The Efficiency of Artificial Pollination on the Hazelnut ‘Tonda Francescana®’ Cultivar and the Xenia Effects of Different Pollinizers
Abstract
1. Introduction
2. Materials and Methods
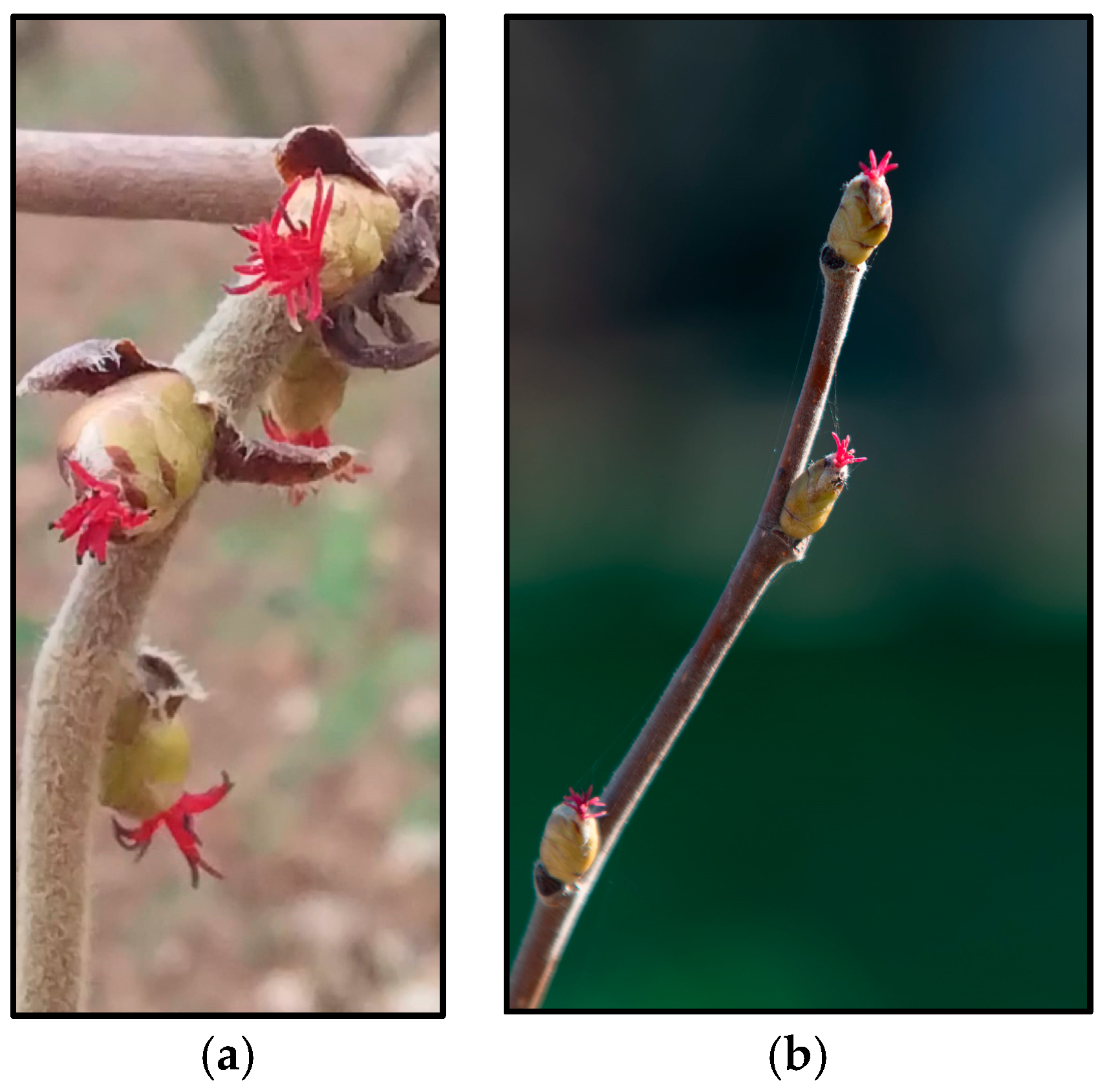
2.1. Plant Material
2.2. Pollen Collection
2.3. Experimental Design and Pollen Application
2.4. Field Recording Data
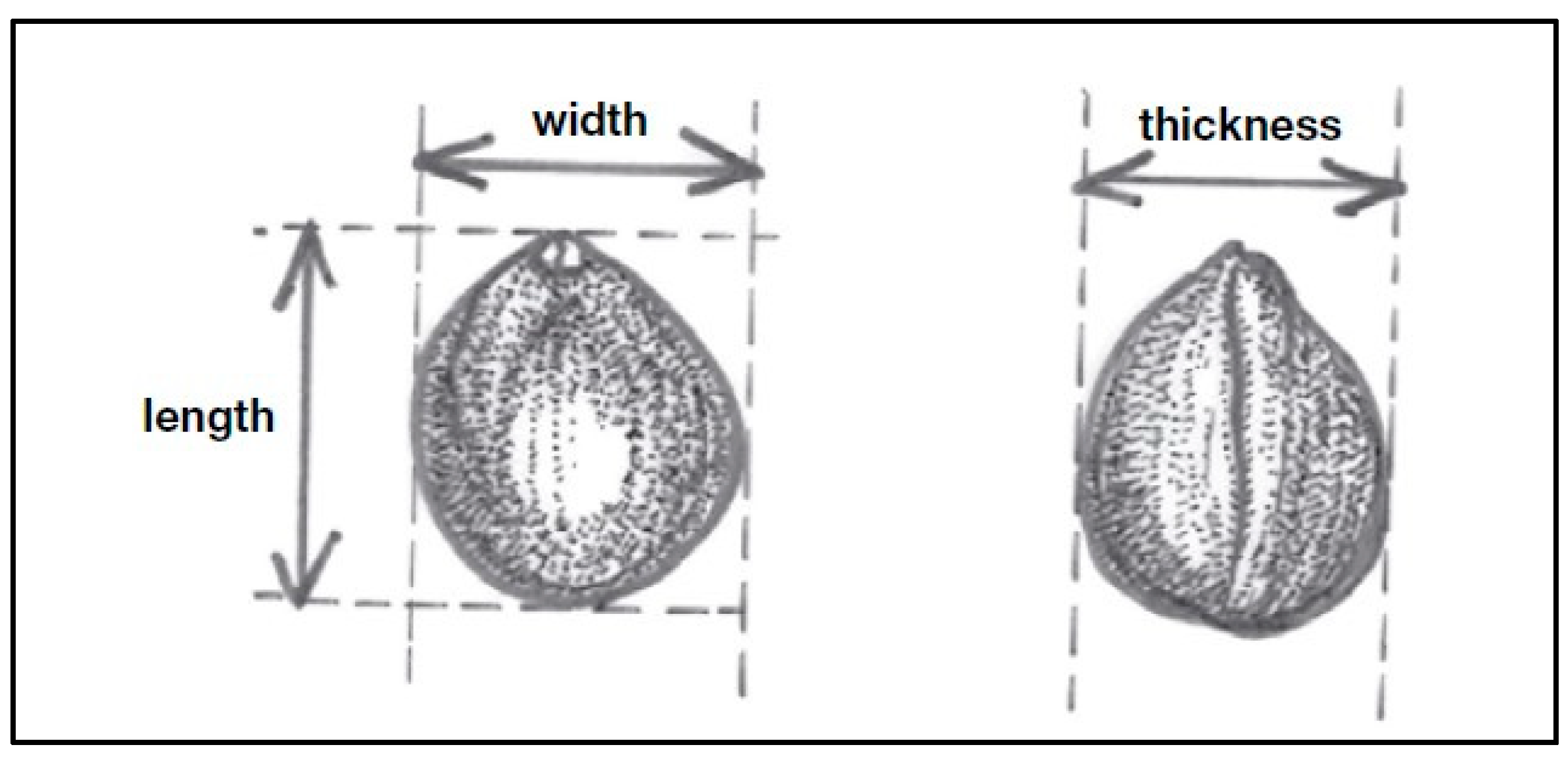
2.5. Nut and Kernel Measurements
2.6. Statistical Analysis
3. Results
3.1. Effect of Artificial Pollination on Fruit Set
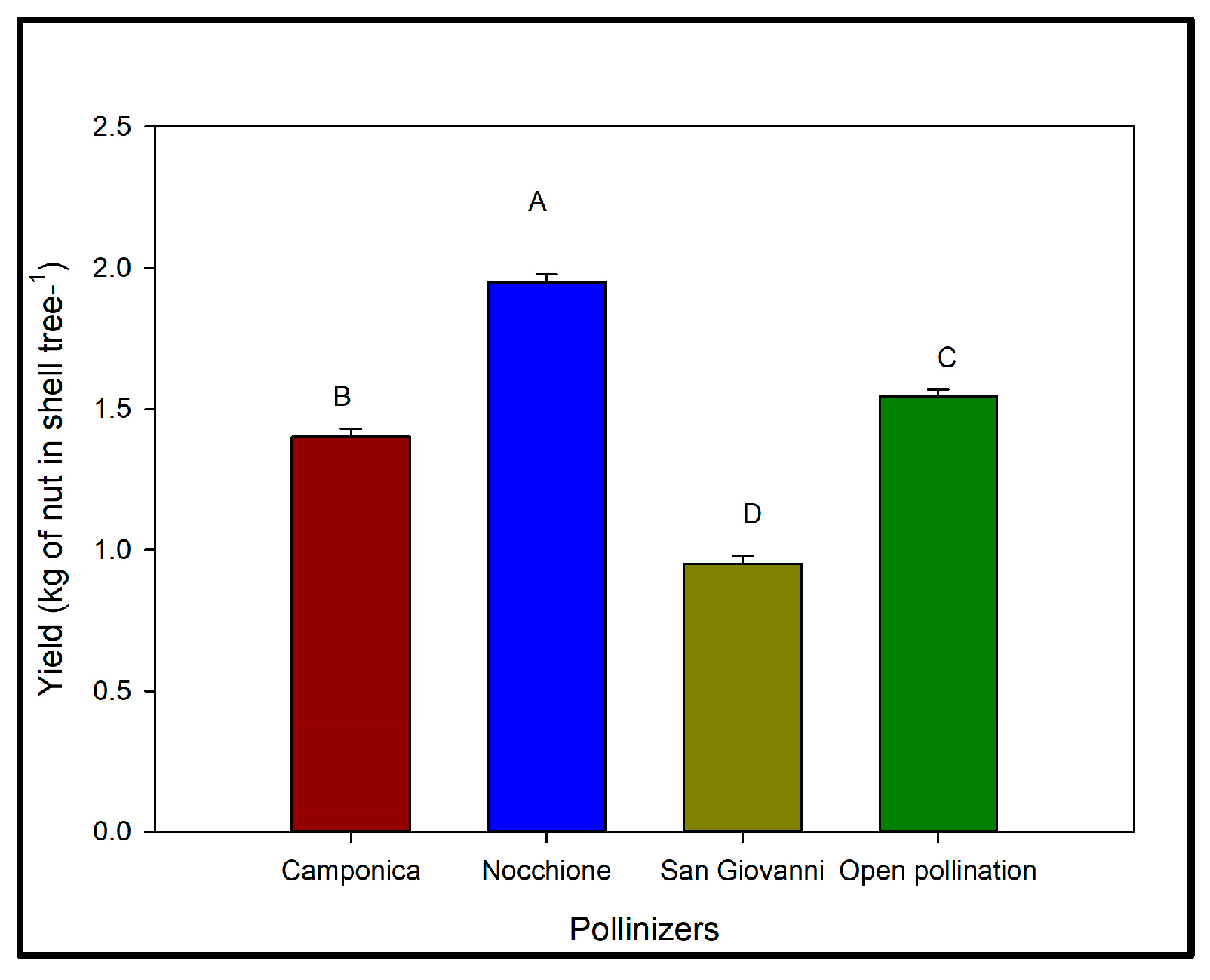
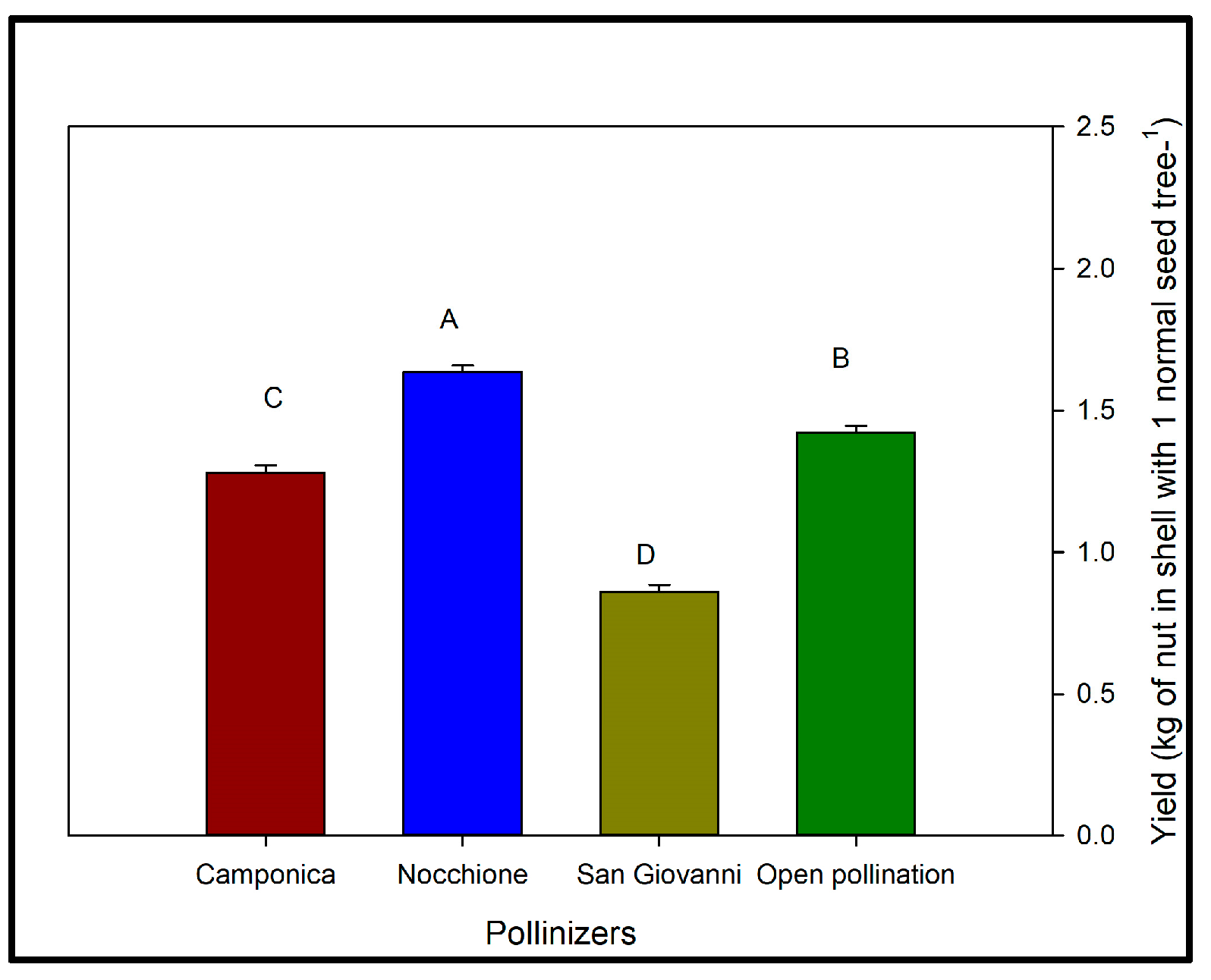
3.2. Effects of Artificial Pollination on Yield
3.3. Paternal Effects on the Number of Normal Seeds per Nut
3.3.1. Effects of Artificial Pollination on Nut Characteristics (Xenia)
3.3.2. Effects of Artificial Pollination on Fruit and Kernel Shape (Xenia)
3.3.3. Effects of Artificial Pollination on Kernel Characteristics (Xenia)
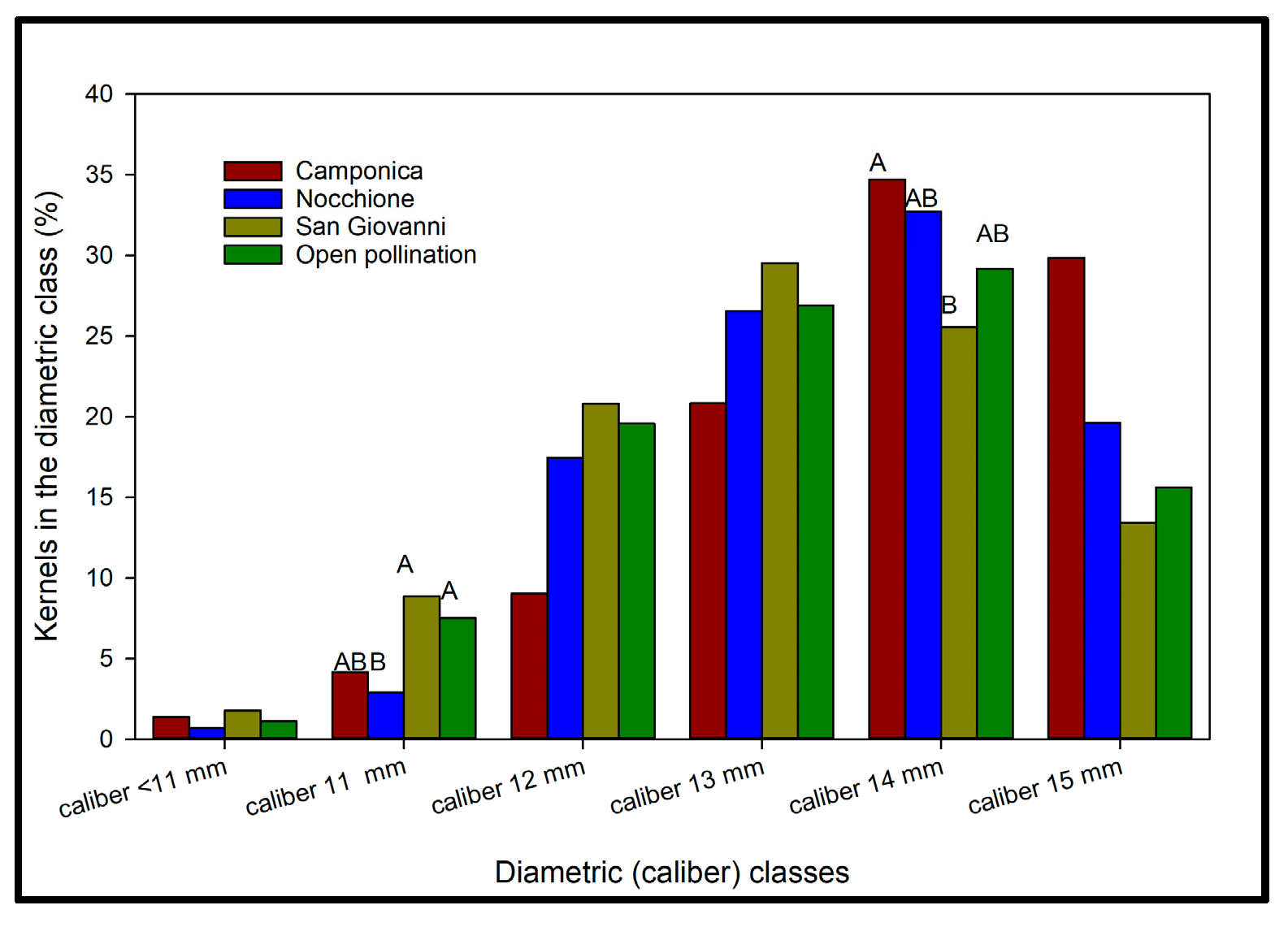
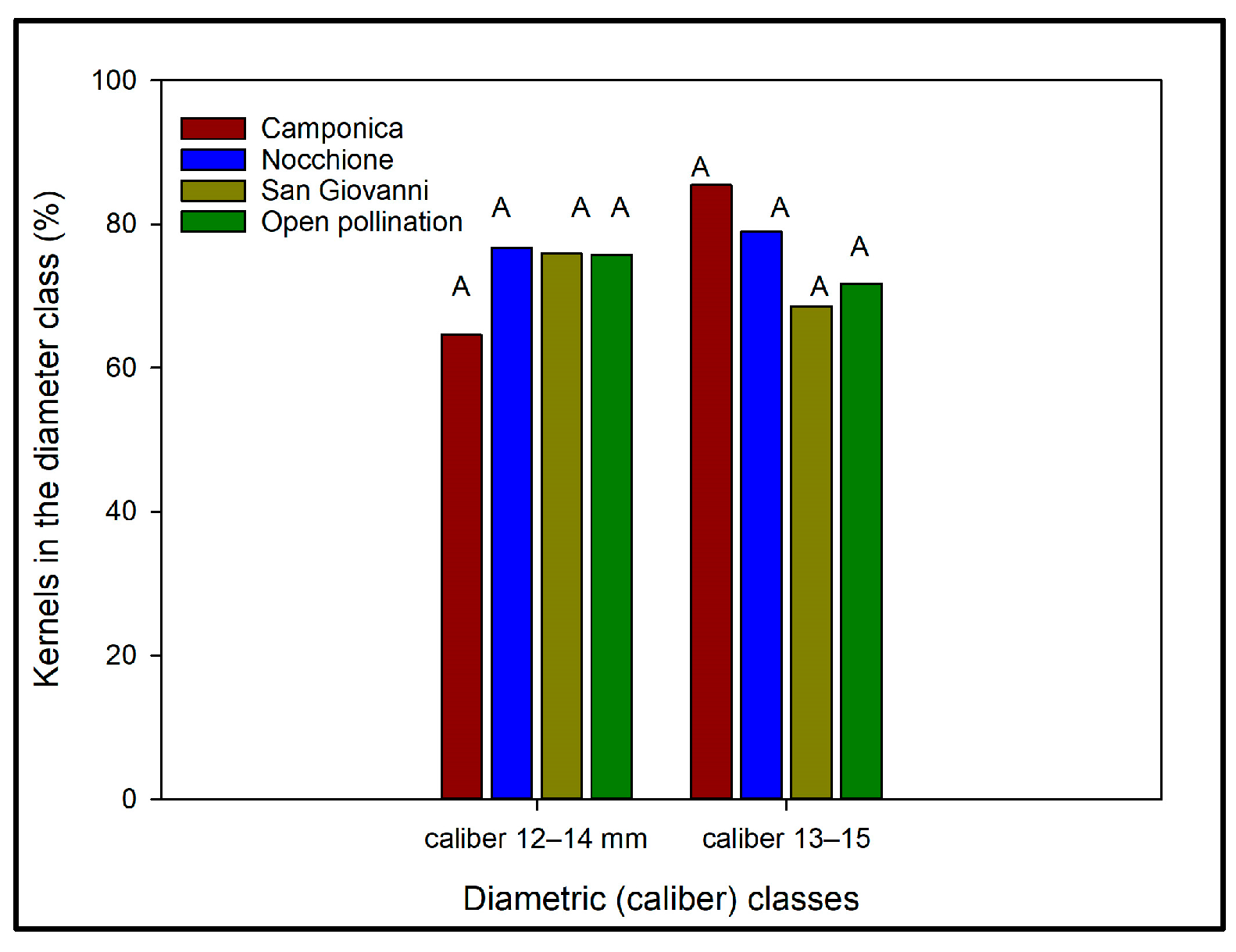
3.3.4. Effects of Artificial Pollination on Kernel Caliber Distribution and Homogeneity
3.4. Pollinizer Effects on Nut Parameters
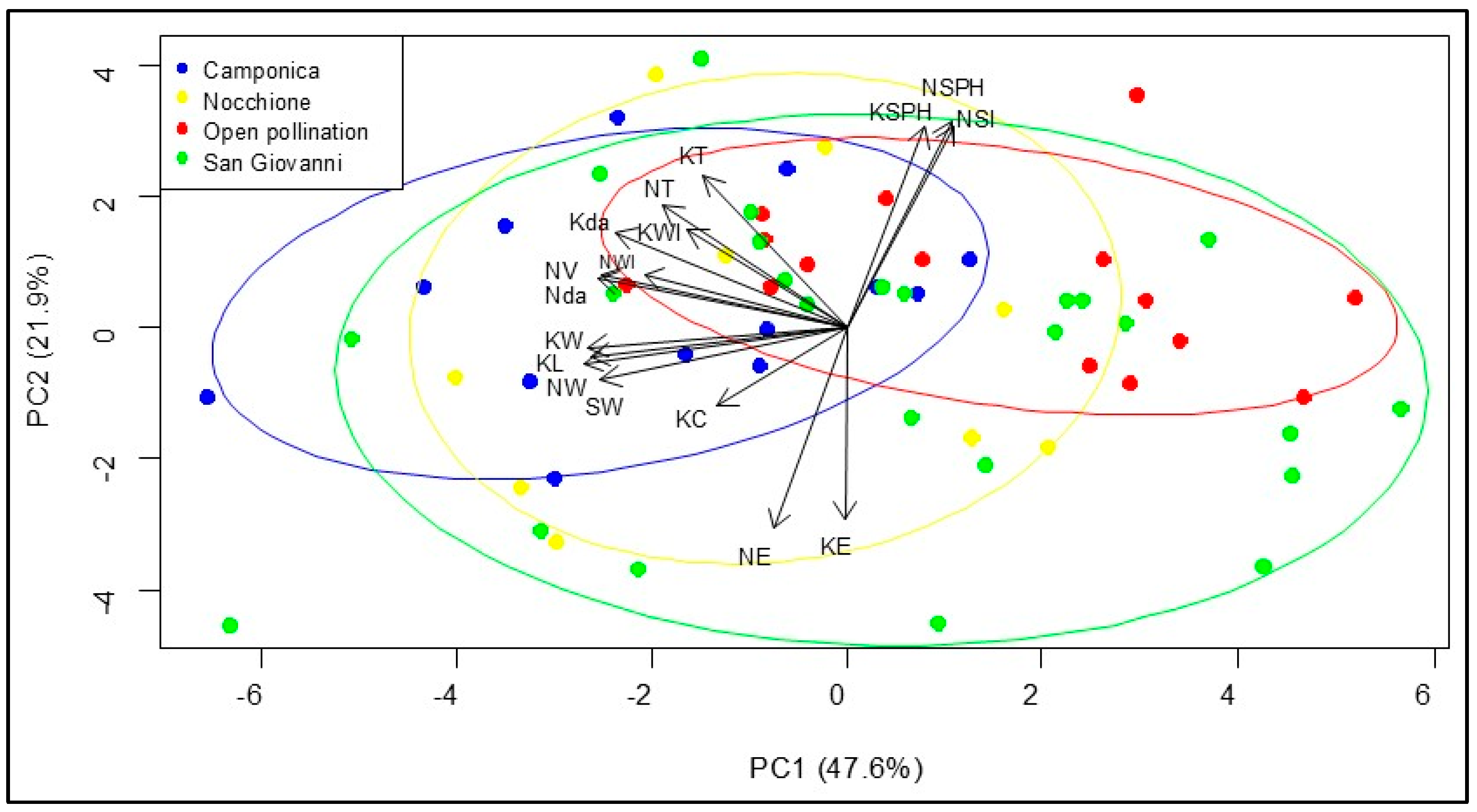
3.5. Relationship Between Nuts and Kernel Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eyles, A.; Close, D.C.; Quarrell, S.R.; Allen, G.R.; Spurr, C.J.; Barry, K.M.; Whiting, M.D.; Gracie, A.J. Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review. Agronomy 2022, 12, 1113. [Google Scholar] [CrossRef]
- Kumar, A.; Rajwar, N.; Tonk, T. Climate Change Effects on Plant-Pollinator Interactions, Reproductive Biology and Ecosystem Services. In Forests and Climate Change: Biological Perspectives on Impact, Adaptation, and Mitigation Strategies; Singh, H., Ed.; Springer Nature: Singapore, 2024; pp. 97–117. ISBN 978-981-9739-05-9. [Google Scholar]
- Portarena, S.; Proietti, S.; Moscatello, S.; Zadra, C.; Cinosi, N.; Traini, C.; Farinelli, D. Effect of Tree Density on Yield and Fruit Quality of the Grafted Hazelnut Cultivar ‘Tonda Francescana®’. Foods 2024, 13, 3307. [Google Scholar] [CrossRef] [PubMed]
- Ascari, L.; Siniscalco, C.; Palestini, G.; Lisperguer, M.J.; Suarez Huerta, E.; De Gregorio, T.; Bregaglio, S. Relationships between Yield and Pollen Concentrations in Chilean Hazelnut Orchards. Eur. J. Agron. 2020, 115, 126036. [Google Scholar] [CrossRef]
- Ahmadov, A. The Impact of Climate Change on Hazelnut Cultivation. Turk. J. Food Agric. Sci. 2024, 6, 106–115. [Google Scholar] [CrossRef]
- Balık, H.İ.; Beyhan, N. Xenia and Metaxenia in Hazelnuts: Effects on Nut Set and Nut Characteristics. Akad. Ziraat Derg. 2019, 8, 9–18. [Google Scholar] [CrossRef]
- Broussard, M.A.; Coates, M.; Martinsen, P. Artificial Pollination Technologies: A Review. Agronomy 2023, 13, 1351. [Google Scholar] [CrossRef]
- Kämper, W.; Wallace, H.M.; Ogbourne, S.M.; Trueman, S.J. Dependence on Cross-Pollination in Macadamia and Challenges for Orchard Management. Proceedings 2020, 36, 76. [Google Scholar] [CrossRef]
- Germain, E. The Reproduction of Hazelnut (Corylus avellana L.): A Review. Acta Hortic. 1994, 351, 195–210. [Google Scholar] [CrossRef]
- Ascari, L.; Cristofori, V.; Macrì, F.; Botta, R.; Silvestri, C.; De Gregorio, T.; Huerta, E.S.; Di Berardino, M.; Kaufmann, S.; Siniscalco, C. Hazelnut Pollen Phenotyping Using Label-Free Impedance Flow Cytometry. Front. Plant Sci. 2020, 11, 615922. [Google Scholar] [CrossRef]
- Di Lena, B.; Curci, G.; Vergni, L.; Farinelli, D. Climatic Suitability of Different Areas in Abruzzo, Central Italy, for the Cultivation of Hazelnut. Horticulturae 2022, 8, 580. [Google Scholar] [CrossRef]
- Farinelli, D.; Bernacchia, C.; Brugnoli, E.; Portarena, S.; Zadra, C. Influence of Pollinizers on Fruit Quality Characteristics in Hazelnut Cultivar ‘Tonda Francescana®’. Acta Hortic. 2023, 1379, 199–206. [Google Scholar] [CrossRef]
- Beyhan, N.; Marangoz, D. An Investigation of the Relationship Between Reproductive Growth and Yield Loss in Hazelnut. Sci. Hortic. 2007, 113, 208–215. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of Different Pollen Sources on Nut and Kernel Characteristics of Hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Kämper, W.; Thorp, G.; Wirthensohn, M.; Brooks, P.; Trueman, S.J. Pollen Paternity Can Affect Kernel Size and Nutritional Composition of Self-Incompatible and New Self-Compatible Almond Cultivars. Agronomy 2021, 11, 326. [Google Scholar] [CrossRef]
- Mascarello, F.B.; de Araújo Neto, S.E.; Silva, N.M.; Machado, L.; Rocha, C.; Uchôa, T.L. Polinização Artificial De Diferentes Números De Estigmas Na Frutificação Do Maracujazeiro Amarelo Em Cultivo Orgânico. Rev. Bras. Ciênc. Amaz. Braz. J. Sci. 2019, 8, 8–14. [Google Scholar] [CrossRef]
- Mokwala, P.W.; Mangena, P. Pollination in Plants; BoD—Books on, Demand; Mokwala, P.W., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-236-3. [Google Scholar]
- Ascari, L.; Guastella, D.; Sigwebela, M.; Engelbrecht, G.; Stubbs, O.; Hills, D.; De Gregorio, T.; Siniscalco, C. Artificial Pollination on Hazelnut in South Africa: Preliminary Data and Perspectives. Acta Hortic. 2018, 1226, 141–148. [Google Scholar] [CrossRef]
- Ellena, M.; Sandoval, P.; Gonzalez, A.; Galdames, R.; Jequier, J.; Contreras, M.; Azocar, G. Preliminary Results of Supplementary Pollination on Hazelnut in South Chile. Acta Hortic. 2014, 1052, 121–127. [Google Scholar] [CrossRef]
- Vinci, A.; Traini, C.; Portarena, S.; Farinelli, D. Assessment of the Midseason Crop Coefficient for the Evaluation of the Water Demand of Young, Grafted Hazelnut Trees in High-Density Orchards. Water 2023, 15, 1683. [Google Scholar] [CrossRef]
- Mazinani, M.; Zarafshan, P.; Dehghani, M.; Vahdati, K.; Etezadi, H. Design and Analysis of an Aerial Pollination System for Walnut Trees. Biosyst. Eng. 2023, 225, 83–98. [Google Scholar] [CrossRef]
- Balık, H.İ.; Demir, T.; Beyhan, Ö. Determination of Pollinator Characteristics of Some Hazelnut Genotypes. Black Sea J. Agric. 2023, 6, 262–268. [Google Scholar] [CrossRef]
- Marco, R.D.; Herter, F.G.; Goldschmidt, R.J.Z.; Martins, C.R.; Crosa, C. Efeitos de fontes de pólen na qualidade de castanhas produzidas por cultivares de noz-pecã Kiowa e Barton. Comun. Sci. 2023, 14, e3696. [Google Scholar] [CrossRef]
- Trueman, S.J.; Nichols, J.; Burwell, C.J.; Kämper, W. Strategic Selection of Polliniser Trees Can Improve Fruit Quality of Lychee, a Crop That Exhibits Mixed-Mating. Basic Appl. Ecol. 2025, 83, 80–87. [Google Scholar] [CrossRef]
- Balık, H.İ.; Beyhan, N. Xenia and Metaxenia Affects Bioactive Compounds of Hazelnut. Turk. J. Food Agric. Sci. 2020, 2, 42–49. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. Revised Dominance Hierarchy for S-Alleles in Corylus avellana L. Theor. Appl. Genet. 1997, 94, 360–366. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. Geographic Distribution of Incompatibility Alleles in Cultivars and Selections of European Hazelnut. J. Am. Soc. Hortic. Sci. 2014, 139, 191–212. [Google Scholar] [CrossRef]
- Gasic, K.; Preece, J.E.; Karp, D. Register of New Fruit and Nut Cultivars List 49. HortScience 2018, 53, 748–776. [Google Scholar] [CrossRef]
- Heard, B. The Phenology and Compatibility of Hazelnut (Corylus avellana) Cultivars in Tennessee. Master’s Thesis, University of Tennessee, Chattanooga, TN, USA, 2016. [Google Scholar]
- Novara, C.; Ascari, L.; La Morgia, V.; Reale, L.; Genre, A.; Siniscalco, C. Viability and Germinability in Long Term Storage of Corylus avellana Pollen. Sci. Hortic. 2017, 214, 295–303. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Seifi, E.; Javadi, D.; Ramezanpour, S.S. A Preliminary Study on Pollen Compatibility of Some Hazelnut Cultivars in Iran. Adv. Hortic. Sci. 2015, 29, 13–16. [Google Scholar] [CrossRef]
- Paradinas, A.; Ramade, L.; Mulot-Greffeuille, C.; Hamidi, R.; Thomas, M.; Toillon, J. Phenological Growth Stages of ‘Barcelona’ Hazelnut (Corylus avellana L.) Described Using an Extended BBCH Scale. Sci. Hortic. 2022, 296, 110902. [Google Scholar] [CrossRef]
- Guastella, D.; Sigwebela, M.; Suarez, E.; Stubbs, O.; Acevedo, J.; Engelbrecht, G. Effect of Photo-Selective Shade Nets on Pollination Process and Nut Development of Corylus avellana L. Front. Plant Sci. 2020, 11, 602766. [Google Scholar] [CrossRef]
- Farinelli, D.; Boco, M.; Tombesi, A. Productive and Organoleptic Evaluation of New Hazelnut Crosses. Acta Hortic. 2009, 845, 651–656. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Determination of Size And Shape Features of Hazelnuts Using Multivariate Analysis. Acta Sci. Pol. Hortorum Cultus 2017, 16, 49–61. [Google Scholar] [CrossRef]
- Bioversity International; FAO; CIHEAM. Descriptors for Hazelnut (Corylus avellana L.); Bioversity International: Rome, Italy; Food and Agriculture Organization of the United Nations: Rome, Italy; International Centre for Advanced Mediterranean Agronomic Studies: Zaragoza, Spain, 2008; Available online: https://qrgj.org/wp-content/uploads/2022/01/Descriptors-for-hazelnut-Corylus-avellana-L (accessed on 15 May 2025).
- Farinelli, D.; Pierantozzi, P.; Palese, A.M. Pollenizer and Cultivar Influence Seed Number and Fruit Characteristics in Olea europaea L. HortScience 2012, 47, 1430–1437. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 2 May 2025).
- Farinelli, D.; Portarena, S.; da Silva, D.F.; Traini, C.; da Silva, G.M.; da Silva, E.C.; da Veiga, J.F.; Pollegioni, P.; Villa, F. Variability of Fruit Quality among 103 Acerola (Malpighia emarginata D. C.) Phenotypes from the Subtropical Region of Brazil. Agriculture 2021, 11, 1078. [Google Scholar] [CrossRef]
- Correia, P.; Rodrigues, C.; Filipe, A.; Guiné, R. Evaluation of Biometric Characteristics of Hazelnuts. Food Biosyst. Eng. Conf. 2019, 11, 1476. [Google Scholar] [CrossRef]
- Tacconi, G.; Michelotti, V. Artificial Pollination in Kiwifruit and Olive Trees. In Pollination in Plants; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, Y.; Liu, Y.; Zhang, T.; Peng, S.; Deng, J. Novel Classification Forms for Xenia. HortScience 2020, 55, 980–987. [Google Scholar] [CrossRef]
- Rahemi, M.; Mojadad, D. Effect of Pollen Source on Nut and Kernel Characteristics of Hazelnut. Acta Hortic. 2001, 556, 371–376. [Google Scholar] [CrossRef]
- Romero, A.; Tous, J.; Plana, J.; Díaz, I.; Boatella, J.; García, J.; López, A. Commercial Quality Characterization of Spanish “Negret” Cultivar. Acta Hortic. 1997, 445, 157–166. [Google Scholar] [CrossRef]
- Yao, Q.; Mehlenbacher, S.A. Heritability, Variance Components and Correlation of Morphological and Phenological Traits in Hazelnut. Plant Breed. 2000, 119, 369–381. [Google Scholar] [CrossRef]




| Technical Characteristics | Technical Specifications |
|---|---|
| Pollen delivers | 100–800 g/h |
| Support backpack weight | 5.5 kg |
| Application speed | 7–8 km/h |
| Air flow maximum speed | 600 m3/h |
| Pollinizers | Fruit Set (n. Fruit/Female Flower Glomeruli) |
|---|---|
| Camponica | 2.28 ± 0.26 b |
| Nocchione | 2.94 ± 0.16 a |
| San Giovanni | 2.25 ± 0.10 b |
| Open pollination (Control) | 2.39 ± 0.13 b |
| Seed Pattern | Pollination Treatments | Camponica | Nocchione | San Giovanni | Open Pollination | Percent/ Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. of Seed | Percent/ Total | N. of Seed | Percent/ Total | N. of Seed | Percent/ Total | N. of Seed | Percent/ Total | |||
| Undeveloped (blank) seed | Observed | 9 | 7.76 | 45 | 15.46 | 66 | 9.23 | 39 | 6.58 | 9.27 |
| Expected | 10.75 | 26.98 | 66.29 | 54.98 | ||||||
| Cell χ2 | 0.29 | 12.04 | 0.00 | 4.64 | ||||||
| One normal seed | Observed | 106 | 91.38 | 244 | 83.85 | 646 | 90.35 | 546 | 92.07 | 89.91 |
| Expected | 104.3 | 261.65 | 642.87 | 533.18 | ||||||
| Cell χ2 | 0.03 | 1.19 | 0.02 | 0.31 | ||||||
| Double seeds | Observed | 1 | 0.89 | 2 | 0.69 | 3 | 0.42 | 8 | 1.35 | 0.82 |
| Expected | 0.95 | 2.38 | 5.84 | 4.84 | ||||||
| Cell χ2 | 0.00 | 0.06 | 1.38 | 2.06 | ||||||
| Pollinizers | Nut Weight (NW) (g) | Kernel Weight (KW) (g) | Shell Weight (SW) (g) | Percentage Kernel (PK) |
|---|---|---|---|---|
| Camponica | 2.81 ± 0.09 a | 1.30 ± 0.04 ab | 1.49 ± 0.05 a | 46.64 ± 0.50 a |
| Nocchione | 2.79 ± 0.07 a | 1.32 ± 0.03 a | 1.49 ± 0.04 a | 47.07 ± 0.35 a |
| San Giovanni | 2.65 ± 0.04 ab | 1.23 ± 0.02 bc | 1.41 ± 0.02 ab | 46.57 ± 0.21 a |
| Open pollination (Control) | 2.53 ± 0.05 b | 1.18 ± 0.02 c | 1.34 ± 0.03 b | 46.71 ± 0.28 a |
| Pollinizers | Nut Width (NWI) (mm) | Nut Thickness (NT) (mm) | Nut Length (NL) (mm) | Nut Shape Index (MSI) |
|---|---|---|---|---|
| Camponica | 19.02 ± 0.17 a | 16.98 ± 0.18 a | 19.37 ± 0.20 a | 0.93 ± 0.010 a |
| Nocchione | 18.87 ± 0.12 ab | 16.42 ± 0.13 b | 19.49 ± 0.14 a | 0.91 ± 0.004 b |
| San Giovanni | 18.58 ± 0.10 b | 16.28 ± 0.08 b | 19.36 ± 0.09 a | 0.90 ± 0.002 b |
| Open pollination (Control) | 18.58 ± 0.07 b | 16.22 ± 0.10 b | 19.15 ± 0.11 a | 0.91 ± 0.003 b |
| Pollinizers | Kernel Width (KWI) (mm) | Kernel Thickness (KT) (mm) | Kernel Length (KL) (mm) | Kernel Elongation (KE) |
|---|---|---|---|---|
| Camponica | 14.26 ± 0.19 a | 12.45 ± 0.27 a | 15.17 ± 0.25 ab | 1.23 ± 0.03 b |
| Nocchione | 13.99 ± 0.14 ab | 11.81 ± 0.19 b | 15.48 ± 0.18 a | 1.33 ± 0.01 a |
| San Giovanni | 13.68 ± 0.08 b | 11.41 ± 0.12 b | 15.01 ± 0.11 ab | 1.33 ± 0.02 a |
| Open pollination (Control) | 13.77 ± 0.11 b | 11.76 ± 0.15 b | 14.76 ± 0.14 b | 1.28 ± 0.02 ab |
| Variable | NW | SW | KW | PK | NWI | NT | NL | NSI | KC | NV | NE | NDa | NDg | NSPH | NRa | NS | KWI | KT | KL | KE | KDa | KDg | KSPH | KRa | KS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NW | 1 | ||||||||||||||||||||||||
| SW | 0.97 | 1 | |||||||||||||||||||||||
| KW | 0.96 | 0.88 | 1 | ||||||||||||||||||||||
| PK | −0.23 | −0.41 | 0.04 | 1 | |||||||||||||||||||||
| NWI | 0.71 | 0.71 | 0.66 | −0.25 | 1 | ||||||||||||||||||||
| NT | 0.67 | 0.65 | 0.65 | −0.19 | 0.52 | 1 | |||||||||||||||||||
| NL | 0.86 | 0.87 | 0.78 | −0.37 | 0.64 | 0.73 | 1 | ||||||||||||||||||
| NSI | −0.28 | −0.32 | −0.21 | 0.25 | 0.19 | 0.05 | −0.50 | 1 | |||||||||||||||||
| KC | 0.51 | 0.39 | 0.65 | 0.45 | 0.07 | −0.03 | 0.14 | −0.20 | 1 | ||||||||||||||||
| NV | 0.86 | 0.85 | 0.80 | −0.30 | 0.81 | 0.88 | 0.91 | −0.11 | 0.07 | 1 | |||||||||||||||
| NE | 0.07 | 0.10 | 0.03 | −0.16 | −0.17 | −0.09 | 0.36 | −0.76 | −0.03 | 0.04 | 1 | ||||||||||||||
| NDa | 0.86 | 0.86 | 0.80 | −0.31 | 0.82 | 0.87 | 0.91 | −0.11 | 0.08 | 1.00 | 0.04 | 1 | |||||||||||||
| NDg | 0.86 | 0.85 | 0.80 | −0.31 | 0.81 | 0.88 | 0.91 | −0.10 | 0.07 | 1.00 | 0.04 | 1.00 | 1 | ||||||||||||
| NSPH | −0.26 | −0.28 | −0.20 | 0.18 | 0.18 | 0.12 | −0.45 | 0.97 | −0.24 | −0.06 | −0.74 | −0.07 | −0.06 | 1 | |||||||||||
| NRa | −0.20 | −0.21 | −0.19 | 0.04 | −0.08 | 0.00 | −0.01 | −0.01 | −0.28 | −0.05 | 0.64 | −0.05 | −0.04 | 0.02 | 1 | ||||||||||
| NS | 0.86 | 0.85 | 0.80 | −0.30 | 0.81 | 0.88 | 0.91 | −0.11 | 0.07 | 1.00 | 0.03 | 1.00 | 1.00 | −0.06 | −0.05 | 1 | |||||||||
| KWI | 0.37 | 0.36 | 0.37 | −0.07 | 0.76 | 0.30 | 0.18 | 0.54 | 0.03 | 0.46 | −0.64 | 0.48 | 0.47 | 0.51 | −0.38 | 0.46 | 1 | ||||||||
| KT | 0.34 | 0.25 | 0.41 | 0.21 | 0.19 | 0.63 | 0.30 | 0.16 | 0.14 | 0.44 | −0.18 | 0.43 | 0.45 | 0.18 | −0.01 | 0.45 | 0.26 | 1 | |||||||
| KL | 0.82 | 0.76 | 0.85 | −0.01 | 0.49 | 0.67 | 0.86 | −0.43 | 0.41 | 0.78 | 0.39 | 0.78 | 0.78 | −0.42 | 0.12 | 0.78 | 0.05 | 0.41 | 1 | ||||||
| KE | 0.17 | 0.22 | 0.11 | −0.25 | 0.15 | −0.18 | 0.26 | −0.44 | 0.04 | 0.08 | 0.44 | 0.09 | 0.08 | −0.46 | 0.10 | 0.08 | −0.20 | −0.76 | 0.22 | 1 | |||||
| KDa | 0.72 | 0.64 | 0.77 | 0.08 | 0.62 | 0.78 | 0.65 | 0.07 | 0.29 | 0.79 | −0.14 | 0.79 | 0.79 | 0.08 | −0.09 | 0.79 | 0.54 | 0.83 | 0.73 | −0.38 | 1 | ||||
| KDg | 0.67 | 0.59 | 0.73 | 0.10 | 0.57 | 0.77 | 0.61 | 0.09 | 0.27 | 0.75 | −0.15 | 0.75 | 0.75 | 0.10 | −0.08 | 0.75 | 0.51 | 0.88 | 0.69 | −0.45 | 1.00 | 1 | |||
| KSPH | −0.37 | −0.37 | −0.34 | 0.13 | −0.02 | −0.10 | −0.51 | 0.71 | −0.23 | −0.25 | −0.72 | −0.25 | −0.24 | 0.70 | −0.26 | −0.24 | 0.51 | 0.36 | −0.62 | −0.76 | 0.09 | 0.13 | 1 | ||
| KRa | −0.39 | −0.35 | −0.41 | −0.04 | 0.12 | −0.32 | −0.55 | 0.70 | −0.29 | −0.30 | −0.74 | −0.29 | −0.30 | 0.68 | −0.35 | −0.30 | 0.62 | −0.17 | −0.75 | −0.29 | −0.22 | −0.22 | 0.82 | 1 | |
| KS | 0.66 | 0.58 | 0.72 | 0.10 | 0.57 | 0.77 | 0.60 | 0.10 | 0.25 | 0.75 | −0.15 | 0.74 | 0.75 | 0.11 | −0.07 | 0.75 | 0.51 | 0.88 | 0.68 | −0.45 | 1.00 | 1.00 | 0.14 | −0.21 | 1 |
| Pollinizers | Nut Volume (NV) (cm3) | Nut Surface Area (NS) (cm2) | Nut Aspect Ratio (NRa) | Kernel Content (KC) |
|---|---|---|---|---|
| Camponica | 3.29 ± 0.08 a | 10.67 ± 0.18 a | 95.82 ± 1.1 a | 0.40 ± 0.010 b |
| Nocchione | 3.19 ± 0.06 ab | 10.45 ± 0.13 ab | 95.19 ± 0.8 a | 0.42 ± 0.010 a |
| San Giovanni | 3.01 ± 0.03 b | 10.23 ± 0.08 b | 95.41 ± 0.5 a | 0.40 ± 0.004 ab |
| Open pollination (Control) | 3.05 ± 0.04 b | 10.12 ± 0.10 b | 96.16 ± 0.6 a | 0.39 ± 0.005 b |
| Pollinizers | Nut Elongation (NE) | Nut Arithmetic Mean Diameter (NDa) (mm) | Nut Geometric Mean Diameter (NDg) (mm) | Nut Sphericity (NSPH) |
|---|---|---|---|---|
| Camponica | 1.11 ± 0.02 b | 18.46 ± 0.16 a | 18.41 ± 0.16 a | 0.95 ± 0.04 a |
| Nocchione | 1.17 ± 0.01 a | 18.28 ± 0.11 ab | 18.21 ± 0.11 ab | 0.93 ± 0.03 b |
| San Giovanni | 1.18 ± 0.01 a | 18.07 ± 0.07 b | 18.01 ± 0.07 b | 0.93 ± 0.03 b |
| Open pollination (Control) | 1.17 ± 0.01 a | 17.98 ± 0.09 b | 17.92 ± 0.09 b | 0.94 ± 0.02 b |
| Pollinizers | Kernel Arithmetic Mean Diameter (KDa) (mm) | Kernel Geometric Mean Diameter (KDg) (mm) | Kernel Aspect Ratio (KRa) | Kernel Sphericity (KSPH) | Kernel Surface Area (KS) (cm2) |
|---|---|---|---|---|---|
| Camponica | 13.96 ± 0.17 a | 13.87 ± 0.17 a | 95.39 ± 2.1 a | 0.92 ± 0.01 a | 6.07 ± 0.14 a |
| Nocchione | 13.76 ± 0.12 ab | 13.64 ± 0.12 ab | 91.02 ± 1.5 a | 0.89 ± 0.01 b | 5.87 ± 0.10 ab |
| San Giovanni | 13.37 ± 0.07 c | 13.25 ± 0.08 c | 91.74 ± 0.9 a | 0.89 ± 0.01 b | 5.55 ± 0.06 c |
| Open pollination (Control) | 13.43 ± 0.09 bc | 13.34 ± 0.10 bc | 93.94 ± 1.2 a | 0.91 ± 0.01 ab | 5.62 ± 0.08 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vargas, R.J.; Facchin, S.L.; Traini, C.; Cinosi, N.; Villa, F.; Portarena, S.; Sánchez-Piñero, M.; Brunetti, M.; Baiocco, A.; Stabile, M.; et al. The Efficiency of Artificial Pollination on the Hazelnut ‘Tonda Francescana®’ Cultivar and the Xenia Effects of Different Pollinizers. Horticulturae 2025, 11, 724. https://doi.org/10.3390/horticulturae11070724
de Vargas RJ, Facchin SL, Traini C, Cinosi N, Villa F, Portarena S, Sánchez-Piñero M, Brunetti M, Baiocco A, Stabile M, et al. The Efficiency of Artificial Pollination on the Hazelnut ‘Tonda Francescana®’ Cultivar and the Xenia Effects of Different Pollinizers. Horticulturae. 2025; 11(7):724. https://doi.org/10.3390/horticulturae11070724
Chicago/Turabian Stylede Vargas, Rodrigo José, Simona Lucia Facchin, Chiara Traini, Nicola Cinosi, Fabiola Villa, Silvia Portarena, Marta Sánchez-Piñero, Mauro Brunetti, Angela Baiocco, Matteo Stabile, and et al. 2025. "The Efficiency of Artificial Pollination on the Hazelnut ‘Tonda Francescana®’ Cultivar and the Xenia Effects of Different Pollinizers" Horticulturae 11, no. 7: 724. https://doi.org/10.3390/horticulturae11070724
APA Stylede Vargas, R. J., Facchin, S. L., Traini, C., Cinosi, N., Villa, F., Portarena, S., Sánchez-Piñero, M., Brunetti, M., Baiocco, A., Stabile, M., & Farinelli, D. (2025). The Efficiency of Artificial Pollination on the Hazelnut ‘Tonda Francescana®’ Cultivar and the Xenia Effects of Different Pollinizers. Horticulturae, 11(7), 724. https://doi.org/10.3390/horticulturae11070724










