Differential Bio-Elicitor Effects on Bioactive Compound Production in Cichorium intybus Root Callus Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Sterilization and Explant Preparation
2.2. Callus Induction
2.3. Fungal Elicitors
2.4. Measurements and Determinations
2.4.1. Characterization of Callus Induction
2.4.2. Determination of Callus Biomass Production
2.4.3. Determination of Total Phenolic Content
2.4.4. Determination of Total Flavonoid Content
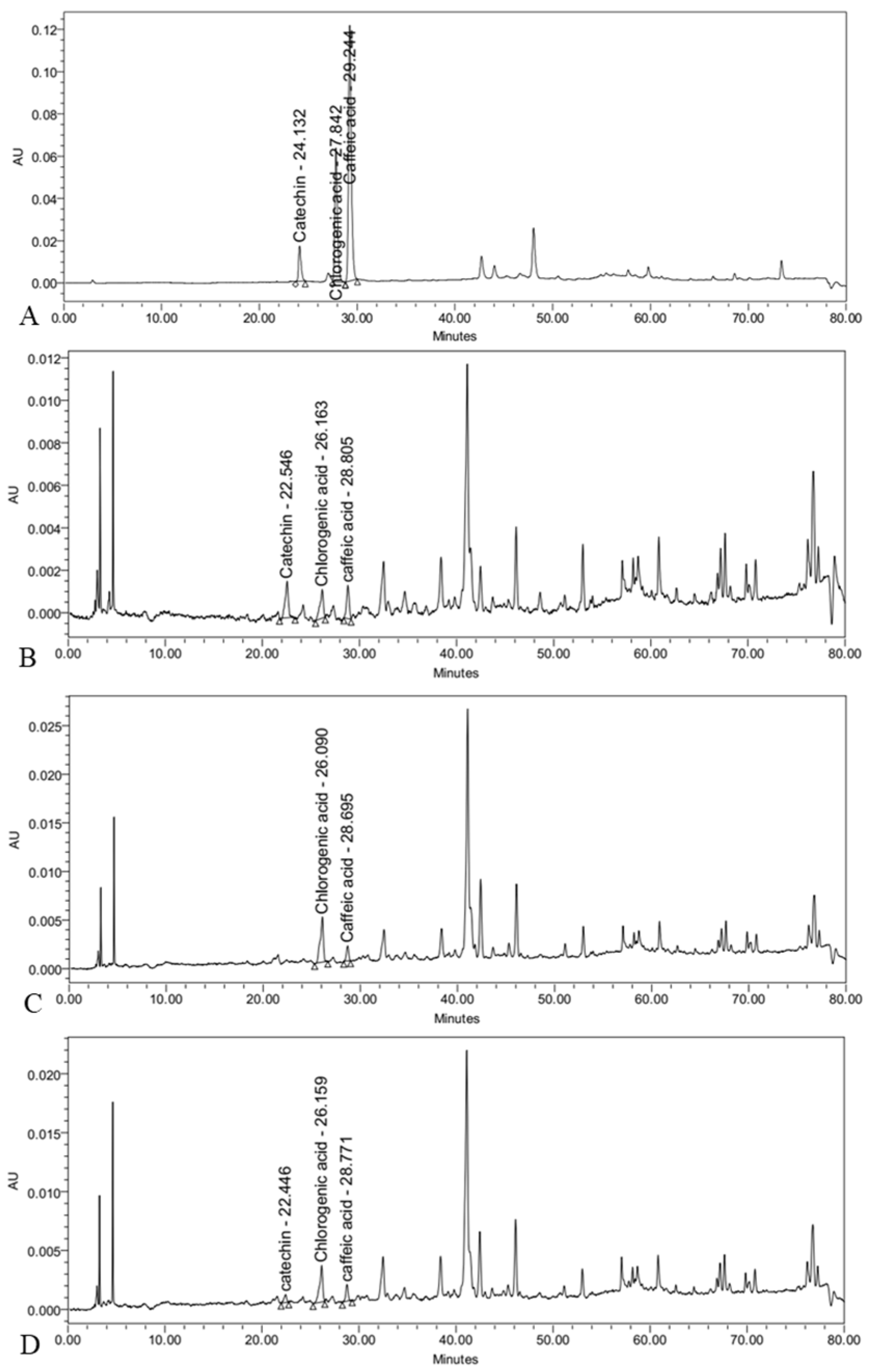
2.4.5. Determination of Chlorogenic Acid, Caffeic Acid, and Catechin
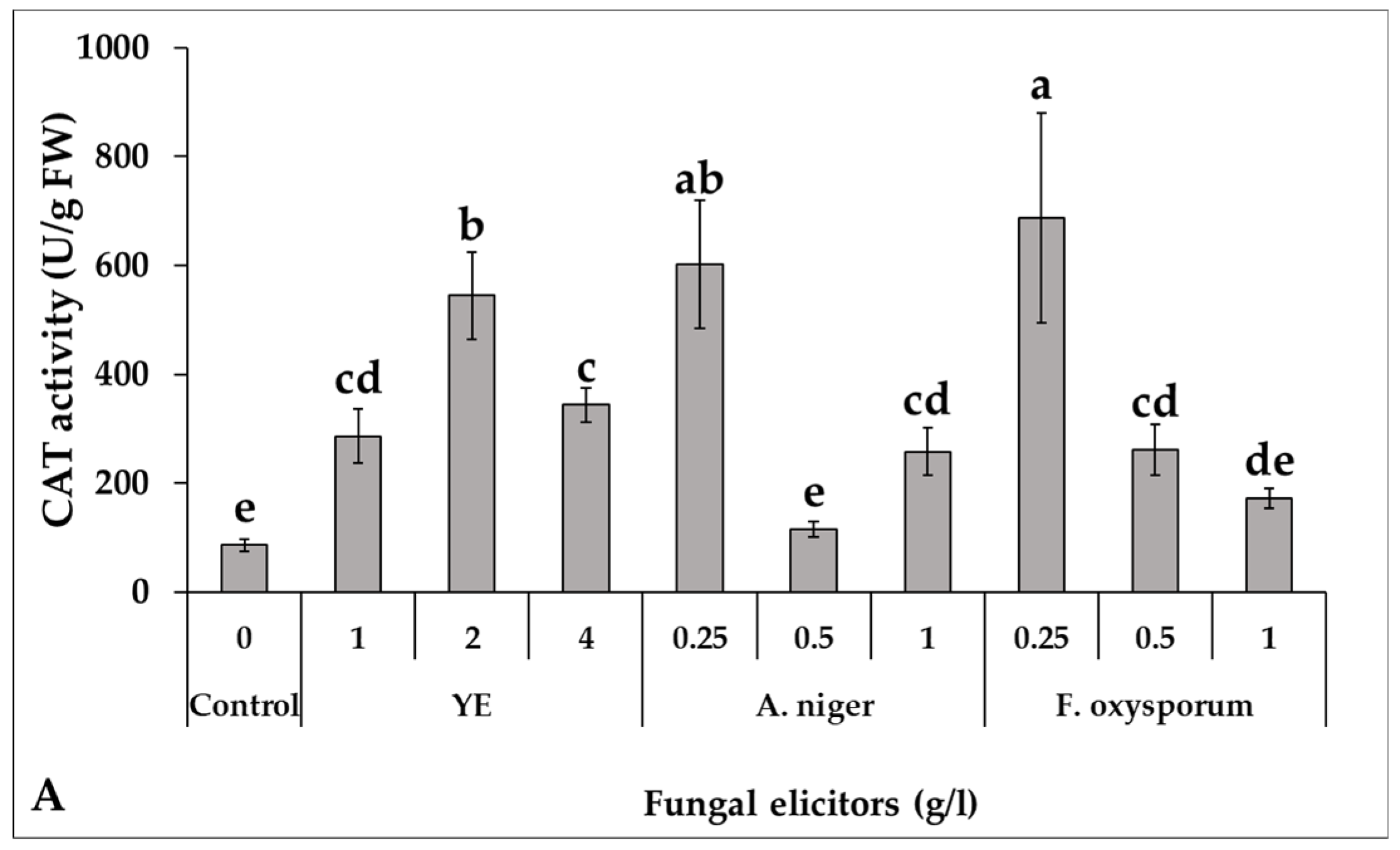
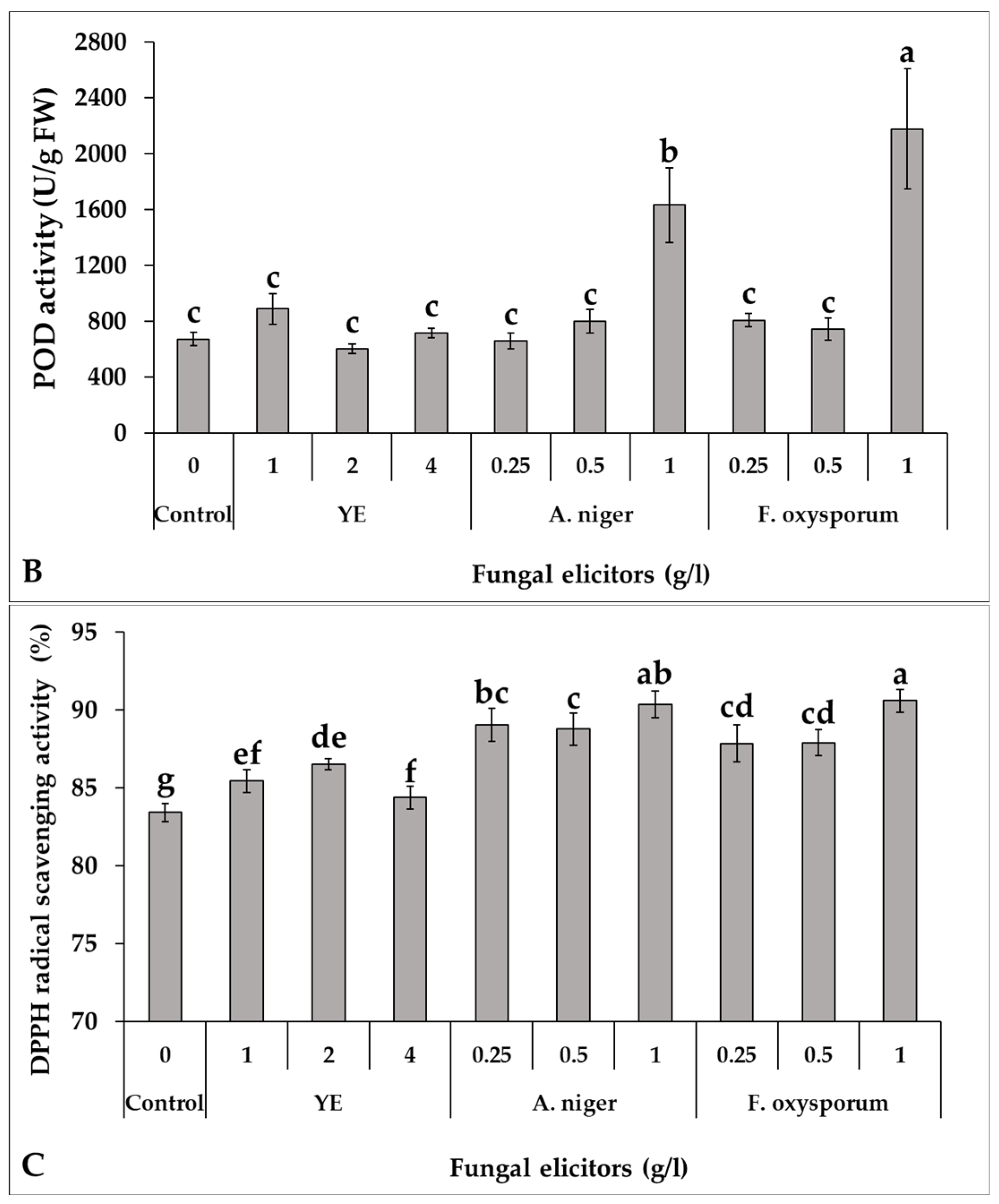
2.4.6. Determination of Antioxidant Enzymes Activity
2.4.7. Determination of Free Radical Scavenging Activity
2.5. The Statistical Analysis
3. Results
3.1. Characterization of Callus Induced by Root Explants Under Different Combinations of PGRs and Light Conditions
3.2. Effect of Fungal Elicitors on Callus Biomass Production
3.3. Effect of Fungal Elicitors on Total Phenolic and Total Flavonoid Production
3.4. Effect of Fungal Elicitors on Phenolic Compounds Content
3.5. Effect of Fungal Elicitors on the Activity of Some Antioxidant Enzymes
3.6. Effect of Fungal Elicitors on Antioxidant Activity
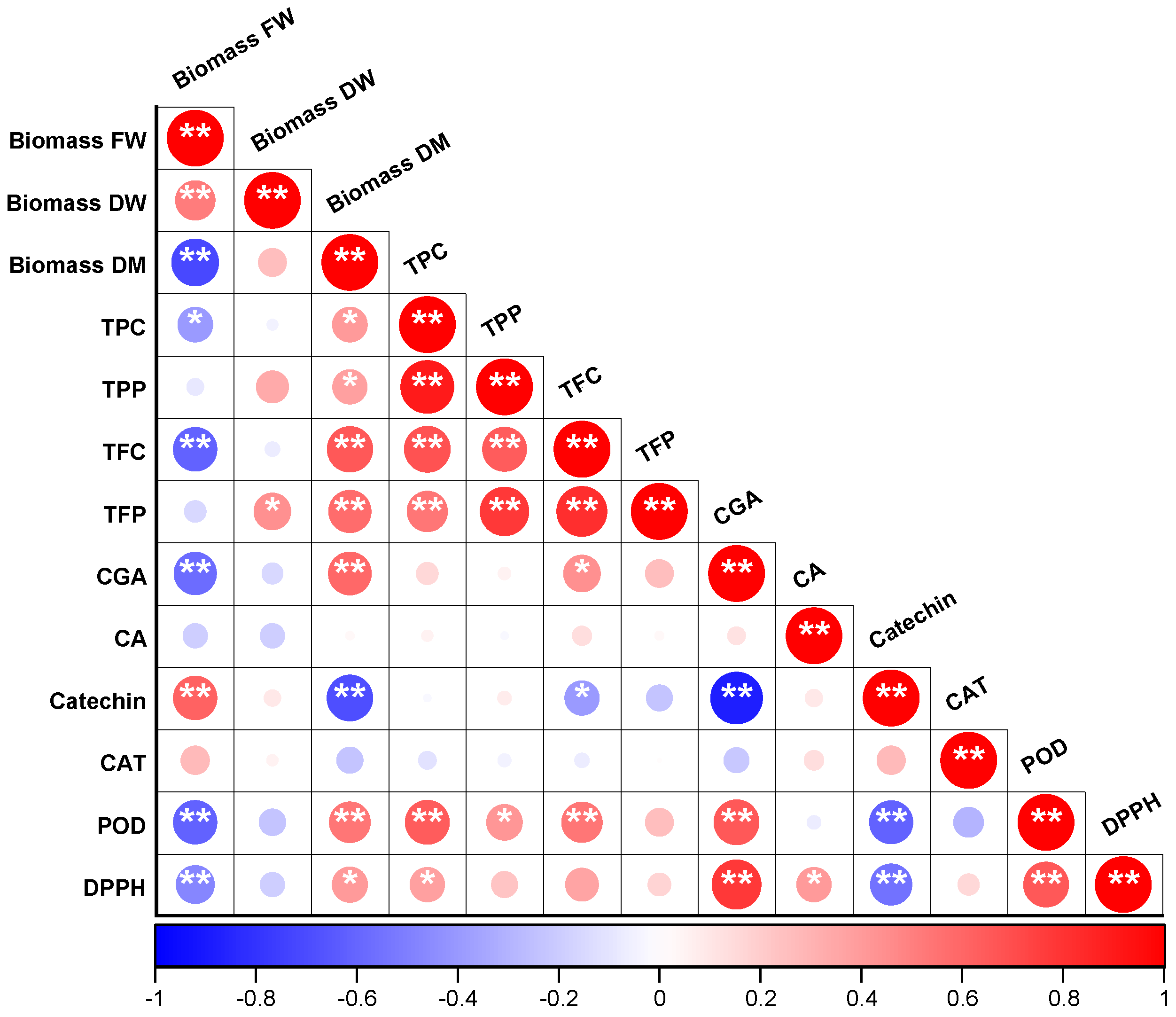
3.7. Pearson’s Correlation Analysis
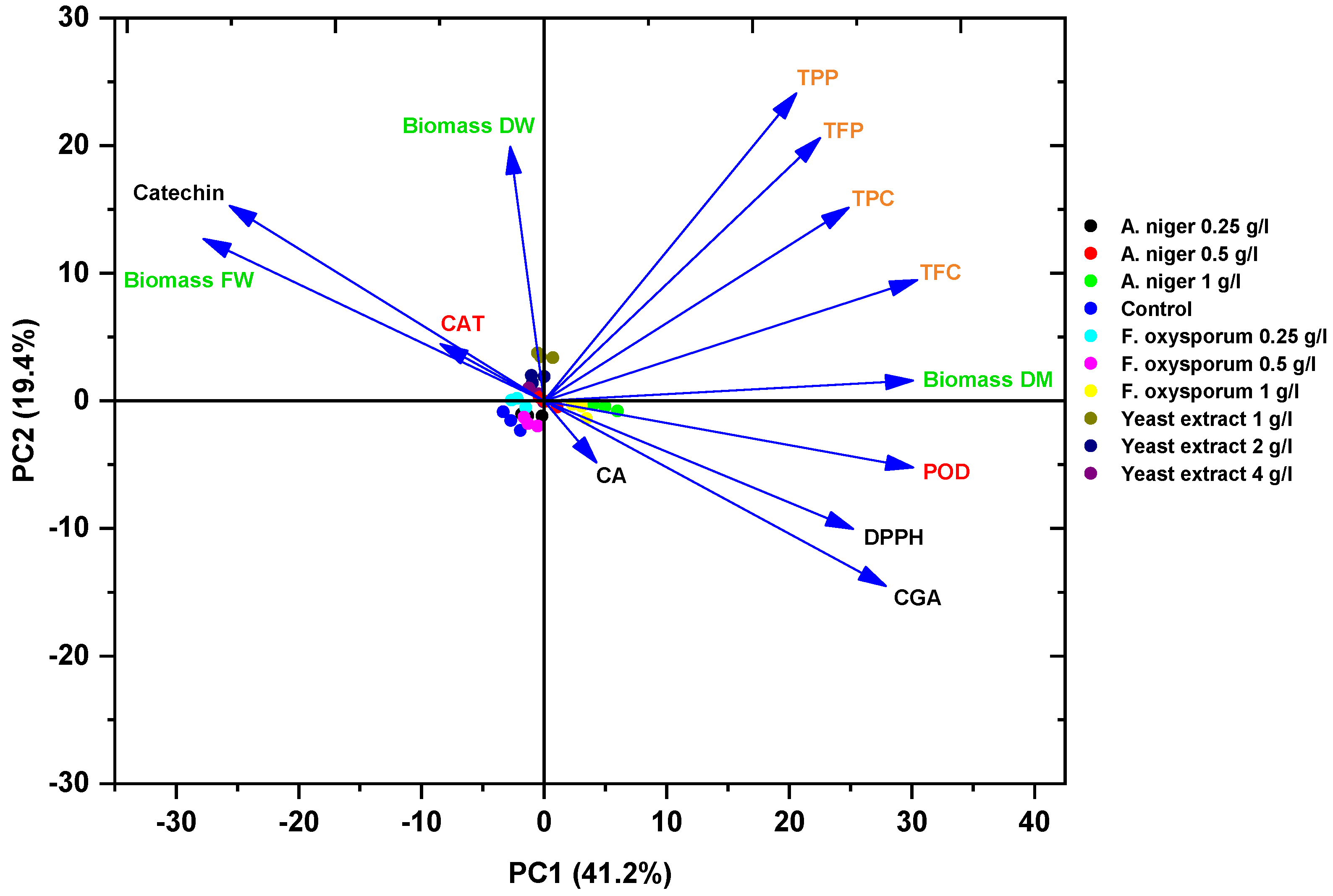
3.8. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Lin, W.; Ling, W.; Wang, D. Established atherosclerosis might be a prerequisite for chicory and its constituent protocatechuic acid to promote endothelium-dependent vasodilation in mice. Mol. Nutr. Food Res. 2016, 60, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, M.-L.; de Vos, W.M. Back to the roots: Revisiting the use of the fiber-rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef]
- Khan, A.M.A.; Chandra, K. Medicinal and Nutritional Importance of Cichorium intybus in Human Health. In Medicinal Plants and Their Bioactive Compounds in Human Health: Volume 1; Ansari, M.A., Shoaib, S., Islam, N., Eds.; Springer: Singapore, 2024; pp. 251–271. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies—Status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Morphological, phytochemical, and essential oil changes induced by different nitrogen supply forms and salinity stress in Echinacea purpurea L. Biocatal. Agric. Biotechnol. 2022, 43, 102396. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Sun, Y. Production of bioactive metabolites in in vitro cultures of saffron (Crocus sativus L.). In Biotechnological Production of Bioactive Phytochemicals of Medicinal Value: A Comprehensive Treatise; Kishor, P.B.K., Pullaiah, T., Suprasanna, P., Rao, A.R., Romano, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 17–65. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Sun, Y.; Nxumalo, W.; Gabr, A.M.M. Biotechnological production of silymarin in Silybum marianum L.: A review. Biocatal. Agric. Biotechnol. 2020, 29, 101775. [Google Scholar] [CrossRef]
- Gabr, A.M.M.; Mabrok, H.B.; Sytar, O.; Smetanska, I. Recent Advances Toward Development of Plant Cell Culture Process for Sustainable Production of Lignans and Their Health Benefits. In Exploring Plant Cells for the Production of Compounds of Interest; Malik, S., Ed.; Springer: Cham, Switzerland, 2021; pp. 249–289. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Arafa, N.M.; Moawad, M.; Aly, U.I. Gas chromatography-mass spectrometry analysis of phytocomponents present in Pimpinella anisum L. callus cultures as affected by yeast and phenylalanine application. Egypt. J. Chem. 2022, 65, 667–675. [Google Scholar] [CrossRef]
- Zafar, R.; Ali, S.M. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J. Ethnopharmacol. 1998, 63, 227–231. [Google Scholar] [CrossRef]
- Wadekar, P.P.; Salunkhe, V.R. Unveiling the therapeutic potential of Cichorium intybus callus: A phytochemical study. Int. J. Chem. Biochem. Sci. 2023, 24, 299–306. [Google Scholar]
- Abas, Y.A.; Eroğlu, A.; Dalar, A.; Türker, M.; Ozdemir, F.A.; Sołowski, G. Phenolic Compound Production Increased In Vitro Regenerated Cichorium intybus L. Appl. Biosci. 2023, 2, 84–93. [Google Scholar] [CrossRef]
- Selwal, N.; Supriadi, K.; Rahayu, F.; Sukmadjaja, D.; Khamidah, A.; Budiaarto, K.; Antarlina, S.S.; Tripatmasari, M.; Wani, A.K. Elicitation strategies for enhanced secondary metabolite synthesis in plant cell cultures and its role in plant defense mechanism. Plant Gene 2025, 41, 100485. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Saad, Z.H.; Eissa, M.A.; Shakir, U. Effect of chitosan and light conditions on the production of callus biomass, total flavonoids and total phenolics in Ginkgo biloba L. Al-Azhar J. Agric. Res. 2021, 46, 28–42. [Google Scholar] [CrossRef]
- Zarad, M.M.; Toaima, N.M.; Refaey, K.A.; Atta, R.F.; Elateeq, A.A. Copper sulfate and Cobalt chloride effect on total phenolics accumulation and antioxidant activity of Artemisia annua L. callus cultures. Al-Azhar J. Agric. Res. 2021, 46, 26–40. [Google Scholar] [CrossRef]
- Khan, T.; Javed, M.U.; Mahmood, T.; Khan, B.; Khan, T.; Asad Ullah, M.; Khurshid, R.; Zaman, G.; Hano, C.; Giglioli-Guivarc’h, N.; et al. Enhancement in the production of phenolic compounds from Fagonia indica callus cultures via Fusarium oxysporum triggered elicitation. Vitr. Cell. Dev. Biol. Plant 2024, 60, 16–27. [Google Scholar] [CrossRef]
- Elshahawy, O.A.M.; Zeawail, M.E.-F.; Hamza, M.A.; Elateeq, A.A.; Omar, M.A. Improving the Production of Total Phenolics and Flavonoids and the Antioxidant Capacity of Echinacea purpurea Callus through Biotic Elicitation. Egypt. J. Chem. 2022, 65, 137–149. [Google Scholar] [CrossRef]
- Lescano, L.; Cziáky, Z.; Custódio, L.; Rodrigues, M.J. Yeast extract elicitation enhances growth and metabolite production in Limonium algarvense callus cultures. Plant Cell Tissue Organ Cult. 2025, 160, 45. [Google Scholar] [CrossRef]
- Maqsood, M.; Abdul, M. Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Rev. Bras. Farmacogn. 2017, 27, 549–556. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Gregersen, P.L.; Mahmood, T. Expression analysis of the polyphenol oxidase gene in response to signaling molecules, herbivory and wounding in antisense transgenic tobacco plants. 3 Biotech 2019, 9, 55. [Google Scholar] [CrossRef]
- Savitha, B.C.; Thimmaraju, R.; Bhagyalakshmi, N.; Ravishankar, G.A. Different biotic and abiotic elicitors influence betalain production in hairy root cultures of Beta vulgaris in shake-flask and bioreactor. Process Biochem. 2006, 41, 50–60. [Google Scholar] [CrossRef]
- Sák, M.; Dokupilová, I.; Kaňuková, Š.; Mrkvová, M.; Mihálik, D.; Hauptvogel, P.; Kraic, J. Biotic and abiotic elicitors of stilbenes production in Vitis vinifera L. cell culture. Plants 2021, 10, 490. [Google Scholar] [CrossRef]
- Rehman, R.; Israr, M.; Srivastava, P.; Bansal, K.; Abdin, M. In vitro regeneration of witloof chicory (Cichorium intybus L.) from leaf explants and accumulation of esculin. Vitr. Cell. Dev. Biol.-Plant 2003, 39, 142–146. [Google Scholar] [CrossRef]
- Dakshayini, K.; Rao, C.V.; Karun, A.; Bhavyashree, U.; Ujwal, P. High-frequency plant regeneration and histological analysis of callus in Cichorium intybus: An important medicinal plant. J. Phytol. 2016, 8, 7–12. [Google Scholar] [CrossRef]
- Koohsari, A.; Chalavi, V.; Akbarpour, V. Effect of Explant Types and Growth Regulators on Callus induction and Secondary Metabolites of Chicory (Cichorium intybus L.). J. Plant Prod. Res. 2020, 27, 59–72. [Google Scholar] [CrossRef]
- Kirakosyan, R.N.; Sumin, A.V.; Polupanova, A.A.; Pankova, M.G.; Degtyareva, I.S.; Sleptsov, N.N.; Khuat, Q.V. Influence of Plant Growth Regulators and Artificial Light on the Growth and Accumulation of Inulin of Dedifferentiated Chicory (Cichorium intybus L.) Callus Cells. Life 2022, 12, 1524. [Google Scholar] [CrossRef]
- Abdelhamid, S.A.; Marzouk, A.I.; Asker, M.S.; El Shabrawi, H.M. RED light promotes flavonoid and phenolic accumulation in Cichorium spp. callus culture as anti-candida agent. Sci. Rep. 2025, 15, 2194. [Google Scholar] [CrossRef]
- Velayutham, P.; Ranjithakumari, B.; Baskaran, P. An efficient in vitro plant regeneration system for Cichorium intybus L.—An important medicinal plant. J. Agric. Technol. 2006, 2, 287–298. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Amako, K.; Chen, G.-X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Gabr, A.M.M.; Abdelkawy, A.M.; Toaima, N.M.; Bosila, H.A.; Zarad, M.M.; Ebrahim, H.S.; Jiao, J.; Pan, H.; Ullah, S.; et al. Establishment of Gypsophila paniculata root culture for biomass, saponin, and flavonoid production. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12886. [Google Scholar] [CrossRef]
- Abdelkawy, A.M.; Alshammari, S.O.; Hussein, H.-A.A.; Kenawy, S.K.M.; Abou El-Enain, I.M.M.; Abdelkhalek, E.S.; Meganid, A.S.; Elateeq, A.A. Hairy root cultures as a potential tool for the biosynthesis of active compounds in Catharanthus roseus. Pak. J. Bot. 2024, 56, 1567–1574. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Zhao, Y.; Sun, Y.; Li, Y.; Peng, Z. How does light regulate plant regeneration? Front. Plant Sci. 2025, 15, 1474431. [Google Scholar] [CrossRef]
- Hadizadeh, H.; Mohebodini, M.; Esmaeilpoor, B.; Chamani, E. Studies on Callus Induction and Regeneration of Medicinal Plant Chicory (Cichorium intybus L.) from Leaf and Petiole Explants. J. Hortic. Sci. 2016, 29, 621–630. [Google Scholar] [CrossRef]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Sudheer, W.N.; Nagella, P. Biotic elicitors influence boeravinone B production from cell suspension cultures of Boerhavia diffusa Linn. S. Afr. J. Bot. 2024, 174, 96–106. [Google Scholar] [CrossRef]
- Warhade, M.I.; Badere, R.S. Fusarium oxysporum cell elicitor enhances betalain content in the cell suspension culture of Celosia cristata. Physiol. Mol. Biol. Plants 2018, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Imbimbo, P.; D’Amelia, V.; Marzocchi, A.; Monti, D.M.; Di Loria, A.; Monti, S.M.; Novellino, E.; Tenore, G.C.; Rigano, M.M. Use of yeast extract to elicit a pulp-derived callus cultures from Annurca apple and potentiate its biological activity. J. Funct. Foods 2024, 112, 105988. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Shourie, A. Yeast extract-mediated elicitation of anti-cancerous compounds licoisoflavone B, licochalcone A, and liquirtigenin in callus cultures of Glycyrrhiza glabra. Biotechnologia 2019, 100, 441–451. [Google Scholar] [CrossRef]
- Zaman, G.; Farooq, U.; Bajwa, M.N.; Jan, H.; Shah, M.; Ahmad, R.; Andleeb, A.; Drouet, S.; Hano, C.; Abbasi, B.H. Effects of yeast extract on the production of phenylpropanoid metabolites in callus culture of purple basil (Ocimum Basilicum L. var purpurascens) and their in-vitro evaluation for antioxidant potential. Plant Cell Tissue Organ Cult. 2022, 150, 543–553. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Mewis, I.; Knorr, D.; Smetanska, I. Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. 2012, 108, 401–409. [Google Scholar] [CrossRef]
- Jasim, H.Y.J.; Habeeb, H.M. Effect of biotic and abiotic elicitors on Salvadora persica callus in vitro. Baghdad Sci. J. 2024, 21, 2829. [Google Scholar] [CrossRef]
- Zhong, L.; Niu, B.; Tang, L.; Chen, F.; Zhao, G.; Zhao, J. Effects of Polysaccharide Elicitors from Endophytic Fusarium oxysporum Fat9 on the Growth, Flavonoid Accumulation and Antioxidant Property of Fagopyrum tataricum Sprout Cultures. Molecules 2016, 21, 1590. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S. Biological elicitors of plant secondary metabolites: Mode of action and use in the production of nutraceutics. In Bio-Farms for Nutraceuticals. Advances in Experimental Medicine and Biology; Giardi, M.T., Rea, G., Berra, B., Eds.; Springer: Boston, MA, USA, 2010; Volume 698, pp. 152–166. [Google Scholar] [CrossRef]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary-secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244. [Google Scholar] [CrossRef]
- Bai, X.; Lee, H.-S.; Han, J.-E.; Murthy, H.N.; Park, S.-Y. Enhancement of Phenolic and Polyacetylene Accumulation in Lobelia chinensis (Chinese lobelia) Plantlet Cultures Through Yeast Extract and Salicylic Acid Elicitation. Horticulturae 2025, 11, 612. [Google Scholar] [CrossRef]
- Nabi, N.; Singh, S.; Saffeullah, P. Responses of in vitro cell cultures to elicitation: Regulatory role of jasmonic acid and methyl jasmonate: A review. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 341–355. [Google Scholar] [CrossRef]
- Chodisetti, B.; Rao, K.; Gandi, S.; Giri, A. Improved gymnemic acid production in the suspension cultures of Gymnema sylvestre through biotic elicitation. Plant Biotechnol. Rep. 2013, 7, 519–525. [Google Scholar] [CrossRef]
- Shilpa, K.; Lakshmi, B.S. Influence of exogenous cinnamic acid on the production of chlorogenic acid in Cichorium intybus L cell culture. S. Afr. J. Bot. 2019, 125, 527–532. [Google Scholar] [CrossRef]
- Sarkate, A.; Banerjee, S.; Mir, J.I.; Roy, P.; Sircar, D. Antioxidant and cytotoxic activity of bioactive phenolic metabolites isolated from the yeast-extract treated cell culture of apple. Plant Cell Tissue Organ Cult. 2017, 130, 641–649. [Google Scholar] [CrossRef]
- Dhiman, V.; Singh, D.; Dhiman, V.K.; Verma, S.K.; Pandey, H. Reactive Oxygen Species (ROS) and Biotic Stress: Evolving Roles Under Climate Change. In Climate Change and Biotic Factors; Singh, A., Pandey, S., Kumar, A., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 201–218. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Garros, L.; Drouet, S.; Zahir, A.; Ahmad, W.; Giglioli-Guivarc’h, N.; Hano, C. Yeast-extract improved biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2018, 135, 347–355. [Google Scholar] [CrossRef]

| PGRs (mg/L) | Callus Color | Callus Texture | Adventitious Root Formation | Adventitious Shoot Formation | |||||
|---|---|---|---|---|---|---|---|---|---|
| NAA | BA | 16/8 h Light/Dark Cycle | Complete Dark | 16/8 h Light/Dark Cycle | Complete Dark | 16/8 h Light/Dark Cycle | Complete Dark | 16/8 h Light/Dark Cycle | Complete Dark |
| 1 | 0 | Light yellow tinged with light violet | Light brown with light violet | Friable | Friable | +++ | ++++ | - | - |
| 1 | 1 | Yellowish green | Light yellow | Friable | Compact | + | + | - | - |
| 1 | 2 | Yellowish white tinged with light violet | Light yellow | Compact | Compact | - | - | ++++ | - |
| 2 | 0 | Light yellow with light violet | Light brown with light violet | Soft | Friable | ++ | ++++ | - | - |
| 2 | 1 | Yellowish green | Light yellow | Friable | Semi-compacted | ++ | - | - | - |
| 2 | 2 | Yellowish white tinged with light violet | Light yellow | Compact | Compact | - | - | ++++ | - |
| Treatments | Fresh Weight (g/Explant) | Dr Weight (g/Explant) | Dry Matter (%) | |||
|---|---|---|---|---|---|---|
| Light conditions (LC) | 16/8 h light/dark cycle | 1.21 ± 0.27 a | 0.083 ± 0.013 a | 7.02 ± 1.00 b | ||
| Complete dark | 0.72 ± 0.25 b | 0.061 ± 0.016 b | 8.58 ± 0.92 a | |||
| PGRs (mg/L) | NAA | BA | ||||
| 1 | 0 | 0.88 ± 0.39 c | 0.067 ± 0.021 cd | 8.19 ± 1.45 b | ||
| 1 | 1 | 0.65 ± 0.09 d | 0.059 ± 0.011 d | 9.04 ± 0.85 a | ||
| 1 | 2 | 0.98 ± 0.25 c | 0.076 ± 0.011 bc | 7.91 ± 1.09 bc | ||
| 2 | 0 | 0.93 ± 0.50 c | 0.062 ± 0.025 d | 7.20 ± 1.23 cd | ||
| 2 | 1 | 1.12 ± 0.39 b | 0.080 ± 0.019 ab | 7.46 ± 1.14 bcd | ||
| 2 | 2 | 1.25 ± 0.12 a | 0.087 ± 0.006 a | 6.97 ± 0.60 d | ||
| LC × PGRs | 16/8 h light/dark cycle | 1 | 0 | 1.23 ± 0.07 bcd | 0.086 ± 0.007 a | 6.96 ± 0.61 de |
| 1 | 1 | 0.67 ± 0.12 ef | 0.060 ± 0.017 bc | 8.78 ± 1.16 ab | ||
| 1 | 2 | 1.20 ± 0.02 cd | 0.084 ± 0.004 a | 6.96 ± 0.40 de | ||
| 2 | 0 | 1.38 ± 0.05 ab | 0.085 ± 0.001 a | 6.15 ± 0.15 e | ||
| 2 | 1 | 1.45 ± 0.11 a | 0.096 ± 0.007 a | 6.62 ± 0.34 de | ||
| 2 | 2 | 1.34 ± 0.06 abc | 0.089 ± 0.003 a | 6.64 ± 0.40 de | ||
| Complete dark | 1 | 0 | 0.52 ± 0.06 fg | 0.049 ± 0.003 cd | 9.43 ± 0.56 a | |
| 1 | 1 | 0.63 ± 0.07 efg | 0.059 ± 0.004 bc | 9.30 ± 0.49 ab | ||
| 1 | 2 | 0.76 ± 0.07 e | 0.067 ± 0.008 b | 8.87 ± 0.30 ab | ||
| 2 | 0 | 0.48 ± 0.08 g | 0.039 ± 0.004 d | 8.26 ± 0.66 bc | ||
| 2 | 1 | 0.80 ± 0.23 e | 0.065 ± 0.011 b | 8.29 ± 1.01 bc | ||
| 2 | 2 | 1.17 ± 0.08 d | 0.085 ± 0.008 a | 7.30 ± 0.64 cd | ||
| Significance | LC | *** | *** | *** | ||
| PGRs | *** | *** | *** | |||
| LC × PGRs | *** | *** | ns | |||
| Fungal Elicitors (g/L) | Callus Biomass FW (g/L Medium) | Callus Biomass DW (g/L Medium) | Callus Biomass DM (%) | |
|---|---|---|---|---|
| Control | 0 | 220.73 ± 29.21 b | 12.38 ± 1.49 c | 5.62 ± 0.31 d |
| Yeast extract | 1 | 251.91 ± 16.73 a | 15.15 ± 0.55 a | 6.03 ± 0.30 cd |
| 2 | 218.90 ± 10.41 b | 13.58 ± 1.46 abc | 6.22 ± 0.76 bcd | |
| 4 | 194.18 ± 18.76 bc | 13.44 ± 0.93 abc | 6.93 ± 0.23 b | |
| Aspergillus niger | 0.25 | 214.29 ± 17.85 b | 12.94 ± 0.57 bc | 6.08 ± 0.70 cd |
| 0.5 | 217.89 ± 15.07 b | 14.64 ± 1.60 ab | 6.71 ± 0.39 bc | |
| 1 | 164.28 ± 13.29 d | 13.86 ± 0.94 abc | 8.44 ± 0.13 a | |
| Fusarium oxysporum | 0.25 | 264.04 ± 21.39 a | 14.58 ± 1.51 ab | 5.52 ± 0.14 d |
| 0.5 | 217.35 ± 10.83 b | 11.94 ± 2.02 c | 5.48 ± 0.74 d | |
| 1 | 177.13 ± 10.31 cd | 12.21 ± 0.90 c | 6.89 ± 0.14 b | |
| Significance | *** | ns | *** | |
| Fungal Elicitors (g/L) | Total Phenolic Content (mg GAE/g DW) | Total Phenolic Productivity (mg GAE/L Medium) | Total Flavonoid Content (mg QE/g DW) | Total Flavonoid Productivity (mg QE/L Medium) | |
|---|---|---|---|---|---|
| Control | 0 | 8.04 ± 0.46 d | 99.28 ± 3.97 g | 4.79 ± 0.23 e | 59.19 ± 2.61 g |
| Yeast extract | 1 | 13.78 ± 0.56 a | 208.59 ± 2.44 a | 5.71 ± 0.23 b | 86.50 ± 0.98 b |
| 2 | 11.98 ± 0.57 b | 162.43 ± 2.12 c | 5.82 ± 0.28 b | 78.97 ± 0.91 c | |
| 4 | 10.77 ± 0.24 c | 144.50 ± 6.88 d | 5.47 ± 0.34 bcd | 73.24 ± 0.88 de | |
| Aspergillus niger | 0.25 | 10.16 ± 0.63 c | 131.31 ± 3.76 e | 4.76 ± 0.22 e | 61.52 ± 1.28 g |
| 0.5 | 10.24 ± 0.50 c | 149.85 ± 3.17 d | 5.19 ± 0.40 cde | 75.85 ± 3.69 cd | |
| 1 | 11.83 ± 0.83 b | 163.79 ± 8.91 c | 6.62 ± 0.21 a | 91.66 ± 1.51 a | |
| Fusarium oxysporum | 0.25 | 8.53 ± 0.49 d | 124.31 ± 3.95 ef | 4.83 ± 0.28 e | 70.32 ± 2.28 ef |
| 0.5 | 10.20 ± 0.53 c | 121.53 ± 2.83 f | 5.02 ± 0.57 de | 59.72 ± 3.58 g | |
| 1 | 14.24 ± 0.78 a | 173.45 ± 5.48 b | 5.61 ± 0.10 bc | 68.38 ± 3.90 f | |
| Significance | *** | *** | *** | *** | |
| Fungal Elicitors (g/L) | Chlorogenic Acid (µg/g DW) | Caffeic Acid (µg/g DW) | Catechin (µg/g DW) | |
|---|---|---|---|---|
| Control | 0 | 173.69 ± 10.60 d | 101.30 ± 6.85 c | 307.24 ± 9.00 c |
| Yeast extract | 1 | 156.22 ± 4.46 e | 105.26 ± 5.41 bc | 403.95 ± 11.70 b |
| 2 | 145.17 ± 7.71 ef | 110.72 ± 4.28 bc | 429.89 ± 5.98 a | |
| 4 | 133.62 ± 9.66 f | 110.56 ± 5.71 bc | 279.39 ± 12.04 de | |
| Aspergillus niger | 0.25 | 199.49 ± 11.49 c | 110.07 ± 7.09 bc | 287.15 ± 6.70 d |
| 0.5 | 206.48 ± 5.47 bc | 123.73 ± 9.30 a | 281.86 ± 4.04 de | |
| 1 | 295.64 ± 15.60 a | 106.65 ± 7.30 bc | - | |
| Fusarium oxysporum | 0.25 | 180.86 ± 4.01 d | 109.74 ± 2.06 bc | 289.47 ± 11.79 cd |
| 0.5 | 198.22 ± 5.45 c | 113.75 ± 3.84 ab | 267.77 ± 8.80 e | |
| 1 | 221.26 ± 6.43 b | 105.74 ± 7.69 bc | 207.50 ± 19.51 f | |
| Significance | *** | * | *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elateeq, A.A.; Zarad, M.M.; Gabr, A.M.M.; Ebrahim, H.S.; Ullah, S.; Elhamamsy, S.M.; Nada, R.S.; Saad, Z.H.; Soliman, M.N.A.; El-khawaga, H.A.; et al. Differential Bio-Elicitor Effects on Bioactive Compound Production in Cichorium intybus Root Callus Cultures. Horticulturae 2025, 11, 678. https://doi.org/10.3390/horticulturae11060678
Elateeq AA, Zarad MM, Gabr AMM, Ebrahim HS, Ullah S, Elhamamsy SM, Nada RS, Saad ZH, Soliman MNA, El-khawaga HA, et al. Differential Bio-Elicitor Effects on Bioactive Compound Production in Cichorium intybus Root Callus Cultures. Horticulturae. 2025; 11(6):678. https://doi.org/10.3390/horticulturae11060678
Chicago/Turabian StyleElateeq, Ahmed A., Mostafa M. Zarad, Ahmed M. M. Gabr, Hanan S. Ebrahim, Shakir Ullah, Sam M. Elhamamsy, Ramy S. Nada, Zakaria H. Saad, Mahmoud N. A. Soliman, Hend A. El-khawaga, and et al. 2025. "Differential Bio-Elicitor Effects on Bioactive Compound Production in Cichorium intybus Root Callus Cultures" Horticulturae 11, no. 6: 678. https://doi.org/10.3390/horticulturae11060678
APA StyleElateeq, A. A., Zarad, M. M., Gabr, A. M. M., Ebrahim, H. S., Ullah, S., Elhamamsy, S. M., Nada, R. S., Saad, Z. H., Soliman, M. N. A., El-khawaga, H. A., Alshammari, W. S., Tanko, W. S., & Hussein, H.-A. A. (2025). Differential Bio-Elicitor Effects on Bioactive Compound Production in Cichorium intybus Root Callus Cultures. Horticulturae, 11(6), 678. https://doi.org/10.3390/horticulturae11060678







