Responses of Biomass and Allometric Growth Equations of Juvenile Mangrove Plants to Salinity, Flooding, and Aboveground Competition
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Site and Materials
2.2. Field Plot Experiment Design
2.3. Measurement of Plant Biomass and Fitting of Allometric Growth Equation
2.4. Data Analysis
3. Results
3.1. Effects of Environmental and Biotic Stresses and Their Interactive Effects on Growth and Biomass of Juvenile Mangrove Plants
3.2. Effects of Environmental and Biotic Stresses and Their Interactive Effects on Biomass Allocation of Juvenile Mangrove Plants
3.3. The Allometric Growth Equation of Juvenile Mangrove Was Fitted Under Different Environmental and Biological Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TB | Total Biomass |
| AGB | Aboveground Biomass |
| BGB | Belowground biomass |
| H | Height |
| D | Basal Diameter |
| Ac | Aegiceras corniculatum |
| Am | Avicennia marina |
| Bg | Bruguiera gymnorhiza |
| Ct | Ceriops tagal |
| Ko | Kandelia obovata |
| Lr | Lumnitzera racemosa |
| Rs | Rhizophora stylosa |
| Sc | Sonneratia caseolaris |
References
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the Most Carbon-Rich Forests in the Tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Sandilyan, S.; Kathiresan, K. Mangrove Conservation: A Global Perspective. Biodivers. Conserv. 2012, 21, 3523–3542. [Google Scholar] [CrossRef]
- Xu, X.; Fu, D.; Su, F.; Lyne, V.; Yu, H.; Tang, J.; Hong, X.; Wang, J. Global Distribution and Decline of Mangrove Coastal Protection Extends Far beyond Area Loss. Nat. Commun. 2024, 15, 10267. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Natural Resources, PRC. 2024 China Natural Resources Bulletin; Ministry of Natural Resources, PRC: Beijing, China, 2025.
- Ministry of Natural Resources, PRC; National Forestry and Grassland Administration, PRC Special Action Plan for Mangrove Protection and Restoration (2020–2025). Available online: https://www.gov.cn/zhengce/zhengceku/2020-08/29/content_5538354.htm (accessed on 23 April 2025).
- Arachchilage, S.; Pulukkutige, P.; Ranasinghe, P.; Madarasinghe, S.; Dahdouh-Guebas, F.; Koedam, N. Stress-Induced Carbon Starvation in Rhizophora mucronata Lam. Seedlings under Conditions of Prolonged Submergence and Water Deficiency: Survive or Succumb. Bot. Serbica 2020, 44, 149–162. [Google Scholar] [CrossRef]
- Wang, W.; Xin, K.; Chen, Y.; Chen, Y.; Jiang, Z.; Sheng, N.; Liao, B.; Xiong, Y. Spatio-Temporal Variation of Water Salinity in Mangroves Revealed by Continuous Monitoring and Its Relationship to Floristic Diversity. Plant Divers. 2024, 46, 134–143. [Google Scholar] [CrossRef]
- Servais, S.; Kominoski, J.; Davis, S.; Gaiser, E.; Pachón, J.; Troxler, T. Effects of Nutrient-Limitation on Disturbance Recovery in Experimental Mangrove Wetlands. Wetlands 2019, 39, 337–347. [Google Scholar] [CrossRef]
- Liang, F.; Hu, J.; Lin, Y.; Li, L.; Yu, Y.; Liu, B.; Meng, Z.; Xiang, Z.; Tan, X. Interspecific Competition and Survival Pressures in Endangered Barringtonia Racemosa Populations of Mainland China. Sci. Rep. 2024, 14, 31190. [Google Scholar] [CrossRef]
- Ahouangan, B.S.C.M.; Koura, B.I.; Sèwadé, C.; Toyi, M.S.; Lesse, A.D.P.; Houinato, M.R.B. Ruminant Keeping around Mangrove Forests in Benin (West Africa): Herders’ Perceptions of Threats and Opportunities for Conservation of Mangroves. Discov. Sustain. 2022, 3, 13. [Google Scholar] [CrossRef]
- Saintilan, N.; Khan, N.S.; Ashe, E.; Kelleway, J.J.; Rogers, K.; Woodroffe, C.D.; Horton, B.P. Thresholds of Mangrove Survival under Rapid Sea Level Rise. Science 2020, 368, 1118–1121. [Google Scholar] [CrossRef]
- Chatting, M.; LeVay, L.; Walton, M.; Skov, M.W.; Kennedy, H.; Wilson, S.; Al-Maslamani, I. Mangrove Carbon Stocks and Biomass Partitioning in an Extreme Environment. Estuar. Coast. Shelf Sci. 2020, 244, 106940. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, A.; Qin, Y. Analysis of Development Trend of International Mangrove Research Based on Web of Science. J. Guangxi Norm. Univ. Sci. Ed. 2024, 42, 1–12. [Google Scholar]
- Gillis, L.G.; Hortua, D.A.S.; Zimmer, M.; Jennerjahn, T.C.; Herbeck, L.S. Interactive Effects of Temperature and Nutrients on Mangrove Seedling Growth and Implications for Establishment. Mar. Environ. Res. 2019, 151, 104750. [Google Scholar] [CrossRef] [PubMed]
- Kimera, F.; Sobhi, B.; Omara, M.; Sewilam, H. Impact of Salinity Gradients on Seed Germination, Establishment, and Growth of Two Dominant Mangrove Species Along the Red Sea Coastline. Plants 2024, 13, 3471. [Google Scholar] [CrossRef]
- Abdul-Hamid, H.; Mohamed, J.; Abiri, R.; Naji, H.; Jalil, M. Allometric Equation for Aboveground Biomass Estimation of Mangroves Mixed Mature Mangrove Forest. Forests 2022, 13, 325. [Google Scholar] [CrossRef]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, Biomass, and Productivity of Mangrove Forests: A Review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Kusmana, C.; Hidayat, T.; Tiryana, T.; Rusdiana, O. Istomo Allometric Models for Above- and below-Ground Biomass of Sonneratia Spp. Glob. Ecol. Conserv. 2018, 15, e00417. [Google Scholar] [CrossRef]
- Hu, X.; Xiong, L.; CHEN, S.; Zhang, H.; Zou, Y.; Zhang, J.; Liu, D.; He, J.; Wu, Y.; Zhu, Z. Study on Biomass Models of Juvenile Mangroves and Carbon Storage of Young Mangrove Ecosystem. J. Trop. Oceanogr. 2024. Available online: https://www.jto.ac.cn/CN/10.11978/2024141 (accessed on 23 April 2025).
- Smith, T.J.; Whelan, K.R.T. Development of Allometric Relations for Three Mangrove Species in South Florida for Use in the Greater Everglades Ecosystem Restoration. Wetl. Ecol. Manag. 2006, 14, 409–419. [Google Scholar] [CrossRef]
- Ball, M.C. Ecophysiology of Mangroves. Trees 1988, 2, 129–142. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource Limitation in Plants-An Economic Analogy. Annu. Rev. Ecol. Evol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Thornley, J.H.M. A Balanced Quantitative Model for Root: Shoot Ratios in Vegetative Plants. Ann. Bot. 1972, 36, 431–441. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global Allocation Rules for Patterns of Biomass Partitioning in Seed Plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Falster, D.S.; Duursma, R.A.; Ishihara, M.I.; Barneche, D.R.; FitzJohn, R.G.; Vårhammar, A.; Aiba, M.; Ando, M.; Anten, N.; Aspinwall, M.J.; et al. BAAD: A Biomass And Allometry Database for Woody Plants. Ecology 2015, 96, 1445. [Google Scholar] [CrossRef]
- Xiong, Y.; Liao, B.; Proffitt, E.; Guan, W.; Sun, Y.; Wang, F.; Liu, X. Soil Carbon Storage in Mangroves Is Primarily Controlled by Soil Properties: A Study at Dongzhai Bay, China. Sci. Total Environ. 2018, 619, 1226–1235. [Google Scholar] [CrossRef]
- Picard, N.; Saint-André, L.; Henry, M. Manual for Building Tree Volume and Biomass Allometric Equations: From Field Measurement to Prediction; CIRAD: Paris, France, 2012. [Google Scholar]
- Ye, Y.; Gu, Y.T.; Gao, H.Y.; Lu, C.Y. Combined Effects of Simulated Tidal Sea-Level Rise and Salinity on Seedlings of a Mangrove Species, Kandelia candel (L.) Druce. Hydrobiologia 2010, 641, 287–300. [Google Scholar] [CrossRef]
- Yang, S.; Shih, S.; Hwang, G.; Adams, J.B.; Lee, H.; Chen, C. The Salinity Gradient Influences on the Inundation Tolerance Thresholds of Mangrove Forests. Ecol. Eng. 2013, 51, 59–65. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W. Ecophysiological Responses of Viviparous Mangrove Rhizophora stylosa Seedlings to Simulated Sea-Level Rise. J. Coast. Res. 2016, 33, 1333–1340. [Google Scholar] [CrossRef]
- Hassan, N. Salinity Stress in Plants: Growth, Photosynthesis and Adaptation Review. GSC Adv. Res. Rev. 2024, 20, 231–243. [Google Scholar] [CrossRef]
- Miyama, M.; Tada, Y. Transcriptional and Physiological Study of the Response of Burma Mangrove (Bruguiera gymnorhiza) to Salt and Osmotic Stress. Plant Mol. Biol. 2008, 68, 119–129. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Huai, J.; Peng, F.; Wu, J.; Lin, R.; Fang, X. Condensation of SEUSS Promotes Hyperosmotic Stress Tolerance in Arabidopsis. Nat. Chem. Biol. 2022, 18, 1361–1369. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Yuan, Z. Plant Responses and Adaptations to Salt Stress: A Review. Horticulturae 2024, 10, 1221. [Google Scholar] [CrossRef]
- Zhu, J. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Medina, C.; Alseekh, S.; Fernie, A.R.; Rambla, J.L.; Pérez-Clemente, R.M.; Gómez-Cádenas, A.; Zandalinas, S.I. Abscisic Acid Promotes Plant Acclimation to the Combination of Salinity and High Light Stress. Plant Physiol. Biochem. 2023, 203, 108008. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Bengtson, P.; Rousk, J. Comparative Toxicities of Salts on Microbial Processes in Soil. Appl. Environ. Microbiol. 2016, 82, 2012–2020. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Xue, R.; Wang, L. Effects of Salinity on the Soil Microbial Community and Soil Fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Alongi, D.M. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Xiao, Y.; Jie, Z.; Wang, M.; Lin, G.; Wang, W. Leaf and Stem Anatomical Responses to Periodical Waterlogging in Simulated Tidal Floods in Mangrove Avicennia marina Seedlings. Aquat. Bot. 2009, 91, 231–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Geng, S.; Zhang, X. A Review of Soil Waterlogging Impacts, Mechanisms, and Adaptive Strategies. Front. Plant Sci. 2025, 16, 1545912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Bai, T.; Wang, Y.; Wu, T.; Zhang, X.; Xu, X.; Han, Z. Morpholoical and Enzymatic Responses to Waterlogging in Three Prunus Species. Sci. Hortic. 2017, 221, 62–67. [Google Scholar] [CrossRef]
- Liu, Y.; Jafari, F.; Wang, H. Integration of Light and Hormone Signaling Pathways in the Regulation of Plant Shade Avoidance Syndrome. aBIOTECH 2021, 2, 131–145. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.L. Control of Plant Growth and Defense by Photoreceptors: From Mechanisms to Opportunities in Agriculture. Mol. Plant 2021, 14, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.E.; Danneberger, T.K.; McMahon, M.J. Spectral Irradiance Available for Turfgrass Growth in Sun and Shade. Crop Sci. 2000, 40, 189–195. [Google Scholar] [CrossRef]
- Fraser, D.P.; Hayes, S.; Franklin, K.A. Photoreceptor Crosstalk in Shade Avoidance. Curr. Opin. Plant Biol. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Wang, Z. Root System Characters in Growth and Distribution Among Three Littoral Halophytes. Acta Ecol. Sin. 2011, 31, 1195–1202. [Google Scholar]
- Guo, W.; Wang, G.; Gou, Q. Effects of Sodium Salt Stress on the Growth and Biomass Allocation of Chenopodiaceae annuals. Acta Ecol. Sin. 2020, 41, 6633–6643. [Google Scholar]
- Wei, M.; Li, H.; Zhang, L.; Guo, Z.; Liu, J.; Ding, Q.; Zhong, Y.; Li, J.; Ma, D.; Zheng, H. Exogenous Hydrogen Sulfide Mediates Na+ and K+ Fluxes of Salt Gland in Salt-Secreting Mangrove Plant Avicennia Marina. Tree Physiol. 2022, 42, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Mishra, A. Chapter 18—Flooding Stress in Plants and Approaches to Overcome. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 355–366. ISBN 978-0-12-812689-9. [Google Scholar]
- Setter, T.L.; Waters, I. Review of Prospects for Germplasm Improvement for Waterlogging Tolerance in Wheat, Barley and Oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Silvestro, R.; Deslauriers, A.; Prislan, P.; Rademacher, T.; Rezaie, N.; Richardson, A.D.; Vitasse, Y.; Rossi, S. From Roots to Leaves: Tree Growth Phenology in Forest Ecosystems. Curr. For. Rep. 2025, 11, 12. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Wang, Q.; Zhou, R.; Gaffney, P.P.J.; Liu, M.; Shi, R.; Gao, Z.; Chu, H.; Niu, S.; et al. Restoration Recovers Plant Diversity but Changes Species Composition and Biomass Allocation in an Alpine Peatland. Ecol. Process. 2025, 14, 24. [Google Scholar] [CrossRef]
- Yang, P.; Niu, M.; Fu, Q.; Qian, L.; Huang, M.; Li, Z.; Sun, H.; Chen, J. Ecosystem Engineers Can Regulate Resource Allocation Strategies in Associated Plant Species. Front. Plant Sci. 2024, 15, 1387951. [Google Scholar] [CrossRef]
- Mccarthy, M.C.; Enquist, B.J. Consistency between an Allometric Approach and Optimal Partitioning Theory in Global Patterns of Plant Biomass Allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]

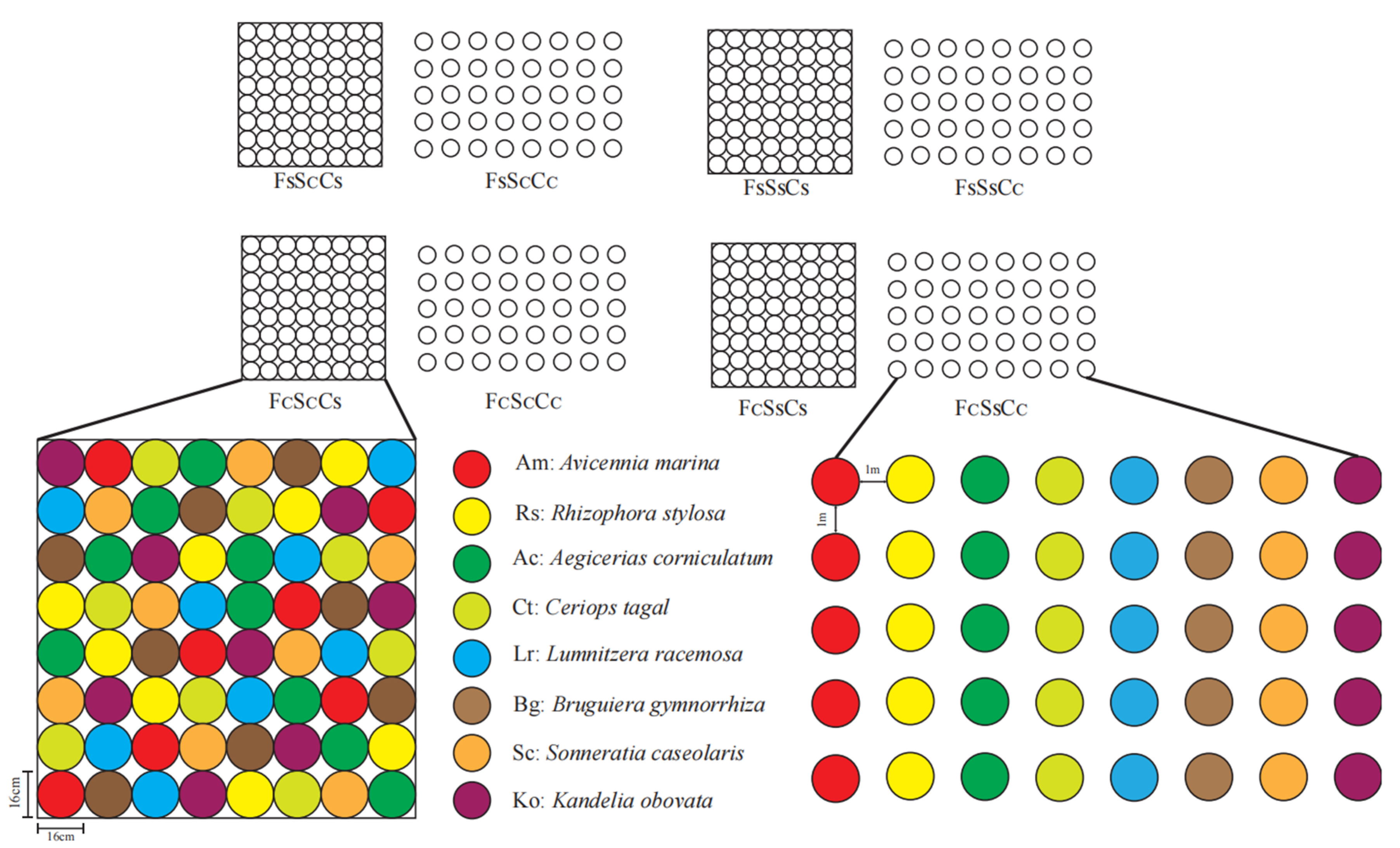
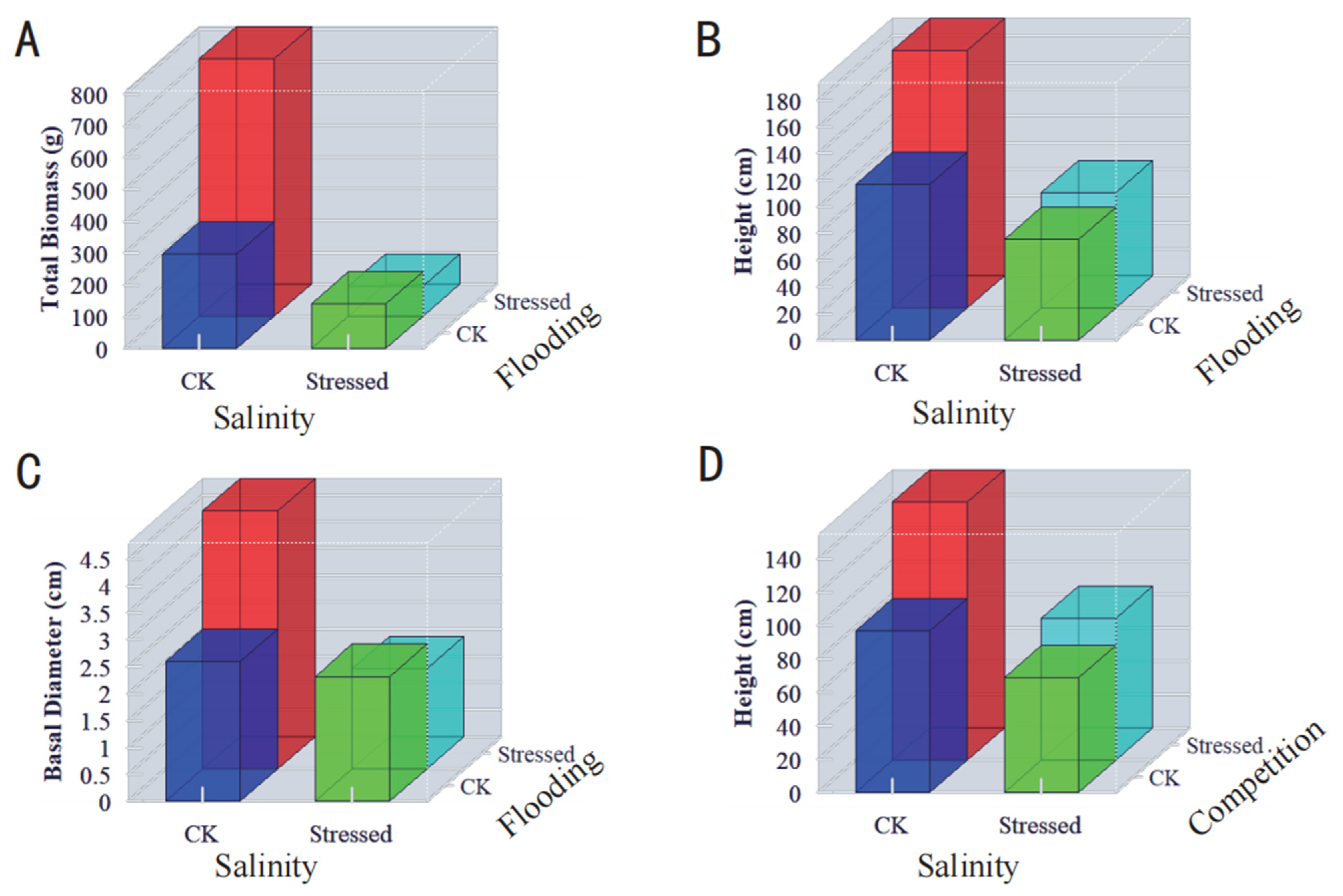

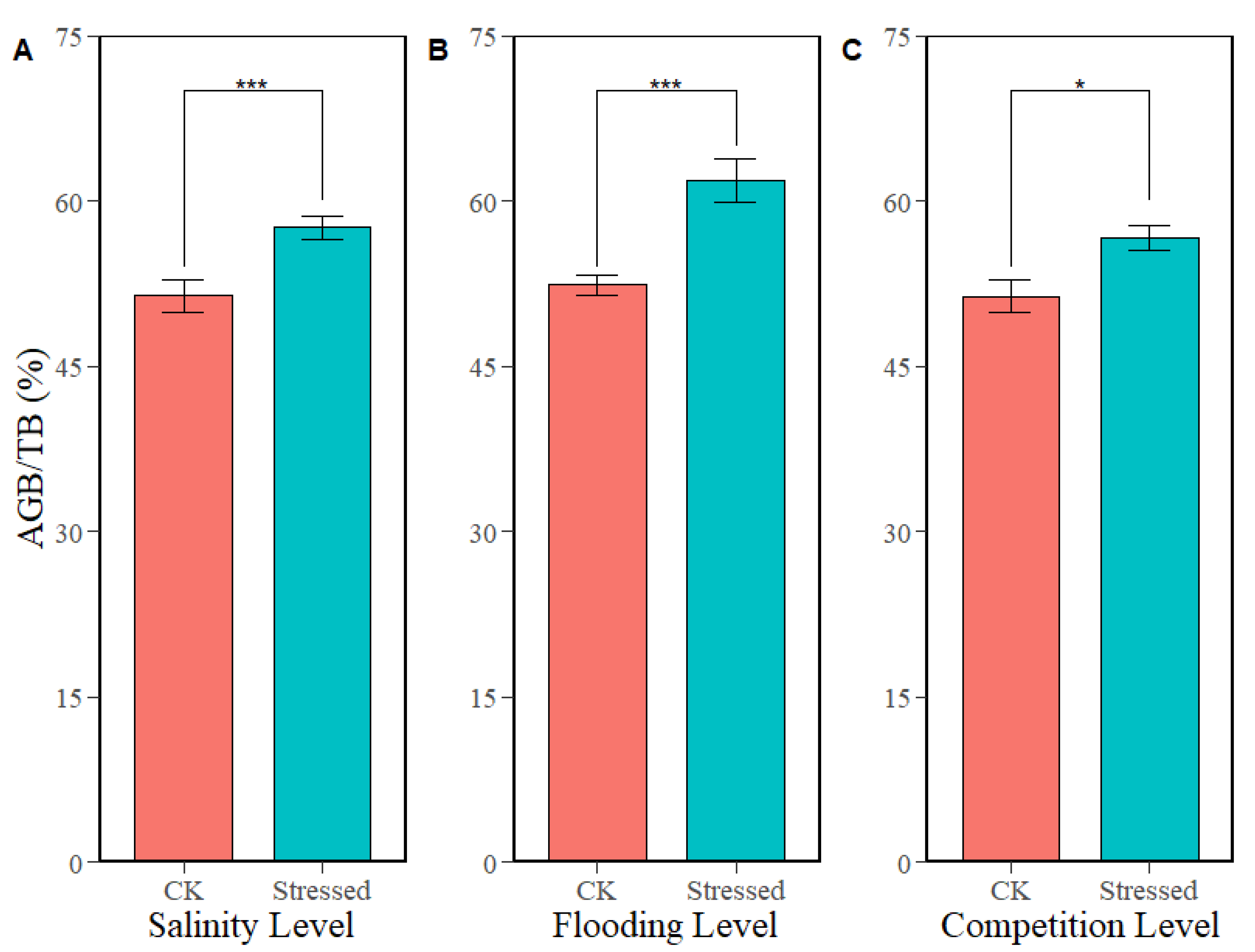
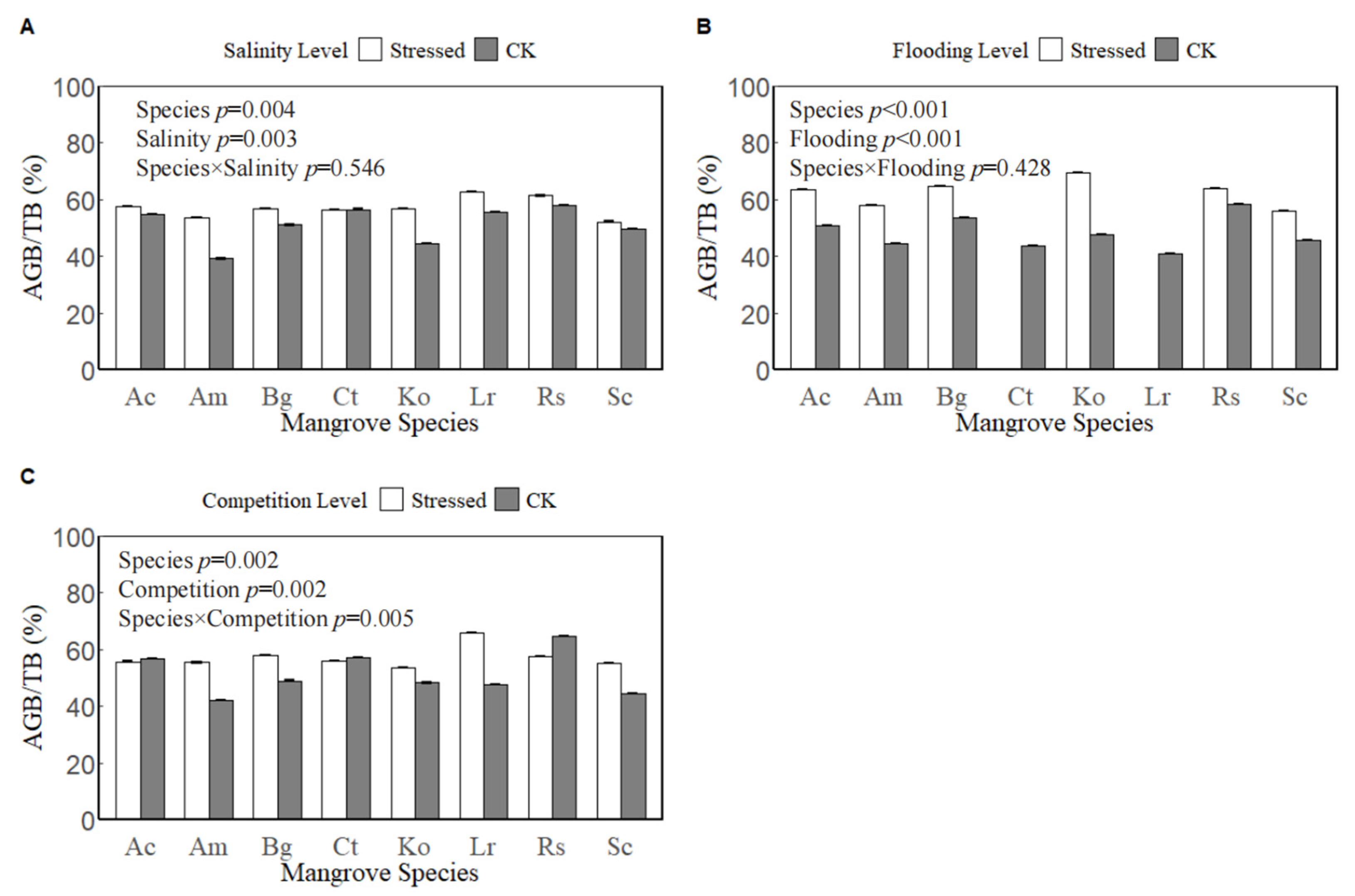

| TB | H | D | |
|---|---|---|---|
| Salinity | <0.001 | <0.001 | <0.001 |
| Flooding | <0.001 | <0.001 | <0.001 |
| Competition | 0.360 | <0.001 | 0.271 |
| Salinity × Flooding | <0.001 | <0.001 | <0.001 |
| Salinity × Competition | 0.167 | 0.002 | 0.732 |
| Flooding × Competition | 0.321 | 0.586 | 0.909 |
| Salinity × Flooding × Competition | 0.453 | 0.374 | 0.257 |
| AGB/TB | |
|---|---|
| Salinity | <0.001 |
| Flooding | <0.001 |
| Competition | 0.034 |
| Salinity × Flooding | 0.330 |
| Salinity × Competition | 0.258 |
| Flooding × Competition | 0.740 |
| Salinity × Flooding × Competition | 0.533 |
| Species | Fitting Model | X | a | b | R2 | p Value | Sample Number |
|---|---|---|---|---|---|---|---|
| A. corniculatum | TB = aX + b | D2H | 0.0584 | 0.0230 | 0.4855 | <0.001 | 43 |
| A. marina | TB = aX + b | H | 0.4059 | −0.2487 | 0.1842 | <0.001 | 25 |
| B. gymnorhiza | TB = aX + b | DH2 | 0.0132 | 0.1068 | 0.3153 | <0.001 | 25 |
| C. tagal | TB = aX + b | D2H | 0.0371 | 0.0256 | 0.7127 | <0.001 | 18 |
| K. obovata | TB = aX + b | D | 0.0575 | 0.0439 | 0.1307 | <0.001 | 32 |
| L. racemosa | TB = aX + b | D2H | 0.0443 | 0.0351 | 0.6460 | <0.001 | 25 |
| R. stylosa | TB = aX + b | D | 0.0210 | 0.0496 | 0.1108 | <0.001 | 36 |
| S. caseolaris | TB = aX + b | D2H | 0.2973 | 0.0067 | 0.9252 | <0.001 | 30 |
| All Species | TB = aX + b | D2H | 0.0584 | 0.0230 | 0.4855 | <0.001 | 234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Wang, W.; Qian, W.; Sheng, N.; Cheng, J.; Xiong, Y. Responses of Biomass and Allometric Growth Equations of Juvenile Mangrove Plants to Salinity, Flooding, and Aboveground Competition. Horticulturae 2025, 11, 712. https://doi.org/10.3390/horticulturae11070712
Hu K, Wang W, Qian W, Sheng N, Cheng J, Xiong Y. Responses of Biomass and Allometric Growth Equations of Juvenile Mangrove Plants to Salinity, Flooding, and Aboveground Competition. Horticulturae. 2025; 11(7):712. https://doi.org/10.3390/horticulturae11070712
Chicago/Turabian StyleHu, Kaijie, Wei Wang, Wei Qian, Nong Sheng, Jiliang Cheng, and Yanmei Xiong. 2025. "Responses of Biomass and Allometric Growth Equations of Juvenile Mangrove Plants to Salinity, Flooding, and Aboveground Competition" Horticulturae 11, no. 7: 712. https://doi.org/10.3390/horticulturae11070712
APA StyleHu, K., Wang, W., Qian, W., Sheng, N., Cheng, J., & Xiong, Y. (2025). Responses of Biomass and Allometric Growth Equations of Juvenile Mangrove Plants to Salinity, Flooding, and Aboveground Competition. Horticulturae, 11(7), 712. https://doi.org/10.3390/horticulturae11070712







