Networking 13 Berry Minerals to Sustain a High Yield of Firm Cranberry Fruits
Abstract
1. Introduction
2. Materials and Methods
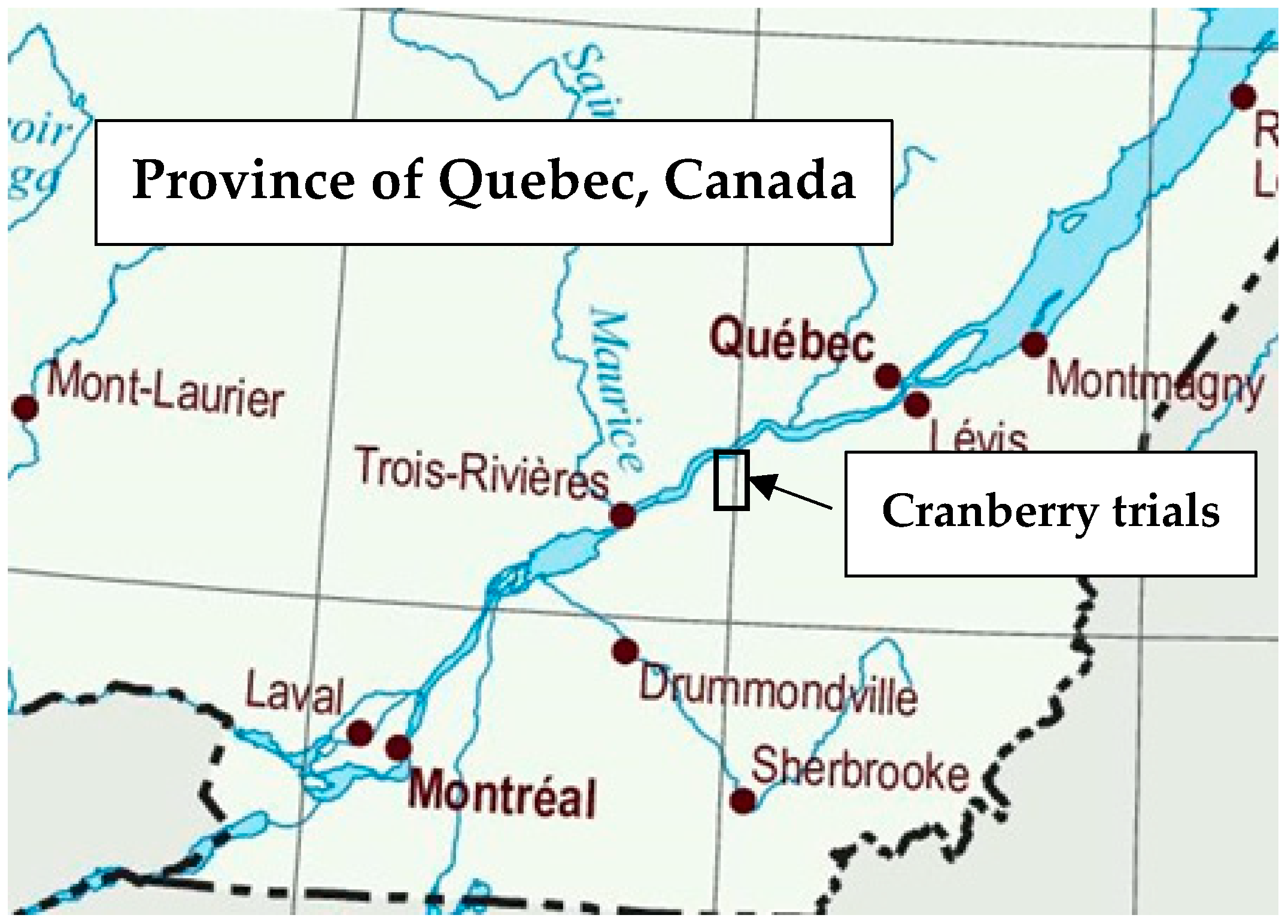
2.1. Database
2.2. Tissue Analyses
2.3. Data Transformation
2.4. Statistical Analysis
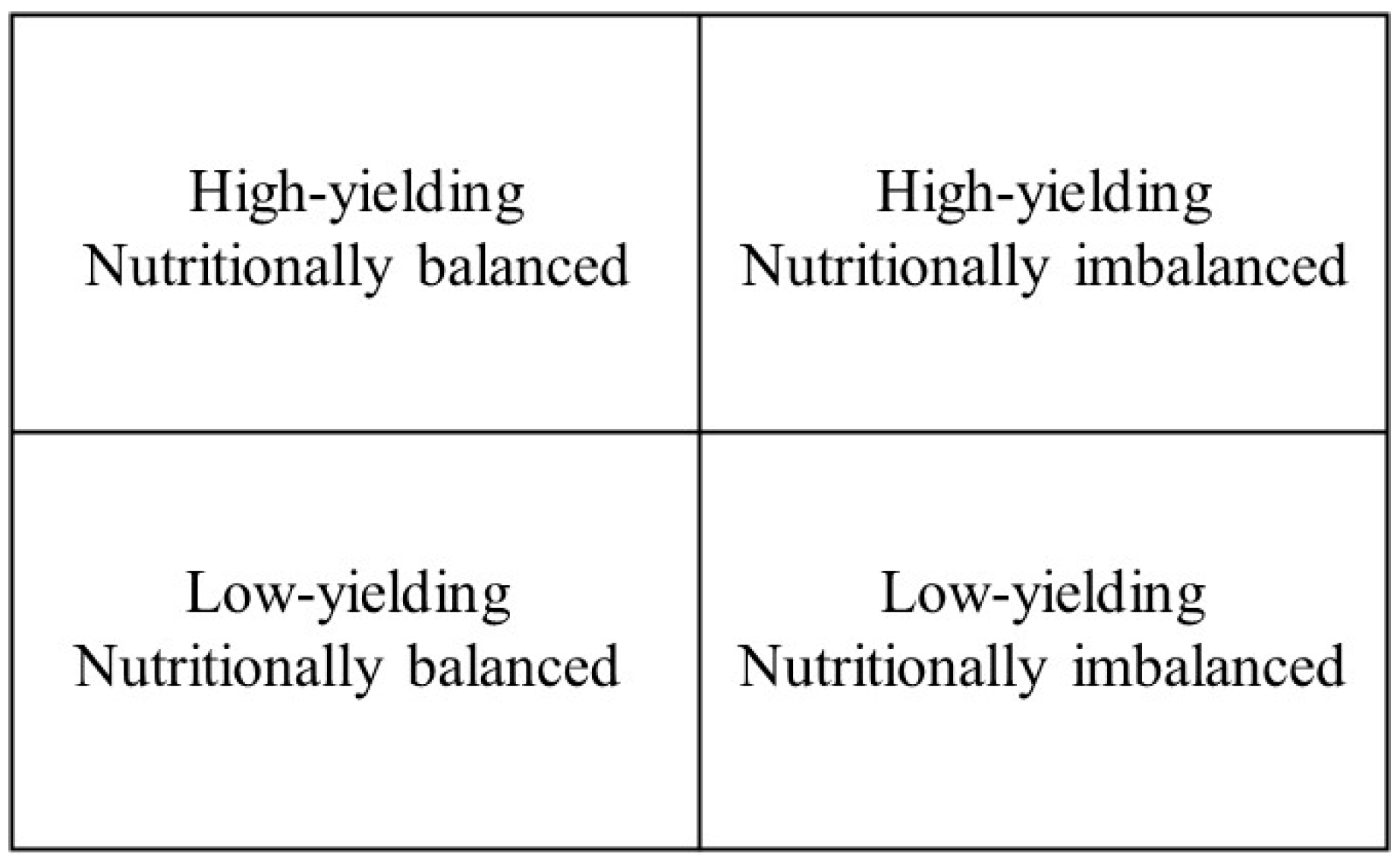
- High-yielding and nutritionally balanced specimens.
- Low-yielding and nutritionally balanced specimens where features other than the ones included in the model limit berry yield or quality.
- High-yielding and nutritionally imbalanced specimens due to luxury consumption, suboptimal concentration, or contamination.
- Low-yielding and nutritionally imbalanced specimens.
3. Results
3.1. Berry Composition, Yield, and Quality
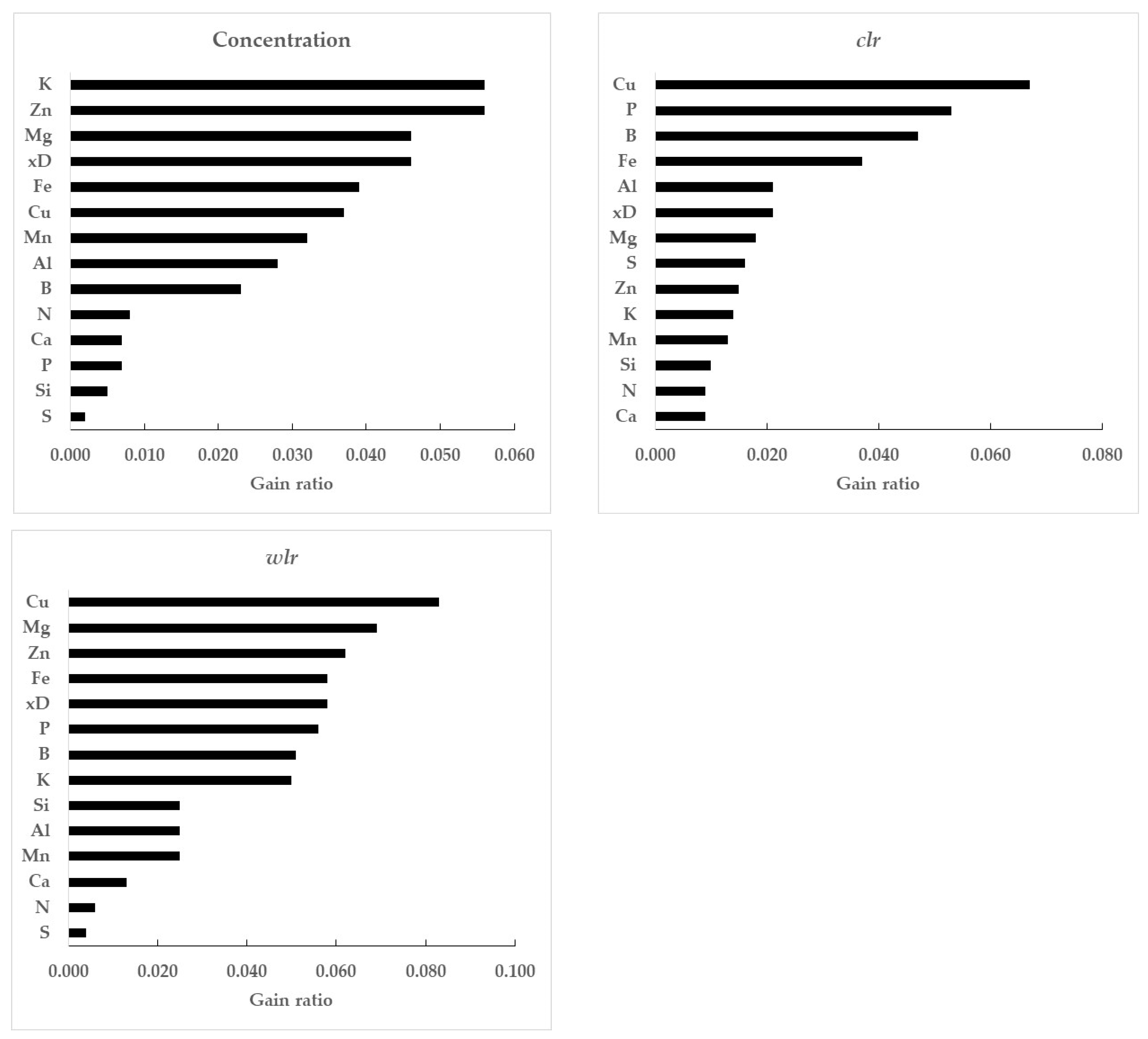
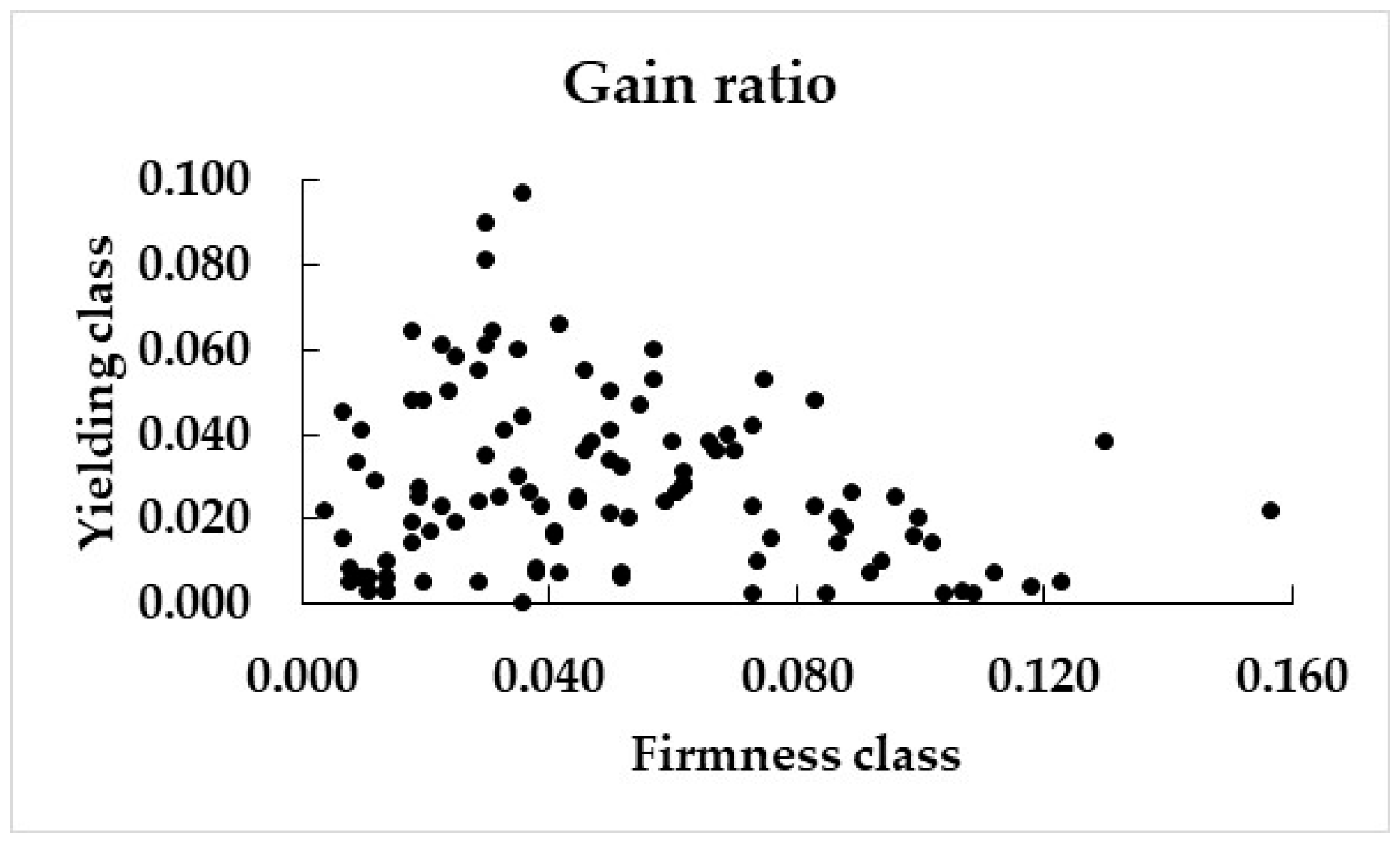
3.2. Gain Ratios
3.3. Classification Models
3.4. Diagnosis of Nutritionally Imbalanced and Low-Yielding Specimens
4. Discussion
4.1. The wlr Expression
4.2. Plant Elemental Composition
4.3. Soil Quality
4.4. Suggestive Corrective Measures
4.5. Limitations of This Study
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sandler, H.; DeMoranville, C. Cranberry Production: A Guide for Massachusetts—Summary Edition; UMass-Amherst College of Natural Resources and the Environment: Amherst, MA, USA, 2008; Available online: https://hdl.handle.net/20.500.14394/9230 (accessed on 13 May 2025).
- Gallardo, R.K.; Klingthong, P.; Zhang, Q.; Polashok, J.; Atucha, A.; Zalapa, J.; Rodriguez-Saona, C.; Vorsa, N.; Iorizzo, M. Breeding Trait Priorities of the Cranberry Industry in the United States and Canada. HortScience 2018, 53, 1467–1474. [Google Scholar] [CrossRef]
- Diaz-Garcia, L.; Rodriguez-Bonilla, L.; Phillips, M.; Lopez-Hernandez, A.; Grygleski, E.; Atucha, A.; Zalapa, J.; Ezura, H. Comprehensive analysis of the internal structure and firmness in American cranberry (Vaccinium macrocarpon Ait.) fruit. PLoS ONE 2019, 14, e0222451. [Google Scholar] [CrossRef] [PubMed]
- Lasa, R.; Tadeo, E.; Dinorin, L.A.; Lime, I.; Williams, T. Fruit firmness, superficial damage, and location modulate infestation by Drosophila suzukii and Zaprionus indianus: The case of guava in Veracruz, Mexico. Entomoligia Expir. Et Appl. 2017, 162, 4–12. [Google Scholar] [CrossRef]
- Weißinger, L.; Arand, K.; Bieler, E.; Kassemeyer, H.H.; Breuer, M.; Müller, C. Physical and Chemical Traits of Grape Varieties Influence Drosophila suzukii Preferences and Performance. Front. Plant Sci. 2021, 12, 664636. [Google Scholar] [CrossRef]
- Marles, R. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Sharma, J.; Dua, V.K.; Sharma, S.; Choudhary, A.K.; Kumar, P.; Sharma, A. Role of Plant Nutrition in Disease Development and Management. In Sustainable Management of Potato Pests and Diseases; Chakrabarti, S.K., Sharma, S., Shah, M.A., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Agric. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Jamaly, R.; Parent, S.-É.; Parent, L.E. Fertilization and Soil Nutrients Impact Differentially Cranberry Yield and Quality in Eastern Canada. Horticulturae 2021, 7, 191. [Google Scholar] [CrossRef]
- Chawla, R.; Sharma, S.K. Nitrogen fertilization of stone fruits: A comprehensive review. J. Plant Nutr. 2025, 48, 445–485. [Google Scholar] [CrossRef]
- Martinez, D.A.; Loening, U.E.; Graham, M.C.; Gathorne-Hardy, A. When the Medicine Feeds the Problem; Do Nitrogen Fertilizers and Pesticides Enhance the Nutritional Quality of Crops for Their Pests and Pathogens? Front. Sustain. Food Syst. 2021, 5, 701310. [Google Scholar] [CrossRef]
- Davenport, J.; DeMoranville, C. Best Management Practices. Nitrogen Management in Cranberry Systems; University of Massachusetts Cranberry Experimental Station: Amherst, MA, USA, 1997; Available online: https://hdl.handle.net/20.500.14394/9267 (accessed on 16 June 2025).
- Jaime, M.; Fischer, G.; Alvarez-Herrera, J.G. Effect of calcium on fruit quality: A review. Agron. Colomb. 2024, 42, e112026. [Google Scholar] [CrossRef]
- Wójcik, P.; Mika, A.; Cieslinski, G. Effect of boron fertilization on the storage ability of apples (Malus domestica Borth.). Acta Hort. 1999, 485, 393–398. [Google Scholar] [CrossRef]
- Thomidis, T.; Karagiannidis, N.; Stefanou, S.; Paresidou, M.; Prodromou, I. Influence of boron applications on preharvest and postharvest nectarine fruit rot caused by brown rot. Australas. Plant Pathol. 2017, 46, 177–181. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Padula, M.P.; Zeng, R.; Gurr, G.M. Silicon: Potential to Promote Direct and Indirect Effects on Plant Defense Against Arthropod Pests in Agriculture. Front. Plant Sci. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef]
- Laing, M.D.; Gatarayiha, M.C.; Adandonon, A. Silicon use for pest control in agriculture: A review. Proc. S. Afr. Sug. Technol. Ass. 2006, 80, 278–286. [Google Scholar]
- Yang, L.; Han, Y.; Li, P.; Ali, S.; Hou, M. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Scientif. Rep. 2017, 7, 4232. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzicker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Deshmukh, R.; Ma, J.F.; Bélanger, R.R. Editorial: Role of Silicon in Plants. Front. Plant Sci. 2017, 8, 1858. [Google Scholar] [CrossRef]
- França, L.L.; Dierings, C.A.; Almeida, A.C.D.S.; Aráujo, M.D.S.; Heinrichs, E.A.; Silva, A.R.D.; Barrigossi, J.A.F.; Jedsus, F.F.D. Resistance in rice to Tibraca limbativentris (Hemiptera: Pentatomidae) influenced by plant silicon content. Fla. Entomol. 2018, 101, 587–591. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.r.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Xue, X.; Geng, T.; Liu, H.; Yang, W.; Zhong, W.; Zhang, Z.; Zhu, C.; Chu, Z. Foliar Application of Silicon Enhances Resistance Against Phytophthora infestans Through the ET/JA- and NPR1-Dependent Signaling Pathways in Potato. Front. Plant Sci. 2012, 12, 609870. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef] [PubMed]
- Zebarth, B.J.; Forge, T.A.; Goyer, C.; Brin, L.D. Effect of soil acidification on nitrification in soil. Can. J. Soil Sci. 2015, 95, 359–363. [Google Scholar] [CrossRef]
- Jamaly, R.; Parent, S.E.; Ziadi, N.; Parent, L.E. Short-Term Impact of Elemental Sulfur on Cranberry Nutrition and Crop Performance. Open J. Soil Sci. 2023, 13, 83–96. [Google Scholar] [CrossRef]
- O’Kennedy, S.O. Soil pH and its impact on nutrient availability and crop growth. Interational J. Geogr. Geol. Environ. 2022, 4, 236–238. [Google Scholar]
- Rout, G.R.; Samantaray, S.; Das, P. Aluminium Toxicity in Plants: A Review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Vardar, F.; Unal, M. Aluminum Toxicity and Resistance in Higher Plants. Adv. Mol. Biol. 2007, 1, 1–12. [Google Scholar]
- Cárcamo, M.P.; Reyes-Diaz, M.; Rengel, Z.; Alberdi, M.; Omena-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Aluminum stress differentially affects physiological performance and metabolic compounds in cultivars of highbush blueberry. Sci. Rep. 2019, 9, 11275. [Google Scholar] [CrossRef]
- Munson, R.D.; Nelson, W.L. Principles and Practices in Plant Analysis. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1990; pp. 359–387. [Google Scholar]
- Bates, T.E. Factors affecting critical nutrient concentrations in plants and their environment: A review. Soil Sci. 1971, 112, 116–130. [Google Scholar] [CrossRef]
- Sumner, M.E. Interpretation of nutrient ratios in plant tissue. Commun. Soil Sci. Plant Anal. 1978, 9, 335–345. [Google Scholar] [CrossRef]
- Courbet, G.; Gallardo, K.; Vigani, G.; Brunel-Muguet, S.; Trouverie, J.; Salon, C.; Ourry, A. Disentangling the complexity and diversity of crosstalk between sulfur and other mineral nutrients in cultivated plants. J. Exp. Bot. 2019, 70, 4183–4196. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-Talks Between Macro- and Micronutrient Uptake and Signaling in Plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, W.M.; Beverly, R.B. The Dilution Effect in Plant Nutrition Studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar] [CrossRef]
- Righetti, T.L.; Wilder, K.L.; Cummings, G.A. Plant Analysis as an aid in fertilizing orchards. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1990; pp. 563–601. [Google Scholar]
- Baxter, I. Ionomics: Studying the Social Network of Mineral Nutrients. Curr. Opin. Plant Biol. 2009, 12, 381–386. [Google Scholar] [CrossRef]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the Study of the Plant Ionome. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef]
- Beaufils, E.R. Diagnosis and recommendation integrated system (DRIS). In Soil Science Bulletin 132; University of Natal: Pietermaritzburg, South Africa, 1973. [Google Scholar]
- Parent, L.E.; Dafir, M. A theoretical concept of compositional nutrient diagnosis. J. Am. Soc. Hortic. Sci. 1992, 117, 239–242. [Google Scholar] [CrossRef]
- Parent, E. Why we should use balances and machine learning to diagnose ionomes. Authorea 2020, 1, 20. [Google Scholar] [CrossRef]
- Baxter, I. Should we treat the ionome as a combination of individual elements, or should we be deriving novel combined traits? J. Exp. Bot. 2015, 66, 2127–2131. [Google Scholar] [CrossRef]
- Aitchison, J. The Statistical Analysis of Compositional Data; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics. A Biometricla Approach; McGraw-Hill: New-York, NY, USA, 1996. [Google Scholar]
- Greenacre, M.; Gunsky, E.; Bacon-Shone, J.; Erb, I.; Quinn, T. Aitchison’s Compositional Data Analysis 40 Years On: A Reappraisal. Stat. Sci. 2023, 38, 386–410. [Google Scholar] [CrossRef]
- Parent, L.E. Compositional and machine learning tools to model plant nutrition: Overview and perspectives. Horticulturae 2025, 11, 161. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Grunes, D.L.; Sumner, M.E. Nutrient interactions in soil and plant nutrition. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. D89–D112. [Google Scholar]
- Srivastava, A.K.; Huchche, A.D.; Parent, L.E.; Malhotra, S.K.; Ziogas Vasileios, Z.; Baishya, L.K. Mineral nutrition of Citrus across the mandrain belts of India. Horticulturae 2025, 11, 254. [Google Scholar] [CrossRef]
- Caron, J.; Pelletier, V.; Kennedy, C.D.; Gallichand, J.; Gumiere, S.; Bonin, S.; Bland, W.L.; Pepin, S. Guidelines of irrigation and drainage management strategies to enhance cranberry production and optimize water use in North America. Can. J. Soil Sci. 2017, 97, 82–91. [Google Scholar] [CrossRef]
- Barnhisel, R.; Bertsch, P.M. Digestion with Perchloric and Nitric Acids. In Methods of Soil Analysis; Page, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 279–280. [Google Scholar]
- Mert, M.C.; Filzmoser, P.; Hron, K. Error Propagation in Isometric Log-ratio Coordinates for Compositional Data: Theoretical and Practical Considerations. Math. Geosci. 2016, 48, 941–961. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, A.L. Plant analysis and interpretation of analysis for horticultural crops. In Soil Testing and Plant Analysis, Part II; Stelly, M., Hamilton, H., Eds.; Soil Science Society of America: Madison, WI, USA, 1967; pp. 59–75. [Google Scholar]
- Walworth, J.L.; Sumner, M.E. The Diagnosis and Recommendation Integrated System (DRIS). Adv. Soil Sci. 1987, 6, 149–188. [Google Scholar] [CrossRef]
- Alarcón-Poblete, E.; Inostroza-Blancheteau, C.; Alberdi, M.; Rengel, Z.; Reyes-Díaz, M. Molecular regulation of aluminum resistance and sulfur nutrition during root growth. Planta 2018, 247, 27–39. [Google Scholar] [CrossRef]
- Couturier, J.; Touraine, B.; Briat, J.F.; Gaymard, F.; Rouhier, N. The iron-sulfur cluster assembly machineries in plants: Current knowledge and open questions. Front. Plant Sci. 2013, 4, 259. [Google Scholar] [CrossRef]
- Zuchi, S.; Watanabe, M.; Hubberten, H.M.; Bromke, M.; Osorio, S.; Fernie, A.R.; Celletti, S.; Paolacci, A.R.; Catarcione, G.; Ciaffi, M.; et al. The interplay between sulfur and iron nutrition in tomato. Plant Physiol. 2015, 169, 2624–2639. [Google Scholar] [CrossRef]
- Astolfi, S.; Celletti, S.; Vigani, G.; Mimmo, T.; Cesco, S. Interaction Between Sulfur and Iron in Plants. Front. Plant Sci. 2021, 12, 670308. [Google Scholar] [CrossRef]
- Pandey, R.; Singh, C.; Mishra, S.; Abdulraheem, M.I.; Vyas, D. Silicon uptake and transport mechanisms in plants: Processes, applications and challenges in sustainable plant management. Biol. Futur. 2024, 76, 19–31. [Google Scholar] [CrossRef]
- Davenport, J.R.; Vorsa, N. Cultivar Fruiting and Vegetative Response to Nitrogen Fertilizer in Cranberry. J. Amer. Soc. Hort. Sci. 1999, 124, 90–93. [Google Scholar] [CrossRef]
- Peng, Q.; Shrestha, A.; Zhang, Y.; Fan, J.; Yu, F.; Wang, G. How lignin biosynthesis responds to nitrogen in plants: A scoping review. Plant Biol. 2024, 26, 881–895. [Google Scholar] [CrossRef]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Carreiro, B.; Mia, M.S.; Paetau-Robinson, I.; Khoo, C.; Neto, C. Pentacyclic Triterpenoid Content in Cranberry Raw Materials and Products. Foods 2024, 13, 3136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, J.; Zang, N.; Yin, Z.; Wang, A. Effects of calcium application on apple fruit softening during storage revealed by proteomics and phosphoproteomics. Hortic. Plant J. 2022, 8, 408–422. [Google Scholar] [CrossRef]
- Gustafson, J.P.; Bhattacharya, P.; Bain, D.C.; Fraser, A.R.; McHardy, W.J. Podzolisation mechanisms and the synthesis of imogolite in northern Scandinavia. Geoderma 1995, 66, 167–184. [Google Scholar] [CrossRef]
- Lundström, U.S.; van Breemen, N.; Bain, D. The podzolization process. A review. Geoderma 2000, 94, 91–107. [Google Scholar] [CrossRef]
- Sanborn, P.; Lamontagne, L.; Hendershot, W. Podzolic soils of Canada: Genesis, distribution, and classification. Can. J. Soil Sci. 2011, 91, 843880. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, J.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Elgagawhary, S.M.; Lindsay, W.L. Solubility of silica in soils. Soil Sci. Soc. Amer. Proc. 1972, 36, 439–442. [Google Scholar] [CrossRef]
- Jones, L.H.P.; Handreck, K.A. Effects of iron & aluminum oxides on silica in solution in soils. Nature 1963, 198, 852–853. [Google Scholar]
- Schaller, J.; Kleber, M.; Puppe, D.; Stein, M.; Sommer, M.; Rillig, M. The importance of reactive silica for maintaining soil health. Plant Soil 2025, 1–12. [Google Scholar] [CrossRef]
- McKeague, J.A.; Cline, M.G. Silica in soil solutions. 1. The form and concentration of dissolved silica in aqueous extracts of some soils. Can. J. Soil Sci. 1963, 43, 70–82. [Google Scholar] [CrossRef]
- Tubana, B.S. Dynamics of Silicon in Soil and Plant to Establish Silicate Fertilization. In Benefits of Silicon in the Nutrition of Plants; de Mello Prado, R., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 57–73. [Google Scholar] [CrossRef]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Van der Paauw, F. Periodic fluctuations of soil fertility, crop yields and of response to fertilization effected by alternating of low and high rainfall. Plant Soil 1962, 17, 154–182. [Google Scholar] [CrossRef]
- Collins, J.B.; Whiteside, E.P.; Cress, C.E. Seasonal variability of pH and lime requirements in several southern Michigan soils when measured in different ways. Soil Sci. Soc. Am. Proc. 1970, 34, 56–61. [Google Scholar] [CrossRef]
- Medappa, K.C.; Dana, M.N. Tolerance of Cranberry Plants to Manganese, Iron and Aluminum. J. Amer. Soc. Hort. Sci. 1970, 95, 107–110. [Google Scholar] [CrossRef]
- Reyes-Diaz, M.; Blancheteau, C.I.; Millaleo, R.; Cruces, E.; Zottele, C.W.; Alberdi, M.; dela Kuz Mora, M. Long-term Aluminum Exposure Effects on Physiological and Biochemical Features of Highbush Blueberry Cultivars. J. Amer. Soc. Hort. Sci. 2010, 135, 212–222. [Google Scholar] [CrossRef]
- Sandhya, K.; Prakash, N.B.; Meunier, J.D. Diatomaceous earth as source of silicon on the growth and yield of rice in contrasted soils of Southern India. J. Soil Sci. Plant Nutr. 2018, 18, 344–360. [Google Scholar] [CrossRef]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon Nutrition and Sugarcane Production: A Review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- USDA. National Organic Program. Potassium Silicate for Use in Crop Production; University of California, Sustainable Agriculture Research and Education Program (UC SAREP): Davis, CA, USA, 2003. Available online: https://www.ams.usda.gov/sites/default/files/media/Pot%20sil%20technical%20advisory%20panel%20report%202003.pdf (accessed on 4 April 2025).
- USEPA. Potassium Silicate. Biopesticides Registration Action Document; PC Code 072606; Office of Pesticide Programs, United State Environmental Protection Agency: Washington DC, USA, 2007. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-072606_7-Oct-07.pdf (accessed on 4 April 2025).
- Thakral, V.; Raturi, G.; Sudhakaran, S.; Mandlik, R.; Sharma, Y.; Shivaraj, S.M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Silicon, a quasi-essential element: Availability in soil, fertilizer regime, optimum dosage, and uptake in plants. Plant Physiol. Biochem. 2024, 208, 108459. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.E. Gypsum and Acid Soils. The World Scene. Adv. Agron. 1993, 51, 1–32. [Google Scholar]
- Liu, H.; Ma, M.; Huang, H.; Shaikh, A.S.; Bai, R.; Yang, Y.; Liu, Y.; Han, X. Research progress on the mechanism of magnesium nutrition alleviating aluminum toxicity in plants. J. Plant Nutr. Fertil. 2025, 31, 810–818. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Snyder, G.H. Methods for silicon analysis in plants, soils, and fertilizers. Stud. Plant Sci. 2011, 8, 185–196. [Google Scholar] [CrossRef]
- Wang, J.J.; Dodla, S.K.; Henderson, R.E. Soil silicon extractability with seven selected extractants in relation to colorimetric and ICP determination. Soil Sci. 2004, 169, 861–870. [Google Scholar] [CrossRef]
- Parent, L.E.; Marchand, S. Response to Phosphorus of Cranberry on High Phosphorus Testing Acid Sandy Soils. Soil Sci. Soc. Am. J. 2006, 70, 1914–1921. [Google Scholar] [CrossRef]
- Ketterings, Q.; Miyamoto, C.; Mathur, R.R.; Dietzel, K.; Gami, S. A Comparison of Soil Sulfur Extraction Methods. Soil Sci. Soc. Am. J. 2011, 75940, 1578–1583. [Google Scholar] [CrossRef]
- Zbíral, J.; Smatanová, M.; Němec, P. Sulphur status in agricultural soils determined using the Mehlich 3 method. Plant Soil Environ. 2018, 64, 255–259. [Google Scholar] [CrossRef]
- Kosola, K.R.; Workmaster, B.A.A. Mycorrhizal Colonization of Cranberry: Effects of Cultivar, Soil Type, and Leaf Litter Composition. J. Amer. Soc. Hort. Sci. 2007, 132, 134–141. [Google Scholar] [CrossRef]

| Element | 2016 | 2017 | 2018 | |||
|---|---|---|---|---|---|---|
| mean | sd | mean | sd | mean | sd | |
| S | 0.380 | 0.064 | 0.435 | 0.053 | 0.481 | 0.104 |
| N | 3.300 | 1.602 | 3.567 | 0.425 | 3.513 | 0.720 |
| P | 0.715 | 0.122 | 0.914 | 0.116 | 0.834 | 0.152 |
| K | 8.372 | 1.268 | 5.791 | 0.495 | 7.860 | 1.217 |
| Ca | 0.559 | 0.182 | 0.518 | 0.099 | 0.627 | 0.201 |
| Mg | 0.475 | 0.055 | 0.327 | 0.045 | 0.542 | 0.087 |
| B | 0.006 | 0.004 | 0.007 | 0.002 | 0.006 | 0.001 |
| Cu | 0.003 | 0.001 | 0.005 | 0.001 | 0.004 | 0.001 |
| Zn | 0.006 | 0.001 | 0.005 | 0.000 | 0.007 | 0.001 |
| Mn | 0.018 | 0.007 | 0.011 | 0.005 | 0.021 | 0.007 |
| Fe | 0.020 | 0.006 | 0.011 | 0.004 | 0.027 | 0.010 |
| Al | 0.248 | 0.355 | 0.015 | 0.007 | 0.018 | 0.006 |
| Si | 0.060 | 0.032 | 0.046 | 0.013 | 0.195 | 0.032 |
| Mg ha−1 | ||||||
| Yield | 38.6 | 12.2 | 20.1 | 11.0 | 37.1 | 10.3 |
| g (compression force) | ||||||
| Firmness | 5390 | 347 | 5521 | 467 | 6333 | 533 |
| Class | Machine Learning Model Relating the Target Class to wlr Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Random Forest | XGBoost | |||||||
| AUC | Accuracy | HYNB | LYNI | AUC | Accuracy | HYNB | LYNI | |
| Yielding | 0.832 | 0.791 | 47 | 264 | 0.851 | 0.796 | 47 | 266 |
| Firmness | 0.917 | 0.873 | 67 | 276 | 0.926 | 0.891 | 72 | 279 |
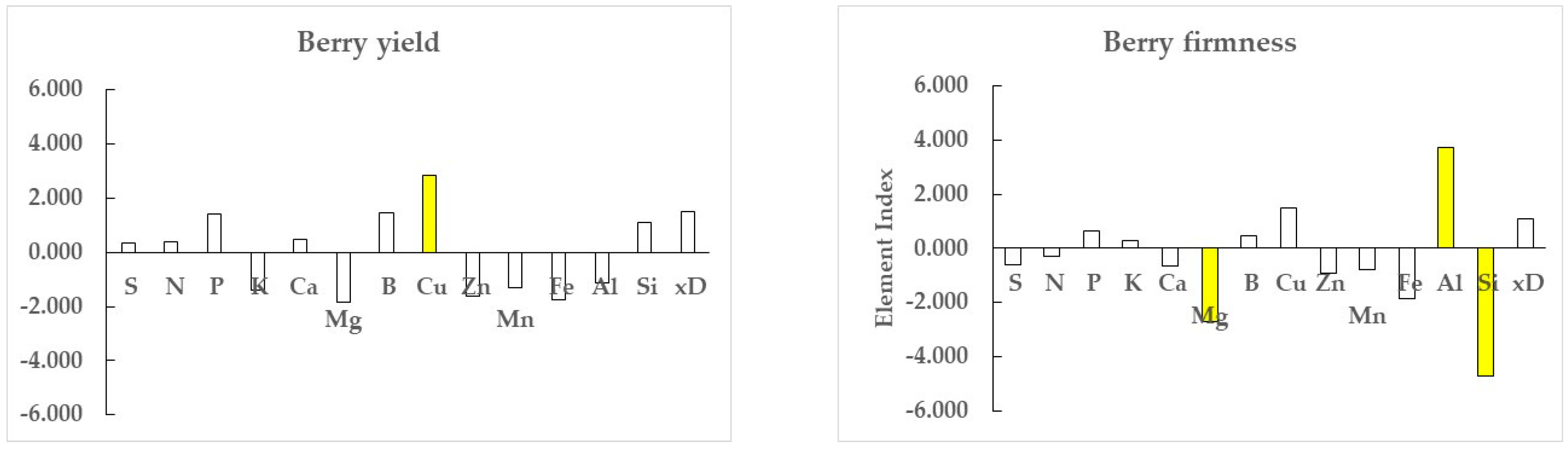
| Element | Firmness | Yield | Mean Difference | t-Test § | ||
|---|---|---|---|---|---|---|
| wlr mean | wlr sd | wlr mean | wlr sd | wlrfirmness − wlryield | Bilateral | |
| S | 0.059312 | 0.003225 | 0.056439 | 0.004698 | +0.002874 | *** |
| N | 0.089563 | 0.002888 | 0.087108 | 0.006188 | +0.002455 | * |
| P | 0.072344 | 0.003266 | 0.067212 | 0.006429 | +0.005132 | *** |
| K | 0.077613 | 0.002570 | 0.082549 | 0.004216 | −0.004936 | *** |
| Ca | 0.056079 | 0.003397 | 0.052858 | 0.004714 | +0.003221 | *** |
| Mg | 0.034284 | 0.002247 | 0.034209 | 0.003444 | +0.000075 | ns |
| B | −0.119389 | 0.004682 | −0.138039 | 0.017744 | +0.018650 | *** |
| Cu | −0.222593 | 0.005790 | −0.232493 | 0.008016 | +0.009900 | *** |
| Zn | −0.113897 | 0.002938 | −0.111281 | 0.003733 | −0.002616 | *** |
| Mn | −0.043308 | 0.005606 | −0.040380 | 0.006240 | −0.002928 | * |
| Fe | −0.065753 | 0.008715 | −0.069454 | 0.006994 | +0.003701 | * |
| Al | −0.068227 | 0.008426 | −0.010906 | 0.036754 | −0.057321 | *** |
| Si | 0.015177 | 0.002899 | −0.005233 | 0.010066 | +0.020410 | *** |
| xD | 0.223028 | 0.003215 | 0.221800 | 0.003634 | +0.001227 | † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parent, L.E. Networking 13 Berry Minerals to Sustain a High Yield of Firm Cranberry Fruits. Horticulturae 2025, 11, 705. https://doi.org/10.3390/horticulturae11060705
Parent LE. Networking 13 Berry Minerals to Sustain a High Yield of Firm Cranberry Fruits. Horticulturae. 2025; 11(6):705. https://doi.org/10.3390/horticulturae11060705
Chicago/Turabian StyleParent, Leon Etienne. 2025. "Networking 13 Berry Minerals to Sustain a High Yield of Firm Cranberry Fruits" Horticulturae 11, no. 6: 705. https://doi.org/10.3390/horticulturae11060705
APA StyleParent, L. E. (2025). Networking 13 Berry Minerals to Sustain a High Yield of Firm Cranberry Fruits. Horticulturae, 11(6), 705. https://doi.org/10.3390/horticulturae11060705






