [Google Scholar] [CrossRef]
- Imada, K.; Sakai, S.; Kajihara, H.; Tanaka, S.; Ito, S. Magnesium Oxide Nanoparticles Induce Systemic Resistance in Tomato against Bacterial Wilt Disease. Plant Pathol. 2016, 65, 551–560. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Balabel, N.M.; Attia, M.S.; El-Sayyad, G.S. Antibacterial and Antibiofilm Potential of Mono-Dispersed Stable Copper Oxide Nanoparticles-Streptomycin Nano-Drug: Implications for Some Potato Plant Bacterial Pathogen Treatment. J. Clust. Sci. 2020, 31, 1021–1040. [Google Scholar] [CrossRef]
- Abdelghany, W.A.; Mohamedin, A.H.; Abo-Elyousr, K.A.M.; Hussein, M.A.M. Control of Bacterial Soft Rot Disease of Potato Caused by Pectobacterium carotovorum subsp. Carotovorum Using Different Nanoparticles. Arch. Phytopathol. Plant Prot. 2022, 55, 1638–1660. [Google Scholar] [CrossRef]
- Paret, M.L.; Vallad, G.E.; Averett, D.R.; Jones, J.B.; Olson, S.M. Photocatalysis: Effect of Light-Activated Nanoscale Formulations of TiO2 on Xanthomonas perforans and Control of Bacterial Spot of Tomato. Phytopathology® 2013, 103, 228–236. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica Nanoparticles Enhance Disease Resistance in Arabidopsis Plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Vera-Reyes, I.; Esparza-Arredondo, I.J.E.; Lira-Saldivar, R.H.; Granados-Echegoyen, C.A.; Alvarez-Roman, R.; Vásquez-López, A.; De Los Santos-Villarreal, G.; Díaz-Barriga Castro, E. In Vitro Antimicrobial Effect of Metallic Nanoparticles on Phytopathogenic Strains of Crop Plants. J. Phytopathol. 2019, 167, 461–469. [Google Scholar] [CrossRef]
- Baldassarre, F.; De Stradis, A.; Altamura, G.; Vergaro, V.; Citti, C.; Cannazza, G.; Capodilupo, A.L.; Dini, L.; Ciccarella, G. Application of Calcium Carbonate Nanocarriers for Controlled Release of Phytodrugs against Xylella fastidiosa Pathogen. Pure Appl. Chem. 2020, 92, 429–444. [Google Scholar] [CrossRef]
- Doyle, R.J.; Dziarski, R. The Bacterial Cell: Peptidoglycan. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 137–154. [Google Scholar]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline Antibiotics and Resistance Mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Allison, D.G.; Lambert, P.A. Modes of Action of Antibacterial Agents. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 597–614. [Google Scholar]
- Bozcal, E.; Dagdeviren, M. Toxicity of β-Lactam Antibiotics: Pathophysiology, Molecular Biology and Possible Recovery Strategies. In Poisoning-From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; IntechOpen: London, UK, 2017. [Google Scholar]
- Naveed, M.; Chaudhry, Z.; Bukhari, S.A.; Meer, B.; Ashraf, H. Antibiotics Resistance Mechanism. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 292–312. [Google Scholar]
- Jerinic, O.; Joseph, S. Conformational Changes in the Ribosome Induced by Translational Miscoding Agents. J. Mol. Biol. 2000, 304, 707–713. [Google Scholar] [CrossRef]
- Champney, W.S. Antibiotics Targeting Bacterial Ribosomal Subunit Biogenesis. J. Antimicrob. Chemother. 2020, 75, 787–806. [Google Scholar] [CrossRef]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef]
- Spencer, A.C.; Panda, S.S. DNA Gyrase as a Target for Quinolones. Biomedicines 2023, 11, 371. [Google Scholar] [CrossRef]
- Reece, R.J.; Maxwell, A. DNA Gyrase: Structure and Function. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 335–375. [Google Scholar] [CrossRef] [PubMed]
- Sojo Martinez, V.R. Membrane Bioenergetics at Major Transitions in Evolution. Ph.D. Thesis, UCL (University College London), London, UK, 2016. [Google Scholar]
- Zou, F.; Tan, C.; Shinali, T.S.; Zhang, B.; Zhang, L.; Han, Z.; Shang, N. Plant Antimicrobial Peptides: A Comprehensive Review of Their Classification, Production, Mode of Action, Functions, Applications, and Challenges. Food Funct. 2023, 14, 5492–5515. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.S.; Henderson, J.P. Pathogenic Adaptations to Host-Derived Antibacterial Copper. Front. Cell. Infect. Microbiol. 2014, 4, 3. [Google Scholar] [CrossRef]
- Wendehenne, D.; Durner, J.; Chen, Z.; Klessig, D.F. Benzothiadiazole, an Inducer of Plant Defenses, Inhibits Catalase and Ascorbate Peroxidase. Phytochemistry 1998, 47, 651–657. [Google Scholar] [CrossRef]
- Kuźniak, E.; Głowacki, R.; Chwatko, G.; Kopczewski, T.; Wielanek, M.; Gajewska, E.; Skłodowska, M. Involvement of Ascorbate, Glutathione, Protein S-Thiolation and Salicylic Acid in Benzothiadiazole-Inducible Defence Response of Cucumber against Pseudomonas syringae pv lachrymans. Physiol. Mol. Plant Pathol. 2014, 86, 89–97. [Google Scholar] [CrossRef]
- Chandra, S.; Sharma, I.P. Elicitins as Microbe-Associated Molecular Patterns and Their Role in Plant Defense. In Unravelling Plant-Microbe Synergy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 77–86. [Google Scholar]
- Belete, T. A Critical Review on Defense Mechanismof Plants against Bacterial Pathogens: From Morphological to Molecular Levels. J. Plant Pathol. Microbiol. 2021, 12, 534. [Google Scholar]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Johnson, K.B.; Smith, T.J.; Temple, T.N.; Gutierrez, E.; Elkins, R.B.; Castagnoli, S.P. Integration of Acibenzolar-S-Methyl with Antibiotics for Protection of Pear and Apple from Fire Blight Caused by Erwinia amylovora. Crop Prot. 2016, 88, 149–154. [Google Scholar] [CrossRef]
- Oh, C.-S.; Beer, S.V. Molecular Genetics of Erwinia Amylovora Involved in the Development of Fire Blight. FEMS Microbiol. Lett. 2005, 253, 185–192. [Google Scholar] [CrossRef]
- Hussein, A.; Mohamed, R.; Amein, T. Biological Control of Fire Blight Disease on Pear Caused by Erwinia amylovora in Erbil Province/Iraq. Tikrit J. Agric. Sci. 2019, 19, 65–71. [Google Scholar] [CrossRef]
- Ait Bahadou, S.; Ouijja, A.; Karfach, A.; Tahiri, A.; Lahlali, R. New Potential Bacterial Antagonists for the Biocontrol of Fire Blight Disease (Erwinia amylovora) in Morocco. Microb. Pathog. 2018, 117, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Strayer-Scherer, A.; Liao, Y.Y.; Young, M.; Ritchie, L.; Vallad, G.E.; Santra, S.; Freeman, J.H.; Clark, D.; Jones, J.B.; Paret, M.L. Advanced Copper Composites Against Copper-Tolerant Xanthomonas perforans and Tomato Bacterial Spot. Phytopathology® 2018, 108, 196–205. [Google Scholar] [CrossRef]
- Dewey, K.A.; Gaw, S.K.; Northcott, G.L.; Lauren, D.R.; Hackenburg, S. The Effects of Copper on Microbial Activity and the Degradation of Atrazine and Indoxacarb in a New Zealand Soil. Soil Biol. Biochem. 2012, 52, 64–74. [Google Scholar] [CrossRef]
- Bravo, G.; Vega-Celedón, P.; Macaya, C.; Vasconez, I.-N.; Seeger, M. Nitrogen Cycle Bacteria in Agricultural Soils: Effects of Nitrogen Fertilizers, Heavy Metals, Pesticides and Bioremediation Approaches. In Rhizomicrobiome Dynamics in Bioremediation; CRC Press: Boca Raton, FL, USA, 2021; pp. 335–356. [Google Scholar]
- Sindhu, S.S.; Dua, S.; Verma, M.K.; Khandelwal, A. Growth Promotion of Legumes by Inoculation of Rhizosphere Bacteria. In Microbes for Legume Improvement; Khan, M.S., Musarrat, J., Zaidi, A., Eds.; Springer: Vienna, Austria, 2010; pp. 195–235. ISBN 978-3-211-99752-9. [Google Scholar]
- Vallad, G.E.; Pernezny, K.L.; Balogh, B.; Wen, A.; Figueiredo, J.F.L.; Jones, J.B.; Momol, T.; Muchovej, R.M.; Havranek, N.; Abdallah, N. Comparison of Kasugamycin to Traditional Bactericides for the Management of Bacterial Spot on Tomato. HortScience 2010, 45, 1834–1840. [Google Scholar] [CrossRef]
- Tahir, H.A.; Sahi, S.T.; Habib, A.; Haq, I.U.; Ahmad, A.; Ashraf, W. Evaluation of Plant Extracts as Biocontrol Agents against Xanthomonas axonopodis pv Citri the Cause of Citrus Canker. Pak. J. Phytopathol. 2016, 28, 35–43. [Google Scholar]
- Anandan, R.; Dharumadurai, D.; Manogaran, G.P. An Introduction to Actinobacteria. Actinobacteria-Basics Biotechnol. Appl. 2016, 1, 388. [Google Scholar]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important Ecophysiological Roles of Non-Dominant Actinobacteria in Plant Residue Decomposition, Especially in Less Fertile Soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef]
- Lee, L.S.; Carmosini, N.; Sassman, S.A.; Dion, H.M.; Sepúlveda, M.S. Agricultural Contributions of Antimicrobials and Hormones on Soil and Water Quality. Adv. Agron. 2007, 93, 1–68. [Google Scholar]
- Segura, P.A.; François, M.; Gagnon, C.; Sauvé, S. Review of the Occurrence of Anti-Infectives in Contaminated Wastewaters and Natural and Drinking Waters. Environ. Health Perspect. 2009, 117, 675–684. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in Agriculture: Benefits and Challenge in Agricultural Productivity, Toxicological Risks to Human Health and Environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.; Meisel, L.; Lopes, A.; de Jesus, R.; Sarmento, G.; Duarte, S.; Sepodes, B.; Fernandes, A.; Dos Santos, M.M.C.; Almeida, A. Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study. Antibiotics 2021, 10, 888. [Google Scholar] [CrossRef]
- Wang, W.; Luo, T.; Zhao, Y.; Yang, X.; Wang, D.; Yang, G.; Jin, Y. Antibiotic Resistance Gene Distribution in Shine Muscat Grapes and Health Risk Assessment of Streptomycin Residues in Mice. J. Hazard. Mater. 2024, 465, 133254. [Google Scholar] [CrossRef]
- DyhrMaN, S.T.; Ammerman, J.W.; Van Mooy, B.A. Microbes and the Marine Phosphorus Cycle. Oceanography 2007, 20, 110–116. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, J.; Chu, Y.; Liu, W.; Yang, L.; Ma, L.; Zhang, Y.; Qiu, D.; Wu, Z.; He, F. Triclosan Weakens the Nitrification Process of Activated Sludge and Increases the Risk of the Spread of Antibiotic Resistance Genes. J. Hazard. Mater. 2021, 416, 126085. [Google Scholar] [CrossRef]
- Chauhan, B.; Dodamani, S.; Malik, S.; Almalki, W.H.; Haque, S.; Sayyed, R.Z. Microbial Approaches for Pharmaceutical Wastewater Recycling and Management for Sustainable Development: A Multicomponent Approach. Environ. Res. 2023, 237, 116983. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and Solutions. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–15. ISBN 978-94-007-7813-9. [Google Scholar]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, Eutrophication and Harmful Algal Blooms along the Freshwater to Marine Continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public. Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the Aquatic Environments: A Review of Lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The Public Health Issue of Antibiotic Residues in Food and Feed: Causes, Consequences, and Potential Solutions. Vet. World 2022, 15, 662. [Google Scholar] [CrossRef]
- Angulo, F.J.; Baker, N.L.; Olsen, S.J.; Anderson, A.; Barrett, T.J. Antimicrobial Use in Agriculture: Controlling the Transfer of Antimicrobial Resistance to Humans. In Proceedings of the Seminars in Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2004; Volume 15, pp. 78–85. [Google Scholar]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic Use in Plant Agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Shaffer, W.J.; Goodman, R.N. Effectiveness of an Extended Agri-Mycin-17 Spray Schedule against Fireblight. Plant Dis. Rep. 1969, 53, 669–672. [Google Scholar]
- Mayerhofer, G.; Schwaiger-Nemirova, I.; Kuhn, T.; Girsch, L.; Allerberger, F. Detecting Streptomycin in Apples from Orchards Treated for Fire Blight. J. Antimicrob. Chemother. 2009, 63, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Bohm, D.A.; Stachel, C.S.; Gowik, P. Confirmatory Method for the Determination of Streptomycin in Apples by LC–MS/MS. Anal. Chim. Acta 2010, 672, 103–106. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.E. Uptake, Translocation, and Persistence of Oxytetracycline in Coconut Palm. Phytopathology 1976, 66, 1039–1042. [Google Scholar] [CrossRef]
- Christiano, R.S.C.; Reilly, C.C.; Miller, W.P.; Scherm, H. Oxytetracycline Dynamics on Peach Leaves in Relation to Temperature, Sunlight, and Simulated Rain. Plant Dis. 2010, 94, 1213–1218. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut Microbiota: An Underestimated and Unintended Recipient for Pesticide-Induced Toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Andleeb, S.; Majid, M.; Sardar, S. Environmental and Public Health Effects of Antibiotics and AMR/ARGs. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–291. [Google Scholar]
- Vidaver, A.K. Uses of Antimicrobials in Plant Agriculture. Clin. Infect. Dis. 2002, 34, S107–S110. [Google Scholar] [CrossRef]
- Adaskaveg, J.; Förster, H.; Holtz, B.A.; Hoffman, E.; Gubler, D.; Erickson, E. Evaluation of Bactericides for Control of Fire Blight of Pears and Apples Caused by Erwinia amylovora. In Proceedings of the X International Workshop on Fire Blight, Bologna, Italy, 5–9 July 2004; Volume 704, pp. 277–282. [Google Scholar]
- Halling-Sørensen, B. Inhibition of Aerobic Growth and Nitrification of Bacteria in Sewage Sludge by Antibacterial Agents. Arch. Environ. Contam. Toxicol. 2001, 40, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, L. Biodiversity and Ecosystem Functioning in Soil. Ambio 1997, 26, 563–570. [Google Scholar]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in Plant Protection: Current Situation and Prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Griffin, K.; Gambley, C.; Brown, P.; Li, Y. Copper-Tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the Control of Diseases Associated with These Pathogens in Tomato and Pepper. A Systematic Literature Review. Crop Prot. 2017, 96, 144–150. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Al-Ghabshi, A.; Al-Mazrooei, N.; Al-Habsi, S. Comparative Pathogenomics of Bacteria Causing Infectious Diseases in Fish. Int. J. Evol. Biol. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of Sulfonamide and Tetracycline-Resistant Bacteria and Resistance Genes in Aquaculture Environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Mdegela, R.H.; Mwakapeje, E.R.; Rubegwa, B.; Gebeyehu, D.T.; Niyigena, S.; Msambichaka, V.; Nonga, H.E.; Antoine-Moussiaux, N.; Fasina, F.O. Antimicrobial Use, Residues, Resistance and Governance in the Food and Agriculture Sectors, Tanzania. Antibiotics 2021, 10, 454. [Google Scholar] [CrossRef]
- Joshi, T.; Sharma, P.; Joshi, T.; Pandey, S.C.; Pande, V.; Pandey, A.; Joshi, D.; Maiti, P.; Nand, M.; Chandra, S. A Spotlight on the Recent Advances in Bacterial Plant Diseases and Their Footprint on Crop Production. Recent Adv. Microb. Divers. 2020, 37–69. [Google Scholar]
- Ding, H.; Luo, C.; Li, Y.; Li, Q.; Dong, Y. Impact of Bacillus Subtilis and Pseudomonas Fluorescens Beneficial Bacterial Agents on Soil-Borne Diseases, Growth, and Economics of Continuous Cropping of Flue-Cured Tobacco. Crop Prot. 2024, 177, 106556. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastnỳ, V.; Bordas, F.; Bollinger, J.-C. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Temple, T.N.; Johnson, K.B.; Loper, J.E. Integrated Control of Fire Blight with Antagonists and Oxytetracycline. In Proceedings of the XI International Workshop on Fire Blight, Portland, OR, USA, 12–17 August 2007; Volume 793, pp. 383–390. [Google Scholar]
- McGhee, G.C.; Sundin, G.W. Evaluation of Kasugamycin for Fire Blight Management, Effect on Nontarget Bacteria, and Assessment of Kasugamycin Resistance Potential in Erwinia amylovora. Phytopathology® 2011, 101, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Sundin, G.W.; Peng, J.; Brown, L.E.; Zeng, Q.; Förster, H.; Adaskaveg, J.E. A Novel IncX Plasmid Mediates High-Level Oxytetracycline and Streptomycin Resistance in Erwinia amylovora from Commercial Pear Orchards in California. Phytopathology® 2023, 113, 2165–2173. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen Decades of Antimicrobial Copper Compounds Applied in Agriculture. A Review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Bastas, K.K.; Kannan, V.R. Modern Trends of Plant Pathogenic Bacteria Control. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; CRC Press: Boca Raton, FL, USA, 2015; p. 358. [Google Scholar]
- Clark, R.B.; Zeto, S.K. Mineral Acquisition by Arbuscular Mycorrhizal Plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Taylor, P.; Reeder, R. Antibiotic Use on Crops in Low and Middle-Income Countries Based on Recommendations Made by Agricultural Advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- Gulzar, R.M.A.; Javed, I.; Abbas, M. Emerging Trends In Plant Disease Management: A Review Of Sustainable And Innovative Approaches. J. Surv. Fish. Sci. 2023, 10, 504–509. [Google Scholar] [CrossRef]
- Riudavets, J.; Moerman, E.; Vila, E. Implementation of Integrated Pest and Disease Management in Greenhouses: From Research to the Consumer. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 457–485. ISBN 978-3-030-22303-8. [Google Scholar]
- Razdan, V.K.; Sabitha, M. Integrated Disease Management: Concepts and Practices. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 369–389. ISBN 978-1-4020-8991-6. [Google Scholar]
- Dubey, S.C.; Gupta, K.; Akhtar, J.; Chalam, V.C.; Singh, M.C.; Khan, Z.; Singh, S.P.; Kumar, P.; Gawade, B.H.; Kiran, R. Plant Quarantine for Biosecurity during Transboundary Movement of Plant Genetic Resources. Indian Phytopathol. 2021, 74, 495–508. [Google Scholar] [CrossRef]
- Fry, W.E. Principles of Plant Disease Management; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Sosnowski, M.R.; Fletcher, J.D.; Daly, A.M.; Rodoni, B.C.; Viljanen-Rollinson, S.L.H. Techniques for the Treatment, Removal and Disposal of Host Material during Programmes for Plant Pathogen Eradication. Plant Pathol. 2009, 58, 621–635. [Google Scholar] [CrossRef]
- Coletta-Filho, H.D.; Castillo, A.I.; Laranjeira, F.F.; de Andrade, E.C.; Silva, N.T.; de Souza, A.A.; Bossi, M.E.; Almeida, R.P.; Lopes, J.R. Citrus Variegated Chlorosis: An Overview of 30 Years of Research and Disease Management. Trop. Plant Pathol. 2020, 45, 175–191. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sain, S.K.; Singh, P. A Perspective on Integrated Disease Management in Agriculture. Bio Bull 2016, 2, 13–29. [Google Scholar]
- Wang, J.-F.; Hanson, P.; Barnes, J.A. Worldwide Evaluation of an International Set of Resistance Sources to Bacterial Wilt in Tomato. In Bacterial Wilt Disease; Prior, P., Allen, C., Elphinstone, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 269–275. ISBN 978-3-642-08361-7. [Google Scholar]
- Wang, J.-F.; Ho, F.-I.; Truong, H.T.H.; Huang, S.-M.; Balatero, C.H.; Dittapongpitch, V.; Hidayati, N. Identification of Major QTLs Associated with Stable Resistance of Tomato Cultivar ‘Hawaii 7996’to Ralstonia solanacearum. Euphytica 2013, 190, 241–252. [Google Scholar] [CrossRef]
- Balogh, B.; Jones, J.B.; Iriarte, F.B.; Momol, M.T. Phage Therapy for Plant Disease Control. Curr. Pharm. Biotechnol. 2010, 11, 48–57. [Google Scholar] [CrossRef]
- Nutter, F.F.W. The Role of Plant Disease Epidemiology in Developing Successful Integrated Disease Management Programs. In General Concepts in Integrated Pest and Disease Management; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 45–79. ISBN 978-1-4020-6060-1. [Google Scholar]
- Li, S.; Wu, F.; Duan, Y.; Singerman, A.; Guan, Z. Citrus Greening: Management Strategies and Their Economic Impact. HortScience 2020, 55, 604–612. [Google Scholar] [CrossRef]
- Ogle, H.; Dale, M. Disease Management: Cultural Practices. In Plant Pathogens and Plant Diseases; Rockvale Publishers: College Grove, TN, USA, 1997; pp. 390–404. [Google Scholar]
- Das, A.K. Citrus Canker-A Review. J. Appl. Hortic. 2003, 5, 52–60. [Google Scholar] [CrossRef]
- Lapwood, D.H. The Effects of Soil Moisture at the Time Potato Tubers Are Forming on the Incidence of Common Scab (Streptomyces scabies). Ann. Appl. Biol. 1966, 58, 447–456. [Google Scholar] [CrossRef]
- Kopecky, J.; Rapoport, D.; Sarikhani, E.; Stovicek, A.; Patrmanova, T.; Sagova-Mareckova, M. Micronutrients and Soil Microorganisms in the Suppression of Potato Common Scab. Agronomy 2021, 11, 383. [Google Scholar] [CrossRef]
- Tein, B.; Kauer, K.; Runno-Paurson, E.; Eremeev, V.; Luik, A.; Selge, A.; Loit, E. The Potato Tuber Disease Occurrence as Affected by Conventional and Organic Farming Systems. Am. J. Potato Res. 2015, 92, 662–672. [Google Scholar] [CrossRef]
- Ioannou, N. Management of Soil-Borne Pathogens of Tomato with Soil Solarization. 1999. Available online: http://publications.ari.gov.cy/tb/1999/tb205-n.ioannou.pdf (accessed on 8 April 2025).
- Bari, M.L.; Enomoto, K.; Nei, D.; Kawamoto, S. Development of Effective Seed Decontamination Technology to Inactivate Pathogens on Mung Bean Seeds and Its Practical Application in Japan. Jpn. Agric. Res. Q. JARQ 2011, 45, 153–161. [Google Scholar] [CrossRef]
- Wright, P.J.; Clark, G.E.; McLachlan, A.R.G. Effects of Wounding, Inoculation with Erwinia carotovora subsp. carotovora, Water-Logging, and Temperature on Incidence of Bacterial Soft Rot in Calla Plants. In Proceedings of the X International Symposium on Flower Bulbs and Herbaceous Perennials 886, Lisse, The Netherlands, 20–24 April 2008; pp. 401–407. [Google Scholar]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current Status of Probiotic and Related Health Benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Spence, C.; Alff, E.; Shantharaj, D.; Bais, H. Probiotics for Plants: Importance of Rhizobacteria on Aboveground Fitness in Plants. In Bacteria in Agrobiology: Plant Probiotics; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–14. ISBN 978-3-642-27514-2. [Google Scholar]
- Sohrabi, F.; Sheikholeslami, M.; Heydari, R.; Rezaee, S.; Sharifi, R. Investigating the Effect of Glomus mosseae, Bacillus subtilis and Trichoderma harzianum on Plant Growth and Controlling Meloidogyne javanica in Tomato. Indian Phytopathol. 2020, 73, 293–300. [Google Scholar] [CrossRef]
- Nair, M.S.; Amalaradjou, M.A.; Venkitanarayanan, K. Antivirulence Properties of Probiotics in Combating Microbial Pathogenesis. Adv. Appl. Microbiol. 2017, 98, 1–29. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Gutarra, L.; Herrera, J.; Fernandez, E.; Kreuze, J.; Lindqvist-Kreuze, H. Diversity, Pathogenicity, and Current Occurrence of Bacterial Wilt Bacterium Ralstonia solanacearum in Peru. Front. Plant Sci. 2017, 8, 1221. [Google Scholar] [CrossRef]
- Yendyo, S.; Ramesh, G.C.; Pandey, B.R. Evaluation of Trichoderma spp., Pseudomonas fluorescens and Bacillus subtilis for Biological Control of Ralstonia Wilt of Tomato. F1000Research 2018, 6, 2028. [Google Scholar] [CrossRef]
- Kariuki, C.K.; Mutitu, E.W.; Muiru, W.M. Effect of Bacillus and Trichoderma Species in the Management of the Bacterial Wilt of Tomato (Lycopersicum esculentum) in the Field. Egypt. J. Biol. Pest Control 2020, 30, 109. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.K.; Golec, M.; Milanowski, J. Pantoea Agglomerans: A Mysterious Bacterium of Evil and Good. Part IV. Beneficial Effects. Ann. Agric. Environ. Med. 2016, 23, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Kebede, G.G. Development of Resistance to Bacillus Thuringiensis (Bt) Toxin by Insect Pests. Development 2023, 14, 25–41. [Google Scholar]
- Goyal, R.K.; Mattoo, A.K. Multitasking Antimicrobial Peptides in Plant Development and Host Defense against Biotic/Abiotic Stress. Plant Sci. 2014, 228, 135–149. [Google Scholar] [CrossRef]
- Darrah, K.E.; Albright, S.; Kumbhare, R.; Tsang, M.; Chen, J.K.; Deiters, A. Antisense Oligonucleotide Activation via Enzymatic Antibiotic Resistance Mechanism. ACS Chem. Biol. 2023, 18, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Sundin, G.W.; Yang, C.-H.; Wang, J.; Huntley, R.B.; Yuan, X.; Zeng, Q. Exploration of Using Antisense Peptide Nucleic Acid (PNA)-Cell Penetrating Peptide (CPP) as a Novel Bactericide against Fire Blight Pathogen Erwinia amylovora. Front. Microbiol. 2017, 8, 687. [Google Scholar] [CrossRef]
- Lacombe, S.; Rougon-Cardoso, A.; Sherwood, E.; Peeters, N.; Dahlbeck, D.; Van Esse, H.P.; Smoker, M.; Rallapalli, G.; Thomma, B.P.; Staskawicz, B. Interfamily Transfer of a Plant Pattern-Recognition Receptor Confers Broad-Spectrum Bacterial Resistance. Nat. Biotechnol. 2010, 28, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.H.; Dahlbeck, D.; Clark, E.T.; Gajiwala, P.; Pasion, R.; Whalen, M.C.; Stall, R.E.; Staskawicz, B.J. Expression of the Bs2 Pepper Gene Confers Resistance to Bacterial Spot Disease in Tomato. Proc. Natl. Acad. Sci. USA 1999, 96, 14153–14158. [Google Scholar] [CrossRef]
- Scofield, S.R.; Tobias, C.M.; Rathjen, J.P.; Chang, J.H.; Lavelle, D.T.; Michelmore, R.W.; Staskawicz, B.J. Molecular Basis of Gene-for-Gene Specificity in Bacterial Speck Disease of Tomato. Science 1996, 274, 2063–2065. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.A.; Civerolo, E.L.; Summerfelt, K.R.; Dandekar, A.M. RNAi-Mediated Oncogene Silencing Confers Resistance to Crown Gall Tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 13437–13442. [Google Scholar] [CrossRef]
- Romero, F.M.; Gatica-Arias, A. CRISPR/Cas9: Development and Application in Rice Breeding. Rice Sci. 2019, 26, 265–281. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Deivamani, M.; Shobhana, V.G.; Sudha, M.; Anandhan, T. RNA Interference: Evolutions and Applications in Plant Disease Management. Arch. Phytopathol. Plant Prot. 2013, 46, 1430–1441. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Mysore, K.S. Virus-induced Gene Silencing Can Persist for More than 2 Years and Also Be Transmitted to Progeny Seedlings in Nicotiana benthamiana and Tomato. Plant Biotechnol. J. 2011, 9, 797–806. [Google Scholar] [CrossRef]
- Majumdar, R.; Rajasekaran, K.; Cary, J.W. RNA Interference (RNAi) as a Potential Tool for Control of Mycotoxin Contamination in Crop Plants: Concepts and Considerations. Front. Plant Sci. 2017, 8, 200. [Google Scholar] [CrossRef]
- Hunter, W.B.; Larson, N.R. Invention of RNAi Biopesticides to Psyllid Vectors and Bacterial Pathogens of Citrus: Future Technologies for Gene Targeting. In RNA Interference in Agriculture: Basic Science to Applications; Smagghe, G., Palli, S.R., Swevers, L., Eds.; Springer: Cham, Switzerland, 2025; pp. 383–432. ISBN 978-3-031-81548-5. [Google Scholar]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Zhang, N.; Roberts, H.M.; Van Eck, J.; Martin, G.B. Generation and Molecular Characterization of CRISPR/Cas9-Induced Mutations in 63 Immunity-Associated Genes in Tomato Reveals Specificity and a Range of Gene Modifications. Front. Plant Sci. 2020, 11, 10. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Sankhyan, S.; Ray, S. Metabolic Engineering of Lactic Acid Bacteria for Antimicrobial Peptides Production. In Antimicrobial Peptides from Lactic Acid Bacteria; Ray, S., Kumar, P., Mandal, M., Eds.; Springer: Singapore, 2024; pp. 67–95. ISBN 978-981-97-3412-2. [Google Scholar]
- Li, L.; He, Z.-Y.; Wei, X.-W.; Gao, G.-P.; Wei, Y.-Q. Challenges in CRISPR/CAS9 Delivery: Potential Roles of Nonviral Vectors. Hum. Gene Ther. 2015, 26, 452–462. [Google Scholar] [CrossRef]
- Gan, W.C.; Ling, A.P. CRISPR/Cas9 in Plant Biotechnology: Applications and Challenges. Biotechnologia 2022, 103, 81. [Google Scholar]
- Tavakoli, K.; Pour-Aboughadareh, A.; Kianersi, F.; Poczai, P.; Etminan, A.; Shooshtari, L. Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences. BioTech 2021, 10, 14. [Google Scholar] [CrossRef]
- Ongadi, P.A. A Comprehensive Examination of Security and Privacy in Precision Agriculture Technologies. GSC Adv. Res. Rev. 2024, 18, 336–363. [Google Scholar] [CrossRef]
- Raj, E.F.I.; Appadurai, M.; Athiappan, K. Precision Farming in Modern Agriculture. In Smart Agriculture Automation Using Advanced Technologies; Choudhury, A., Biswas, A., Singh, T.P., Ghosh, S.K., Eds.; Transactions on Computer Systems and Networks; Springer: Singapore, 2021; pp. 61–87. ISBN 978-981-16-6123-5. [Google Scholar]
- Cowan, T.; Zinn, J.A. Precision Agriculture and Site-Specific Management: Current Status and Emerging Policy Issues; Congressional Research Service, Library of Congress: Washington, DC, USA, 2000.
- Akhter, R.; Sofi, S.A. Precision Agriculture Using IoT Data Analytics and Machine Learning. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 5602–5618. [Google Scholar] [CrossRef]
- Garg, S.; Rumjit, N.P.; Roy, S. Smart Agriculture and Nanotechnology: Technology, Challenges, and New Perspective. Adv. Agrochem 2024, 3, 115–125. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol Potentiality of Plant Growth Promoting Bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A Review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Arif, M.; Ullah, R.; Ahmad, M.; Ali, A.; Ullah, Z.; Ali, M.; Al-Joufi, F.A.; Zahoor, M.; Sher, H. Green Synthesis of Silver Nanoparticles Using Euphorbia wallichii Leaf Extract: Its Antibacterial Action against Citrus Canker Causal Agent and Antioxidant Potential. Molecules 2022, 27, 3525. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Abd-Elsalam, K.A.; Tmam, A.M.; Sofy, M.R. Silver-Based Nanomaterials for Plant Diseases Management: Today and Future Perspectives. In Silver Nanomaterials for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 495–526. [Google Scholar]

| Biological Antibacterial Agent | Bacterial Pathogen | Disease | Host Crop | Reference |
|---|---|---|---|---|
| Pantoea agglomerans | Erwinia amylovora | Fire blight | Apple, pear | [24] |
| Pseudomonas graminis | [25] | |||
| Lysobacter enzymogenes | Pseudomonas syringae | Bacterial speck | Tomato | [26] |
| Trichoderma asperellum | [27] | |||
| Bacillus amyloliquefaciens | Ralstonia solanacearum | Bacterial wilt | [28] | |
| B. subtilis | [29] | |||
| Trichoderma harzianum, T. asperellum | [30,31] | |||
| P. fluorescens | Xanthomonas oryzae | Bacterial blight | [32] | |
| P. fluorescens | X. campestris | Black rot | Crucifers | [33] |
| Streptomyces spp. | Brassicas | [34] | ||
| B. velezensis | Cabbage | [35] | ||
| B. velezensis | Agrobacterium tumefaciens | Crown gall | Japanese rose | [36] |
| B. thuringiensis | X. euvesicatora | Bacterial spot | Pepper | [37] |
| Lactobacillus plantarum | Xanthomonas arboricola | Stone fruits | [38] |
| Chemical Antibacterial Agent | Bacterial Pathogen | Disease | Host Crop | Reference |
|---|---|---|---|---|
| Streptomycin | Erwinia amylovora | Fire blight | Apple, pear | [41] |
| Tetracycline | Candidatus liberibacter | Huanglongbing (Citrus greening) | Citrus | [41] |
| Oxytetracycline | X. axonopodis pv. citri | Citrus canker | [42] | |
| Copper hydroxide | X. campestris pv. campestris | Black rot | Cabbage | [43] |
| Kasugamycin | Pseudomonas syringae | Black spot | Stone fruits | [44] |
| Trichloroisocyanuric acid | Bacterial canker | Kiwifruits | [45] | |
| Erythromycin | X. campestris | Canker | Mango | [46] |
| Validamycin A | P. solanacearum | Bacterial wilt | Tomato | [47] |
| Quinolones | Ralstonia solanacearum | Potato | [48] | |
| Thiabendazole | Erwinia carotovora | Soft rot | [49] |
| Nanoparticle | Type | Shape | Pathogen | Host Crop | Reference |
|---|---|---|---|---|---|
| Ag | Metallic (Inorganic) | Spherical | Xanthomonas perforan (Bacterial spot) | Tomato | [68] |
| Pectobacterium carotovorum (Soft rot) | Pepper | [69] | |||
| Au | X. axonopodis (Citrus canker) | Citrus | [68] | ||
| Silica | X. campestris pv. Vesicatoria (Bacterial spot) | Tomato | [70] | ||
| Selenium | Ralstonia solanacearum (Bacterial wilt) | Potato | [71] | ||
| Bovine submaxillary mucin silver nanoparticles (BSM-Ag NPs) | - | Acidovorax citrulli (Bacterial fruit blotch) | Melon | [72] | |
| MgO | Metallic oxide (Inorganic) | Cubic | R. solanacearum (Bacterial wilt) | Tomato | [73] |
| CuO | Cubic | Clavibacter michiganensis subsp. Sepedonicus (Bacterial ring rot) | Potato | [74] | |
| ZnO | Spherical | Pectobacterium carotovorum (Soft rot) | [75] | ||
| TiO2 | X. perforans (Bacterial spot) | Tomato | [76] | ||
| SiO2 | P. syringae | Arabidopsis | [77] | ||
| Fe3O4 | - | [78] | |||
| Calcium carbonate nanocrystals | Non-metallic (Inorganic) | - | Xylella fastidiosa (Quick decline syndrome) | Olive | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, M.A.; Iqbal, M.M.; Ashfaq, M.; Raza, N.; Wang, J.; Hafeez, A.; Kayani, S.B.; Ali, Q. Impact of Antibacterial Agents in Horticulture: Risks to Non-Target Organisms and Sustainable Alternatives. Horticulturae 2025, 11, 753. https://doi.org/10.3390/horticulturae11070753
Mehmood MA, Iqbal MM, Ashfaq M, Raza N, Wang J, Hafeez A, Kayani SB, Ali Q. Impact of Antibacterial Agents in Horticulture: Risks to Non-Target Organisms and Sustainable Alternatives. Horticulturae. 2025; 11(7):753. https://doi.org/10.3390/horticulturae11070753
Chicago/Turabian StyleMehmood, Mirza Abid, Muhammad Mazhar Iqbal, Muhammad Ashfaq, Nighat Raza, Jianguang Wang, Abdul Hafeez, Samah Bashir Kayani, and Qurban Ali. 2025. "Impact of Antibacterial Agents in Horticulture: Risks to Non-Target Organisms and Sustainable Alternatives" Horticulturae 11, no. 7: 753. https://doi.org/10.3390/horticulturae11070753
APA StyleMehmood, M. A., Iqbal, M. M., Ashfaq, M., Raza, N., Wang, J., Hafeez, A., Kayani, S. B., & Ali, Q. (2025). Impact of Antibacterial Agents in Horticulture: Risks to Non-Target Organisms and Sustainable Alternatives. Horticulturae, 11(7), 753. https://doi.org/10.3390/horticulturae11070753






