Dynamics of Phytohormones in Persistent Versus Deciduous Calyx Development in Pear Revealed by Targeted Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Site and Materials
2.2. Sample Collection
2.3. Absolute Quantitative and Qualitative Analysis of Endogenous Hormones in Calyx
2.4. Instrument and Equipment
2.5. Sample Treatment
2.6. Preparation of the Standard Solution
2.7. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
2.8. Flow Phase Conditions
2.9. Mass Spectrometric Conditions
2.10. Venn Diagram
2.11. Data Analysis
2.12. Statistical Analysis
3. Results
3.1. Appearance Morphological Characteristics of Different Position Sepals of Korla Fragrant Pear
3.2. Determination of Metabolic Groups of Calyx in Different Positions
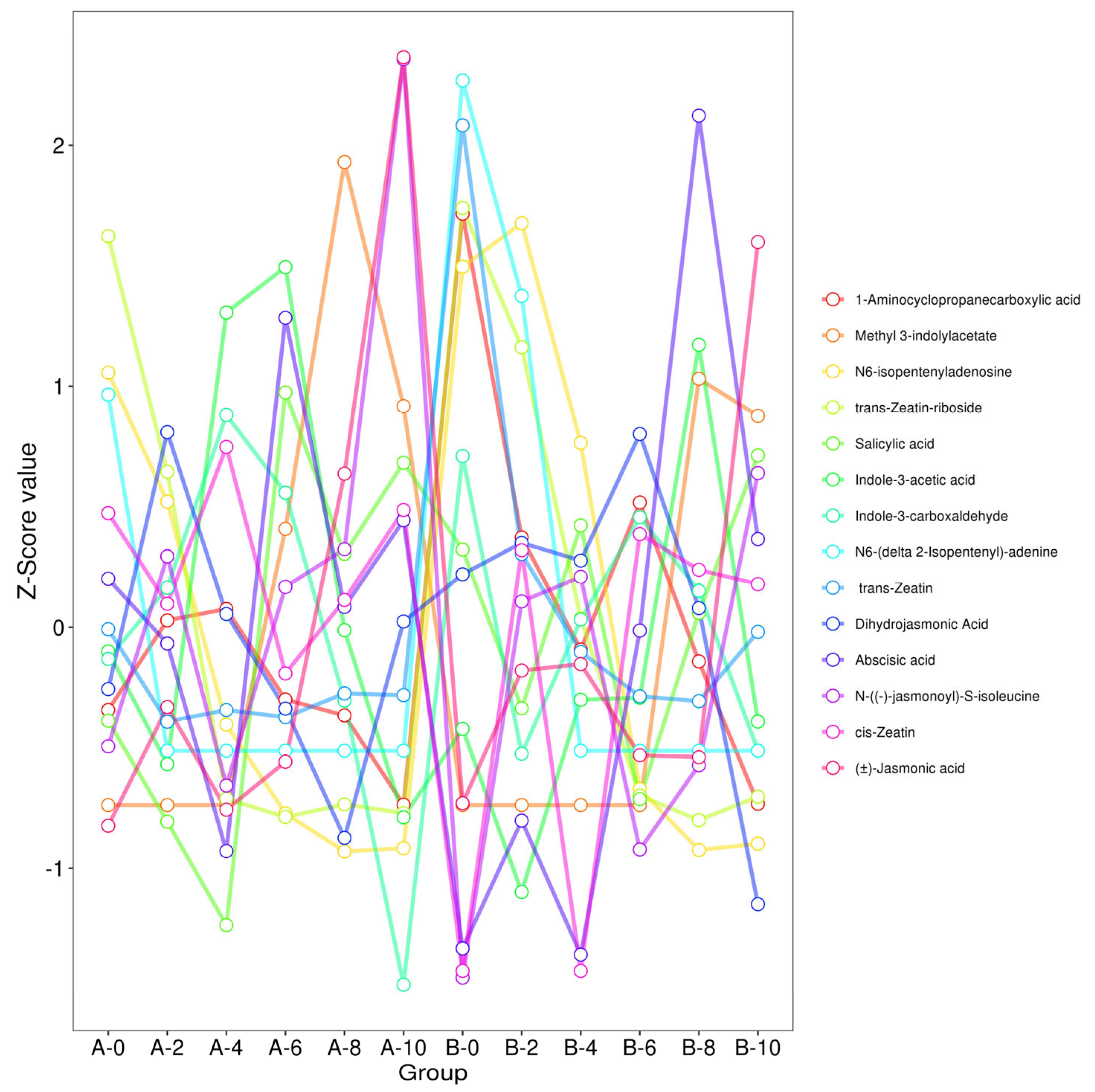
3.3. Trend Analysis
3.4. Heat Map of Different Metabolites
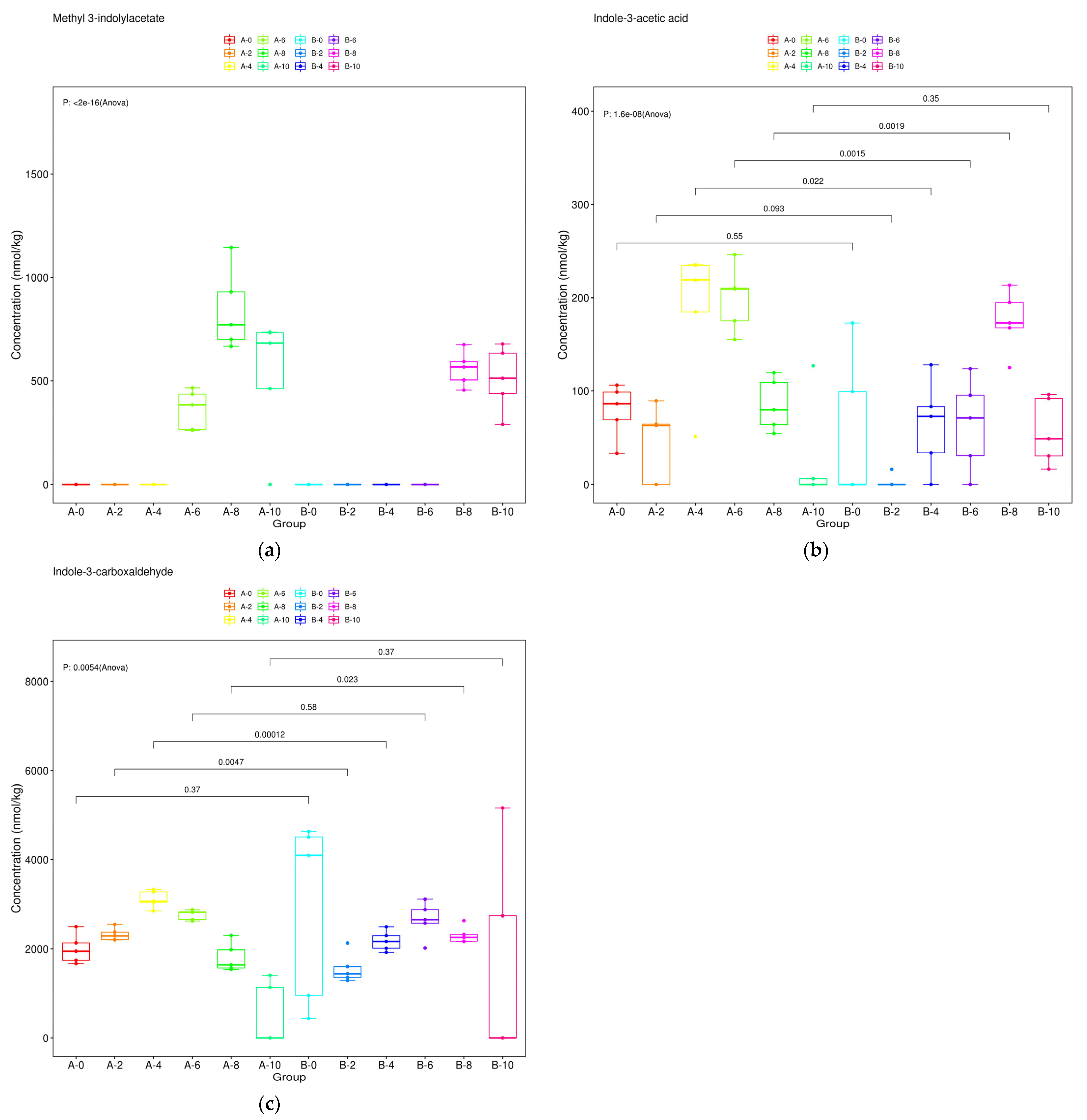
3.5. Dynamic Changes of Auxin Metabolites
3.6. Dynamic Changes of Cytokinin Metabolites
3.7. Dynamic Changes of Jasmonic Acid Metabolites
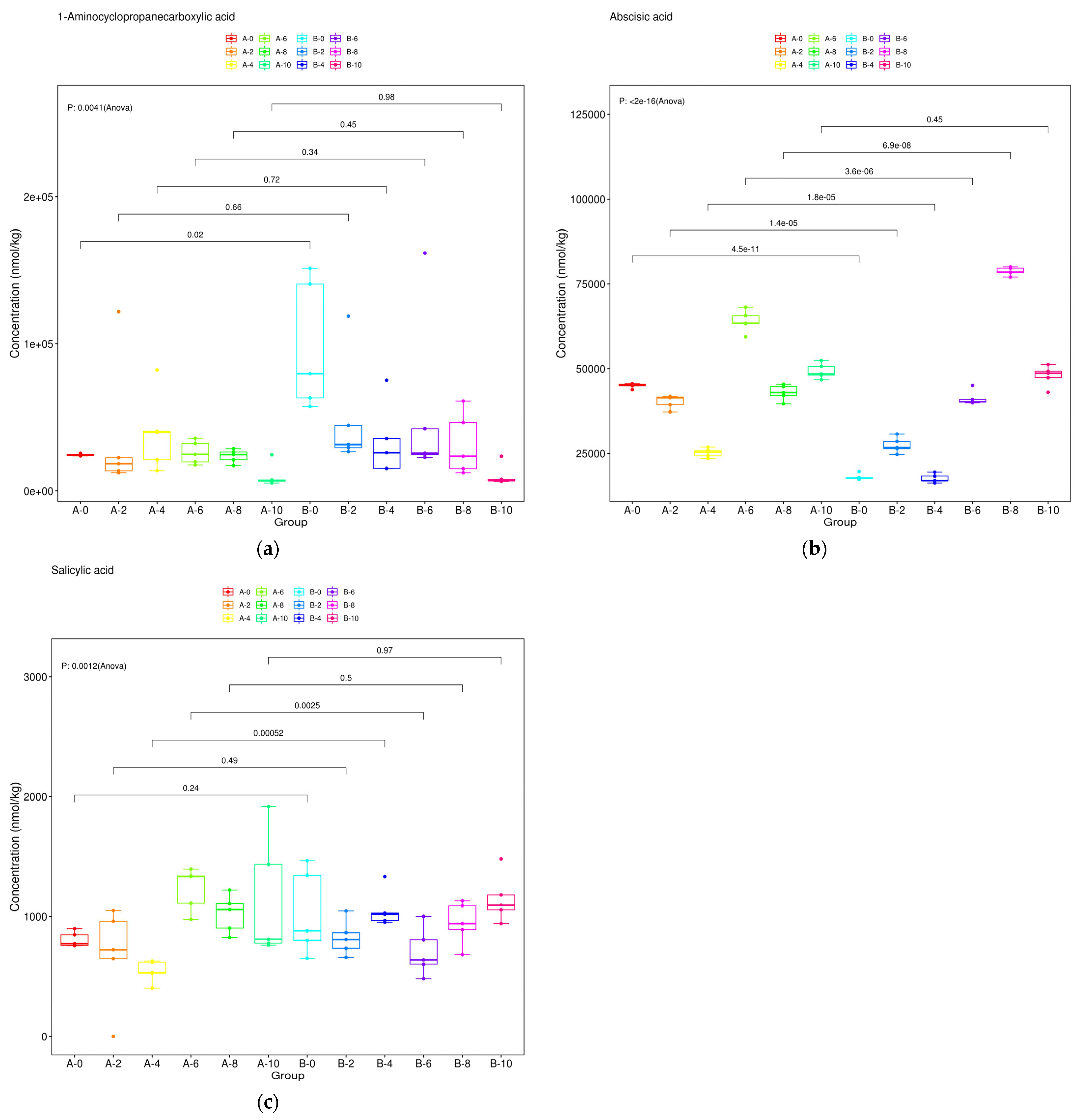
3.8. Dynamic Changes of Metabolites of Ethylene, Abscisic Acid and Salicylic Acid
3.9. Metabolite Correlation Heatmap Across Different Floral Positions in Calyx Tissue
3.10. The Proportion of Different Metabolites in Calyx Targeted Metabolism Group at Different Stages
3.11. Venn Diagram Analyse
4. Discussion
4.1. Sequence-Specific Dynamics and Abscission Layer Differentiation of Auxin Metabolism
4.2. Cytokinin Homeostasis Regulates Sepal Function Maintenance
4.3. JA-ABA-Ethylene Synergistic Network Drives Terminal Segregation in the Abscission Zone
4.4. Hierarchical Characteristics of Resource Allocation Policies
4.5. IP in Layer-Specificity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | 1-Aminocyclopropanecarboxylic acid. |
| ME-IAA | Methyl 3-indolylacetate. |
| IPA | N6-isopentenyladenosine. |
| TZR | trans-Zeatin-riboside. |
| SA | Salicylic acid. |
| IAA | Indole-3-acetic acid. |
| ICA | Indole-3-carboxaldehyde. |
| IP | N6-(delta 2-Isopentenyl)-adenine. |
| tZ | trans-Zeatin. |
| DHJA | Dihydrojasmonic Acid. |
| ABA | Abscisic acid. |
| JA-Ile | N-((-)-jasmonoyl)-S-isoleucine. |
| cZ | cis-Zeatin. |
| JA | (±)-Jasmonic acid. |
| GA | gibberellin |
| ETH | ethylene |
| GA1 | Gibberellin A1 |
| GA3 | Gibberellin A3 |
| GA4 | Gibberellin A4 |
| GA7 | Gibberellin A7 |
| ZR | riboside |
References
- Pan, Y.; Meng, X.; Che, F.; Xue, S.; Zhang, T.; Zhao, S.; Liao, K. Metabolic Profiles of Sugar Metabolism and Respiratory Metabolism of Korla Pear (Pyrus sinkiangensis Yu) Throughout Fruit Development and Ripening. Sci. Agric. Sin. 2016, 49, 3391–3412. [Google Scholar] [CrossRef]
- Guo, K.; Wang, L.; Yu, M.; Fan, W.; Bao, J. Effects of nitrogen application period on fruit setting rate and lignin accumulation during sepal development of Korla fragrant pear. Pomol. J. 2025, 42, 568–576. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Chen, Y.; Zheng, Q.; Wang, Z.; Wang, W.; Bao, J. Effects of pre-anthesis and anthesis irrigation on active oxygen metabolism and programmed cell death in calyx tube of Korla fragrant pear. J. Pomol. 2023, 40, 2124–2135. [Google Scholar] [CrossRef]
- Muhetar, Z.; Abdul, X.Y.; Yushan, K.; Li, J. Effects of plant endogenous hormone content in different parts of young fruit of Korla fragrant pear on fruit sepal abscission. Plant Physiol. 2020, 56, 2179–2186. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Gong, X.; Bao, J. Effects of Different Foliar Fertilizer Treatments on Fruit Quality of the Korla Fragrant Pear. Horticulturae 2024, 10, 51. [Google Scholar] [CrossRef]
- Wang, B.-H.; Sun, X.-X.; Niu, J.-X. Cloning of kfpMYB Gene and Its Promoter in Korla Fragrant Pear (Pyrus sinkiangensis) and Determination Their Responses to Hormones. ACTA Hortic. Sin. 2015, 42, 1448–1456. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, M.; Wu, C.-Y.; Bao, J.-P. Effects of Plant Growth Regulators on the Endogenous Hormone Content of Calyx Development in ‘Korla’ Fragrant Pear. HortScience 2022, 57, 497–503. [Google Scholar] [CrossRef]
- Nakano, R.; Ogura, E.; Kubo, Y.; Inaba, A. Ethylene Biosynthesis in Detached Young Persimmon Fruit Is Initiated in Calyx and Modulated by Water Loss from the Fruit. Plant Physiol. 2003, 131, 276–286. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Duan, N.; Jia, Y.K.; Xu, J.; Chen, H.L.; Sun, P. Research Progress on Plant Endogenous Hormones. Chin. Agric. Sci. Bull. 2015, 031, 159–165. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal Regulation of Plant Growth and Development. PLoS Biol. 2004, 2, e311. [Google Scholar] [CrossRef]
- Kim, H.U. Lipid Metabolism in Plants. Plants 2020, 9, 871. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Yamada, M. The Roles of Peptide Hormones and Their Receptors during Plant Root Development. Genes 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zang, Y.; Ma, H.; Xu, K.; Zhang, W.; Zheng, W. Advances in studies on organ abscission and cell wall metabolism in abscission zone of fruit trees. Plant Physiol. 2023, 59, 281–291. [Google Scholar] [CrossRef]
- Zheng, L.; Wen, Y.; Lin, Y.; Tian, J.; Shaobai, J.; Hao, Z.; Wang, C.; Sun, T.; Wang, L.; Chen, C. Phytohormonal dynamics in the abscission zone of Korla fragrant pear during calyx abscission: A visual study. Front. Plant Sci. 2024, 15, 1452072. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Niu, J.; Li, C.; Cao, F.; Quan, S. Identification and expression analysis of genes related to calyx persistence in Korla fragrant pear. BMC Genom. 2016, 17, 132. [Google Scholar] [CrossRef]
- Hao, Z.; Wen, Y.; Tian, J.; Shao, B.; Yang, D.; Zhang, F. The relationship between sepal abscission of Korla fragrant pear and phytohormones in different parts of abscission zone. Plant Physiol. 2022, 58, 1369–1380. [Google Scholar] [CrossRef]
- Wong, C.; Alabadí, D.; Blázquez, M.A. Spatial regulation of plant hormone action. J. Exp. Bot. 2023, 74, 6089–6103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, G.; Duan, X.; Zhang, K.; Zhang, X.; Zhou, Y.; Wu, C.; Zhang, X.; Tan, S.; Hua, X.; et al. Research Progress and Trends in Metabolomics of Fruit Trees. Front. Plant Sci. 2022, 13, 881856. [Google Scholar] [CrossRef]
- Furch, A.C.U.; Zimmermann, M.R.; Kogel, K.; Reichelt, M.; Mithöfer, A. Direct and individual analysis of stress-related phytohormone dispersion in the vascular system of Cucurbita maxima after flagellin 22 treatment. New Phytol. 2014, 201, 1176–1182. [Google Scholar] [CrossRef]
- Martinelli, F.; Basile, B.; Morelli, G.; D’andria, R.; Tonutti, P. ().Effects of irrigation on fruit ripening behavior and metabolic changes in olive. Sci. Hortic. 2012, 144, 201–207. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Wang, D.; Ma, Y.; Shan, Y.; Ren, X.; Hu, H.; Wu, C.; Yang, J.; Ma, Y. Simultaneous determination of five plant hormones in cotton leaves using QuEChERS combined with HPLC–MS/MS. J. Cotton Res. 2024, 7, 18. [Google Scholar] [CrossRef]
- de Falco, B.; Giannino, F.; Carteni, F.; Mazzoleni, S.; Kim, D.-H. Metabolic flux analysis: A comprehensive review on sample preparation, analytical techniques, data analysis, computational modelling, and main application areas. RSC Adv. 2022, 12, 25528–25548. [Google Scholar] [CrossRef] [PubMed]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chen, H.; Lee, G.; Emonet, A.; Kim, S.-G.; Shim, D.; Lee, Y. MSD2-mediated ROS metabolism fine-tunes the timing of floral organ abscission in Arabidopsis. New Phytol. 2022, 235, 2466–2480. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, S. The Interconnected Relationship between Auxin Concentration Gradient Changes in Chinese Fir Radial Stems and Dynamic Cambial Activity. Forests 2022, 13, 1698. [Google Scholar] [CrossRef]
- Gibson, C.L.; Isley, J.W.; Falbel, T.G.; Mattox, C.T.; Lewis, D.R.; Metcalf, K.E.; Muday, G.K. A Conditional Mutation in SCD1 Reveals Linkage Between PIN Protein Trafficking, Auxin Transport, Gravitropism, and Lateral Root Initiation. Front. Plant Sci. 2020, 11, 910. [Google Scholar] [CrossRef]
- Tang, Q.; Tillmann, M.; Cohen, J.D. Analytical methods for stable isotope labeling to elucidate rapid auxin kinetics in Arabidopsis thaliana. PLoS ONE 2024, 19, e0303992. [Google Scholar] [CrossRef]
- González-García, M.P.; Sáez, A.; Lanza, M.; Hoyos, P.; Bustillo-Avendaño, E.; Pacios, L.F.; Gradillas, A.; A Moreno-Risueno, M.; Hernaiz, M.J.; del Pozo, J.C. Synthetically derived BiAux modulates auxin co-receptor activity to stimulate lateral root formation. Plant Physiol. 2024, 195, 1694–1711. [Google Scholar] [CrossRef]
- Li, X.; Han, B.; Yang, Y. Research Progress on the Regulatory Network and Crosstalk of Plant Hormones in Plant Organ Abolition. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2023, 44, 92–100. [Google Scholar] [CrossRef]
- Gao, J.; Zhuang, S.; Zhang, W. Advances in Plant Auxin Biology: Synthesis, Metabolism, Signaling, Interaction with Other Hormones, and Roles under Abiotic Stress. Plants 2024, 13, 2523. [Google Scholar] [CrossRef]
- Le Bail, A.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Le Panse, S.; Stewart, S.; Scornet, D.; Cock, J.M.; Ljung, K.; et al. Auxin Metabolism and Function in the Multicellular Brown Alga Ectocarpus siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef]
- Que, Y.; Huang, D.; Gong, S.; Zhang, X.; Yuan, B.; Xue, M.; Shi, W.; Zeng, F.; Liu, M.; Chen, T.; et al. Indole-3-Carboxylic Acid From the Endophytic Fungus Lasiodiplodia pseudotheobromae LPS-1 as a Synergist Enhancing the Antagonism of Jasmonic Acid Against Blumeria graminis on Wheat. Front. Cell. Infect. Microbiol. 2022, 12, 898500. [Google Scholar] [CrossRef]
- Liu, J.; Shi, M.; Wang, J.; Zhang, B.; Li, Y.; Wang, J.; El-Sappah, A.H.; Liang, Y. Comparative Transcriptomic Analysis of the Development of Sepal Morphology in Tomato (Solanum Lycopersicum L.). Int. J. Mol. Sci. 2020, 21, 5914. [Google Scholar] [CrossRef]
- Maio, K.A.; Moubayidin, L. ‘Organ’ising Floral Organ Development. Plants 2024, 13, 1595. [Google Scholar] [CrossRef]
- E Jameson, P.; Song, J. Will cytokinins underpin the second ‘Green Revolution’? J. Exp. Bot. 2020, 71, 6872–6875. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Liu, J.; Zhang, P.; Kudoyarova, G.; Liu, C.-J.; Zhang, K. Spatially distributed cytokinins: Metabolism, signaling, and transport. Plant Commun. 2024, 5, 100936. [Google Scholar] [CrossRef]
- Ismael, S.; Rodrigues, C.; Santos, G.M.; Castela, I.; Barreiros-Mota, I.; Almeida, M.J.; Calhau, C.; Faria, A.; Araújo, J.R. IPA and its precursors differently modulate the proliferation, differentiation, and integrity of intestinal epithelial cells. Nutr. Res. Pract. 2023, 17, 616–630. [Google Scholar] [CrossRef]
- Pokimica, N.; Ćosić, T.; Uzelac, B.; Ninković, S.; Raspor, M. Dissecting the Roles of the Cytokinin Signaling Network: The Case of De Novo Shoot Apical Meristem Formation. Biomolecules 2024, 14, 381. [Google Scholar] [CrossRef]
- Hluska, T.; Hlusková, L.; Emery, R.J.N. The Hulks and the Deadpools of the Cytokinin Universe: A Dual Strategy for Cytokinin Production, Translocation, and Signal Transduction. Biomolecules 2021, 11, 209. [Google Scholar] [CrossRef]
- Kućko, A.; Florkiewicz, A.B.; Wolska, M.; Miętki, J.; Kapusta, M.; Domagalski, K.; Wilmowicz, E. Jasmonate-Dependent Response of the Flower Abscission Zone Cells to Drought in Yellow Lupine. Plants 2022, 11, 527. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Sawicki, M.; Barka, E.A.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Morianou, G.; Kourgialas, N. Drought and Salinity in Citriculture: Optimal Practices to Alleviate Salinity and Water Stress. Agronomy 2021, 11, 1283. [Google Scholar] [CrossRef]
- Polko, J.K.; Kieber, J.J. 1-Aminocyclopropane 1-Carboxylic Acid and Its Emerging Role as an Ethylene-Independent Growth Regulator. Front. Plant Sci. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Bu, J.; Jiang, Y.; Khan, Z.U.; Luo, Z.; Mao, L.; Ying, T. Comprehensive Analysis of ABA Effects on Ethylene Biosynthesis and Signaling during Tomato Fruit Ripening. PLoS ONE 2016, 11, e0154072. [Google Scholar] [CrossRef]
- Galindo-Trigo, S.; Khandare, V.; Roosjen, M.; Adams, J.; Wangler, A.-M.; Bayer, M.; Borst, J.W.; Smakowska-Luzan, E.; Butenko, M.A. A multifaceted kinase axis regulates plant organ abscission through conserved signaling mechanisms. Curr. Biol. 2024, 34, 3020–3030.e7. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Maeda, H.; Chang, C.Y.; San Miguel, P.; Baxter, I.; Cooper, B.; Perera, M.A.; Nikolau, B.J.; Vitek, O.; Morgan, J.A.; et al. Developmental changes in the metabolic network of snapdragon flowers. PLoS ONE 2012, 7, e40381. [Google Scholar] [CrossRef]
- Ding, L.-N.; Li, Y.-T.; Wu, Y.-Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Fooyontphanich, K.; Morcillo, F.; Joët, T.; Dussert, S.; Serret, J.; Collin, M.; Amblard, P.; Tangphatsornruang, S.; Roongsattham, P.; Jantasuriyarat, C.; et al. Multi-scale comparative transcriptome analysis reveals key genes and metabolic reprogramming processes associated with oil palm fruit abscission. BMC Plant Biol. 2021, 21, 92. [Google Scholar] [CrossRef]
- Wang, J.; Cai, Y.; Zhang, N.; Zhang, Y. Regulatory Factors and Molecular Mechanism of Sucrose Transporters’ Expressions in Plant. Biotechnol. Bull. 2021, 37, 115–124. [Google Scholar] [CrossRef]
- Yuan, Y.; Khourchi, S.; Li, S.; Du, Y.; Delaplace, P. Unlocking the Multifaceted Mechanisms of Bud Outgrowth: Advances in Understanding Shoot Branching. Plants 2023, 12, 3628. [Google Scholar] [CrossRef]
- Kućko, A.; Wilmowicz, E.; Pokora, W.; Alché, J.D.D. Disruption of the Auxin Gradient in the Abscission Zone Area Evokes Asymmetrical Changes Leading to Flower Separation in Yellow Lupine. Int. J. Mol. Sci. 2020, 21, 3815. [Google Scholar] [CrossRef]
- Gundesli, M.A.; Kafkas, S.; Guney, M.; Kafkas, N.E. Identification of the profile of endogenous cytokinin-like compounds during different plant growth stages and their effects on flower bud abscission in pistachio (Pistacia vera L.). Folia Hortic. 2020, 32, 21–35. [Google Scholar] [CrossRef]

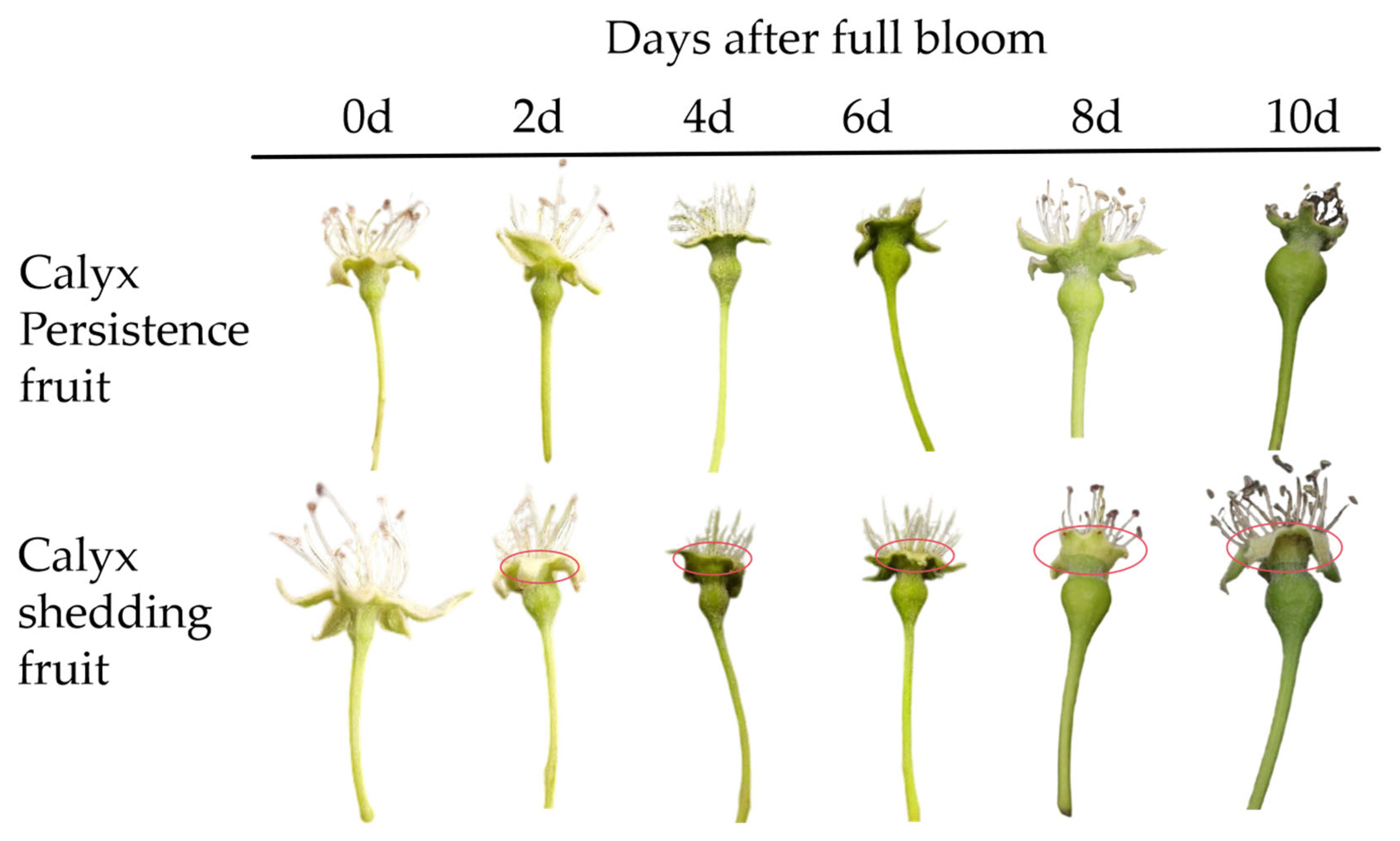







| Sampling Time The First Position | Group Name | Sampling Time The Fourth Position | Group Name |
|---|---|---|---|
| 0 DAF | A-0 | 0 DAF | B-0 |
| 2 DAF | A-2 | 2 DAF | B-2 |
| 4 DAF | A-4 | 4 DAF | B-4 |
| 6 DAF | A-6 | 6 DAF | B-6 |
| 8 DAF | A-8 | 8 DAF | B-8 |
| 10 DAF | A-10 | 10 DAF | B-10 |
| Abbr. | Concentration (nmol/L) | Recovery | RSD |
|---|---|---|---|
| ACC | 200.00 | 106.1 | 3.9% |
| ME-IAA | 200.00 | 98.4 | 4.1% |
| IPA | 200.00 | 93.4 | 7.0% |
| K | 31.25 | 99.8 | 4.9% |
| tZR | 200.00 | 95.0 | 5.1% |
| Me-JA | 31.25 | 97.8 | 3.8% |
| SA | 200.00 | 100.0 | 3.1% |
| IAA | 200.00 | 93.5 | 5.4% |
| IBA | 200.00 | 92.6 | 7.3% |
| ICA | 200.00 | 86.0 | 1.9% |
| IP | 31.25 | 105.0 | 5.1% |
| tZ | 31.25 | 103.1 | 2.0% |
| H2-JA | 31.25 | 100.5 | 2.0% |
| ABA | 200.00 | 95.7 | 2.7% |
| JA-Ile | 15.63 | 99.5 | 2.8% |
| cZ | 31.25 | 98.3 | 4.3% |
| dh-Z | 31.25 | 98.6 | 2.7% |
| GA1 | 31.25 | 95.8 | 3.1% |
| GA3 | 31.25 | 101.5 | 1.1% |
| GA4 | 31.25 | 99.7 | 6.0% |
| GA7 | 31.25 | 102.4 | 3.1% |
| Me-SA | 200.00 | 93.1 | 5.3% |
| JA | 200.00 | 97.8 | 3.6% |
| BL | 31.25 | 113.1 | 1.2% |
| Compounds Name | Abbr. | Formula | Test Results |
|---|---|---|---|
| 1-Aminocyclopropanecarboxylic acid. | ACC. | C4H7NO2 | △ |
| Methyl 3-indolylacetate. | ME-IAA. | C11H11NO2 | △ |
| N6-isopentenyladenosine. | IPA. | C15H21N5O4 | △ |
| Kinetin. | 6-KT. | C10H9N5O | — |
| trans-Zeatin-riboside. | TZR. | C15H21N5O5 | △ |
| Methyl jasmonate. | MeJA. | C13H20O3 | — |
| Salicylic acid. | SA. | C7H6O3 | △ |
| Indole-3-acetic acid. | IAA. | C10H9NO2 | △ |
| 3-Indolebutyric acid. | IBA. | C12H13NO2 | — |
| Indole-3-carboxaldehyde. | ICA. | C9H7NO | △ |
| N6-(delta 2-Isopentenyl)-adenine. | IP. | C10H13N5 | △ |
| trans-Zeatin. | tZ. | C10H13N5O | △ |
| Dihydrojasmonic Acid. | DHJA. | C12H20O3 | △ |
| Abscisic acid. | ABA. | C15H20O4 | △ |
| N-((-)-jasmonoyl)-S-isoleucine. | JA-Ile. | C18H29NO4 | △ |
| cis-Zeatin. | cZ. | C10H13N5O | △ |
| DL-Dihydrozeatin. | DL-DHZ. | C10H15N5O | — |
| Gibberellin A1. | GA1. | C19H24O6 | — |
| Gibberellin A3. | GA3. | C19H22O6 | — |
| Gibberellin A4. | GA4. | C19H24O5 | — |
| Gibberellin A7. | GA7. | C19H22O5 | — |
| Methyl salicylate. | MESA. | C8H8O3 | — |
| (±)-Jasmonic acid. | JA. | C12H18O3 | △ |
| Brassinolide | BL | C28H48O6 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Han, F.; Zhou, N.; Wang, L.; Li, Y.; Fan, W.; Zhang, T.; Bao, J. Dynamics of Phytohormones in Persistent Versus Deciduous Calyx Development in Pear Revealed by Targeted Metabolomics. Horticulturae 2025, 11, 642. https://doi.org/10.3390/horticulturae11060642
Yu M, Han F, Zhou N, Wang L, Li Y, Fan W, Zhang T, Bao J. Dynamics of Phytohormones in Persistent Versus Deciduous Calyx Development in Pear Revealed by Targeted Metabolomics. Horticulturae. 2025; 11(6):642. https://doi.org/10.3390/horticulturae11060642
Chicago/Turabian StyleYu, Mingyang, Feng Han, Nana Zhou, Lanfei Wang, Yang Li, Weifan Fan, Tianzheng Zhang, and Jianping Bao. 2025. "Dynamics of Phytohormones in Persistent Versus Deciduous Calyx Development in Pear Revealed by Targeted Metabolomics" Horticulturae 11, no. 6: 642. https://doi.org/10.3390/horticulturae11060642
APA StyleYu, M., Han, F., Zhou, N., Wang, L., Li, Y., Fan, W., Zhang, T., & Bao, J. (2025). Dynamics of Phytohormones in Persistent Versus Deciduous Calyx Development in Pear Revealed by Targeted Metabolomics. Horticulturae, 11(6), 642. https://doi.org/10.3390/horticulturae11060642






