Abstract
Anthocyanins, as natural pigments belonging to the flavonoid group, play a crucial role in plant reproduction, stress resistance and human fitness. Kiwifruit, which is rich in anthocyanins, demonstrates significant potential for promoting health benefits. Although light is widely recognized as an inducer of anthocyanin accumulation, we observed that kiwifruit accumulates more anthocyanin after bagging treatment. This unexpected finding suggests that anthocyanin biosynthesis in kiwifruit may also be regulated by other environmental or physiological factors influenced by bagging, such as humidity, temperature, or gas exchange. This implies that bagging may trigger specific regulatory pathways that promote anthocyanin accumulation through multiple environmental cues beyond light. Therefore, RNA-seq was performed to find the potential pathway. A total of 260 differentially expressed genes were found, including 8 transcription factors and 1 anthocyanin biosynthesis gene F3GT1 (glucosyltransferase). Dual-luciferase reporter assays revealed that bHLH transcription factors could activate the promoter of F3GT1 by 2.45-fold. We infer that bagging treatment increases the kiwifruit anthocyanin content through the bHLH291-F3GT1 pathway. This study not only highlights the potential agricultural applications and commercial value of bagging treatment but also provides new theoretical support for improving fruit coloration and optimizing breeding strategies.
1. Introduction
Anthocyanins are a crucial group of secondary metabolites in plants, also recognized as one of the most important water-soluble plant pigments. These compounds typically accumulate in specific plant tissues, including leaves, fruits, flowers and seeds, where they provide pigmentation [1,2]. Anthocyanins are responsible for a wide range of colors in plants, from orange and red to blue and purple. In addition to their role in coloration, anthocyanins assist plants in defending against ultraviolet light, excessive sunlight, and certain pathogens [3]. In nutritive tissues, anthocyanins serve a photoprotective function by providing a light absorption barrier for photosynthetic cells. The accumulation of anthocyanins for this protective function is balanced with the light requirements for optimal photosynthesis. In addition, anthocyanins play a critical role in eliminating reactive oxygen species under stress conditions [4]. Moreover, anthocyanins are utilized in the development of color-based visual assessment methods for identifying various compounds. Their role in plant responses to a wide range of environmental stresses, such as drought, cold, and UV radiation, is well established [5]. Furthermore, anthocyanins serve as visual cues for pollinators and seed dispersers, which are essential for the plant’s reproductive success [6]. In addition to their ecological roles, anthocyanins in fruits are potent dietary antioxidants, providing health benefits to humans by mitigating oxidative stress, reducing inflammation, and lowering the risk of chronic diseases such as cardiovascular disorders and diabetes [7,8,9]. Therefore, increasing the anthocyanin content in kiwifruit (Actinidia spp.) holds significant importance from both nutritional and commercial perspectives [10].
Recent studies have widely revealed that anthocyanin accumulation is tightly regulated by environmental factors, including hormone, temperature, abiotic stresses and so on [11,12,13]. Among these, light is an essential factor influencing athocyanin accumulation during fruit development [14,15]. The light-induced regulation of anthocyanin biosynthesis in the callus of red-fleshed apples is primarily attributed to alterations in MYB10 transcriptional levels [16]. Under high-intensity white light conditions, the accumulation of anthocyanins in rough bluegrass can increase up to 100 times compared to normal light conditions [17]. Strong light promotes the expression of structural genes involved in anthocyanin biosynthesis in Arabidopsis thaliana, including AtCHS, AtF3H, and AtDFR, as well as regulatory genes AtPAP1 and AtPAP2 (both of which are MYB transcription factors), thereby enhancing anthocyanin content in seedlings and rosette leaves [18].
Bagging treatment is a widely adopted horticultural practice aimed at enhancing fruit appearance, protecting against pests and sunburn, and minimizing pesticide residue. The biosynthetic pathway of anthocyanins has been well characterized, proceeding through the flavonoid branch of the phenylpropanoid pathway, with key enzymes including dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), and flavonoid 3-O-glucosyltransferase (F3GT). Among these, glucosyltransferase plays a pivotal role in contributing to the stability, solubility, and functional diversity of anthocyanins [19]. Glycosyltransferase F3GT has been identified as a major regulatory point in anthocyanin production in litchis [20]. Similarly, the glycosylation-related gene LcF3GT1 is essential for red pigmentation in litchi pericarp, supported by a significant positive relationship between F3GT enzyme activity and anthocyanin levels observed in 15 different litchi varieties [21].
Anthocyanin biosynthesis is primarily regulated by a central regulatory complex consisting of MYB, bHLH and WD40 proteins (MBW) [22]. In addition to MYB transcription factors, bHLH transcription factors have also been extensively reported to be involved in the metabolism of anthocyanins in fruits [23,24]. Proteins in this family typically contain two conserved domains, containing a basic DNA-binding domain and a helix–loop–helix (HLH) domain [25]. bHLH can regulate the structural genes of the anthocyanin synthesis pathway by forming protein complexes with MYB transcription factors. For instance, the co-expression of MdMYBPA1 and MdbHLH33 in apple calli increases anthocyanin production under low-temperature conditions because MdbHLH33 can directly bind to the low-temperature-responsive (LTR) cis-element of the MdMYBPA1 promoter and promotes its activity [26]. What is more, MdMYB308L’s interaction with MdbHLH33 enables MdMYB308L to positively regulate both cold tolerance and anthocyanin accumulation in apple [27]. Our investigation unexpectedly discovered that the inner pericarp of bagged kiwifruit turned redder. The anthocyanin content of bagged kiwifruit is also higher than that of unbagged ones. RNA-seq was performed to explore the possible regulatory mechanisms. The RNA-seq results revealed that F3GT1 was up-regulated in the bagged kiwifruit. Moreover, we found that the promoter of F3GT1 was activated by bHLH291. EMSA further confirmed that the bHLH291 protein directly binds to the E-box motif within the F3GT1 promoter. Hence, we concluded that bagging treatment up-regulates anthocyanin content in kiwifruit via the bHLH291-F3GT1 pathway. Accordingly, this study aimed to uncover the regulatory pathway by which bagging enhances anthocyanin accumulation, particularly through identifying key transcription factors and their target genes.
2. Materials and Methods
2.1. Plant Material and Treatment
The ‘Hongshi’ (HS) kiwifruit (Actinidia chinensis Planch) at the color-changing stage (initiated around 5 weeks after pollination, with visible red pigmentation in the flesh, at which stage the bagging treatment applied) was used in this study due to its high anthocyanin content and consistent coloration, making it a suitable model for investigating pigment regulation. It was collected from a commercial orchard located in Chengdu, Sichuan Province (at 31.25° N, 104.11° E), in 2022. The region has a humid subtropical monsoon climate, with an average temperature of 16.2 °C and annual precipitation of around 1000 mm, which is an ideal environment for kiwifruit growth. The color-changing stage in kiwifruit development has been linked to dynamic changes in anthocyanin content, especially pelargonidin and cyanidin derivatives, as observed in yellow-fleshed cultivars [28]. Double-layer light-blocking paper bags made of natural wood pulp paper were used for the bagging treatment. These bags not only block light and protect against rain but also allow for air permeability and ventilation, which is essential for the healthy growth of kiwifruit. The treatment was initiated at 35 days after full bloom, which corresponds to approximately 5 weeks after pollination, and continued through to subsequent sampling stages. Fruits with similar sizes and consistent growth conditions and free from physiological diseases and mechanical damage were selected for subsequent experiments. Fruits were sampled at multiple post-pollination stages, including 9, 11, 13, 15, 17, 19, 21, and 23 weeks after pollination, for both bagged and non-bagged treated fruit. At each sampling point, there were eighteen bagged and eighteen unbagged fruits, respectively, which were divided into three groups as biological replicates. The inner pericarp of kiwifruit was cut into small pieces, rapidly frozen in liquid nitrogen, and stored at −80 °C for further experiments.
2.2. Extraction and Quantification of Anthocyanins
Kiwifruit samples were ground into powder using liquid nitrogen, and approximately 0.2 g of the samples were weighed and transferred to a centrifuge tube. Subsequently, we added 600 μL of acidified methanol solution (containing 1% HCl) to tubes and mixed. The mixtures were then incubated overnight at 4 °C in the dark. The next day, 400 μL of ddH2O was added to the tubes to dilute the methanol solution to 60% concentration, followed by the addition of 1 mL of chloroform. The mixtures were then thoroughly vortexed. Then, they were centrifuged at 6000 rpm for 5 min at 4 °C. After centrifugation, we transferred 800 μL of the supernatant to a clean tube and diluted it with 60% methanol solution (containing 1% HCl). A standard curve was prepared using purified cyanidin-3-O-glucoside (Shanghai Macklin Biochemical Co., Ltd., Shanghai, China). The absorbance (A) of the samples was measured at wavelengths of 530 nm and 657 nm using a spectrophotometer (Synergy H1, Bio Tek, Winooski, VT, USA). The anthocyanin content was calculated using the following formula: [(A530 − A657) × dilution factor]/(mg fresh weight tissue) × 1000 [6].
2.3. Extraction of Total RNA from Kiwifruit Samples
The total RNA of the flesh of kiwifruit was extracted by using the CTAB (cetyltrimethylammonium bromide) method [29]. Approximately 0.5 g kiwifruit samples were weighed and transferred to a centrifuge tube. Subsequently, 4 ml of pre-heated CTAB buffer (containing 5% β-mercaptoethanol) was added and placed at 65 °C for 10 min. The samples were then centrifuged at 12,000 rpm for 10 min at room temperature. The supernatant was transferred to a new tube and mixed with an equal volume of chloroform/isopentanol (24:1, v/v) and then centrifuged at 12,000 rpm for 10 min at 4 °C. This chloroform/isopentanol extraction step (mixing and centrifugation) was repeated once. And then the supernatant was carefully transferred to a new tube, followed by the addition of an equal volume of isopropanol. The mixtures were incubated at −20 °C for 2 h, followed by centrifugation at 12,000 rpm for 15 min at 4 °C. And then the supernatant was removed. The RNA pellets were washed by 75% ethanol and centrifuged at 12,000 rpm for 5 min at 4 °C. The 75% ethanol was subsequently removed, and the RNA pellets were dried. Finally, 20 μL of RNase-free H2O was added to the tubes to obtain the RNA. RNA concentration and purity were assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), with absorbance ratios A260/A280 and A260/A230 used as quality indicators. RNA integrity was further confirmed by 1.2% agarose gel electrophoresis.
2.4. Transcriptome Sequencing
Fruit samples with and without bagging at 9, 11 and 13 weeks after pollination were selected for RNA sequencing, with each point including three biological replicates. The RNA-seq libraries were constructed by BGI (Wuhan, China) and sequenced using the DNBSEQ sequencing platform. Libraries were sequenced with 150 bp paired-end reads. Each RNA-seq library yielded between 38.92 and 42.9 million clean reads, with total clean bases ranging from 5.87 to 6.43 Gb. The Q20 scores for clean reads exceeded 95.83% for all samples, indicating high sequencing quality. The Red5 (Actinidia chinensis) genome was used as the reference genome [30]. Clean reads were aligned to the Red5 genome using HISAT v2.1.0, with an average mapping rate of 95.18%. The gene expression was represented by FPKM (Fragments Per kb Per Million Reads). Differentially expressed genes (DEGs) were analyzed by DEseq with |Log2FC| ≥ 1 and FDR ≤ 0.001.
2.5. Phylogenetic Tree Analysis
Phylogenetic trees are made by using identified kiwifruit bHLH and MYB genes as well as arabidopsis bHLH and MYB genes. The bHLH and MYB amino acid sequences of arabidopsis were obtained from the PlantTFDB database (https://planttfdb.gao-lab.org/, accessed on 7 June 2024). Amino acid sequences were aligned using the MUSCLE algorithm in MEGA 11 software. Evolutionary relationships among species and genes were inferred by calculating evolutionary distances using the Neighbor-Joining method. The reliability of the phylogenetic trees was assessed through a bootstrapping approach with 1000 replicates. Midpoint rooting was applied to the tree for an improved visualization and interpretation of evolutionary distances. Phylogenetic trees were constructed using MEGA11 software [31] and visualized with the iTOL platform (https://itol.embl.de/, accessed on 11 March 2025) [32].
2.6. First-Strand cDNA Synthesis and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
First-strand cDNA synthesis was performed using the PrimeScript RT Reagent Kit with gDNA Eraser (RR047A, Takara, Beijing, China) according to the manufacturer’s guidelines. To remove residual genomic DNA, 2 µL of gDNA Eraser was added to 1 ug of total RNA and we made up the volume to 10 µL using DEPC H2O, and the mixture was incubated at 42 °C for 2 min. Subsequently, we added 1 µL of PrimerScript RT Enzyme Mix I, 1 µL of RT Primer Mix, 4 µL of PrimerScript Buffer 2 and 4 µL of DEPC H2O to generate first-strand cDNA.
A real-time quantitative polymerase chain reaction (RT-qPCR) was performed using LightCycler® 480 (Roche, Boston, MA, USA) and LightCycler 480 SYBR Green I Master mix (Roche). Each 20 µL reaction mixture contained 10 µL LightCycler 480 SYBR Green I Master mix, 2 µL of diluted cDNA, 1 µL of forward primer (10 µM), 1 µL of reverse primer (10 µM), and 6 µL of DEPC-treated water. The RT-qPCR was run by the following program: 95 °C for 5 min, followed by 37 cycles of 95 °C for 10 s, 60 °C for 10 s, and 75 °C for 15 s and an automatic melting curve analysis.
Gene-specific primers were designed using Primer3 (https://ginkgo.zju.edu.cn/genome/tools/primer3/ accessed on 5 June 2025). Their amplification specificity were validated by melt curve analysis and confirmed by sequencing to ensure the PCR products matched the expected fragment. The kiwifruit Actin gene was used as a housekeeping gene based on previous studies demonstrating its stable expression across various tissues in kiwifruit [33], and the gene expression level was analyzed by the 2−△CT method. All reactions were performed in triplicate for each biological replicate. All primers used for RT-qPCR, including the housekeeping gene Actin, are provided in Table A1.
2.7. Dual-Luciferase Reporter Assay
The full-length coding sequence of eight transcription factors (bHLH82, bHLH141, bHLH162, bHLH287, bHLH291, MYB61, MYB223, and MYBR123), identified from the transcriptome result, were cloned. And these transcription factors were inserted into the pSAK277 vector, which was driven by the CaMV 35S promoter [34]. Moreover, a 2 kb fragment upstream of the F3GT1 start codon was cloned as the proximal promoter region and inserted into the pGreenII 0800-LUC vector [35]. The promoter was amplified from genomic DNA. The recombinant plasmids were transformed into the Agrobacterium tumefaciens strain GV3101 using the heat-shock method. Recombinant strain GV3101 carrying the LUC reporter plasmid was grown in LB medium containing 50 mg/L kanamycin and 20 mg/L rifampicin, while those carrying the pSAK effector plasmid were cultured in LB medium containing 50 mg/L spectinomycin and 20 mg/L rifampicin. All cultures were incubated at 28 °C for two days. And then cultured bacteria were diluted to an OD600 of 0.75 in infiltration buffer (10 mM MES, pH 5.6; 10 mM MgCl2; 150 μM acetosyringone). The TF/promoter ratio was set at 10: 1, and the mixtures were infiltrated into the leaves of Nicotiana benthamiana using a needleless syringe. Empty vector pSAK277 (EV) + promoter at the same ratio was set as the negative control. After infiltrated, the plants were placed in greenhouse for a 16 h light/8 h dark photoperiod. Leaves were harvested 72 h after infiltration. The Firefly luciferase and Renilla luciferase signals were detected by using the Dual-Luciferase Reporter Assay Kit (Vazyme, Nanjing, China) [36]. Luciferase was normalized by the Firefly luciferase/Renilla luciferase (LUC/REN) ratio. And the negative control was set to 1. The primers used for constructing the pSAK277 and pGreenII 0800-LUC vectors are provided in Table A2.
2.8. Statistical Analyses
Figures were produced with GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). All data were obtained with at least three replicates, and error bars indicate standard error (SE). The unpaired Student’s t-test was used for calculating significant differences in transcriptome data, and a two-way analysis of variance (ANOVA) followed by least significant difference (LSD) post hoc tests were used for the data of the experimental measurements.
3. Results
3.1. Anthocyanin Content in Kiwifruit at Different Developmental Stages
The treatment group was bagged at the fifth week after pollination (WAP), while the control group remained unbagged. Figure 1a shows the color change stage of ‘Hongshi’ kiwifruit across eight different developmental stages after pollination. Numerous studies have reported that the accumulation of red pigment in kiwifruit is attributed to the accumulation of anthocyanin [37]. Therefore we measured the anthocyanin content of kiwifruit inner pericarp in both the control and treatment groups. The measurement results revealed that anthocyanin content in bagged kiwifruit is slightly higher than unbagged ones from 9 W to 17 W (Figure 1b). After 17 W, anthocyanin levels in the fruits of both the control and treatment groups exhibited a declining trend, with no significant differences observed between the groups (Figure 1b). Overall, in our investigation, we observed that the anthocyanin levels in the inner flesh of kiwifruit increased after bagging in the color changing stage, despite the fact that bagging treatment usually reduces light intensity. This phenomenon encourages us to continue investigating the underlying mechanisms responsible for the significant accumulation of anthocyanins in bagged kiwifruits.
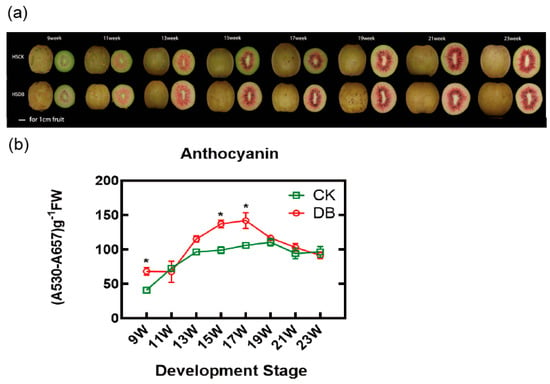
Figure 1.
Color changes in kiwifruits in the control and bagged groups. (a) The color change stage of ‘Hongshi’ kiwifruit across eight different developmental stages following pollination: 9 weeks (9W), 11 weeks (11W), 13 weeks (13W), 15 weeks (15W), 17 weeks (17W), 19 weeks (19W), 21 weeks (21W), and 23 weeks (23W). (b) The anthocyanin content both in the control and bagged kiwifruit. A repeated-measures two-way ANOVA was conducted and significant differences were detected, LSD tests were applied for pairwise comparisons. A p value < 0.05 was considered statistically significant. LSD0.05 = 19.58 in (b). Asterisks indicate statistically significant differences between the control and bagged treatment group. The error bars in panels (b) represent the standard error (SE) of at least three biological replicates. “CK” indicates the control group and “DB” indicates the double-bagged treatment group in the figure.
3.2. Identification of Anthocyanin Biosynthesis Structural Genes Responding to Bagging Treatment
To further investigate the molecular mechanisms underlying anthocyanin accumulation in kiwifruit under bagging treatment, RNA high-throughput sequencing (RNA-seq) was performed. The previous results in Section 3.1 above showed that bagging treatment increases anthocyanin content during the color-changing stages. We analyzed all anthocyanin biosynthesis genes in kiwifruit according to [38] (Figure 2a). The RNA-seq result revealed that only two structure genes, ANS1 (Acc16762) and F3GT1 (Acc20131), exhibited differential expression across two growth stages (Figure 2b). Further analysis revealed that the expression (FPKM) of ANS1 was less than 3 (Figure 2b), whereas its homologous gene ANS4, which has been reported to be involved in kiwifruit anthocyanin accumulation [39], had an expression higher than 200 (Figure A1). Hence, ANS1 is not the key gene contributing to anthocyanin accumulation under bagging treatment. As for F3GT1, which has been reported to be involved in anthocyanin accumulation in kiwifruit inner pericarp [37], its expression was significantly higher in bagged kiwifruit, particularly at 9W and 11W. Therefore, F3GT1 was selected for further analysis.
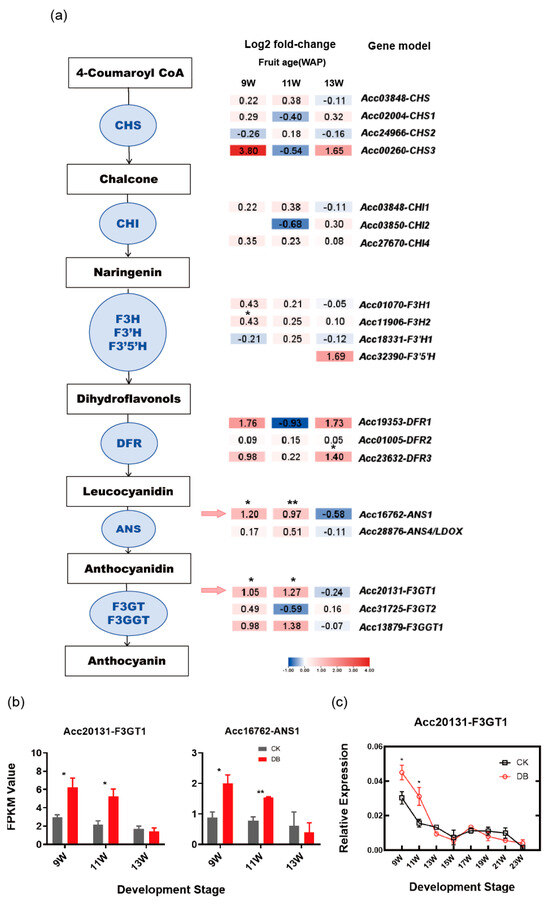
Figure 2.
The differentially expressed F3GT1 was identified based on transcriptomic data. (a) A schematic representation of the anthocyanin biosynthesis pathway in kiwifruit and the expression levels of the structural genes involved. (b) The relative expression levels of the critical biosynthetic genes F3GT1 and ANS1 in anthocyanin biosynthesis, showing significant differences in FPKM values between the control and bagging treatment groups across two growth stages. Unpaired t-tests were applied to compare the control and treatment groups at each time point. Asterisks indicate significant differences between the two groups (* p < 0.05; ** p < 0.01). (c) An expression pattern analysis of the key structural gene F3GT1 validated using RT-qPCR during the color change development stage of fruit. A repeated-measures two-way ANOVA was conducted and significant differences were detected, and LSD tests were applied for pairwise comparisons. A p value < 0.05 was considered statistically significant. LSD0.05 = 0.0077. The error bars in (b,c) represent the SE of three biological replicates.
A real-time quantitative PCR (RT-qPCR) was carried out to detected the expression of F3GT1 during the developmental stages (Figure 2c). The RT-qPCR result was consistent with the RNA-seq result, showing that the expression of F3GT1 was significantly higher in the bagged group compared to the control group at 9 and 11W. We propose that the increased expression of F3GT1 contributes to anthocyanin accumulation under bagging treatment during the early color-changing stages.
3.3. Determination of Potential Transcriptional Regulators for F3GT1
To investigate the potential transcriptional regulatory mechanism of F3GT1, we performed an in-depth analysis of the transcriptome. We found 1089 differentially expressed genes (DEGs) in our transcriptome. A total of 260 genes exhibited common differential expression across any two sampling points. Among these, 248 genes exhibited differential expression at both 9W and 11W, and only 6 genes showed common differential expression in three stages (Figure 3a). The 9W and 11W stages exhibited the highest number of shared differentially expressed genes between the bagged and control groups. This result prompted us to focus our subsequent analysis on these stages. These 260 DEGs were used for further analysis. Plant Transcription Factor Database (http://planttfdb.gao-lab.org/index.php accessed on 5 June 2025) analysis was carried out to predict transcription factors, and eight transcription factors were found. Interestingly, these transcription factors belong to two families, bHLH and MYB (Figure 3b). The five bHLH and three MYB genes identified do not include MYB10, MYB110, and bHLH5/42, which have been previously reported to be involved in anthocyanin metabolism [40,41,42,43]. Among these, four genes appeared to have a higher expression in bagged kiwifruit, while the expression of the remaining four genes was repressed by bagging treatment (Figure 3b). These eight candidate TFs were used for further experiments.
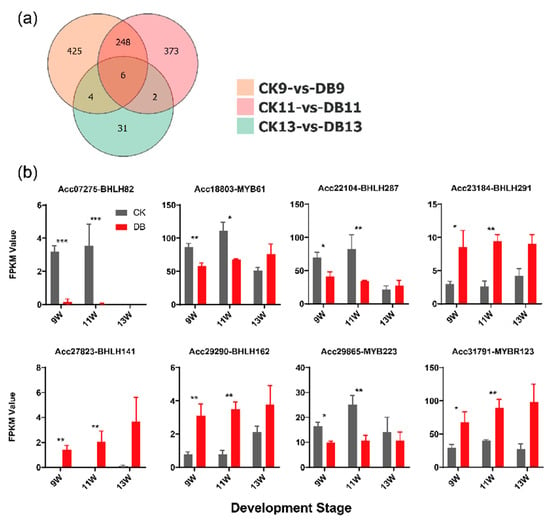
Figure 3.
The identification of candidate differentially expressed TFs. (a) A Venn diagram of differentially expressed genes (DEGs) between the control and bagging treatment groups in three developmental stages. (b) The FPKM value of the eight differentially expressed TFs. Unpaired t-tests were applied to compare the control and treatment groups at each time point. Asterisks indicate significant differences between the two groups (* p < 0.05; ** p < 0.01; *** p < 0.001). Error bars represent the SE of three biological replicates.
3.4. bHLH291 Positively Regulates F3GT1 Promoter Activity
In order to study the regulatory mechanism of the eight candidate TFs, the full coding sequences of TFs and the promoter of F3GT1 were inserted into the pSAK277 and pGREEN 0800 LUC vectors, respectively. A dual-luciferase assay was carried out to test the regulatory effects of the TFs on the F3GT1 promoter (Figure 4). We found that bHLH291 significantly activated the F3GT1 promoter, and promoter activity was increased to 2.45-fold (Figure 4). Nevertheless, MYB transcription factors MYB61, MYB223, and MYBR123 did not show any obvious activation or repression effects on the F3GT1 promoter.
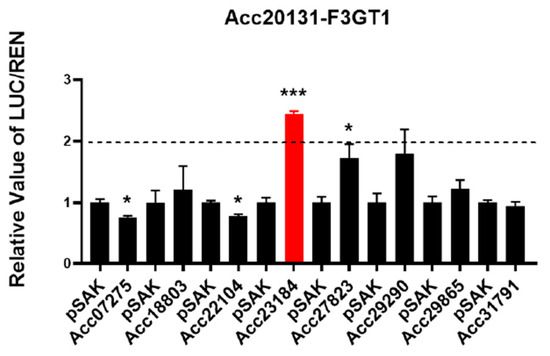
Figure 4.
The transcriptional regulation of F3GT1 by bHLH291. The regulatory effects of eight candidate TFs on the F3GT1 promoter. The Firefly luciferase/Renilla luciferase (LUC/REN) ratio of the empty pSAK vector mixed with the F3GT1 promoter was set as 1. Unpaired t-tests were applied to compare the empty vector and treatment groups regarding different genes. Asterisks indicate significant differences between the two groups (* p < 0.05; *** p < 0.001). Error bars represent the SE of three biological replicates.
3.5. Phylogenetic Tree of bHLH and MYB Transcription Factor
A phylogenetic tree of bHLH genes revealed that five kiwifruit bHLH genes belong to IVb, Va, Vb and XII subgroups [44]. Among these, bHLH141 (Acc27823) belongs to the Vb subfamily, bHLH82 (Acc07275) to IVb, and bHLH162 (Acc29290) to Va. Additionally, bHLH287 (Acc22104) and bHLH291 (Acc23184) are members of the XII subfamily (Figure 5a). We found that these five bHLH members are not homologous to TT8, EGL3 and GL3 [45,46], which are involved in anthocyanin metabolism [47]. A phylogenetic tree of MYB revealed that MYB223 (Acc29865) belongs to subgroup S1 [48], which is associated with MYB30, a regulator known to inhibit sucrose-induced anthocyanin biosynthesis in Arabidopsis seedlings [49]. In addition, MYB61 (Acc18803) is part of subgroup S13, which typically includes MYB86 which is involved in the metabolic regulation of anthocyanins in plants [50]. Moreover, MYB61 of Paphiopedilum hirsutissimum may regulate structural genes involved in flower color formation [48]. MYBR123 (Acc31791) was not included in the phylogenetic tree because it does not belong to the R2R3-MYB family (Figure 5b).
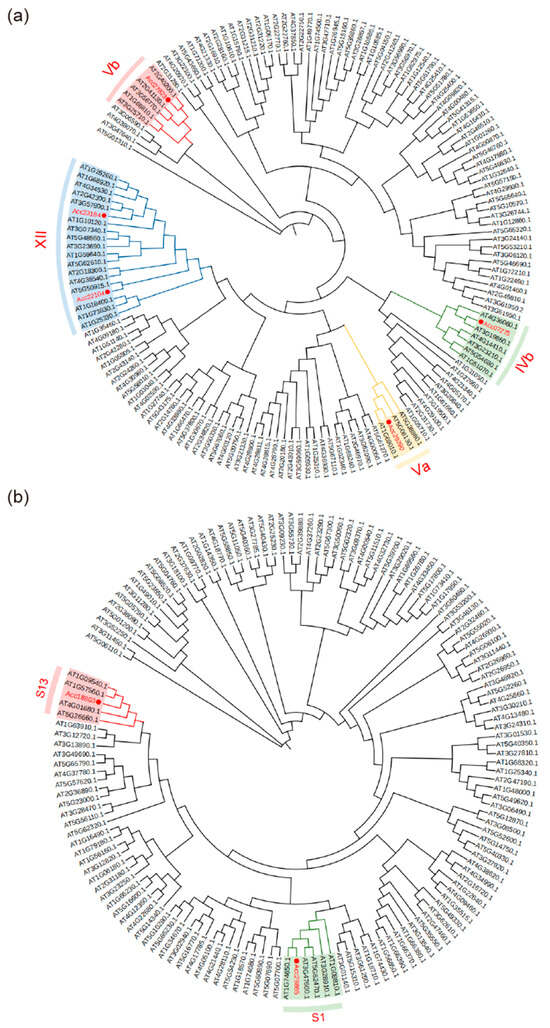
Figure 5.
Phylogenetic trees of bHLH and R2R3-MYB families. Circles in (a,b) represent kiwifruit bHLH or MYB members.
4. Discussion
4.1. Bagging Treatment Increases Anthocyanin Content in Kiwifruit Flesh
Bagging is a widely used technique in fruit cultivation that effectively prevents the occurrence of diseases and pests. However, by reducing light intensity, bagging often results in decreased anthocyanin accumulation in the fruit. Dark treatment during the developmental process of Gerbera hybrida results in an irreversible inhibition of anthocyanin accumulation [51]. High light intensity enhances anthocyanin accumulation by modulating the levels of endogenous hormones and activating key enzymes in blueberry leaves [52]. The application of one- to three-layered paper bags for apple fruits can exert varying degrees of inhibitory effects on anthocyanin accumulation in fruit peel [53]. It is reported that after using bags of different colors and materials to treat ‘Kyoho’ (Vitis labruscana) berries, white, green and yellow bags and combinations of umbrellas led to significant increases in the proportions of certain stable anthocyanins in the berry skins, such as coumaroylated and methylated anthocyanins [54]. However, we observed that after bagging treatment, anthocyanin content in the flesh of kiwifruit increased, contrary to the commonly reported reduction in anthocyanin accumulation under bagging. This discovery indicates that there is a pathway promoting the synthesis of anthocyanins in kiwifruit under bagging.
4.2. F3GT1 Is a Candidate Structural Gene Responsive to Anthocyanin Accumulation Under Bagging Treatment
Anthocyanins are synthesized via the phenylpropanoid pathway, which has been well-characterized [55]. Several anthocyanin-related structure genes have been identified in kiwifruit [42]. Our RNA-seq and RT-qPCR results suggested that F3GT1 is a candidate anthocyanin structural gene associated with anthocyanin biosynthesis and may contribute to anthocyanin accumulation in bagged kiwifruit. In the white mutant Malay apple fruit skin, the absence of anthocyanins was attributed to the lack of F3GT expression and activity, despite the presence of transcripts for other anthocyanin biosynthetic genes [56]. F3GT is often referred to as UFGT. The name UFGT is derived from its substrate (UDP-glucose) and the type of reaction it catalyzes, while F3GT is named based on its catalytic reaction (3-O-glucosylation). In essence, they are different names for the same class of enzyme, catalyzing identical reactions that convert anthocyanin precursors into stable anthocyanin-3-O-glucosides. In different plant species, the specific name may vary depending on the research context and species. For example, the UDP-glucose: flavonoid 3-O-glucosyltransferase isolated from the seed coats of black soybean (Glycine max (L.) Merr.) is referred to as UGT78K1 [57]. Under strong light conditions, the levels of UFGT in blueberry exhibit a significant positive correlation with anthocyanin content [58]. The application of exogenous ethylene to cabbage effectively suppresses the expression of UFGT and reduces anthocyanin accumulation [59]. The accumulation of anthocyanins in the red petals of kiwifruit hybrid families is primarily due to the activation of F3GT1 by the MBW complex [60]. Moreover, F3GT1 has been reported to regulate anthocyanin accumulation in red-fleshed kiwifruit cultivars [37]. Our experimental results showed that the expression of F3GT1 was significantly upregulated under bagging treatment, indicating that this enhanced expression of F3GT1 may contribute to the increased anthocyanin content observed in bagged fruits.
4.3. bHLH291 Targets F3GT1 and Enhances Its Expression
The bHLH family, the second-largest family of plant transcription factors, is widely involved in various important activities during plant growth and development. bHLH transcription factors have been reported to regulate anthocyanin metabolism in many horticultural corps. For instance, MdMYBPA1 and MdbHLH33 forms a protein complex that results in increased anthocyanin in the red-fleshed apple calli [26]. bHLH transcription factors often act as positive regulators that promote anthocyanin accumulation by upregulating structural genes in the biosynthetic pathway, as exemplified by bHLH291 in our study. This regulatory mechanism has also been experimentally confirmed in strawberry: FvbHLH9 acts as a positive regulator of anthocyanin biosynthesis in strawberry fruit, mediating its regulatory effect by forming a heterodimer with FvHY5 to activate the expression of FvDFR [61]. Furthermore, bHLH transcription factors can regulate anthocyanin pathway genes directly. The cold-induced transcription factor MdbHLH3 binds to the promoters of anthocyanin biosynthesis genes MdDFR and MdF3GT activating their expression in apple [62]. PsbHLH1 regulates anthocyanin biosynthesis in Paeonia suffruticosa by directly binding to the promoters of PsDFR and PsANS, thereby transcriptionally activating their expression [63]. In this study, we found that bagging treatment up-regulates the expression of bHLH291. The dual-luciferase assay revealed that bHLH291 can activate the expression of the F3GT1 promotor. These results indicate a new regulatory pathway of anthocyanins and also provide new insights into the accumulation of anthocyanins under bagging (Figure 6).
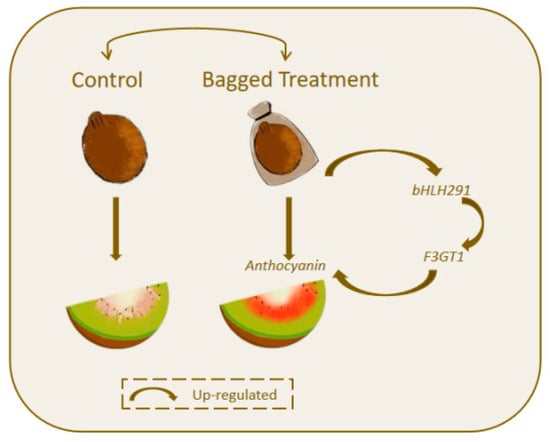
Figure 6.
A schematic model of bagging-promoted anthocyanin accumulation in kiwifruit. Bagging treatment promotes the expression of bHLH291, which subsequently activates F3GT1 expression and enhances anthocyanin accumulation in the inner flesh.
5. Conclusions
Bagging technology has been widely applied in agriculture with substantial effects. However, bagging treatment typically inhibits light signals, leading to suppressed anthocyanin accumulation in fruits. Surprisingly, we observed an increase in anthocyanin content in the inner pericarp of kiwifruit during the early stage of color change following bagging treatment. This finding provides a novel approach for enhancing kiwifruit quality. Additionally, through RNA-seq, dual-luciferase assays, and other experiments, we discovered that bagging induces bHLH291 expression, which subsequently up-regulates the expression of the anthocyanin synthesis gene F3GT1 promotor. These results reveal a synthetic pathway for anthocyanin accumulation under low-light conditions. Our findings could be applied to breeding strategies and bagging protocols aiming to enhance pigment accumulation in commercial kiwifruit and provide a molecular basis for understanding how bagging influences anthocyanin accumulation in kiwifruit flesh. Critically, this research demonstrates the applicability of fruit bagging as a horticultural practice to boost the nutraceutical properties and market competitiveness of red-fleshed kiwifruit cultivars. We also acknowledge certain limitations in the current study. Specifically, the experimental validation of the bHLH291 gene function necessitates the generation of stably transformed kiwifruit plants through plant transgenic techniques for consistent fruit phenotype analysis. Future investigations should further elucidate the synergistic effects of bHLH291 with other transcription factors (e.g., MYB, WD40 proteins) on anthocyanin accumulation in kiwifruit.
Author Contributions
Conceptualization, X.-R.Y. and W.-Q.W.; methodology, K.-Y.Z.; validation, X.-Y.K.; formal analysis, X.-Y.K.; investigation, X.-Y.K. and M.-Y.T.; resources, X.-R.Y.; data curation, M.-Y.T.; writing original draft preparation, X.-Y.K.; Writing, review and editing, W.-Q.W.; supervision, W.-Q.W.; project administration, X.-R.Y.; funding acquisition, X.-R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation grant number LZ23C150001; the Fruit New Varieties Breeding Project of Zhejiang Province grant number 2021C02066-8. And The APC was funded by the Zhejiang Provincial Natural Science Foundation grant number LZ23C150001.
Data Availability Statement
Transcriptome sequencing data are available at NCBI PRJNA1219817.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Primers used for quantitative real-time PCR.
Table A1.
Primers used for quantitative real-time PCR.
| Gene | Purpose | Primer Sequence 5′-3′ |
|---|---|---|
| F3GT1(Acc20131)-FP | RT-qPCR | TAGCCAAGCAGAGATCCGCTTCC |
| F3GT1(Acc20131)-RP | RT-qPCR | CAAGAATCCTTCTGGTAAGTACTGTTTCGA |
| Actin-FP | RT-qPCR | TGTCCCATGTCTGGTTGATGACT |
| Actin-RP | RT-qPCR | TGCATGAGCGATCAAGTTTCAAG |

Table A2.
Primers used for vector construction.
Table A2.
Primers used for vector construction.
| Purpose | Gene | Primer Sequence 5′-3′ |
|---|---|---|
| pSAK277 | bHLH82 (Acc07275)-FP | ACTAGTGGATCCAAAGAATTCATGGATCACCTAAATCACGACGC |
| pSAK277 | bHLH82 (Acc07275)-RP | GACTCTAGAAGTACTCTCGAGTCAATCATCAGCCTTCCCACC |
| pSAK277 | MYB61 (Acc18803)-FP | ACTAGTGGATCCAAAGAATTCATGGGGAGGCACTCTTGCTGC |
| pSAK277 | MYB61 (Acc18803)-RP | GACTCTAGAAGTACTCTCGAGCTAAGAATAGTGTCCAAAAGTGGC |
| pSAK277 | bHLH287 (Acc22104)-FP | ACTAGTGGATCCAAAGAATTCATGGCAGCTTTCCCAAGCACCC |
| pSAK277 | bHLH287 (Acc22104)-RP | GACTCTAGAAGTACTCTCGAGTTAATGGAATGAACACAAGTTG |
| pSAK277 | bHLH291 (Acc23184)-FP | ACTAGTGGATCCAAAGAATTCATGGAGCCAATTTCAGGAACAG |
| pSAK277 | bHLH291 (Acc23184)-RP | GACTCTAGAAGTACTCTCGAGTTAGCTAATAAAGCTGGGATTG |
| pSAK277 | bHLH141 (Acc27823)-FP | ACTAGTGGATCCAAAGAATTCATGGCATGTAAATCAGATCTTG |
| pSAK277 | bHLH141 (Acc27823)-RP | GACTCTAGAAGTACTCTCGAGCTAATGAGAAGGCAAGAAGAACC |
| pSAK277 | bHLH162 (Acc29290)-FP | ACTAGTGGATCCAAAGAATTCATGGAGCTTCCTCAACCCCG |
| pSAK277 | bHLH162 (Acc29290)-RP | GACTCTAGAAGTACTCTCGAGTTAGCTAGTCTTGAGCCTCTTTAGGG |
| pSAK277 | MYB223 (Acc29865)-FP | ACTAGTGGATCCAAAGAATTCATGGGAAGACCACCGTGCTGCG |
| pSAK277 | MYB223 (Acc29865)-RP | GACTCTAGAAGTACTCTCGAGTCAAAACAGATCATCACTTCCCCC |
| pSAK277 | MYBR123 (Acc31791)-FP | ACTAGTGGATCCAAAGAATTCATGGAGATTCTCTCACCGGCG |
| pSAK277 | MYBR123 (Acc31791)-RP | GACTCTAGAAGTACTCTCGAGTCAAAAACTTCTCCATCGATCACCGG |
| LUC | F3GT1 (Acc20131)-FP | GTCGACGGTATCGATAAGCTTGACCGACTTAGATCAACAACTGATCT |
| LUC | F3GT1 (Acc20131)-RP | TGTTTTTGGCGTCTTCCATGGTTTTTTAAGGCTTTGATTTTGG |
Appendix B
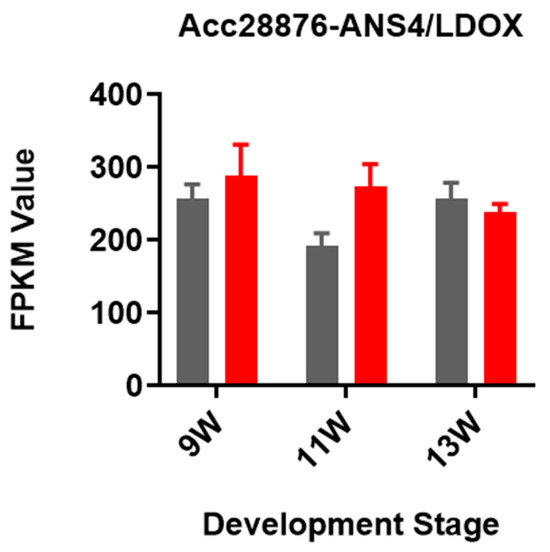
Figure A1.
The relative expression level of the anthocyanin biosynthesis pathway gene ANS4. Gray bars represent the control group (CK), and red bars represent the double bagging treatment group (DB).
References
- Rahim, M.A.; Busatto, N.; Trainotti, L. Regulation of anthocyanin biosynthesis in peach fruits. Planta 2014, 240, 913–929. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, S.K.; Choi, H.C.; Kim, S.U. Identification of anthocyanins from pigmented rice seeds. Agric. Chem. Biotechnol. 1998, 41, 257–260. [Google Scholar]
- Li, B.Q.; Xia, Y.X.; Wang, Y.Y.; Qin, G.Z.; Tian, S.P. Characterization of Genes Encoding Key Enzymes Involved in Anthocyanin Metabolism of Kiwifruit during Storage Period. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Lewis, D.H.; Zhang, H.B.; Montefiori, M.; Brendolise, C.; Boase, M.R.; Ngo, H.; Jameson, P.E.; Schwinn, K.E. A Conserved Network of Transcriptional Activators and Repressors Regulates Anthocyanin Pigmentation in Eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef]
- Sytar, O.; Kosyan, A.; Taran, N.; Smetanska, I. Anthocyanin’s as marker for selection of buckwheat plants with high rutin content. Gesunde Pflanz. 2014, 66, 165–169. [Google Scholar] [CrossRef]
- Wang, W.Q.; Moss, S.M.A.; Zeng, L.H.; Espley, R.; Wang, T.C.; Kui, L.W.; Fu, B.L.; Schwinn, K.E.; Allan, A.C.; Yin, X.R. The red flesh of kiwifruit is differentially controlled by specific activation-repression systems. New Phytol. 2022, 235, 630–645. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef]
- Maierdan, Y.S.; Nulibiya, M.H.T.; Zhou, W.T. Research progress of anthocyanins against cardiovascular diseases. J. Food Saf. Qualit. 2020, 11, 8844–8848. [Google Scholar]
- Navas, M.J.; Jiménez-Moreno, A.M.; Bueno, J.M.; Sáez-Plaza, P.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part IV: Extraction of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, J.L.; Cherono, S.; An, J.P.; Allan, A.C.; Han, Y.P. Colorful hues: Insight into the mechanisms of anthocyanin pigmentation in fruit. Plant Physiol. 2023, 192, 1718–1732. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- He, J.P.; Yao, L.; Pecoraro, L.; Liu, C.X.; Wang, J.; Huang, L.Q.; Gao, W.Y. Cold stress regulates accumulation of flavonoids and terpenoids in plants by phytohormone, transcription process, functional enzyme, and epigenetics. Crit. Rev. Biotechnol. 2023, 43, 680–697. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Yu, M.; Man, Y.P.; Wang, Y.C. Light- and Temperature-Induced Expression of an R2R3-MYB Gene Regulates Anthocyanin Biosynthesis in Red-Fleshed Kiwifruit. Int. J. Mol. Sci. 2019, 20, 5228. [Google Scholar] [CrossRef]
- Ngoc, A.T.; Naing, A.H.; Kim, C.K. Influences of different light sources and light/dark cycles on anthocyanin accumulation and plant growth in Petunia. J. Plant Biotechnol. 2016, 43, 119–124. [Google Scholar]
- Wang, N.; Zhang, Z.Y.; Jiang, S.H.; Xu, H.F.; Wang, Y.C.; Feng, S.Q.; Chen, X.S. Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2016, 127, 217–227. [Google Scholar] [CrossRef]
- Petrella, D.P.; Metzger, J.D.; Blakeslee, J.J.; Nangle, E.J.; Gardner, D.S. Anthocyanin Production Using Rough Bluegrass Treated with High-Intensity Light. HortScience 2016, 51, 1111–1120. [Google Scholar] [CrossRef]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef]
- Wang, J.D.; Zhao, Y.Q.; Sun, B.; Yang, Y.T.; Wang, S.P.; Feng, Z.R.; Li, J.Y. The structure of anthocyanins and the copigmentation by common micromolecular copigments: A review. Food Res. Int. 2024, 176, 15. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Hu, G.B.; Hu, F.C.; Wang, H.C.; Yang, Z.Y.; Lai, B. The UDP glucose: Flavonoid-3-O-glucosyltransferase (UFGT) gene regulates anthocyanin biosynthesis in litchi (Litchi chinesis Sonn.) during fruit coloration. Mol. Biol. Rep. 2012, 39, 6409–6415. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, J.Q.; Wu, Z.C.; Lai, B.; Huang, X.M.; Qin, Y.H.; Wang, H.C. Hu Functional characterization of a glucosyltransferase gene, LcUFGT1, involved in the formation of cyanidin glucoside in the pericarp of Litchi chinensis. Physiol. Plant. 2016, 156, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Ma, K.X.; Qi, Y.W.; Lv, G.W.; Ren, X.L.; Liu, Z.D.; Ma, F.W. Transcriptional Regulation of Anthocyanin Synthesis by MYB-bHLH-WDR Complexes in Kiwifruit (Actinidia chinensis). J. Agric. Food Chem. 2021, 69, 3677–3691. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Jiang, Y.B.; Tang, G.C.; Guo, L.Y.; Qiao, G.Z.; Liu, S.H.; Tan, B.; Cheng, J.; Zhang, L.L.; et al. The basic helix-loop-helix transcription factor PpeUNE12 regulates peach ripening by promoting polyamine catabolism and anthocyanin synthesis. Plant Physiol. Biochem. 2025, 220, 109537. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.X.; Li, W.F.; Chu, M.Y.; Ma, Z.H.; Wang, P.; Mao, J.; Chen, B.H. MdbHLH51 plays a positive role in anthocyanin accumulation in ‘Red Delicious’ apples. Trees 2022, 36, 1687–1695. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Zong, X.M.; Ren, P.; Qian, Y.Q.; Fu, A.G. Basic Helix-Loop-Helix (bHLH) Transcription Factors Regulate a Wide Range of Functions in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.Z.; Jiang, S.H.; Chen, Z.J.; Xu, H.F.; Fang, H.C.; Su, M.Y.; Zhang, J.; Wang, Y.C.; Liu, W.J.; et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018, 96, 39–55. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Xu, H.F.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef]
- Xiong, Y.; He, J.; Li, M.; Du, K.; Lang, H.; Gao, P.; Xie, Y. Integrative Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Yellow-Fleshed Kiwifruit. Int. J. Mol. Sci. 2023, 24, 1573. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, X.F.; Zhu, Y.J.; Zhu, J.Z.; Liu, C.; Wang, Z.Y.; Shen, X.X.; Allan, A.C.; Yin, X.R. Identification of miRNA858 long-loop precursors in seed plants. Plant Cell 2024, 36, 1637–1654. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Crowhurst, R.; Hilario, E.; Nardozza, S.; Fraser, L.; Peng, Y.Y.; Gunaseelan, K.; Simpson, R.; Tahir, J.; Deroles, S.C.; et al. A manually annotated Actinidia chinensis var. chinensis (kiwifruit) genome highlights the challenges associated with draft genomes and gene prediction in plants. BMC Genom. 2018, 19, 257. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Ferradás, Y.; Rey, L.; Martínez, Ó.; Rey, M.; González, M.V. Identification and validation of reference genes for accurate normalization of real-time quantitative PCR data in kiwifruit. Plant Physiol. Biochem. 2016, 102, 27–36. [Google Scholar] [CrossRef]
- Kamo, K.; Blowers, A.; McElroy, D. Effect of the cauliflower mosaic virus 35S, actin, and ubiquitin promoters on uidA expression from a bar-uidA-fusion gene in transgenic Gladiolus plants. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 13–20. [Google Scholar] [CrossRef]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 14. [Google Scholar] [CrossRef]
- Fu, B.L.; Wang, W.Q.; Liu, X.F.; Duan, X.W.; Allan, A.C.; Grierson, D.; Yin, X.R. An ethylene-hypersensitive methionine sulfoxide reductase regulated by NAC transcription factors increases methionine pool size and ethylene production during kiwifruit ripening. New Phytol. 2021, 232, 237–251. [Google Scholar] [CrossRef]
- Montefiori, M.; Espley, R.V.; Stevenson, D.; Cooney, J.; Datson, P.M.; Saiz, A.; Atkinson, R.G.; Hellens, R.P.; Allan, A.C. Identification and characterisation of F3GT1 and F3GGT1, two glycosyltransferases responsible for anthocyanin biosynthesis in red-fleshed kiwifruit (Actinidia chinensis). Plant J. 2011, 65, 106–118. [Google Scholar] [CrossRef]
- Nardozza, S.; Boldingh, H.L.; Kashuba, M.P.; Feil, R.; Jones, D.; Thrimawithana, A.H.; Ireland, H.S.; Philippe, M.; Wohlers, M.W.; McGhie, T.K.; et al. Carbon starvation reduces carbohydrate and anthocyanin accumulation in red-fleshed fruit via trehalose 6-phosphate and MYB27. Plant Cell Environ. 2020, 43, 819–835. [Google Scholar] [CrossRef]
- Wang, L.; Tang, W.; Hu, Y.; Zhang, Y.; Sun, J.; Guo, X.; Lu, H.; Yang, Y.; Fang, C.; Niu, X.; et al. A MYB/bHLH complex regulates tissue-specific anthocyanin biosynthesis in the inner pericarp of red-centered kiwifruit Actinidia chinensis cv. Hongyang. Plant J. 2019, 99, 359–378. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Sun, L.X.; Xi, D.D.; Li, X.F.; Gao, L.; Miao, L.M.; Luo, Y.; Tian, M.M.; Zhu, H.F. Methyl jasmonate-induced bHLH42 mediates tissue-specific accumulation of anthocyanins via regulating flavonoid metabolism-related pathways in Caitai. Physiol. Plant. 2024, 176, 13. [Google Scholar] [CrossRef]
- Castillejo, C.; Waurich, V.; Wagner, H.; Ramos, R.; Oiza, N.; Muñoz, P.; Triviño, J.C.; Caruana, J.; Liu, Z.C.; Cobo, N.; et al. Allelic Variation of MYB10 Is the Major Force Controlling Natural Variation in Skin and Flesh Color in Strawberry (Fragaria spp.) Fruit. Plant Cell 2020, 32, 3723–3749. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Kui, L.W.; Cooney, J.M.; Wang, T.; Espley, R.V.; Allan, A.C. Differential regulation of the anthocyanin profile in purple kiwifruit (Actinidia species). Hortic. Res. 2019, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.; Wu, Y.W.; Bai, T.; Li, Y.; Li, X.J.; Li, N.B.; Zhou, Y.; Zhang, J.L. Molecular and Metabolic Regulation of Flavonoid Biosynthesis in Two Varieties of Dendrobium devonianum. Curr. Issues Mol. Biol. 2024, 46, 14270–14290. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Dubos, C. The arabidopsis bHLH transcription factor family. Trends Plant Sci. 2024, 29, 13. [Google Scholar] [CrossRef]
- Baudry, A.; Caboche, M.; Lepiniec, L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. 2006, 46, 768–779. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Wang, X.C.; Wu, J.; Guan, M.L.; Zhao, C.H.; Geng, P.; Zhao, Q. Arabidopsis MYB4 plays dual roles in flavonoid biosynthesis. Plant J. 2020, 101, 637–652. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Zhou, H.; He, J.; Zhang, Y.; Zhao, H.; Sun, X.; Chen, X.; Liu, X.; Zheng, Y.; Lin, H. RHA2b-mediated MYB30 degradation facilitates MYB75-regulated, sucrose-induced anthocyanin biosynthesis in Arabidopsis seedlings. Plant Commun. 2024, 5, 100744. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Q.; Tang, D.B.; Chen, Y.Q.; Zang, J.W.; Zhao, W.J.; Chen, J.G.; Zhang, Q.F.; Yin, Z.P. Development of suspension culture technology and hormone effects on anthocyanin biosynthesis for red Cyclocarya paliurus cells. Plant Cell Tissue Organ Cult. 2022, 149, 175–195. [Google Scholar] [CrossRef]
- Meng, X.C.; Wang, X.J. Regulation of flower development and anthocyanin accumulation in Gerbera hybrida. J. Horticult. Sci. Biotechnol. 2004, 79, 131–137. [Google Scholar] [CrossRef]
- An, X.; Tan, T.; Zhang, X.; Guo, X.; Zhu, Y.; Song, Z.; Wang, D. Effects of Light Intensity on Endogenous Hormones and Key Enzyme Activities of Anthocyanin Synthesis in Blueberry Leaves. Horticulturae 2023, 9, 618. [Google Scholar] [CrossRef]
- Ju, Z.G. Fruit bagging, a useful method for studying anthocyanin synthesis and gene expression in apples. Sci. Hortic. 1998, 77, 155–164. [Google Scholar] [CrossRef]
- Zhou, S.H.; Guo, R.R.; Wei, R.F.; Liu, J.B.; Yu, H.; Shi, X.F.; Zhang, Y.; Xie, T.L.; Cheng, G. Effects of bagging or the combination of umbrella and bag treatments on anthocyanin accumulation in the berry skin of ‘Kyoho’ (Petunia Vitis labruscana) Grape. Food Sci. Technol. 2020, 40, 394–400. [Google Scholar] [CrossRef]
- Pazmiño-Durán, E.A.; Giusti, M.M.; Wrolstad, R.E.; Glória, M.B.A. Anthocyanins from Oxalis triangularis as potential food colorants. Food Chem. 2001, 75, 211–216. [Google Scholar] [CrossRef]
- Kotepong, P.; Ketsa, S.; van Doorn, W.G. A white mutant of Malay apple fruit (Syzygium malaccense) lacks transcript expression and activity for the last enzyme of anthocyanin synthesis, and the normal expression of a MYB transcription factor. Funct. Plant Biol. 2011, 38, 75–86. [Google Scholar] [CrossRef]
- Kovinich, N.; Saleem, A.; Arnason, J.T.; Miki, B. Functional characterization of a UDP-glucose:flavonoid 3-O-glucosyltransferase from the seed coat of black soybean (Glycine max (L.) Merr.). Phytochemistry 2010, 71, 1253–1263. [Google Scholar] [CrossRef]
- An, X.L.; Tan, T.Y.; Song, Z.J.; Guo, X.L.; Zhang, X.Y.; Zhu, Y.Z.; Wang, D.L. Physiological response of anthocyanin synthesis to different light intensities in blueberry. PLoS ONE 2023, 18, e0283284. [Google Scholar] [CrossRef]
- Wang, F.H.; Ahammed, G.J.; Li, G.Y.; Bai, P.T.; Jiang, Y.; Wang, S.X.; Chen, S.C. Ethylene is involved in red light-induced anthocyanin biosynthesis in cabbage (Brassica oleracea). Int. J. Agric. Biol. 2019, 21, 955–963. [Google Scholar]
- Fraser, L.G.; Seal, A.G.; Montefiori, M.; McGhie, T.K.; Tsang, G.K.; Datson, P.M.; Hilario, E.; Marsh, H.E.; Dunn, J.K.; Hellens, R.P.; et al. An R2R3 MYB transcription factor determines red petal colour in an Actinidia (kiwifruit) hybrid population. BMC Genom. 2013, 14, 28. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.B.; Chen, G.Q.; Wu, J.; Liu, Z.C.; Lian, H.L. FvhHLH9 Functions as a positive regulator of anthocyanin biosynthesis by forming a HY5-bHLH9 Transcription Complex in Strawberry Fruits. Plant Cell Physiol. 2020, 61, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhou, L.; Han, L.L.; Zou, H.Z.; Miao, K.; Wang, Y. PsbHLH1, a novel transcription factor involved in regulating anthocyanin biosynthesis in tree peony (Paeonia suffruticosa). Plant Physiol. Biochem. 2020, 154, 396–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).