Characterization of Polyploid Embryoid Lines Induced via Unfertilized Ovule Culture of Loquat (Eriobotrya japonica Lindl.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Unfertilized Ovules Culture
2.2. Ploidy Analysis and Chromosomal Cytogenetic Analysis
2.3. Molecular and Genome Characterization
2.4. Metabolic Substance Determination
2.5. Data Analysis
3. Results
3.1. Embryoid Induction from Unfertilized Ovules of Loquat
3.2. Ploidy Analysis of Embryoid Lines Derived from Unfertilized Ovules
3.3. SSR Analysis Coupled with Genomic Sequencing Provides Molecular Evidence for Ovule-Derived Polyploids Formation
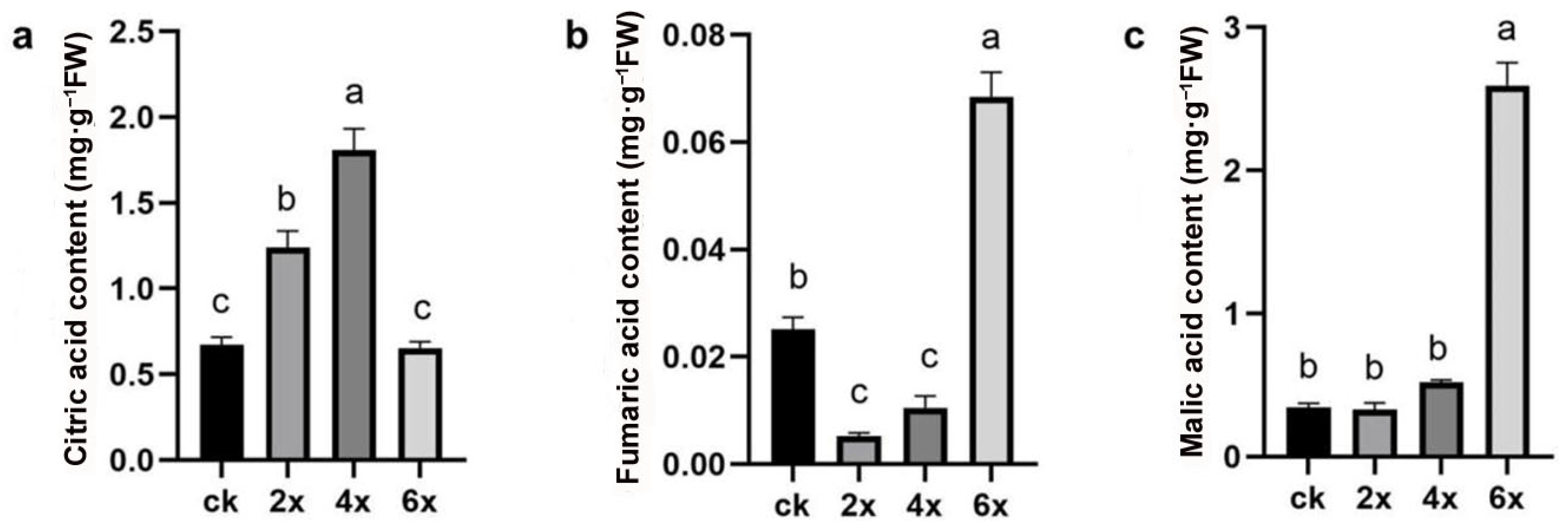
3.4. Soluble Sugar and Organic Acid Detection in Different Ploidy Embryoids Derived from Ovules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral Polyploidy in Seed Plants and Angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.E.; Takebayashi, N.; Barker, M.S.; Mayrose, I.; Greenspoon, P.B.; Rieseberg, L.H. The Frequency of Polyploid Speciation in Vascular Plants. Proc. Natl. Acad. Sci. USA 2009, 106, 13875–13879. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhu, Z.; Huang, L.; Luo, X.; Li, Y.; Xiao, C.; Yang, J.; Wang, J.; Zou, Q.; Tao, L.; et al. DNA Repair- and Nucleotide Metabolism-Related Genes Exhibit Differential CHG Methylation Patterns in Natural and Synthetic Polyploids (Brassica napus L.). Hortic. Res. 2021, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Y.; Ni, P.; Ni, Z.; Sun, Q.; Zong, Y. CRISPR-Mediated Acceleration of Wheat Improvement: Advances and Perspectives. J. Gene. Genom. 2023, 50, 815–834. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Q.; Huang, J.; Zhang, S.; Yao, W.; Yu, Z.; Deng, Z.; Yu, J.; Kong, W.; Yu, X.; et al. A Chromosomal-Scale Genome Assembly of Modern Cultivated Hybrid Sugarcane Provides Insights into Origination and Evolution. Nat. Commun. 2024, 15, 3041. [Google Scholar] [CrossRef]
- Serra Mari, R.; Schrinner, S.; Finkers, R.; Ziegler, F.M.R.; Arens, P.; Schmidt, M.H.-W.; Usadel, B.; Klau, G.W.; Marschall, T. Haplotype-Resolved Assembly of a Tetraploid Potato Genome Using Long Reads and Low-Depth Offspring Data. Genome Biol. 2024, 25, 26. [Google Scholar] [CrossRef]
- Manivannan, A.; Cheeran Amal, T. Deciphering the Complex Cotton Genome for Improving Fiber Traits and Abiotic Stress Resilience in Sustainable Agriculture. Mol. Biol. Rep. 2023, 50, 6937–6953. [Google Scholar] [CrossRef]
- Zhuang, W.; Chen, H.; Yang, M.; Wang, J.; Pandey, M.K.; Zhang, C.; Chang, W.-C.; Zhang, L.; Zhang, X.; Tang, R.; et al. The Genome of Cultivated Peanut Provides Insight into Legume Karyotypes, Polyploid Evolution and Crop Domestication. Nat. Genet. 2019, 51, 865–876. [Google Scholar] [CrossRef]
- Rijzaani, H.; Bayer, P.E.; Rouard, M.; Doležel, J.; Batley, J.; Edwards, D. The Pangenome of Banana Highlights Differences between Genera and Genomes. Plant Genome 2022, 15, e20100. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Zhang, Z.; Edwards, E.J.; Gathunga, E.K.; Fan, P.; Duan, W.; Li, S.; Liang, Z. Gene Body Demethylation Increases Expression and Is Associated with Self-Pruning during Grape Genome Duplication. Hortic. Res. 2020, 7, 84. [Google Scholar] [CrossRef]
- Popowski, E.; Thomson, S.J.; Knäbel, M.; Tahir, J.; Crowhurst, R.N.; Davy, M.; Foster, T.M.; Schaffer, R.J.; Tustin, D.S.; Allan, A.C.; et al. Construction of a High-Density Genetic Map for Hexaploid Kiwifruit (Actinidia chinensis Var. Deliciosa) Using Genotyping by Sequencing. G3-Genes Genom. Genet. 2021, 11, jkab142. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Bi, G.; Wang, H.; Hui, R.; Chen, J.; Lian, Q.; Wang, X.; Fang, W.; Zhang, J.; Dong, Z.; et al. Genomes of Autotetraploid Wild and Cultivated Ziziphus Mauritiana Reveal Polyploid Evolution and Crop Domestication. Plant Physiol. 2024, 196, 2701–2720. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Du, H.; Zhu, C.; Wan, H.; Liu, F.; Ruan, J.; Mower, J.P.; Zhu, A. Haplotype-Resolved Genomes of Wild Octoploid Progenitors Illuminate Genomic Diversifications from Wild Relatives to Cultivated Strawberry. Nat. Plants 2023, 9, 1252–1266. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Shi, C.; Zhang, X.; Duan, K.; Luo, J. Morphological, Cytological and Fertility Consequences of a Spontaneous Tetraploid of the Diploid Pear (Pyrus pyrifolia Nakai) Cultivar ‘Cuiguan.’. Sci. Hortic. 2015, 189, 59–65. [Google Scholar] [CrossRef]
- Liu, X.; Song, L.; Xue, B.; Chi, Z.; Wang, Y.; Wen, S.; Lv, W.; Hu, Q.; Guo, Q.; Wang, S.; et al. Organic Acid and Sugar Components Accumulation and Flavor Associated Metabolites Dynamic Changes in Yellow- and White-Fleshed Seedless Loquats (Eriobotrya japonica). Food Chem. X 2024, 21, 101046. [Google Scholar] [CrossRef]
- Van De Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An Evolutionary and Ecological Force in Stressful Times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Wang, T.; Duan, Y.; Ren, J.; Gao, H.; Fan, Y.; Xia, Q.; Cao, H.; Xie, K.; et al. Polyploidization Leads to Salt Stress Resilience via Ethylene Signaling in Citrus Plants. New Phytolo. 2025, 246, 176–191. [Google Scholar] [CrossRef]
- Meeus, S.; Semberova, K.; Storme, N.D.; Geelen, D.; Vallejo-Marı’n, M. Effect of Whole-Genome Duplication on the Evolutionary Rescue of Sterile Hybrid Monkeyflowers. Plant Commun. 2020, 1, 100093. [Google Scholar] [CrossRef]
- Soomro, S.R.; Soomro, S.N.; Altaf, M.T.; Liaqat, W.; Nadeem, M.A.; Baloch, F.S.; Aasim, M.; Mohamed, H.I. Development of Tetraploids in Tissue Culture: Modern Techniques and Biotechnological Innovations. Plant Cell Tiss. Organ. Cult. 2025, 160, 51. [Google Scholar] [CrossRef]
- Xie, K.D.; Yuan, D.Y.; Wang, W.; Xia, Q.M.; Wu, X.M.; Chen, C.W.; Chen, C.L.; Grosser, J.W.; Guo, W.W. Citrus Triploid Recovery Based on 2x × 4x Crosses via an Optimized Embryo Rescue Approach. Sci. Hortic. 2019, 252, 104–109. [Google Scholar] [CrossRef]
- Liu, M.; Liu, P. Artificial Autopolyploidization in Jujube. Sci. Hortic. 2023, 314, 111916. [Google Scholar] [CrossRef]
- Narukulla, V.; Lahane, Y.; Uge, P.; Pandey, S.; Fiske, K.; Kawale, K.; Jagannadham, P.T.K.; Ziogas, V. Production of Triploid Seedless Sweet Orange [Citrus sinensis (L.) Osbeck] Cv. Mosambi: A Success Story. Agronomy 2024, 14, 829. [Google Scholar] [CrossRef]
- Narukulla, V.; Lahane, Y.; Fiske, K.; Pandey, S.; Ziogas, V. Induction of Polyploidy in Citrus Rootstocks through In Vitro Colchicine Treatment of Seed-Derived Explants. Agronomy 2023, 13, 1442. [Google Scholar] [CrossRef]
- Guo, W.W.; Deng, X. Intertribal Hexaploid Somatic Hybrid Plants Regeneration from Electrofusion between Diploids of Citrus sinensis and Its Sexually Incompatible Relative, Clausena lansium. Theor. Appl. Genet. 1999, 98, 581–585. [Google Scholar] [CrossRef]
- Grosser, J.W.; Gmitter, F.G. Protoplast Fusion for Production of Tetraploids and Triploids: Applications for Scion and Rootstock Breeding in Citrus. Plant Cell Tiss. Organ. Cult. 2011, 104, 343–357. [Google Scholar] [CrossRef]
- Abdullah, M.; Sliwinska, E.; Góralski, G.; Latocha, P.; Tuleja, M.; Widyna, P.; Popielarska-Konieczna, M. Effect of Medium Composition, Genotype and Age of Explant on the Regeneration of Hexaploid Plants from Endosperm Culture of Tetraploid Kiwiberry (Actinidia arguta). Plant Cell Tiss. Organ. Cult. 2021, 147, 569–582. [Google Scholar] [CrossRef]
- Marin-Montes, I.M.; Rodríguez-Pérez, J.E.; Robledo-Paz, A.; De La Cruz-Torres, E.; Peña-Lomelí, A.; Sahagún-Castellanos, J. Haploid Induction in Tomato (Solanum lycopersicum L.) via Gynogenesis. Plants 2022, 11, 1595. [Google Scholar] [CrossRef]
- Zou, T.; Min, Z.; Song, H.; Gong, S.; Tong, L.; Sun, L.; Yang, H.; Zhang, J.; Sun, X. Whole-Transcriptome Sequence Analysis of Pumpkin Ovules in Response to Heat Shock during Unfertilized Ovary Culture. Sci. Hortic. 2024, 329, 113007. [Google Scholar] [CrossRef]
- Wang, P.; Yang, S.; Chen, M.; Liu, Y.; He, Q.; Sun, H.; Wu, D.; Xiang, S.; Jing, D.; Wang, S.; et al. Karyotype Variation Patterns and Phenotypic Responses of Hybrid Progenies of Triploid Loquat (Eriobotrya japonica) Provide New Insight into Aneuploid Germplasm Innovation. Hortic. Res. 2025, 12, uhaf023. [Google Scholar] [CrossRef]
- Chi, Z.; Liu, X.; Wen, S.; Wang, Y.; Lv, W.; Guo, Q.; Xia, Y.; Jing, D.; Liang, G. Integrated Metabolomic Profiling and Transcriptome Analysis of Fruit Quality and Ripeness in Early-Maturing Seedless Triploid Loquat. Sci. Hortic. 2023, 316, 112012. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Suo, X.; Liu, M.; Jing, D.; Zhang, Y.; Dang, J.; Wu, D.; He, Q.; Xia, Y.; et al. EjFAD8 Enhances the Low-Temperature Tolerance of Loquat by Desaturation of Sulfoquinovosyl Diacylglycerol (SQDG). Int. J. Mol. Sci. 2023, 24, 6946. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Wang, L.; Guo, Q.; Liang, G. Extensive Genetic and DNA Methylation Variation Contribute to Heterosis in Triploid Loquat Hybrids. Genome 2018, 61, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Liu, Z.; Xia, Y.; Jing, D.; Guo, Q.; Liang, G.; He, Q. An Efficient System for Mesophyll Protoplast Isolation, Purification, and Transformation in Loquat: Studies on Fluorescent Marker Analysis and Subcellular Localization. Horticulturae 2025, 11, 391. [Google Scholar] [CrossRef]
- Wang, H.; Dang, J.; Wu, D.; Xie, Z.; Yan, S.; Luo, J.; Guo, Q.; Liang, G. Genotyping of Polyploid Plants Using Quantitative PCR: Application in the Breeding of White-Fleshed Triploid Loquats (Eriobotrya japonica). Plant Methods 2021, 17, 93. [Google Scholar] [CrossRef]
- Gamborg, O.; Miller, R.; Ojima, K. Nutrient Requirement Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Lei, C.; Xia, Q.; Wu, D.; He, Q.; Jing, D.; Guo, Q.; Liang, G.; Dang, J. A Female Fertile Triploid Loquat Line Produces Fruits with Less Seed and Aneuploid Germplasm. Sci. Hortic. 2023, 319, 112141. [Google Scholar] [CrossRef]
- Dang, J.; Wu, T.; Liang, G.; Wu, D.; He, Q.; Guo, Q. Identification and Characterization of a Loquat Aneuploid with Novel Leaf Phenotypes. Hortscience 2019, 54, 804–808. [Google Scholar] [CrossRef]
- Wen, G.; Dang, J.; Xie, Z.; Wang, J.; Jiang, P.; Guo, Q.; Liang, G. Molecular Karyotypes of Loquat (Eriobotrya japonica) Aneuploids Can Be Detected by Using SSR Markers Combined with Quantitative PCR Irrespective of Heterozygosity. Plant Methods 2020, 16, 22. [Google Scholar] [CrossRef]
- Ruiz, C.; Breto, M.P.; Asıns, M.J. A Quick Methodology to Identify Sexual Seedlings in Citrus Breeding Programs Using SSR Markers. Euphytica 2000, 112, 89–94. [Google Scholar] [CrossRef]
- Jing, D.; Liu, X.; He, Q.; Dang, J.; Hu, R.; Xia, Y.; Wu, D.; Wang, S.; Zhang, Y.; Xia, Q.; et al. Genome Assembly of Wild Loquat (Eriobotrya japonica) and Resequencing Provide New Insights into the Genomic Evolution and Fruit Domestication in Loquat. Hortic. Res. 2023, 10, uhac265. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; He, L.; Li, Y.; Huang, W.; Xi, F.; Lin, L.; Zhi, Q.; Zhang, W.; Yang, Y.T.; Geng, C.; et al. OTG-Snpcaller: An Optimized Pipeline Based on TMAP and GATK for SNP Calling from Ion Torrent Data. PLoS ONE 2014, 9, 97507. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Negoro, T.; Orihara, K.; Irahara, T.; Nishiyama, H.; Hagiwara, K.; Nishida, R.; Takagi, H.; Satoh, K.; Yamamoto, Y.; Shimizu, S.; et al. Influence of SNPs in Cytokine-related Genes on the Severity of Food Allergy and Atopic Eczema in Children. Pediatric Allergy Immunol. 2006, 17, 583–590. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Metin, D.; Atakul, Z.; Kurtar, E.S.; Seymen, M.; Alan, A.R.; Çelebi Toprak, F. Callogenesis, Embryogenesis, and Plantlet Initiation in Citron Watermelon (Citrullus lanatus Var. Citroides) via Anther and Unfertilized Ovary Culture. Sci. Hortic. 2024, 337, 113493. [Google Scholar] [CrossRef]
- Wijowska, M.; Kuta, E.; Przywara, L. In Vitro Culture of Unfertilized Ovules of Viola odorata L. Acta Biol. Cracov. Bot. 1999, 41, 95–101. [Google Scholar]
- Liu, X.; Li, D.; Zhang, Y.; Zhou, X.; Wang, S.; Zhao, J.; Guo, J.; Jiang, Q.; Ren, F. The Cytological Mechanism of the Peach Haploid Producing Triploid Offspring. Hortic. Res. 2025, 12, uhae316. [Google Scholar] [CrossRef]
- Zhan, N.; Li, L.; Zhang, L.; He, W.; Yang, Q.; Bi, F.; Deng, G.; Kiggundu, A.; Yi, G.; Sheng, O. Transcriptome and Metabolome Profiling Provide Insights into Hormone-Mediated Enhanced Growth in Autotetraploid Seedlings of Banana (Musa Spp.). Front. Sustain. Food Syst. 2023, 6, 1070108. [Google Scholar] [CrossRef]
- Ren, J.; Lu, X.; Duan, Y.-Y.; Xiao, G.-A.; Xie, K.-D.; Wu, X.-M.; Guo, W.-W. In Vivo Tetraploid Induction of Mono-Embryonic Citrus Genotypes by Colchicine Treatment. Sci. Hortic. 2024, 338, 113701. [Google Scholar] [CrossRef]
- Khalid, M.F.; Vincent, C.; Morillon, R.; Anjum, M.A.; Ahmad, S.; Hussain, S. Different Strategies Lead to a Common Outcome: Different Water-Deficit Scenarios Highlight Physiological and Biochemical Strategies of Water-Deficit Tolerance in Diploid versus Tetraploid Volkamer Lemon. Tree Physiol. 2021, 41, 2359–2374. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Zhang, Y.; Peng, J.; Liu, C.; Li, D.; Guo, W. Divergence in Cold Tolerance Promotes Niche Differentiation between Diploid and Polyploid Kiwifruits along an Altitudinal Gradient in Southwest China. Oikos 2024, 5, e10181. [Google Scholar] [CrossRef]
- Hui, T.; Bao, L.; Shi, X.; Zhang, H.; Xu, K.; Wei, X.; Liang, J.; Zhang, R.; Qian, W.; Zhang, M.; et al. Grafting Seedling Rootstock Strengthens Tolerance to Drought Stress in Polyploid Mulberry (Morus alba L.). Plant Physiol. Bioch. 2024, 208, 108441. [Google Scholar] [CrossRef]
- Rugini, E.; Silvestri, C.; Ceccarelli, M.; Muleo, R.; Cristofori, V. Mutagenesis and Biotechnology Techniques as Tools for Selecting New Stable Diploid and Tetraploid Olive Genotypes and Their Dwarfing Agronomical Characterization. Hortscience 2016, 51, 799–804. [Google Scholar] [CrossRef]
- Liu, R.; Gao, C.; Jin, J.; Wang, Y.; Jia, X.; Ma, H.; Zhang, Y.; Zhang, H.; Qi, B.; Xu, J. Induction and Identification of Tetraploids of Pear Plants (Pyrus bretschneideri and Pyrus betulaefolia). Sci. Hortic. 2022, 304, 111322. [Google Scholar] [CrossRef]
- Shi, X.; Wang, C.; Li, W.; Xiao, H.; Li, Y.; Niu, B. Analysis of Seed Phenotypic and Metabolic Characteristics of Diploid and Tetraploid Tartary Buckwheat. Phyton-Int. J. Exp. Bot. 2022, 91, 1973–1986. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.R.; Zhang, X.J.; Duan, S.D.; Su, X.; Fan, Z.H.; Hao, L.H.; Xiang, D.Y.; Chen, D.F.; Niu, S.C. Integrated Metabolomic and Transcriptomic Analysis Reveals the Mechanism of High Polysaccharide Content in Tetraploid Dendrobium Catenatum Lindl. Ind. Crops Prod. 2024, 212, 118391. [Google Scholar] [CrossRef]
- Tan, F.Q.; Zhang, M.; Xie, K.D.; Fan, Y.J.; Song, X.; Wang, R.; Wu, X.M.; Zhang, H.Y.; Guo, W.W. Polyploidy Remodels Fruit Metabolism by Modifying Carbon Source Utilization and Metabolic Flux in Ponkan Mandarin (Citrus reticulata Blanco). Plant Sci. 2019, 289, 110276. [Google Scholar] [CrossRef]






| Varieties | No. of Inoculated Unfertilized Ovules | No. of Gynogenesis Line | Gynogenesis Rate (GR) (%) | No. of Derived Embryoid Line | Embryoid Induction Rate (EIR) (%) |
|---|---|---|---|---|---|
| ‘Senwei Zaosheng’ | 560 | 35 | 11.05 ± 4.30 b | 0 | 0 |
| ‘Huabai 1’ | 680 | 98 | 13.62 ± 2.61 b | 12 | 12.81 ± 3.13 b |
| ‘Xingning 1’ | 880 | 158 | 21.63 ± 3.35 a | 76 | 54.37 ± 6.84 a |
| ‘Zaozhong 6’ | 160 | 37 | 20.63 ± 2.26 a | 0 | 0 |
| ‘Daduhe’ | 180 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ruan, T.; Zhang, Y.; Wang, P.; Dang, J.; Xia, Y.; Jing, D.; Liang, G.; Guo, Q.; He, Q. Characterization of Polyploid Embryoid Lines Induced via Unfertilized Ovule Culture of Loquat (Eriobotrya japonica Lindl.). Horticulturae 2025, 11, 727. https://doi.org/10.3390/horticulturae11070727
Wang S, Ruan T, Zhang Y, Wang P, Dang J, Xia Y, Jing D, Liang G, Guo Q, He Q. Characterization of Polyploid Embryoid Lines Induced via Unfertilized Ovule Culture of Loquat (Eriobotrya japonica Lindl.). Horticulturae. 2025; 11(7):727. https://doi.org/10.3390/horticulturae11070727
Chicago/Turabian StyleWang, Shuming, Tingting Ruan, Yin Zhang, Peng Wang, Jiangbo Dang, Yan Xia, Danlong Jing, Guolu Liang, Qigao Guo, and Qiao He. 2025. "Characterization of Polyploid Embryoid Lines Induced via Unfertilized Ovule Culture of Loquat (Eriobotrya japonica Lindl.)" Horticulturae 11, no. 7: 727. https://doi.org/10.3390/horticulturae11070727
APA StyleWang, S., Ruan, T., Zhang, Y., Wang, P., Dang, J., Xia, Y., Jing, D., Liang, G., Guo, Q., & He, Q. (2025). Characterization of Polyploid Embryoid Lines Induced via Unfertilized Ovule Culture of Loquat (Eriobotrya japonica Lindl.). Horticulturae, 11(7), 727. https://doi.org/10.3390/horticulturae11070727






