Morphometric and Biochemical Analysis with Seed Protein Profiling of Passiflora Species Found in the Northeastern Himalayan Region of India
Abstract
1. Introduction
2. Materials and Methods
2.1. Details of Genotypes
2.2. Morphological Characterisation
2.3. Sample Preparation
2.4. Biochemical Characterisation
2.5. Protein Extraction and Estimation
2.6. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Staining Solution
2.8. Statistical Analysis
3. Results
3.1. Morphological Variations
3.2. Contributions of Morphological Characteristics Towards Diversity in Passiflora Species
3.3. Biochemical Characteristics of Fruit Juice, Leaves, Petioles, and Tendrils
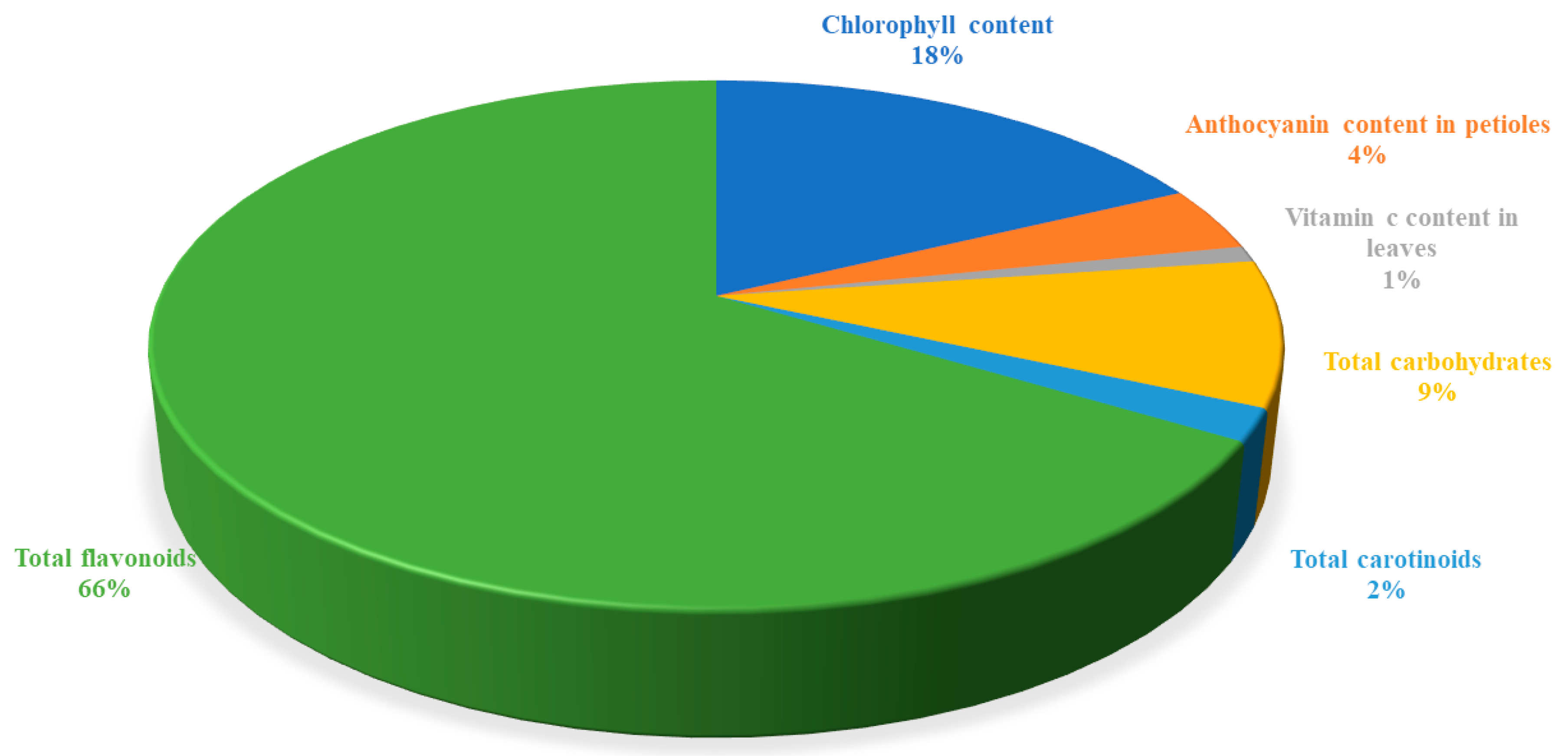
3.4. Total Flavonoids Share the Highest Percentage Towards Diversity in Passion Fruit Species
3.5. Characterisation of Passiflora Species Through Seed Protein Profiles (SDS-PAGE)
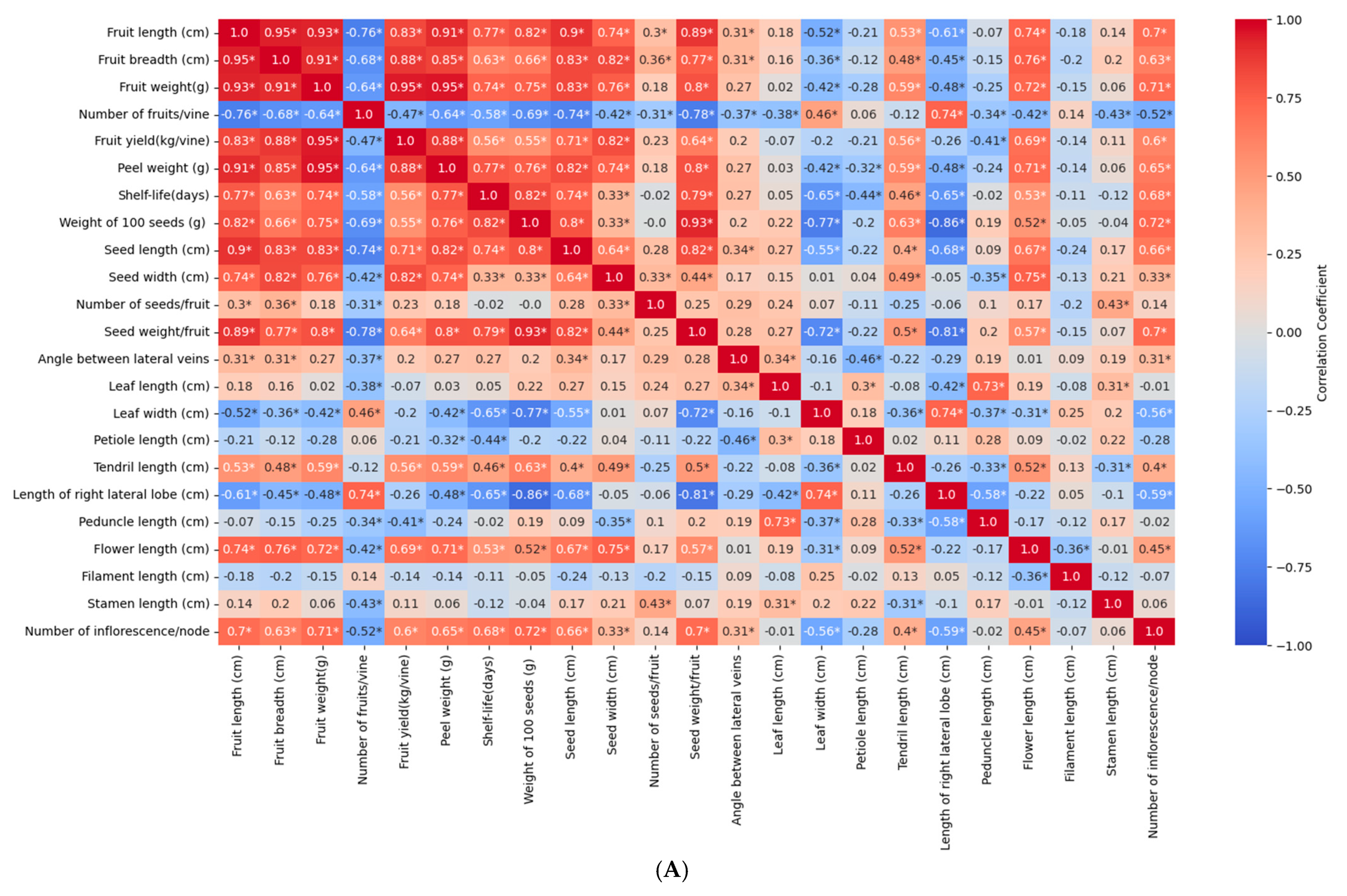
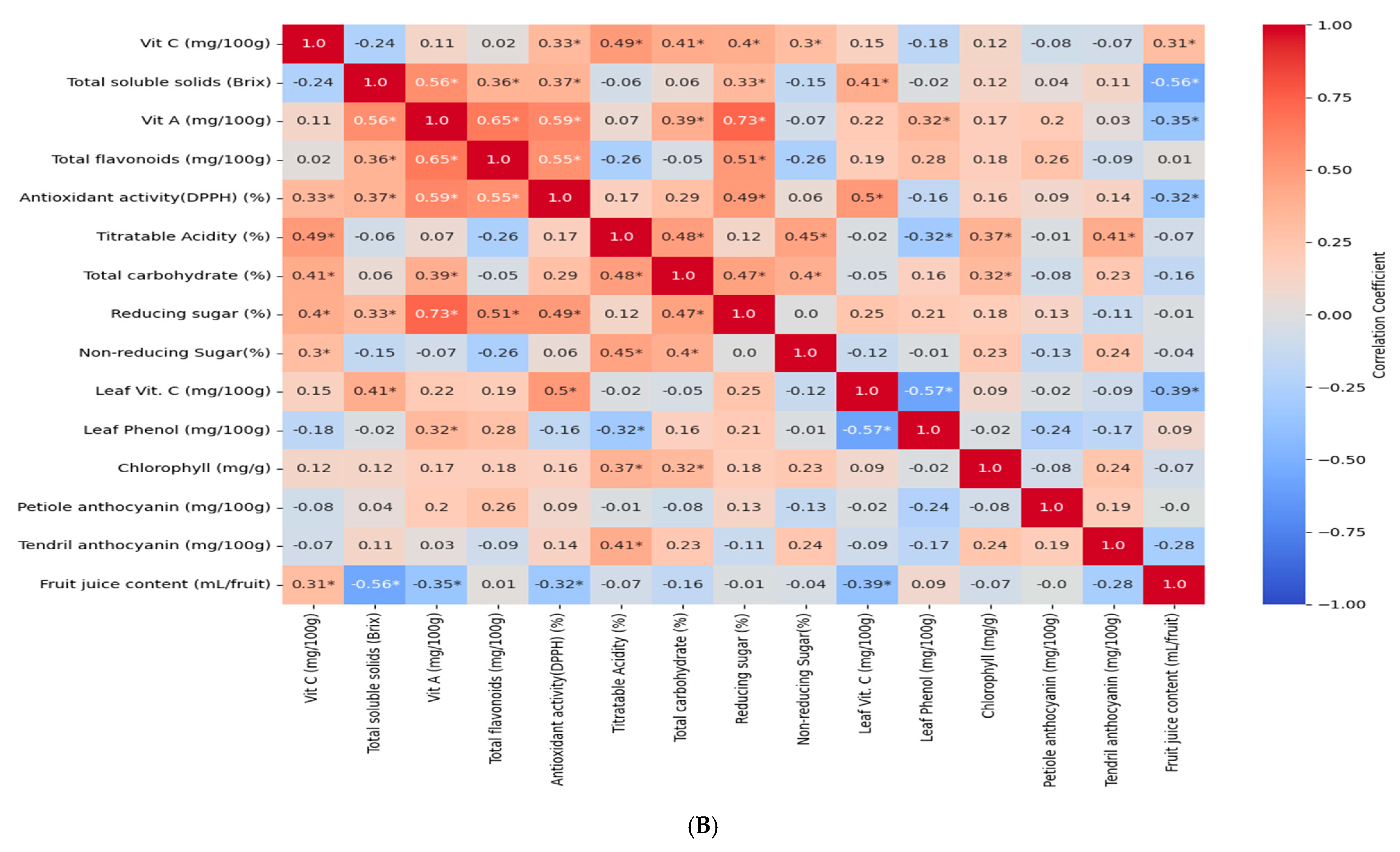
3.6. Correlation Analysis of Different Traits
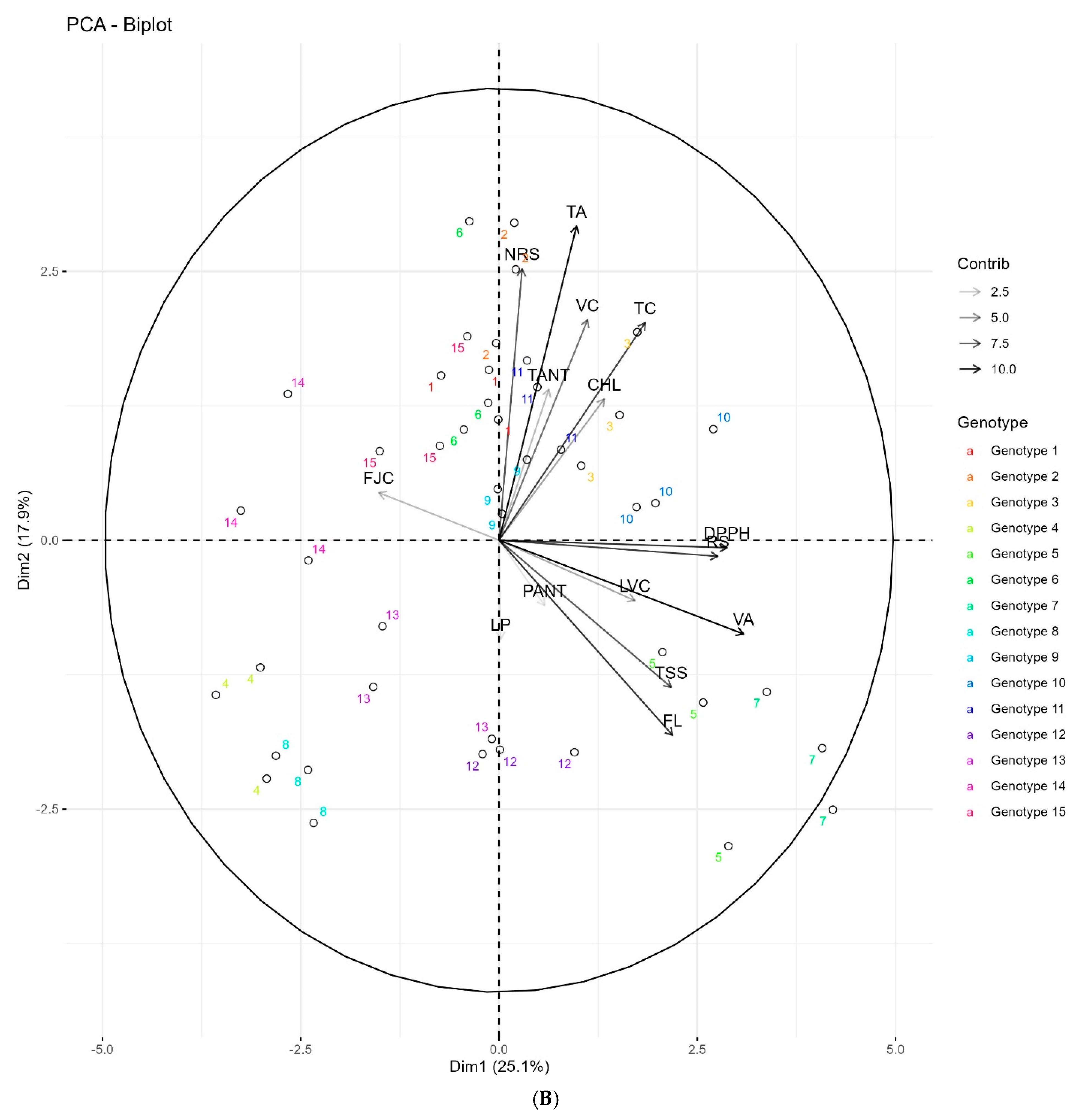
3.7. Principal Components Analysis of Different Traits
4. Discussion
4.1. Morphological Characterisation
4.2. Biochemical Characterisation
4.3. Seed Protein Profiling
4.4. Correlation and PCA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P. | Passiflora |
| Cm | centimeter |
| g | gram |
| PCA | principal component analysis |
| TSS | total soluble solid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SDS-PAGE | sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| SLS | sodium lauryl sulphate |
| TEMED | tetramethylethylenediamine |
References
- Shankar, K.; Singh, S.R.; Hazarika, B.N.; Wangchu, L.; Singh, B. Cultivated Passiflora sp. in North East region of India. Indian Hortic. 2021, 66, 50–52. [Google Scholar]
- Bailey, M.; Sarkhosh, A.; Rezazadeh, A.; Anderson, J.; Chambers, A.; Crane, J.H. The passion fruit in Florida: HS1406, 1/2021. Edis 2021, 2021. Available online: https://edis.ifas.ufl.edu/publication/HS1406 (accessed on 27 May 2025). [CrossRef]
- Thokchom, R.; Mandal, G. Production preference and importance of passion fruit (Passiflora edulis): A review. J. Agric. Eng. Food Technol. 2017, 4, 27–30. [Google Scholar]
- Feuillet, C. Passifloraceae (Passion flower family). In Flowering Plants of the Neotropics; Mori, N., Henderson, S.A., Stevenson, D.W., Heald, S.D., Eds.; Oxford University Press: Oxford, MI, USA, 2004; pp. 286–287. [Google Scholar]
- Ulmer, T.; MacDougal, J.M. Passiflora: Passion Flowers of the World; Timber Press: Portland, OR, USA, 2004; p. 430. [Google Scholar]
- Fajardo, D.; Angel, F.; Grum, M.; Tohme, J.; Lobo, M.; Roca, W.M.; Sanchez, I. Genetic variation analysis of the genus Passiflora L using RAPD markers. Euphytica 1998, 101, 341–347. [Google Scholar] [CrossRef]
- Viana, A.J.C.; Souza, M.M.; Araújo, I.S.; Corrêa, R.X.; Ahnert, D. Genetic diversity in Passiflora species determined by morphological and molecular characteristics. Biol. Plant. 2010, 54, 535–538. [Google Scholar] [CrossRef]
- Viana, A.P.; Pereira, T.S.; Pereira, M.G.; de Souza, M.M.; Maldonado, J.M.; Do Amaral Junior, A.T. Genetic diversity among yellow passion fruit commercial genotypes and among Passiflora species using RAPD. Rev. Bras. Frutic. 2003, 25, 489–493. [Google Scholar]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H. Genetic diversity in Passiflora species assessed by morphological and ITS sequence analysis. Sci. World J. 2014, 2014, 598313. [Google Scholar] [CrossRef]
- Joy, P.P. Passion fruit (Passiflora edulis Sims): Passifloraceae; Pineapple Research Station (Kerala Agricultural University): Kerala, India, 2010. [Google Scholar]
- Aizza, L.C.B.; Sawaya, A.C.H.F.; Dornelas, M.C. Identification of anthocyanins in the corona of two species of Passiflora and their hybrid by UHPLC-ESI-MS/MS. Biochem. Syst. Ecol. 2019, 85, 60–67. [Google Scholar] [CrossRef]
- Reis, L.C.R.D.; Facco, E.M.P.; Salvador, M.; Flores, S.H.; De Oliveira Rios, A. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Lucci, P.; Nunez, O.; Tundis, R.; Balzano, M.; Frega, N.G.; Lanfranco, C.; Sabrina, M.; Daria, F.; Encarnacion, M.; et al. Native Colombian fruits and their by-products: Phenolic profile, antioxidant activity and hypoglycaemic potential. Foods 2019, 8, 89. [Google Scholar] [CrossRef]
- Beena, V.L.; Beevy, S.S. Genetic diversity in two species of Passiflora L. (Passifloraceae) by karyotype and protein profiling. Nucl. 2015, 58, 101–106. [Google Scholar] [CrossRef]
- Shankar, K.; Singh, S.R.; Annu, T. Existence of Passiflora ligularis Juss in North Eastern Himalayan Region of India. Res. J. Agric. Sci. 2021, 12, 2276–2280. [Google Scholar]
- Ministry of Agriculture & Farmers Welfare. Agricultural Statistics at a Glance; Ministry of Agriculture & Farmers Welfare, Government of India: New Dehli, India, 2022; p. 92.
- Da Silva, M.A.P.; Placido, G.R.; Caliari, M.; Carvalho, B.S.; Da Silva, R.M.; Cagnin, C.; De Lima, M.S.; do Carmo, R.M.; Da Silva, R.C.F. Physical and chemical characteristics and instrumental colour parameters of passion fruit (Passiflora edulis Sims). Afr. J. Agric. Res. 2015, 10, 1119–1126. [Google Scholar] [CrossRef]
- Jamir, T.T.; Sharma, H.K.; Dolui, A.K. Folklore medicinal plants of Nagaland, India. Fitoterapia 1999, 70, 395–401. [Google Scholar] [CrossRef]
- Mowrey, D. Herbal Tonic Therapies; Keats Publishing Inc.: New Canaan, CT, USA, 1993; p. 400. [Google Scholar]
- Patel, R.K.; Singh, A.; Prakash, J.; Nath, A.; Deka, B.C. Physico-biochemical changes during fruit growth, development and maturity in passion fruit genotypes. Indian J. Hort. 2014, 71, 486–493. [Google Scholar]
- Swaminathan, M.S. Enlarging the basis of food security. In Proceedings of the International Workshop on the Role of Underutilized Species, Chennai, India, 17–19 February 1999; M.S. Swaminathan Research Foundation: Chennai, India, 1999. [Google Scholar]
- De Jesus, O.N.; de Oliveira, E.J.; Faleiro, F.G.; TL, S.; Girardi, E.A. Illustrated Morpho-Agronomic Descriptors for Passiflora spp.; Embrapa Mandioca e Fruticultura: Brasília, Brazil, 2017; p. 126. [Google Scholar]
- Collins, T.J. ImageJ for Biotechniques microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Bayfield, R.F.; Cole, E.R. Colorimetric estimation of vitamin A with trichloroacetic acid. Methods Enzymol. 1980, 67, 180–195. [Google Scholar]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Tata McGraw-Hill: New Delhi, India, 1986; pp. 89–90. [Google Scholar]
- Medlicott, A.P.; Reynoso, W.; Thompson, A.K. Modeling of mango ripening for prediction of optimal harvest time and maturity. Acta Hortic. 1988, 269, 215–223. [Google Scholar]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Capocasa, F.; Scalzo, J.; Mezzetti, B. Combining quality and antioxidant content in fruit breeding. Acta Hortic. 2008, 814, 61–66. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B.; Zytkowiak, R. Coupling of respiration, nitrogen, and sugars underlies convergent temperature acclimation in Pinus banksiana across wide-ranging sites and populations. Glob. Change Biol. 2008, 14, 782–797. [Google Scholar] [CrossRef]
- Espinosa, D.S.; Melgarejo, L.M.; Hernandez, M.S.; Melo, S.E.; Fernandez-Trujillo, J.P. Physiological and biochemical characterization of sweet granadilla (Passiflora ligularis Juss) at different locations. In Proceedings of the 8th Postharvest Symposium, Cartagena, Spain, 21–24 June 2016; pp. 1459–1464. [Google Scholar]
- Joseph, A.V.; Sobhana, A.; Joseph, J.; Bhaskar, J.; Vikram, H.C.; Sankar, S.J. Performance evaluation of passion fruit (Passiflora edulis Sims.) genotypes. J. Trop. Agric. 2021, 59, 292–301. [Google Scholar]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Joseph, A.V.; Sobhana, A.; Sankar, S.J. Evaluation of passion fruit (Passiflora edulis Sims) genotypes for yield and quality. J. Trop. Agric. 2015, 53, 165–168. [Google Scholar]
- Viana, A.P.; Freitas, J.C.O.; Santos, C.E.M.; Moreira, S.O.; Paiva, C.L.; Santos, E.A.; Amaral Júnior, A.T. Breeding of passion fruit: A historical overview and future perspectives. Front. Plant Sci. 2021, 12, 712228. [Google Scholar]
- Souza, M.M.; Pereira, M.G. Molecular characterization of genotypes of the genus Passiflora L. using inter-simple sequence repeat (ISSR) markers. Sci. Hortic. 2006, 111, 164–169. [Google Scholar]
- Shankar, K.; Singh, S.R.; Wangchu, L.; Singh, B. Passion fruit in India: Cultivation, utilization, and future prospects. Indian Hortic. 2022, 67, 6–9. [Google Scholar]
- Souza, L.M.D.; Ferreira, K.S.; Chaves, J.B.P.; Teixeira, S.L. L-ascorbic acid, β-carotene and lycopene content in papaya fruits (Carica papaya) with or without physiological skin freckles. Sci. Agric. 2008, 65, 246–250. [Google Scholar] [CrossRef]
- Shinohara, T.; Usui, M.; Higa, Y.; Igarashi, D.; Inoue, T. Effect of accumulated minimum temperature on sugar and organic acid content in passion fruit. J. ISSAAS 2013, 19, 1–7. [Google Scholar]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; Kinga, W.S.; Sahrira, M.A.S. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food Agric. 2012, 93, 1198–1205. [Google Scholar] [CrossRef]
- Lobo, M.; Tohme, J.; Angel, F.; Roca, W. Application of molecular markers for characterization of Passiflora germplasm. Proc. Int. Symp. Trop. Fruits 1996, 1, 34–45. [Google Scholar]
- Muthuswamy, M.; Madanagopal, R.; Durairaj, S.; Elayabalan, S. Evaluation of superior genotypes of passion fruit (Passiflora edulis Sims) under lower Pulney hills of Tamil Nadu. J. Pharmacogn. Phytochem. 2021, 10, 2535–2539. [Google Scholar]
- Silva, R.F.D.; Santos, V.S.; Santos, J.M.D.; Brito, N.V.; Pessoa, R.C.D.; Oliveira, G.M.D.; Soares, A.B.; Viana, A.P. Diversity and structure of the Passiflora edulis gene pool accessed by SSR markers. Acta Sci. Agron. 2018, 40, e39373. [Google Scholar]
- Santos, E.; Andrade, R.; Gouveia, E. Utilization of the pectin and pulp of the passion fruit from Caatinga as probiotic food carriers. Food Biosci. 2017, 20, 56–61. [Google Scholar] [CrossRef]
- Viera, W.; Shinohara, T.; Samaniego, I.; Terada, N.; Sanada, A.; Ron, L.; Koshio, K. Pulp mineral content of passion fruit germplasm grown in Ecuador and its relationship with fruit quality traits. Plants 2022, 11, 697. [Google Scholar] [CrossRef]
- Lopes, B.G.; Rodrigues, G.M.; Vieira, A.M.; Savian, T.V.; Faria, G.A. Relationships between yellow and purple passion fruit variables. Revista Bras. Eng. Agrícola Ambient. 2024, 28, e275006. [Google Scholar] [CrossRef]




| Species | Code | Sources | Latitude (N) | Longitude (E) | Altitude |
|---|---|---|---|---|---|
| P. edulis f. flavicarpa Deg | P1 | Andro, Manipur | 24°73′ | 94°04′ | 815 m |
| P. edulis f. flavicarpa Deg | P2 | West Imphal, Manipur | 24°47′ | 93°58′ | 906 m |
| P. edulis f. flavicarpa Deg | P3 | Sutamura, west Tripura, Tripura | 23°62′ | 91°26′ | 20 m |
| P. edulis f. flavicarpa Deg | P4 | College of Agriculture, Biswanath Cherali, Assam | 26°43′ | 93°08′ | 82 m |
| P. edulis f. flavicarpa Deg | P5 | Notun Basti, Dimapur, Nagaland | 25°55′ | 93°43′ | 154 m |
| P. edulis f. flavicarpa Deg | P6 | CHF, Pasighat, Arunachal Pradesh | 28°04′ | 95°19′ | 162 m |
| P. edulis Sims | P7 | Kangpokpi, Manipur | 24°42′ | 93°46′ | 1510 m |
| P. edulis Sims | P8 | ICAR-NOFRI, East Sikkim | 27°17′ | 88°36′ | 882 m |
| P. edulis Sims | P9 | Aizawl, Mizoram | 23°43′ | 92°44′ | 786 m |
| P. edulis Sims | P10 | CHF, Campus, Pasighat, Arunachal Pradesh | 28°04′ | 95°19′ | 168 m |
| P. edulis Sims | P11 | Ziro, Lower Subansiri, Arunachal Pradesh | 27°32′ | 93°48′ | 1566 m |
| P. edulis Sims | P12 | Pasighat, Arunachal Pradesh | 28°03′ | 95°20′ | 154 m |
| P. ligularis Juss | P13 | Lunghar Village, Ukhrul, Manipur | 25°16′ | 94°42′ | 1633 m |
| P. ligularis Juss | P14 | Sakhabama, Kohima, Nagaland | 25°39′ | 94°11′ | 1077 m |
| P. quadrangularis L. | P15 | Pasighat, Arunachal Pradesh | 28°03′ | 95°20′ | 156 m |
| Plant Part | Characters | References |
|---|---|---|
| Leaf | I. Anthocyanin content (mg/100 g) | [31] |
| II. Vitamin C content (mg/100 g) | [27] | |
| III. Phenol content (mg/100 g) | [25] | |
| IV. Chlorophyll content (mg/g) | [32] | |
| Petiole | V. Anthocyanin content (mg/100 g) | [31] |
| Tendril | VI. Anthocyanin content (mg/100 g) | |
| Fruit | VII. Vitamin C (mg/100 g) | [27] |
| VIII. Total soluble solids (°Brix) | Hand refractometer | |
| IX. Total carotenoid (mg/100 g) | [24] | |
| X. Total flavonoids (mg/100 g) | [25] | |
| XI. Antioxidant activity (DPPH) (%) | [26] | |
| XII. Titratable acidity (%) | [27] | |
| XIII. Total carbohydrates (%) | [28] | |
| XIV. Reducing sugar (%) | [29] | |
| XV. Non-reducing sugar (%) | [30] |
| Formulation for 15% Acrylamide Separating Gel | Formulation for 5% Acrylamide Stacking Gel | |
|---|---|---|
| Water | 6.9 mL | 5.5 mL |
| 30% Acrylamide mixture | 15 mL | 1.3 mL |
| Separating gel buffer (1.5 M Tris-HCl, pH 8.8) | 7.5 mL | 1.0 mL |
| 2% SDS | 0.3 mL | 0.1 mL |
| 10% Ammonium persulphate | 0.3 mL | 0.1 mL |
| TEMED | 0.012 mL | 0.008 mL |
| Sample Preparation | ||
| 0.6 M Tris-HCl | 5.0 mL | |
| 1% SDS | 0.5 g | |
| 0.5% Bromophenol blue solution | 5 mL | |
| 10% sucrose | 5.0 g | |
| A | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Code | Angle Between Lateral Veins (°) | Leaf Length (cm) | Leaf Width (cm) | Petiole Length (cm) | Tendril Length (cm) | Length of Right Lateral Lobe (cm) | Peduncle Length (cm) | Flower Length (cm) | Filament Length (cm) | Stamen Length (cm) | Number of Flowers per Node | |
| P. edulis f. flavicarpa Deg. | P1 | 63.38 ab | 12.56 bc | 16.07 a | 2.21 bc | 13.39 g | 7.17 a | 3.27 de | 6.93 d | 0.83 d | 1.80 abc | 1.00 b | |
| P. edulis f. flavicarpa Deg. | P2 | 63.02 ab | 12.21 bcd | 15.56 ab | 1.94 cd | 12.70 g | 7.03 ab | 3.20 de | 6.87 d | 0.80 d | 1.77 abcd | 1.00 b | |
| P. edulis f. flavicarpa Deg. | P3 | 59.70 abc | 11.23 cdef | 14.10 bcd | 1.78 de | 16.13 e | 6.90 b | 3.27 de | 5.60 i | 0.83 d | 1.63 abcde | 1.00 b | |
| P. edulis f. flavicarpa Deg. | P4 | 63.38 ab | 12.70 b | 15.67 ab | 2.22 bc | 20.23 c | 5.90 de | 3.10 de | 5.60 i | 1.37 a | 2.02 a | 1.00 b | |
| P. edulis f. flavicarpa Deg. | P5 | 57.21 bc | 10.20 f | 12.68 de | 1.76 de | 14.57 f | 6.60 c | 3.07 de | 6.73 d | 0.83 d | 1.50 bcdef | 1.00 b | |
| P. edulis f. flavicarpa Deg. | P6 | 42.24 d | 11.27 cdef | 14.80 abc | 3.02 a | 22.40 b | 5.80 e | 3.23 de | 7.40 c | 0.90 cd | 1.67 abcde | 1.00 b | |
| P. edulis Sims | P7 | 58.32 abc | 10.68 ef | 13.24 cde | 1.73 de | 20.54 c | 5.43 g | 2.93 de | 6.30 e | 1.13 b | 0.70 h | 1.00 b | |
| P. edulis Sims | P8 | 57.12 bc | 10.86 def | 13.92 bcd | 1.79 de | 19.13 d | 5.27 h | 3.30 d | 5.93 fgh | 1.13 b | 0.80 gh | 1.00 b | |
| P. edulis Sims | P9 | 57.10 bc | 10.66 def | 13.80 bcd | 1.75 de | 19.11 d | 5.19 h | 3.27 d | 5.81 fgh | 1.11 b | 0.78 gh | 1.00 b | |
| P. edulis Sims | P10 | 57.97 abc | 15.13 a | 12.07 ef | 2.49 b | 22.13 b | 6.00 d | 5.07 c | 7.73 b | 0.80 d | 1.12 fgh | 1.00 b | |
| P. edulis Sims | P11 | 54.07 c | 10.41 f | 12.64 de | 1.96 cd | 20.00 c | 5.40 gh | 2.93 de | 5.77 hig | 0.67 e | 1.28 def | 1.00 b | |
| P. edulis Sims | P12 | 58.79 abc | 10.42 f | 13.28 cde | 1.89 cde | 19.20 d | 5.30 gh | 2.87 e | 5.87 fgh | 1.13 b | 1.39 cdef | 1.00 b | |
| P. ligularis Juss | P13 | 62.17 ab | 14.45 a | 10.51 fg | 2.42 b | 14.27 f | 0.00 i | 7.23 a | 6.00 f | 0.97 c | 1.57 abcdef | 1.67 c | |
| P. ligularis Juss | P14 | 63.55 ab | 14.38 a | 11.58 ef | 1.88 cde | 16.13 e | 0.00 i | 6.43 b | 5.97 fg | 0.83 d | 1.97 ab | 1.67 c | |
| P. quadrangularis L. | P15 | 64.52 a | 11.95 bcde | 9.44 g | 1.56 e | 28.13 a | 0.00 i | 2.40 f | 9.20 a | 0.83 d | 1.42 cdef | 2.67 a | |
| B | |||||||||||||
| Species | Code | Fruit Length (cm) | Fruit Breadth (cm) | Fruit Weight (g) | Number of Fruits per Vine | Fruit Yield (kg per vine) | Peel Weight (g) | Shelf-life (days) | Weight of 100 Seeds (g) | Seed Length (cm) | Seed Width (cm) | Number of Seeds per Fruit | Seed Weight per Fruit |
| P. edulis f. flavicarpa Deg. | P1 | 6.16 cd | 5.33 cd | 78.75 b | 126.34 ef | 9.94 bc | 42.85 b | 9.33 cde | 1.17 f | 0.54 cd | 0.35 b | 146.33 bcd | 1.92 ef |
| P. edulis f. flavicarpa Deg. | P2 | 6.63 bc | 6.16 b | 65.1 b | 138.33 de | 9.01 bcde | 42.32 b | 8.33 de | 0.90 g | 0.56 bc | 0.38 b | 231.33 a | 2.08 def |
| P. edulis f. flavicarpa Deg. | P3 | 6.16 cd | 5.33 cd | 69.98 b | 144.00 cde | 10.07 bc | 43.34 b | 6.33 e | 1.32 f | 0.54 cd | 0.35 b | 193.00 ab | 2.54 cdef |
| P. edulis f. flavicarpa Deg. | P4 | 6.51 bcd | 5.64 bc | 77.69 b | 118.00 f | 9.15 bcd | 43.29 b | 7.00 e | 2.00 de | 0.52 de | 0.37 b | 166.33 bcd | 3.03 cd |
| P. edulis f. flavicarpa Deg. | P5 | 5.95 cd | 5.02 cd | 45.18 b | 128.00 ef | 5.78 cde | 20.59 b | 11.67 bcd | 1.95 de | 0.52 de | 0.18 c | 171.00 bcd | 3.27 c |
| P. edulis f. flavicarpa Deg. | P6 | 6.51 bcd | 5.64 bc | 77.69 b | 134.92 ef | 10.473 b | 47.96 b | 6.00 e | 2.00 de | 0.52 de | 0.37 b | 152.00 bcd | 3.04 cd |
| P. edulis Sims | P7 | 5.95 cd | 5.02 cd | 45.18 b | 166.67 ab | 7.56 bcde | 24.59 b | 10.33 bcde | 2.31 c | 0.51 de | 0.20 c | 140.00 bcd | 3.23 c |
| P. edulis Sims | P8 | 4.79 e | 4.27 ef | 32.7 b | 159.67 abc | 5.22 de | 16.30 b | 10.00 bcde | 1.88 e | 0.52 de | 0.21 c | 123.33 de | 2.31 cdef |
| P. edulis Sims | P9 | 4.68 e | 4.21 f | 31.78 b | 152.67 bcd | 4.85 de | 15.90 b | 12.00 bcd | 1.85 e | 0.49 e | 0.19 c | 87.67 e | 1.61 f |
| P. edulis Sims | P10 | 6.16 cd | 5.33 cd | 33.08 b | 161.33 abc | 5.35 de | 16.17 b | 9.33 cde | 2.17 cd | 0.54 cd | 0.35 b | 146.33 bcd | 3.15 cd |
| P. edulis Sims | P11 | 5.69 d | 4.86 de | 43.89 b | 157.67 abc | 6.95 bcde | 22.86 b | 9.83 bcde | 2.16 cd | 0.52 de | 0.22 c | 136.33 cde | 2.97 cde |
| P. edulis Sims | P12 | 5.72 d | 4.89 de | 43.99 b | 174.67 a | 7.68 bcde | 22.23 b | 13.00 bc | 2.18 cd | 0.51 de | 0.20 c | 141.67 bcd | 2.92 cde |
| P. ligularis Juss | P13 | 7.17 b | 5.38 cd | 53.41 b | 86.67 g | 4.62 e | 32.31 b | 14.00 b | 3.20 b | 0.60 b | 0.17 c | 164.33 bcd | 5.27 b |
| P. ligularis Juss | P14 | 7.16 b | 5.36 cd | 53.23 b | 87.33 g | 4.67 e | 31.94 b | 12.00 bcd | 3.20 b | 0.60 b | 0.17 c | 180.33 bc | 5.25 b |
| P. quadrangularis L. | P15 | 14.48 a | 9.30 a | 496.67 a | 52.33 h | 26.23 a | 360.00 a | 27.33 a | 5.45 a | 0.79 a | 0.62 a | 172.33 bcd | 9.37 a |
| Species | Genotypes | Vit C (mg g−1) | Total Soluble Solids (°Brix) | Total Carotenoids (mg g−1) | Total Flavonoids (mg g−1) | Antioxidant Activity (DPPH) (%) | Titratable Acidity (%) | Total Carbohydrate (%) | Reducing Sugar (%) | Non-Reducing Sugar (%) | Fruit Juice Content (mL/fruit) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. edulis f. flavicarpa Deg. | P1 | 0.238 bcd | 16.17 d | 0.100 hi | 0.114 def | 10.85 cde | 3.56 ab | 10.14 de | 4.92 cdef | 5.20 abcd | 34.17 b |
| P. edulis f. flavicarpa Deg. | P2 | 0.26.1 abc | 15.97 d | 0.090 ij | 0.113 def | 11.70 bcd | 3.91 a | 11.14 abcd | 4.63 defg | 6.54 a | 20.70 bcd |
| P. edulis f. flavicarpa Deg. | P3 | 0.262 abc | 15.13 e | 0.116 h | 0.09 ef | 12.93 bcd | 3.41 abc | 12.12 ab | 4.93 cdef | 5.79 abc | 24.09 bcd |
| P. edulis f. flavicarpa Deg. | P4 | 0.262 abc | 16.03 d | 0.08.7 ij | 0.11 def | 11.48 bcd | 3.32 bc | 10.31 cde | 4.10 fg | 6.52 a | 31.35 bc |
| P. edulis f. flavicarpa Deg. | P5 | 0.265 abc | 18.13 a | 0.300 c | 0.35 a | 22.15 a | 1.15 gh | 10.84 bcd | 6.88 a | 4.72 abcd | 21.31 bcd |
| P. edulis f. flavicarpa Deg. | P6 | 0.214 cd | 17.53 c | 0.077 j | 0.16 cde | 12.50 bcd | 2.43 e | 7.81 f | 4.35 efg | 3.05 d | 34.20 b |
| P. edulis Sims | P7 | 0.292 ab | 18.07 a | 0.265 f | 0.11 def | 11.96 bcd | 2.78 de | 12.36 ab | 6.36 ab | 6.28 ab | 17.35 d |
| P. edulis Sims | P8 | 0.226 cd | 18.28 a | 0.397 a | 0.24 bc | 12.60 bcd | 2.59 e | 10.59 bcd | 6.92 a | 3.42 cd | 14.09 d |
| P. edulis Sims | P9 | 0.207 cd | 17.94 ab | 0.196 g | 0.07 g | 9.80 def | 2.52 e | 12.06 abc | 5.54 bcd | 5.99 abc | 10.94 d |
| P. edulis Sims | P10 | 0.214 cd | 17.27 c | 0.240 b | 0.26 b | 14.76 b | 3.19 bcd | 12.88 a | 5.98 abc | 6.40 ab | 13.75 d |
| P. edulis Sims | P11 | 0.320 a | 14.70 ef | 0.285 e | 0.10 ef | 13.70 bc | 2.91 cde | 12.18 ab | 6.41 ab | 5.96 abc | 18.14 cd |
| P. edulis Sims | P12 | 0.177 de | 17.53 bc | 0.145 d | 0.20 bcd | 10.33 cdef | 1.20 g | 10.14 de | 5.35 bcde | 3.86 bcd | 18.78 cd |
| P. ligularis Juss | P13 | 0.134 e | 14.43 f | 0.0001 k | 0.11 ef | 7.52 efg | 0.64 h | 8.41 f | 3.51 g | 4.25 abcd | 16.77 d |
| P. ligularis Juss | P14 | 0.127 e | 14.64 bc | 0.0001 k | 0.11 def | 7.17 fg | 0.63 h | 8.80 ef | 4.03 fg | 4.50 abcd | 16.77 d |
| P. quadrangularis L. | P15 | 0.308 a | 13.54 g | 0.0172 k | 0.17 cde | 6.28 g | 1.81 f | 10.32 cde | 5.52 bcde | 4.92 abcd | 117.92 a |
| Species | Genotypes | Leaf Vit. C (mg g−1) | Leaf Phenol (mg g−1) | Leaf Chlorophyll (mg g−1) | Petiole Anthocyanin (µg g−1) | Tendril Anthocyanin (µg g−1) |
|---|---|---|---|---|---|---|
| P. edulis f. flavicarpa Deg. | P1 | 1.151 cd | 1.538 h | 2.84 ab | 20.30 bcde | 28.90 ab |
| P. edulis f. flavicarpa Deg. | P2 | 1.1807 c | 1.527 h | 2.90 a | 18.60 cde | 33.90 a |
| P. edulis f. flavicarpa Deg. | P3 | 0.981 de | 1.432 h | 1.56 de | 30.50 ab | 26.90 abc |
| P. edulis f. flavicarpa Deg. | P4 | 1.218 c | 1.795 g | 1.50 de | 15.70 def | 19.80 abcd |
| P. edulis f. flavicarpa Deg. | P5 | 1.748 a | 1.942 g | 1.87 cde | 28.10 abc | 21.40 abcd |
| P. edulis f. flavicarpa Deg. | P6 | 1.256 c | 1.798 g | 1.16 e | 9.30 ef | 24.50 abcd |
| P. edulis Sims | P7 | 1.172 cd | 2.709 e | 2.16 bcd | 19.0 cde | 31.40 ab |
| P. edulis Sims | P8 | 1.101 cd | 2.813 de | 1.57 de | 35.40 a | 21.10 abcd |
| P. edulis Sims | P9 | 1.183 c | 2.242 f | 1.64 cde | 5.90 f | 20.40 abcd |
| P. edulis Sims | P10 | 489 g | 4.985 a | 2.60 ab | 10.10 def | 31.10 ab |
| P. edulis Sims | P11 | 1.258 c | 2.968 cd | 1.32 e | 4.30 f | 14.10 bcd |
| P. edulis Sims | P12 | 1.500 b | 3.059 c | 2.38 abc | 5.90 f | 6.70 d |
| P. ligularis Juss | P13 | 0.882 ef | 3.108 c | 1.23 e | 21.50 bcd | 26.40 abc |
| P. ligularis Juss | P14 | 0.752 f | 3.113 c | 1.10 e | 20.40 bcde | 16.0 abcd |
| P. quadrangularis L. | P15 | 0.493 g | 3.489 b | 1.65 cde | 17.80 cde | 9.30 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shankar, K.; Singh, S.R.; Wangchu, L.; Phurailatpam, A.; Shantikumar, L.; Mariam Anal, P.; Devachandra, N.; Hazarika, B.N.; Dolatabadian, A. Morphometric and Biochemical Analysis with Seed Protein Profiling of Passiflora Species Found in the Northeastern Himalayan Region of India. Horticulturae 2025, 11, 637. https://doi.org/10.3390/horticulturae11060637
Shankar K, Singh SR, Wangchu L, Phurailatpam A, Shantikumar L, Mariam Anal P, Devachandra N, Hazarika BN, Dolatabadian A. Morphometric and Biochemical Analysis with Seed Protein Profiling of Passiflora Species Found in the Northeastern Himalayan Region of India. Horticulturae. 2025; 11(6):637. https://doi.org/10.3390/horticulturae11060637
Chicago/Turabian StyleShankar, Kripa, Senjam Romen Singh, Lobsang Wangchu, Arunkumar Phurailatpam, Lukram Shantikumar, Ps. Mariam Anal, Nongthombam Devachandra, Budhindra Nath Hazarika, and Aria Dolatabadian. 2025. "Morphometric and Biochemical Analysis with Seed Protein Profiling of Passiflora Species Found in the Northeastern Himalayan Region of India" Horticulturae 11, no. 6: 637. https://doi.org/10.3390/horticulturae11060637
APA StyleShankar, K., Singh, S. R., Wangchu, L., Phurailatpam, A., Shantikumar, L., Mariam Anal, P., Devachandra, N., Hazarika, B. N., & Dolatabadian, A. (2025). Morphometric and Biochemical Analysis with Seed Protein Profiling of Passiflora Species Found in the Northeastern Himalayan Region of India. Horticulturae, 11(6), 637. https://doi.org/10.3390/horticulturae11060637








