Wettability of the Plant Growth Regulator 28-HB on Pepper Leaves at Different Developmental Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
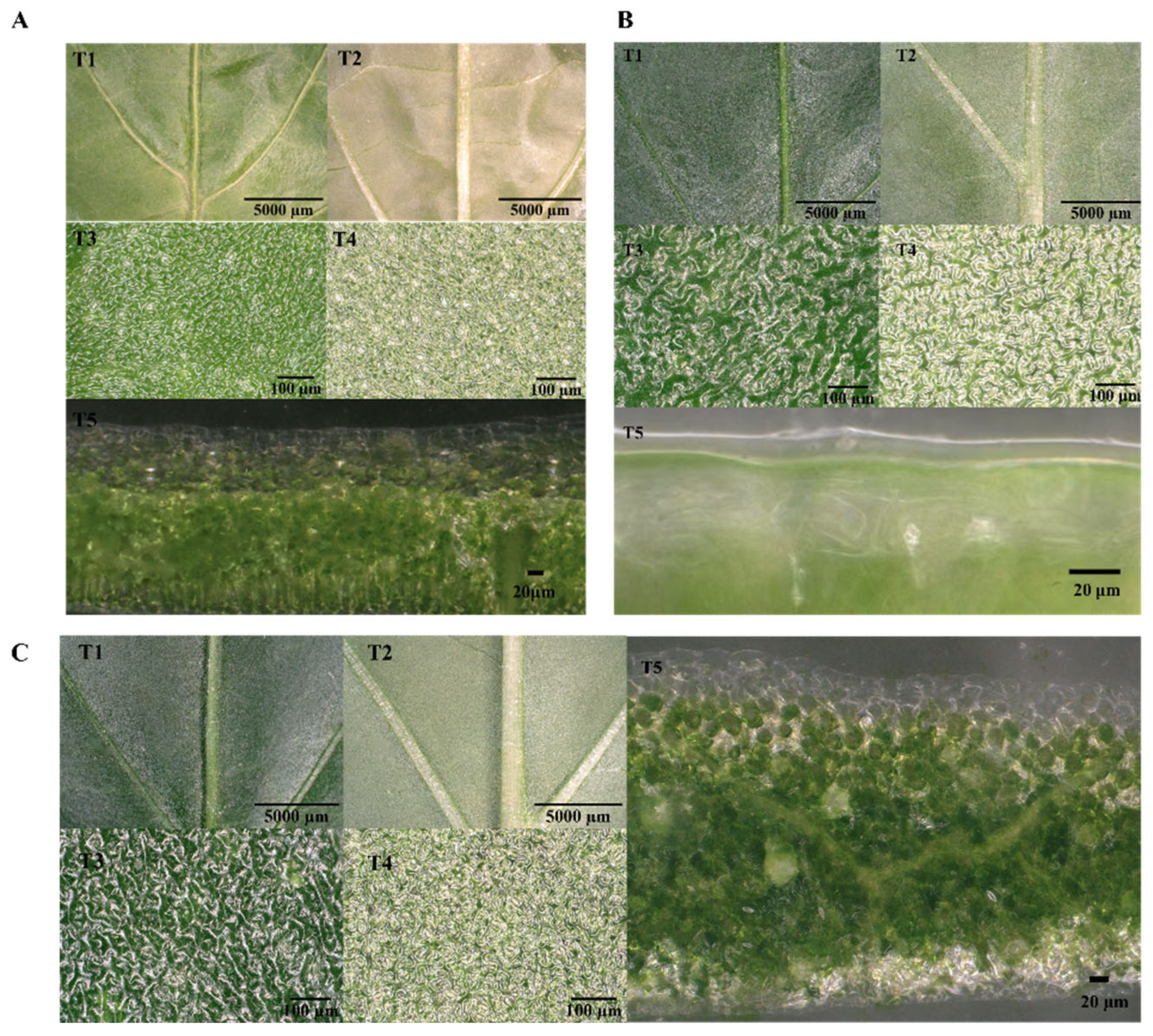
2.2. Leaf Surface Characterization
2.3. Preparation of Reagents and Test Solutions
2.4. Calculation of Leaf Surface Free Energy and Its Components
2.5. Surface Tension and Contact Angle Measurement
2.6. Calculation of Adhesion Tension and Work of Adhesion
2.7. Data Analysis
3. Results and Discussion
3.1. Surface Morphology of Pepper Leaves at Different Growth Stages
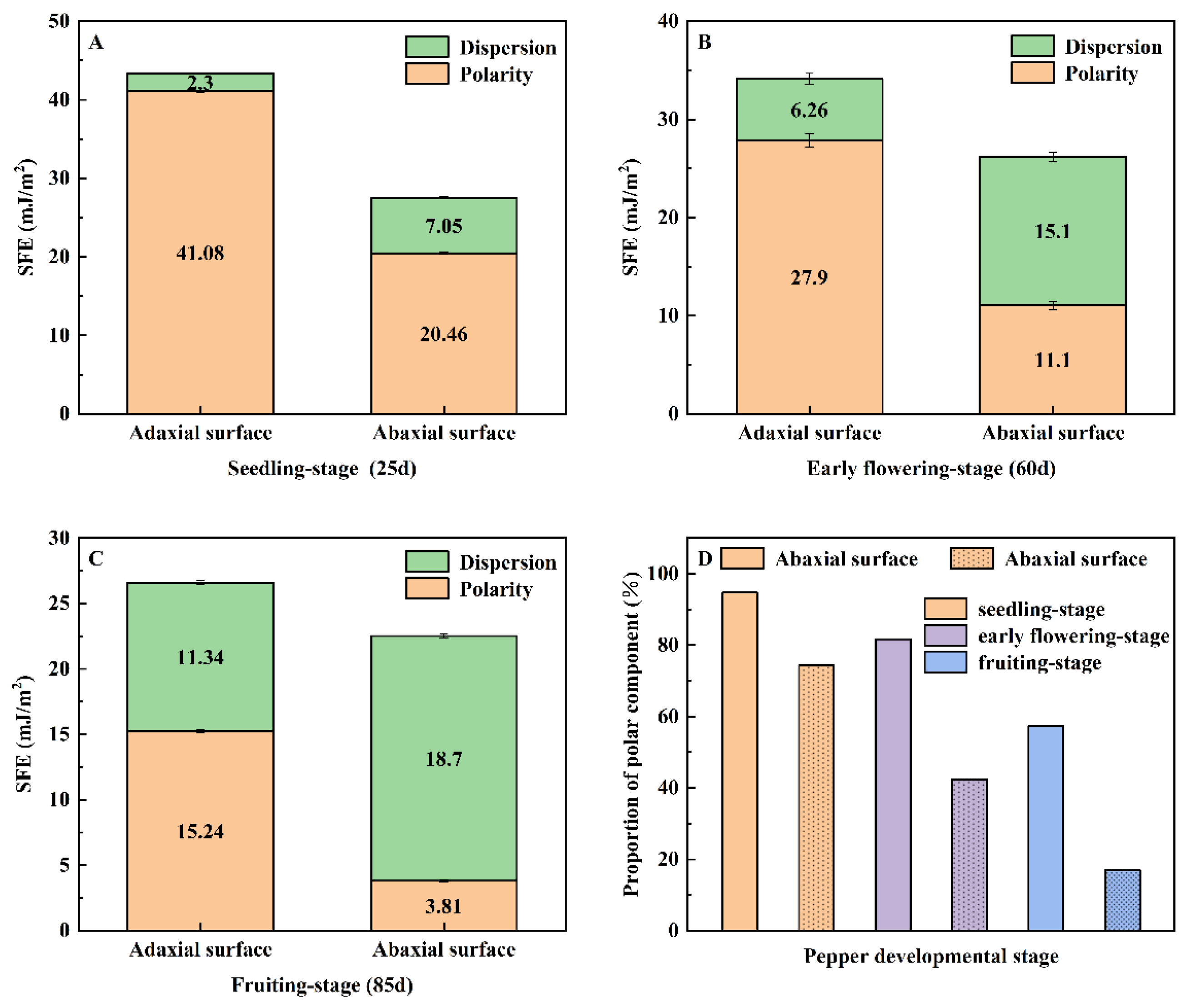
3.2. Surface Free Energy and Its Components in Pepper Leaves
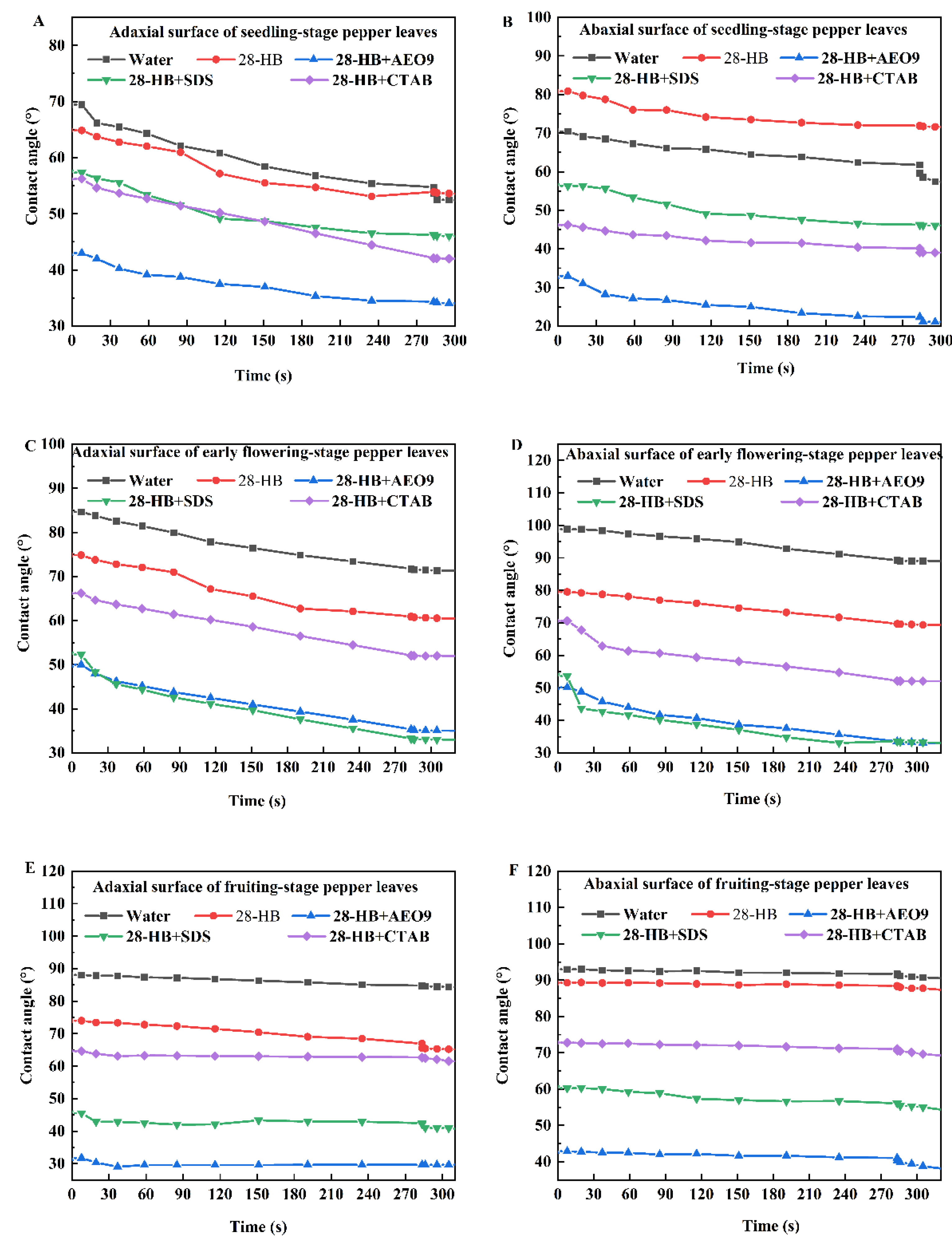
3.3. Changes in Contact Angle of 28-HB on Leaves of Peppers at Different Growth Stages
3.4. Influence of Droplet Surface Tension and Plant Growth Stage on the State of Droplets on Pepper Leaves
3.5. Adhesion of 28-HB to Pepper Leaves
4. Conclusions
- (1)
- With increasing plant maturity, the leaf surface roughness increased markedly from 12.7 μm at the seedling stage to over 50 μm at the fruiting stage, indicating progressively more complex surface structures. At all growth stages, the adaxial surface exhibited higher surface free energy (SFE) than the abaxial surface. However, both the SFE and the ratio of polar components (RP) decreased over time. Specifically, the adaxial SFE declined from 43.4 mJ/m2 at the seedling stage to 26.6 mJ/m2 at the fruiting stage, while the abaxial SFE decreased from 27.5 mJ/m2 to 22.5 mJ/m2. The RP on the adaxial surface dropped significantly from 94.70% to 57.34%. The addition of AEO9 to 28-HB resulted in a significant reduction in the contact angle on pepper leaves, with values approaching 45° or below. In comparison to 28-HB mixed with SDS and CTAB, 28-HB with AEO9 showed better wettability performance on both the adaxial and abaxial surfaces of the leaves across all growth stages.
- (2)
- The addition of surfactants significantly enhanced the wettability of 28-HB on pepper leaf surfaces (p < 0.05). Compared with SDS and CTAB, the mixture of 28-HB with AEO-9 resulted in the greatest improvement, reducing the contact angle to below 45°, the adhesion tension to below 30 mN/m, and the work of adhesion to below 105 mJ/m2. This enhanced wetting effect was observed consistently on both leaf surfaces across all developmental stages.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kondo, S.; Costa, G.; Ohara, H.; Mattheis, J. Usage and action of plant growth regulators in horticultural crop production. Sci. Hortic. 2022, 304, 111293. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, L.; Ji, Y.; Li, S. Seed treatment with iron chlorine E6 enhances germination and seedling growth of rice. Agriculture 2022, 12, 218. [Google Scholar] [CrossRef]
- Hilal, B.; Khan, T.A.; Fariduddin, Q. Recent advances and mechanistic interactions of hydrogen sulfide with plant growth regulators in relation to abiotic stress tolerance in plants. Plant Physiol. Biochem. 2023, 196, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Yuan, S.; Zhu, X. Droplet characterization of a complete fluidic sprinkler with different nozzle dimensions. Biosyst. Eng. 2016, 148, 90–100. [Google Scholar] [CrossRef]
- Hu, B.; Li, H.; Jiang, Y.; Tang, P.; Du, L. Review of the interaction mechanism for droplets and foliage under sprinkler irrigation and water-fertilizer integration. Int. J. Agric. Biol. Eng. 2024, 17, 31–43. [Google Scholar] [CrossRef]
- Appah, S.; Wang, P.; Ou, M.; Gong, C.; Jia, W. Review of electrostatic system parameters, charged droplets characteristics and substrate impact behavior from pesticides spraying. Int. J. Agric. Biol. Eng. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Gong, C.; Jia, F.; Kang, C. Deposition of water and emulsion hollow droplets on hydrophilic and hydrophobic surfaces. Agriculture 2024, 14, 960. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Wang, D.; Hao, R. The motion of strawberry leaves in an air-assisted spray field and its influence on droplet deposition. Trans. Asabe 2021, 64, 83–93. [Google Scholar] [CrossRef]
- Xi, T.; Li, C.; Qiu, W.; Wang, H.; Lv, X.; Han, C.; Ahmad, F.; Wang, H. Droplet deposition behavior on a pear leaf surface under wind-induced vibration. Appl. Eng. Agric. 2020, 36, 913–926. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, C.; Cao, L.; Zheng, L.; Xu, J.; Li, F.; Huang, Q. Effect of surfactant concentration on the evaporation of droplets on cotton (Gossypium hirsutum L.) leaves. Colloids Surf. B 2018, 167, 206–212. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, J.; Xiao, S.; Fang, H.; Zheng, Q. Investigating the wettability of rapeseed leaves. Appl. Eng. Agric. 2021, 37, 399–409. [Google Scholar] [CrossRef]
- Loti, N.N.A.; Noor, M.R.M.; Chang, S. Integrated Analysis of Machine Learning and Deep Learning in Chili Pest and Disease Identification. J. Sci. Food Agric. 2020, 101, 3582–3594. [Google Scholar]
- Ahmed, I.H.M.; Ali, E.F.; Gad, A.A.; Bardisi, A.; El-Tahan, A.M.; Abd Esadek, O.A.; El-Saadony, M.T.; Gendy, A.S. Impact of Plant Growth Regulators Spray on Fruit Quantity and Quality of Pepper (Capsicum annuum L.) Cultivars Grown under Plastic Tunnels. Saudi J. Biol. Sci. 2022, 29, 2291–2298. [Google Scholar] [CrossRef]
- Van Zyl, S.A.; Brink, J.-C.; Calitz, F.J.; Fourie, P.H. Effects of adjuvants on deposition efficiency of fenhexamid sprays applied to chardonnay grapevine foliage. Crop Prot. 2010, 29, 843–852. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Li, Y.; Wang, Y. The effects of leaf roughness, surface free energy and work of adhesion on leaf water drop adhesion. PLoS ONE 2014, 9, e107062. [Google Scholar] [CrossRef]
- Van Maarseveen, C.; Han, H.; Jetter, R. Development of the Cuticular Wax during Growth of Kalanchoe daigremontiana (Hamet et Perr. de La Bathie) Leaves. Plant Cell Environ. 2009, 32, 73–81. [Google Scholar] [CrossRef]
- Ahmed, J.O. Biochemical Investigation of Leaf Development in Capsicum Frutescens. J. Agric. Sci. 2013, 5, 165–175. [Google Scholar] [CrossRef][Green Version]
- Van Maarseveen, C.; Jetter, R. Composition of the Epicuticular and Intracuticular Wax Layers on Kalanchoe daigremontiana (Hamet et Perr. de La Bathie) Leaves. Phytochemistry 2009, 70, 899–906. [Google Scholar] [CrossRef]
- Henningsen, J.N.; Bahamonde, H.A.; Mühling, K.H.; Fernández, V. Tomato and pepper leaf parts contribute differently to the absorption of foliar-applied potassium dihydrogen phosphate. Plants 2023, 12, 2152. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, H.; Yang, X. Characterization of droplet impact and deposit formation on leaf surfaces: Droplet impact and deposit formation on leaf surfaces. Pest. Manag. Sci. 2015, 71, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.; Sijs, R.; de Goede, T.; Bonn, D. Controlling Droplet Deposition with Surfactants. Phys. Rev. Fluids 2021, 6, 033601. [Google Scholar] [CrossRef]
- Li, H.; Travlos, I.; Qi, L.; Kanatas, P.; Wang, P. Optimization of herbicide use: Study on spreading and evaporation characteristics of glyphosate-organic silicone mixture droplets on weed leaves. Agronomy 2019, 9, 547. [Google Scholar] [CrossRef]
- Tredenick, E.C.; Forster, W.A.; Pethiyagoda, R.; van Leeuwen, R.M.; McCue, S.W. Evaporating droplets on inclined plant leaves and synthetic surfaces: Experiments and mathematical models. J. Colloid Interface Sci. 2021, 592, 329–341. [Google Scholar] [CrossRef]
- Hagedorn, O.; Fleute-Schlachter, I.; Mainx, H.G.; Zeisler-Diehl, V.; Koch, K. Surfactant-induced enhancement of droplet adhesion in superhydrophobic soybean leaves. Beilstein J. Nanotechnol. 2017, 8, 2345–2356. [Google Scholar] [CrossRef]
- Tamjidi, S.; Moghadas, B.K.; Esmaeili, H.; Khoo, F.S.; Gholami, G.; Ghasemi, M. Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: A review study. Process Saf. Environ. Prot. 2021, 148, 775–795. [Google Scholar] [CrossRef]
- Bao, Z.; Zeng, A.; Gao, T.; Gao, Y.; He, Q.; Huang, Y.; Chou, J.; Yu, L.; Zhang, C.; Du, F. Controlling impact behavior on superhydrophobic surfaces for droplets of nonionic surfactants by tailoring hydrophilic chain length. J. Mol. Liq. 2022, 346, 117071. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H.E.; Bagley, W.E.; Derksen, R.C.; Krause, C.R. Adjuvant effects on evaporation time and wetted area of droplets on waxy leaves. Trans. ASABE 2010, 53, 13–20. [Google Scholar] [CrossRef]
- Yan, J.-K.; Chen, T.-T.; Wang, Z.-W.; Wang, C.; Liu, C.; Li, L. Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chem. 2022, 389, 133083. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, X.; Bing, L.; Song, J.; Qi, Y.; Han, Q.; Yang, Y.; Lin, D. Responses of growth, enzyme activity, and flower bud differentiation of pepper seedlings to nitrogen concentration at different growth stages. Agronomy 2024, 14, 2270. [Google Scholar] [CrossRef]
- Janssen, D.; De Palma, R.; Verlaak, S.; Heremans, P.; Dehaen, W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Film. 2006, 515, 1433–1438. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Adam, N.K. Use of the Term ‘Young’s Equation’ for Contact Angles. Nature 1957, 180, 809–810. [Google Scholar] [CrossRef]
- Nairn, J.J.; Forster, W.A.; van Leeuwen, R.M. Quantification of physical (roughness) and chemical (dielectric constant) leaf surface properties relevant to wettability and adhesion. Pest Manag. Sci. 2011, 67, 1562–1570. [Google Scholar] [CrossRef]
- Appah, S.; Jia, W.; Ou, M.; Wang, P.; Asante, E.A. Analysis of potential impaction and phytotoxicity of surfactant-plant surface interaction in pesticide application. Crop Prot. 2020, 127, 104961. [Google Scholar] [CrossRef]
- Packham, D.E. Work of Adhesion: Contact Angles and Contact Mechanics. Int. J. Adhes. Adhes. 1996, 16, 121–128. [Google Scholar] [CrossRef]
- Liu, D.-D.; Xu, Z.-C.; Zhang, L.; Luo, L.; Zhang, L.; Wei, T.-X.; Zhao, S. Adsorption behaviors of cationic surfactants and wettability in polytetrafluoroethylene–solution–air systems. Langmuir 2012, 28, 16845–16854. [Google Scholar] [CrossRef]
- Nairn, J.J.; Forster, W.A. Methods for evaluating leaf surface free energy and polarity having accounted for surface roughness. Pest Manag. Sci. 2017, 73, 1854–1865. [Google Scholar] [CrossRef]
- Gizer, S.G.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Low-pressure plasma treated polycarbonate and polymethyl methacrylate (PMMA) sheets with different surface patterns to change their surface properties. Surf. Interfaces 2023, 37, 102646. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, R.; Fan, R.; Liu, Z.; Kong, W.; Zhang, P.; Du, F. Wettability of pear leaves from three regions characterized at different stages after flowering using the OWRK method. Pest Manag. Sci. 2018, 74, 1804–1809. [Google Scholar] [CrossRef]
- Zheng, L.; Cao, C.; Chen, Z.; Cao, L.; Huang, Q.; Song, B. Efficient pesticide formulation and regulation mechanism for improving the deposition of droplets on the leaves of rice. Pest Manag. Sci. 2021, 77, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. The wetting of leaf surfaces. Curr. Opin. Colloid Interface Sci. 2011, 16, 326–334. [Google Scholar] [CrossRef]
- Gaskin, R.E.; Steele, K.D.; Forster, W.A. Characterising plant surfaces for spray adhesion and retention. N. Z. Plant Prot. 2005, 58, 179–183. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M.V. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Membr. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, X.; Pang, M.; Liu, K. Analyses of Droplet Spreading and the Movement of Wetting Line on a Solid Surface. Acta Phys. Sin. 2016, 65, 16801. [Google Scholar] [CrossRef]
- Wang, C.; Xu, G.; Xu, D.; Xu, L.; Gu, Z. Wettability analysis on leaves of huangoubao sweet pepper at different growth stages. Jiangsu J. Agric. Sci. 2020, 36, 882–887. [Google Scholar]
- Zhou, Q.; Wu, Y.; Rosen, M.J. Surfactant−surfactant molecular interactions in mixed monolayers at a highly hydrophobic solid/aqueous solution interface and their relationship to enhanced spreading on the solid substrate. Langmuir 2003, 19, 7955–7962. [Google Scholar] [CrossRef]
- Cao, C.; Liu, M.; Ma, X.; Chen, Y.; Huang, Q. Enhancing droplets deposition on superhydrophobic plant leaves by bio-based surfactant: Experimental characterization and molecular dynamics simulations. J. Mol. Liq. 2023, 387, 122696. [Google Scholar] [CrossRef]
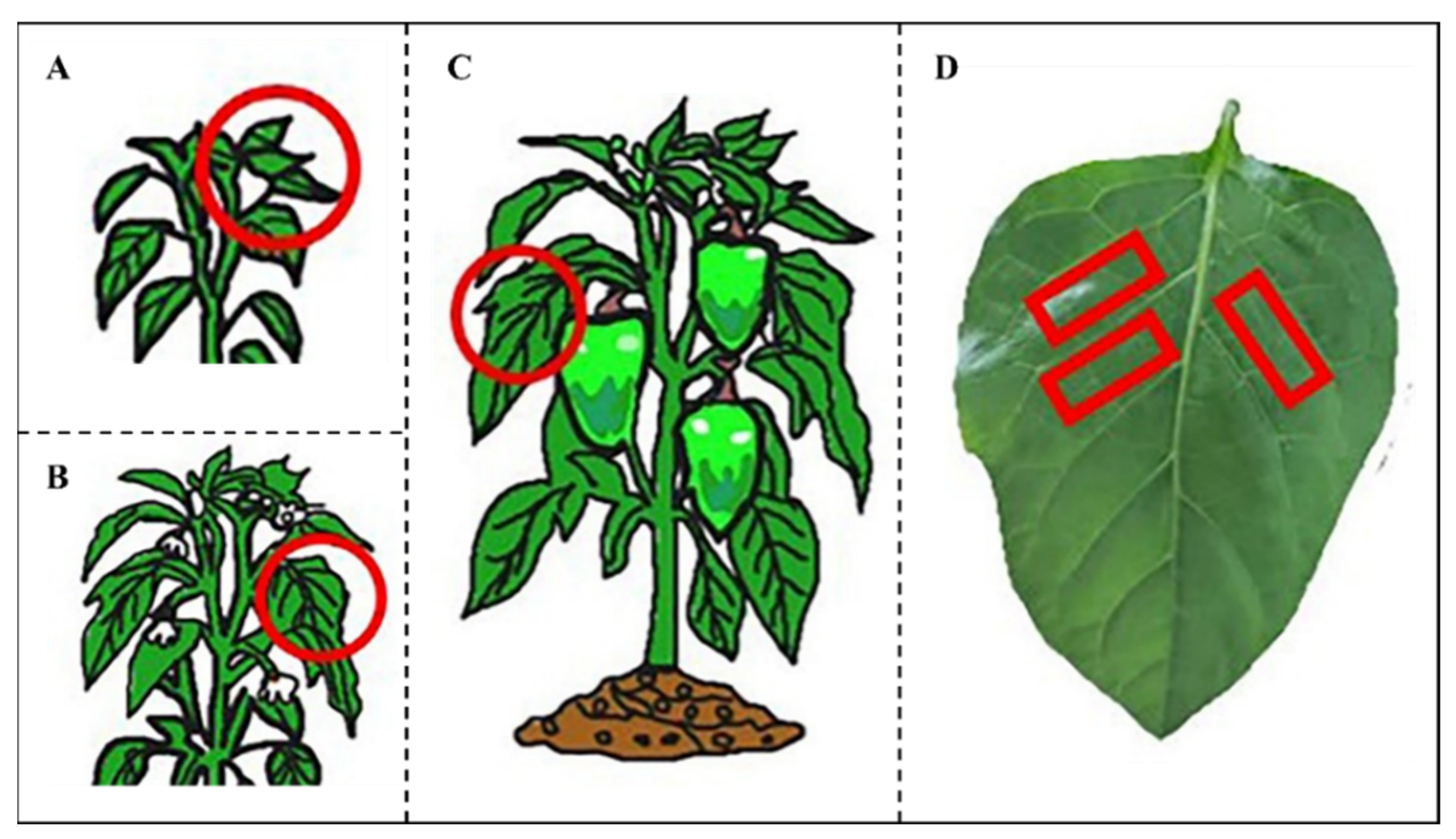

| Growth Stage | Placement | Deionized Water (°) | 28-HB (°) | 28-HB + CTAB (°) | 28-HB + SDS (°) | 28-HB + AEO9 (°) |
|---|---|---|---|---|---|---|
| Seedling | Adaxial | 65.49 ± 1.25 | 62.76 ± 0.75 | 53.66 ± 0.95 | 55.58 ± 2.20 | 40.24 ± 1.17 |
| Abaxial | 68.49 ± 1.20 | 78.76 ± 2.25 | 44.46 ± 0.92 | 55.58 ± 2.23 | 28.24 ± 1.08 | |
| Early flowering | Adaxial | 82.49 ± 1.35 | 72.76 ± 0.72 | 63.66 ± 0.96 | 45.58 ± 1.23 | 46.24 ± 2.57 |
| Abaxial | 98.43 ± 1.80 | 78.78 ± 0.66 | 62.93 ± 1.57 | 42.71 ± 1.51 | 45.75 ± 0.74 | |
| Fruiting | Adaxial | 83.77 ± 0.61 | 73.35 ± 1.59 | 63.11 ± 0.85 | 42.85 ± 0.84 | 29.05 ± 0.54 |
| Abaxial | 92.71 ± 0.30 | 89.19 ± 0.24 | 72.53 ± 0.43 | 60.03 ± 0.78 | 42.49 ± 0.50 |
| Scheme | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Growth stage | 1748.133 | 5 | 349.627 | 209.785 | <0.001 |
| Surface tension | 13,413.949 | 3 | 4471.316 | 2682.904 | <0.001 |
| Growth stage × Surface tension | 2637.957 | 15 | 175.864 | 105.523 | <0.001 |
| Pepper Leaves | Solution | Contact Angle (°) | Adhesion Tension (mN/m) | Work of Adhesion (mJ/m2) |
|---|---|---|---|---|
| Adaxial surface of pepper leaves at seedling stage | 28-HB | 62.76 | 26.28 | 83.69 |
| 28-HB + CTAB | 53.66 | 23.32 | 62.68 | |
| 28-HB + SDS | 55.58 | 19.26 | 53.34 | |
| 28-HB + AEO9 | 40.24 | 24.79 | 57.26 | |
| Abaxial surface of pepper leaves at seedling stage | 28-HB | 78.76 | 11.19 | 68.60 |
| 28-HB + CTAB | 44.46 | 28.09 | 67.45 | |
| 28-HB + SDS | 55.58 | 19.26 | 53.34 | |
| 28-HB + AEO9 | 28.24 | 28.61 | 61.08 | |
| Adaxial surface of pepper leaves at early flowering stage | 28-HB | 72.76 | 17.01 | 74.42 |
| 28 HB + CTAB | 63.66 | 17.46 | 56.82 | |
| 28-HB + SDS | 45.58 | 23.85 | 57.93 | |
| 28 HB + AEO9 | 46.24 | 22.46 | 54.93 | |
| Abaxial surface of pepper leaves at early flowering stage | 28-HB | 78.78 | 11.17 | 68.58 |
| 28 HB + CTAB | 62.93 | 17.91 | 57.27 | |
| 28-HB + SDS | 42.71 | 25.04 | 59.12 | |
| 28 HB + AEO9 | 45.75 | 22.66 | 55.13 | |
| Adaxial surface of pepper leaves at the fruiting stage | 28-HB | 73.35 | 16.45 | 73.86 |
| 28 HB + CTAB | 63.11 | 17.80 | 57.16 | |
| 28-HB + SDS | 42.85 | 24.99 | 59.07 | |
| 28 HB + AEO9 | 29.05 | 28.39 | 60.86 | |
| Abaxial surface of pepper leaves at the fruiting stage | 28-HB | 89.19 | 0.812 | 58.22 |
| 28 HB + CTAB | 72.53 | 11.82 | 51.18 | |
| 28-HB + SDS | 60.03 | 17.02 | 51.10 | |
| 28 HB + AEO9 | 42.49 | 23.94 | 56.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, K.; Gao, Z.; Zhu, C.; Guan, X.; Qiu, B. Wettability of the Plant Growth Regulator 28-HB on Pepper Leaves at Different Developmental Stages. Horticulturae 2025, 11, 661. https://doi.org/10.3390/horticulturae11060661
Dong X, Wang K, Gao Z, Zhu C, Guan X, Qiu B. Wettability of the Plant Growth Regulator 28-HB on Pepper Leaves at Different Developmental Stages. Horticulturae. 2025; 11(6):661. https://doi.org/10.3390/horticulturae11060661
Chicago/Turabian StyleDong, Xiaoya, Kaiyuan Wang, Zhouming Gao, Cuicui Zhu, Xianping Guan, and Baijing Qiu. 2025. "Wettability of the Plant Growth Regulator 28-HB on Pepper Leaves at Different Developmental Stages" Horticulturae 11, no. 6: 661. https://doi.org/10.3390/horticulturae11060661
APA StyleDong, X., Wang, K., Gao, Z., Zhu, C., Guan, X., & Qiu, B. (2025). Wettability of the Plant Growth Regulator 28-HB on Pepper Leaves at Different Developmental Stages. Horticulturae, 11(6), 661. https://doi.org/10.3390/horticulturae11060661







