Impact of Prolonged Frozen Storage on ‘Mejhoul’ Date Palm Cultivar Based on Selected Qualitative Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Freezing Experiments
2.3. Weight Loss Rate and Physical Defects
2.4. Physicochemical Analysis
2.4.1. Total Soluble Solids, pH, Acidity, and Water Activity
2.4.2. Color and Hardness
2.4.3. Sugar Analysis
2.4.4. Polyphenol Determination
2.5. Image Acquisition and Image-Feature Determination
2.6. Statistical Analysis
3. Results
3.1. Weight Loss and Main Physicochemical Attributes
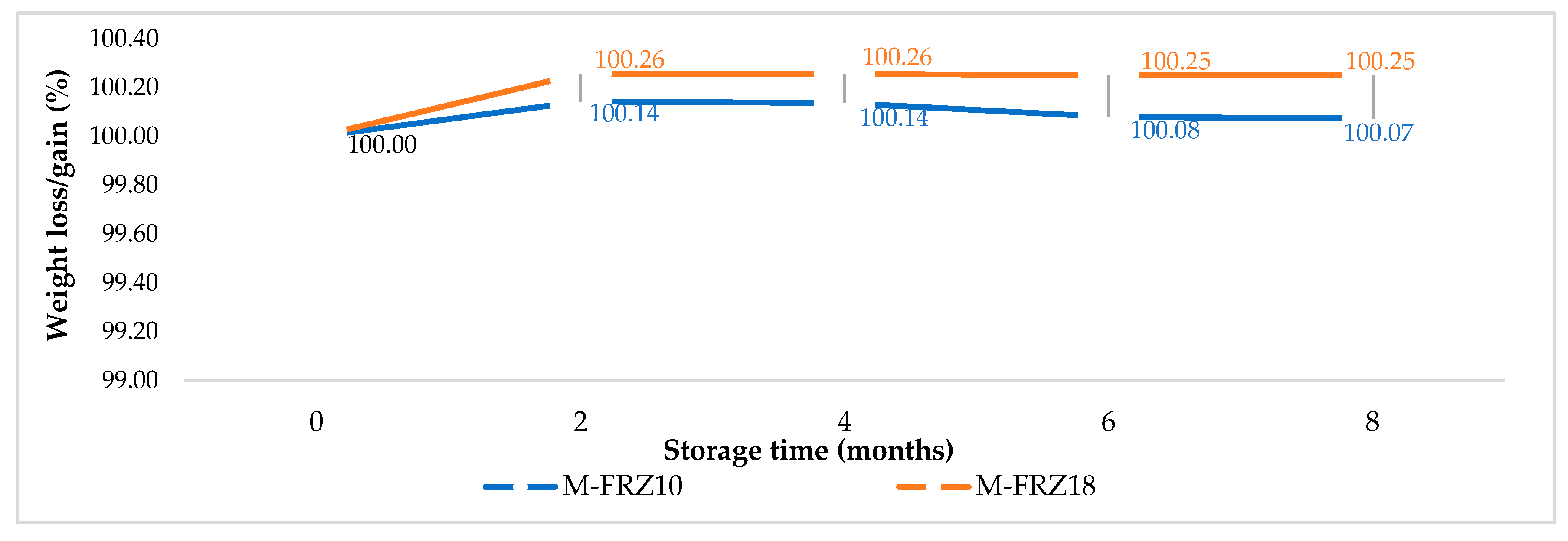
3.1.1. Weight Loss and Physical Defects
3.1.2. Physicochemical Attributes
3.2. Polyphenol Profile and Sugars
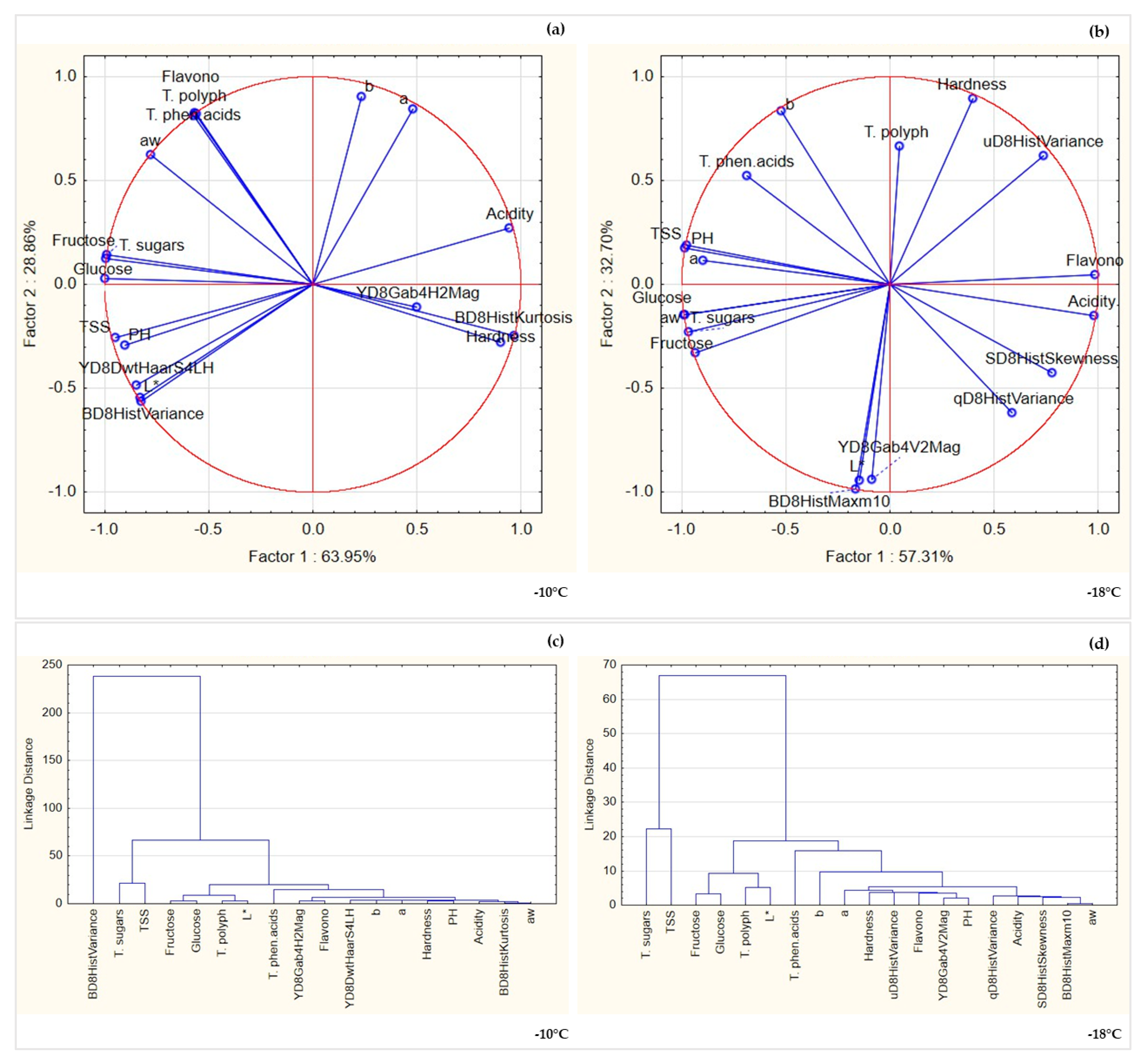
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding

Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alotaibi, K.D.; Alharbi, H.A.; Yaish, M.W.; Ahmed, I.; Alharbi, S.A.; Alotaibi, F.; Kuzyakov, Y. Date palm cultivation: A review of soil and environmental conditions and future challenges. Land Degrad. Dev. 2023, 34, 2431–2444. [Google Scholar] [CrossRef]
- Sarraf, M.; Jemni, M.; Kahramanoğlu, I.; Artés, F.; Shahkoomahally, S.; Namsi, A.; Ihtisham, M.; Brestic, M.; Mohammadi, M.; Rastogi, A. Commercial techniques for preserving date palm (Phoenix dactylifera) fruit quality and safety: A review. Saudi J. Biol. Sci. 2021, 28, 4408–4420. [Google Scholar] [CrossRef] [PubMed]
- Elhoumaizi, M.A.; Jdaini, K.; Alla, F.; Parmar, A. Variations in physicochemical and microbiological characteristics of ‘Mejhoul’ dates (Phoenix dactylifera L.) from Morocco and new countries of its expansion. J. Saudi Soc. Agric. Sci. 2023, 22, 318–326. [Google Scholar] [CrossRef]
- Zaid, A.; Oihabi, A. Origin and geographical distribution of the Mejhoul date variety. In Mejhoul Variety-The Jewel of dates-origin, distribution and International Markets; Khalifa International Award for Date Palm and Agricultural Innovation: Abu Dhabi, United Arab Emirates, 2022; pp. 15–20. [Google Scholar]
- Ramchoun, M.; Alem, C.; Ghafoor, K.; Ennassir, J.; Zegzouti, Y.F. Functional composition and antioxidant activities of eight Moroccan date fruit varieties (Phoenix dactylifera L.). J. Saudi Soc. Agric. Sci. 2017, 16, 257–264. [Google Scholar]
- Salomón-Torres, R.; Ortiz-Uribe, N.; Sol-Uribe, J.A.; Villa-Angulo, C.; Villa-Angulo, R.; Valdez-Salas, B.; García-González, C.; Monroy, C.G.I.; Norzagaray-Plasencia, S. Influence of different sources of pollen on the chemical composition of date (Phoenix dactylifera L.) cultivar Medjool in México. Aust. J. Crop Sci. 2018, 12, 1008–1015. [Google Scholar] [CrossRef]
- Noutfia, Y.; Ropelewska, E. What can artificial intelligence approaches bring to an improved and efficient harvesting and postharvest handling of date fruit (Phoenix dactylifera L.)? A review. Postharvest Biol. Technol. 2024, 213, 112926. [Google Scholar] [CrossRef]
- Alsmairat, N.; Othman, Y.; Ayad, J.; Al-Ajlouni, M.; Sawwan, J.; El-Assi, N. Anatomical assessment of skin separation in date palm (Phoenix dactylifera L. var. Mejhoul) fruit during maturation and ripening stages. Agriculture 2022, 13, 38. [Google Scholar] [CrossRef]
- Misbah, A.; Essarioui, A.; Noutfia, Y. Technologies post-récolte pour la préservation de la qualité des dattes durant le stockage. Afr. Mediterr. Agric. J.-Al Awamia 2022, 134, 30–59. [Google Scholar]
- Abu-Shama, H.S.; Abou-Zaid, F.O.F.; El-Sayed, E.Z. Effect of using edible coatings on fruit quality of Barhi date cultivar. Sci. Hortic. 2020, 265, 109262. [Google Scholar] [CrossRef]
- Jemni, M.; Chniti, S.; Harbaoui, K.; Ferchichi, A.; Artés, F. Partial vacuum and active modified atmosphere packaging for keeping overall quality of dates. J. New Sci. Agric. Biotechnol. 2016, 29, 1656–1665. [Google Scholar]
- Noutfia, Y.; Alem, C.; Filali Zegzouti, Y. Assessment of physico-chemical and sensory properties of two date (Phoenix dactylifera L.) cultivars under commercial cold storage conditions. J. Food Process. Preserv. 2019, 43, e14228. [Google Scholar] [CrossRef]
- Cherif, S.; Le Bourvellec, C.; Bureau, S.; Benabda, J. Effect of storage conditions on ‘Deglet Nour’date palm fruit organoleptic and nutritional quality. LWT 2021, 137, 110343. [Google Scholar] [CrossRef]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Cryogenic freezing of fresh date fruits for quality preservation during frozen storage. J. Saudi Soc. Agric. Sci. 2018, 17, 9–16. [Google Scholar] [CrossRef]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Freezing of fresh Barhi dates for quality preservation during frozen storage. Saudi J. Biol. Sci. 2018, 25, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahyai, R.; Al-Kharusi, L. Physical and chemical quality attributes of freeze-stored dates. Int. J. Agric. Biol. 2012, 14, 97–100. [Google Scholar]
- Jemni, M.; Ramirez, J.G.; Oton, M.; Artés-Hernandez, F.; Harbaoui, K.; Namsi, A.; Ferchichi, A. Chilling and Freezing Storage for Keeping Overall Quality of “Deglet Nour” Dates. J. Agric. Sci. Technol. 2019, 21, 63–76. [Google Scholar]
- Mowafy, S.G.; Sabbah, M.A.; Mostafa, Y.S.; Elansari, A.M. Effect of freezing rate on the quality properties of Medjool dates at the tamr stage. J. Food Process. Preserv. 2020, 44, e14938. [Google Scholar] [CrossRef]
- Noutfia, Y.; Ropelewska, E.; Jóźwiak, Z.; Rutkowski, K. Non-Destructive Monitoring of External Quality of Date Palm Fruit (Phoenix dactylifera L.) During Frozen Storage Using Digital Camera and Flatbed Scanner. Sensors 2024, 24, 7560. [Google Scholar] [CrossRef]
- Liu, D.K.; Xu, C.C.; Guo, C.X.; Zhang, X.X. Sub-zero temperature preservation of fruits and vegetables: A review. J. Food Eng. 2020, 275, 109881. [Google Scholar] [CrossRef]
- Alain, L.B.; Hamdami, N.; Toublanc, C.; Havet, M. An Overview Of Selected “Subzero” Temperature Storage Regimes Of Foods: Regulations And Perspectives About The Expected Impact Of The” 3 Degrees More” Initiative On The Shelf Life Of Frozen Foods. Int. J. Refrig. 2025, 176, 336–344. [Google Scholar]
- Red Book-IIR. Recommendations for the Processing and Handling of Frozen Foods, 4th ed.; Leif, B.-S., Ed.; International Institute of Refrigeration: Paris, France, 2006; ISBN 9782913149526. [Google Scholar]
- Sayin, L.; Peters, T.; Allouche, Y.; Evans, J.; Falagan, N.; Hetterscheid, B. Three Degrees of Change: Frozen Food in a Resilient and Sustainable Food System; Fox, T., Ed.; Summary Report & Initial Findings; International Institute of Refrigeration: Paris, France, 2023. [Google Scholar]
- Lustig, I.; Bernstein, Z.; Gophen, M. Skin separation in Majhul fruits. Int. J. Plant Res. 2014, 4, 29–35. [Google Scholar]
- Younuskunju, S.; Mohamoud, Y.A.; Mathew, L.S.; Mayer, K.F.; Suhre, K.; Malek, J.A. The genetics of fruit skin separation in date palm. BMC Plant Biol. 2024, 24, 1050. [Google Scholar] [CrossRef] [PubMed]
- Antoine, S.; Pailly, O.; Gibon, Y.; Luro, F.; Santini, J.; Giannettini, J.; Berti, L. Short-and long-term effects of carbohydrate limitation on sugar and organic acid accumulation during mandarin fruit growth. J. Sci. Food Agric. 2016, 96, 3906–3914. [Google Scholar] [CrossRef]
- Pozo, L.; Kender, W.J.; Burns, J.K.; Hartmond, U.; Grant, A. Effects of gibberellic acid on ripening and rind puffing in ‘Sunburst’ mandarin. Proc. Fla. State Hortic. Soc. 2000, 13, 102–105. [Google Scholar]
- Xu, F.; An, H.; Zhang, J.; Xu, Z.; Jiang, F. Effects of fruit load on sugar/acid quality and puffiness of delayed-harvest citrus. Horticulturae 2021, 7, 189. [Google Scholar] [CrossRef]
- Gophen, M. Skin separation in date fruits. Int. J. Plant Res. 2014, 4, 11–16. [Google Scholar]
- Krishna, K.R.; Smruthi, J.; Manivannan, S. Packaging and storage of stone fruits. In Production Technology of Stone Fruits; Springer: Berlin/Heidelberg, Germany, 2021; pp. 273–305. [Google Scholar]
- Bonat Celli, G.; Ghanem, A.; Su-Ling Brooks, M. Influence of freezing process and frozen storage on the quality of fruits and fruit products. Food Rev. Int. 2016, 32, 280–304. [Google Scholar] [CrossRef]
- Mohammed, M.; Munir, M.; Aljabr, A. Prediction of date fruit quality attributes during cold storage based on their electrical properties using artificial neural networks models. Foods 2022, 11, 1666. [Google Scholar] [CrossRef]
- Cui, K.; Yang, L.; Shu, C.; Liu, J.; Zhu, Z.; Yang, Z.; Zhu, X.; Jiang, W. Near freezing temperature storage alleviates cell wall polysaccharide degradation and softening of apricot (Prunus armeniaca L.) fruit after simulated transport vibration. Sci. Hortic. 2021, 288, 110296. [Google Scholar] [CrossRef]
- Adhikary, T.; Gill, P.S.; Jawandha, S.K.; Bhardwaj, R.D.; Anurag, R.K. Browning and quality management of pear fruit by salicylic acid treatment during low temperature storage. J. Sci. Food Agric. 2021, 101, 853–862. [Google Scholar] [CrossRef]
- Fagundes, C.; Carciofi, B.A.M.; Monteiro, A.R. Estimate of respiration rate and physicochemical changes of fresh-cut apples stored under different temperatures. Food Sci. Technol. 2013, 33, 60–67. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Combination of chitosan coating and heat shock treatments to maintain postharvest quality and alleviate cracking of Akebia trifoliate fruit during cold storage. Food Chem. 2022, 394, 133330. [Google Scholar] [CrossRef] [PubMed]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Younis, M.; Abdelkarim, D. Quality changes in fresh date fruits (Barhi) during individual quick freezing and conventional slow freezing. Pak. J. Agric. Sci. 2016, 53, 917–924. [Google Scholar]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of chitosan-and alginate-based coatings enriched with cinnamon essential oil microcapsules to improve the postharvest quality of mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.; Zaritzky, N. Cooling and freezing of fruits and fruit products. In Fruit Preservation: Novel and Conventional Technologies; Springer: Berlin/Heidelberg, Germany, 2018; pp. 127–180. [Google Scholar]
- Zuo, X.; Cao, S.; Ji, N.; Li, Y.; Zhang, J.; Jin, P.; Zheng, Y. High relative humidity enhances chilling tolerance of zucchini fruit by regulating sugar and ethanol metabolisms during cold storage. Postharvest Biol. Technol. 2022, 189, 111932. [Google Scholar] [CrossRef]
- Galsurker, O.; Kelly, G.; Doron-Faigenboim, A.; Aruchamy, K.; Salam, B.B.; Teper-Bamnolker, P.; Lers, A.; Eshel, D. Endogenous sugar level is associated with differential heat tolerance in onion bulb scales. Postharvest Biol. Technol. 2020, 163, 111145. [Google Scholar] [CrossRef]
- Allaith Abdulameer, A.; Safa, H.A.; Jafer, F. Effect of different thermal treatments and freezing on the antioxidant constituents and activity of two Bahraini date cultivars (Phoenix dactylifera L.). Int. J. Food Sci. Technol. 2012, 47, 783–792. [Google Scholar] [CrossRef]
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J. Food Compos. Anal. 2008, 21, 241–248. [Google Scholar] [CrossRef]
- Oliveira, A.; Coelho, M.; Alexandre, E.M.; Almeida, D.P.; Pintado, M. Long-term frozen storage and pasteurization effects on strawberry polyphenols content. Food Bioprocess Technol. 2015, 8, 1838–1844. [Google Scholar] [CrossRef]
- Noutfia, Y.; Ropelewska, E.; Szwejda-Grzybowska, J.; Mieszczakowska-Frąc, M.; Siarkowski, S.; Rutkowski, K.P.; Konopacka, D. Effects of mild infrared and convective drying on physicochemical properties, polyphenol compounds, and image features of two date palm cultivars:‘Mejhoul’and ‘Boufeggous’. LWT 2025, 218, 117502. [Google Scholar] [CrossRef]
- Neri, L.; Faieta, M.; Di Mattia, C.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Antioxidant activity in frozen plant foods: Effect of cryoprotectants, freezing process and frozen storage. Foods 2020, 9, 1886. [Google Scholar] [CrossRef] [PubMed]
- Kader, F.; Haluk, J.P.; Nicolas, J.P.; Metche, M. Degradation of cyanidin 3-glucoside by blueberry polyphenol oxidase: Kinetic studies and mechanisms. J. Agric. Food Chem. 1998, 46, 3060–3065. [Google Scholar] [CrossRef]
- Blanda, G.; Cerretani, L.; Bendini, A.; Cardinali, A.; Lercker, G. Phenolic content and antioxidant capacity versus consumer acceptance of soaked and vacuum impregnated frozen nectarines. Eur. Food Res. Technol. 2008, 227, 191–197. [Google Scholar] [CrossRef]


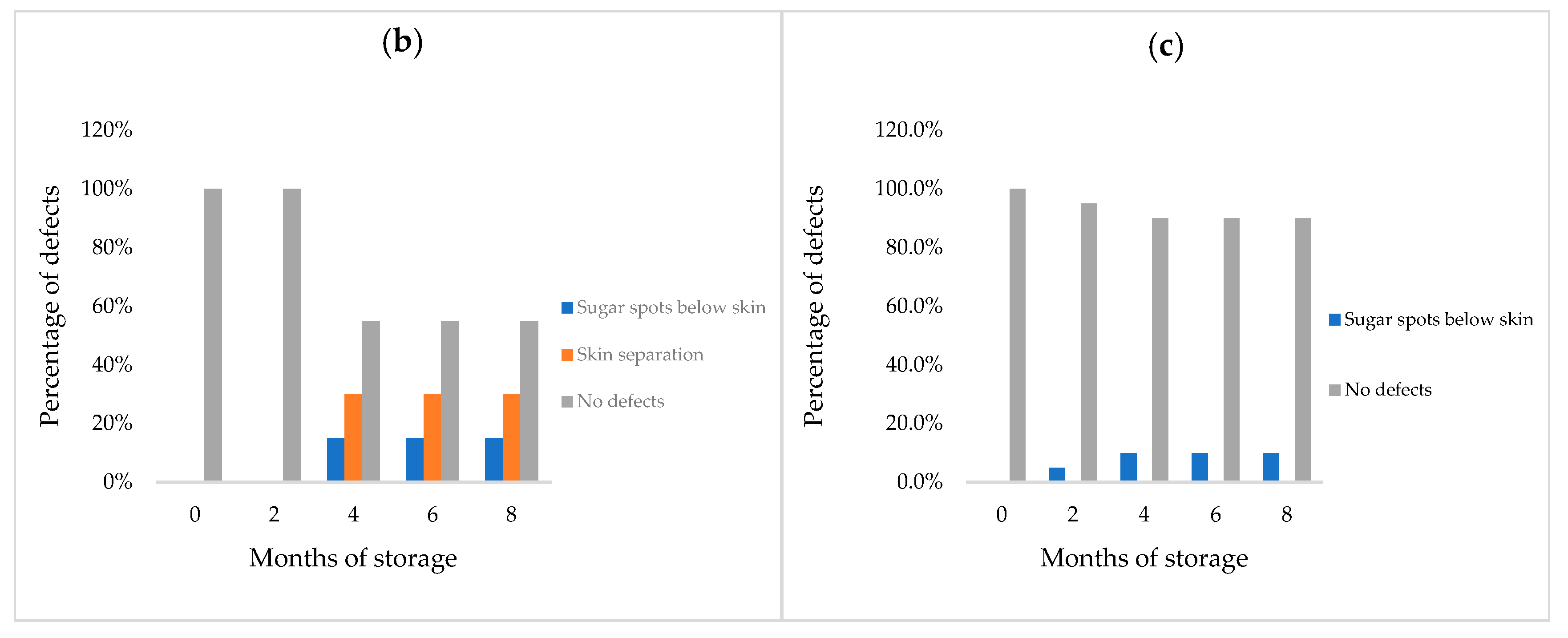
| Quality Attribute | Storage Time (Months) | −10 °C | −18 °C |
|---|---|---|---|
| Water activity | 0 | 0.586 a | 0.586 a |
| 2 | 0.589 a,b | 0.596 a | |
| 4 | 0.584 a | 0.592 a | |
| 6 | 0.616 c | 0.587 a | |
| 8 | 0.606 b,c | 0.620 b | |
| pH | 0 | 6.66 a | 6.66 a |
| 2 | 6.92 b | 6.95 c | |
| 4 | 6.89 b | 6.93 b,c | |
| 6 | 6.84 b | 6.77 a,b | |
| 8 | 6.91 b | 6.98 c | |
| Acidity (%) | 0 | 1.985 a | 1.985 a |
| 2 | 1.445 b | 1.470 b,c | |
| 4 | 1.530 b | 1.495 b,c | |
| 6 | 1.440 b | 1.630 b | |
| 8 | 1.475 b | 1.290 c | |
| TSS (°Bx) | 0 | 77.7 a | 77.7 a |
| 2 | 78.5 a,b | 78.5 a,b | |
| 4 | 78.8 b | 79.8 b | |
| 6 | 78.3 a,b | 77.8 a | |
| 8 | 77.7 a | 78.1 a | |
| Hardness (N) | 0 | 8.49 a | 8.49 a |
| 2 | 7.02 a,b | 6.08 b | |
| 4 | 6.14 b | 6.81 a,b | |
| 6 | 6.46 b | 7.95 a,b | |
| 8 | 5.95 b | 6.58 b |
| Quality Attribute | Storage Time (Months) | −10 °C | −18 °C |
|---|---|---|---|
| L* | 0 | 28.4 a | 28.4 a |
| 2 | 27.4 a | 27.3 a | |
| 4 | 27.6 a | 27.9 a | |
| 6 | 27.9 a | 27.9 a | |
| 8 | 28.5 a | 28.3 a | |
| a* | 0 | 4.95 a | 4.95 a |
| 2 | 4.96 a | 4.82 a | |
| 4 | 5.00 a | 4.58 a | |
| 6 | 4.69 a | 4.98 a | |
| 8 | 4.56 a | 4.81 a | |
| b* | 0 | 4.49 a | 4.49 a |
| 2 | 4.33 a | 4.70 a | |
| 4 | 4.53 a | 4.32 a | |
| 6 | 4.18 a | 4.32 a | |
| 8 | 4.32 a | 4.59 a |
| Functional Compound (g 100 g−1 DM) | Storage Time (Months) | −10 °C | −18 °C |
|---|---|---|---|
| Total sugars | 0 | 82.3 a | 82.3 a |
| 4 | 82.9 a | 83.7 a | |
| 8 | 83.3 a | 83.9 a | |
| Glucose | 0 | 41.5 a | 41.5 a |
| 4 | 41.7 a | 42.4 a | |
| 8 | 42.2 a | 42.0 a | |
| Fructose | 0 | 39.8 a | 39.8 a |
| 4 | 40.0 a | 40.1 a | |
| 8 | 39.8 a | 40.6 a | |
| Total polyphenols | 0 | 33.9 a | 33.9 a |
| 4 | 34.9 a | 34.1 a | |
| 8 | 29.5 a | 31.1 a | |
| Total phenolic acids | 0 | 21.9 a | 21.9 a |
| 4 | 22.4 a | 23.4 a | |
| 8 | 13.9 b | 13.9 b | |
| Total flavonoids | 0 | 12.1 a | 12.1 a |
| 4 | 12.5 a | 10.7 a | |
| 8 | 9.63 a | 10.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noutfia, Y.; Ropelewska, E.; Szwejda-Grzybowska, J.; Jóźwiak, Z.; Mieszczakowska-Frąc, M.; Rutkowski, K.P. Impact of Prolonged Frozen Storage on ‘Mejhoul’ Date Palm Cultivar Based on Selected Qualitative Characteristics. Horticulturae 2025, 11, 731. https://doi.org/10.3390/horticulturae11070731
Noutfia Y, Ropelewska E, Szwejda-Grzybowska J, Jóźwiak Z, Mieszczakowska-Frąc M, Rutkowski KP. Impact of Prolonged Frozen Storage on ‘Mejhoul’ Date Palm Cultivar Based on Selected Qualitative Characteristics. Horticulturae. 2025; 11(7):731. https://doi.org/10.3390/horticulturae11070731
Chicago/Turabian StyleNoutfia, Younes, Ewa Ropelewska, Justyna Szwejda-Grzybowska, Zbigniew Jóźwiak, Monika Mieszczakowska-Frąc, and Krzysztof P. Rutkowski. 2025. "Impact of Prolonged Frozen Storage on ‘Mejhoul’ Date Palm Cultivar Based on Selected Qualitative Characteristics" Horticulturae 11, no. 7: 731. https://doi.org/10.3390/horticulturae11070731
APA StyleNoutfia, Y., Ropelewska, E., Szwejda-Grzybowska, J., Jóźwiak, Z., Mieszczakowska-Frąc, M., & Rutkowski, K. P. (2025). Impact of Prolonged Frozen Storage on ‘Mejhoul’ Date Palm Cultivar Based on Selected Qualitative Characteristics. Horticulturae, 11(7), 731. https://doi.org/10.3390/horticulturae11070731







