Effects of Potassium Supply in Nutrient Solution on Water and Nutrient Absorption of Substrate-Grown Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Management Practices
2.2. Experimental Design
2.3. Measurement Metrics and Methods
2.3.1. Water Absorption Capacity and Water Absorption Efficiency
2.3.2. Continuous Transpiration Measurement
2.3.3. Element Absorption Capacity and Element Absorption Efficiency
2.3.4. Potassium Utilization Efficiency in Tomato Plants
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Effect of K+ Supply in Nutrient Solution on Potassium Utilization Efficiency
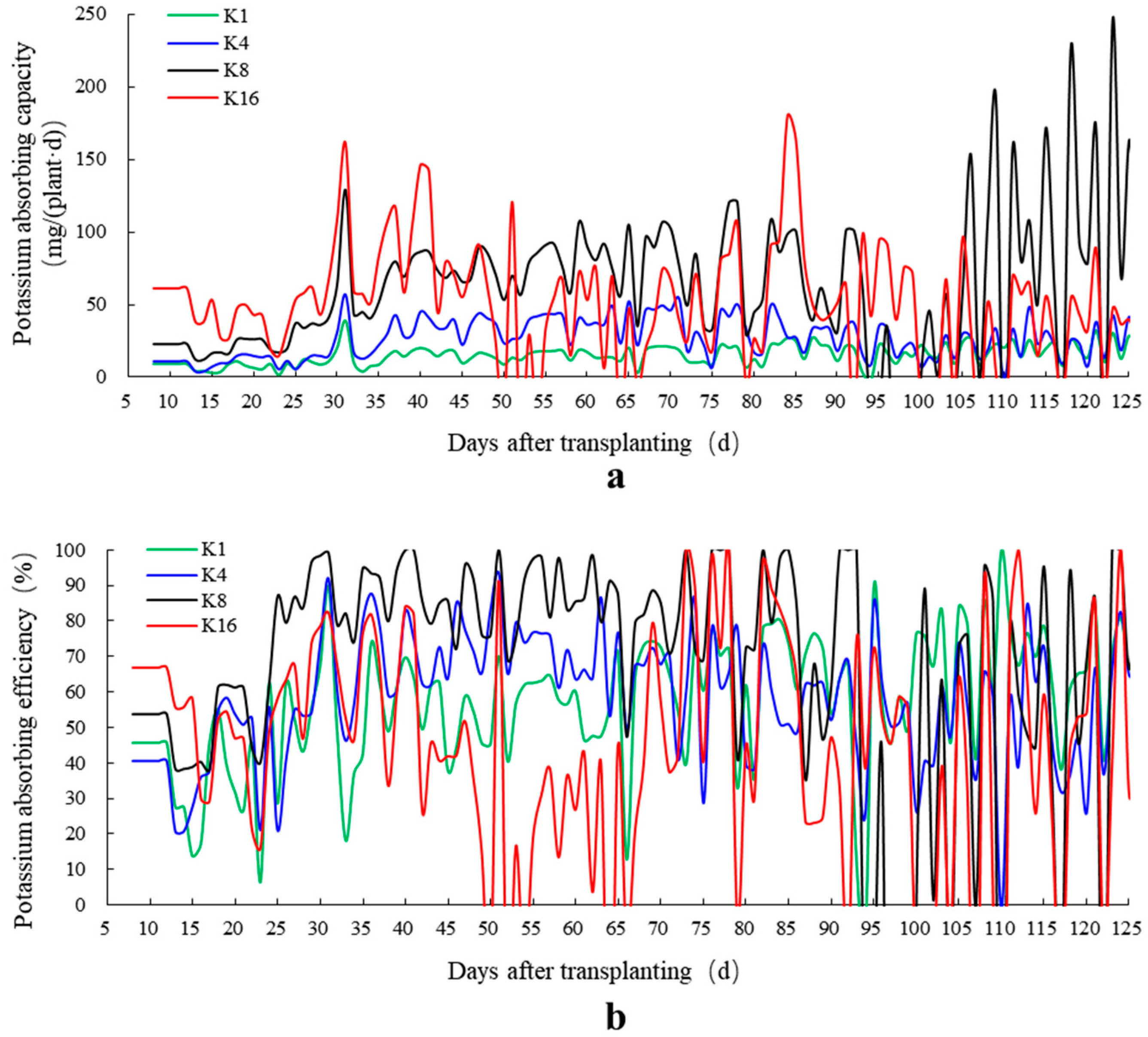
3.2. Effect of K+ Supply in Nutrient Solution on Potassium Absorption Efficiency
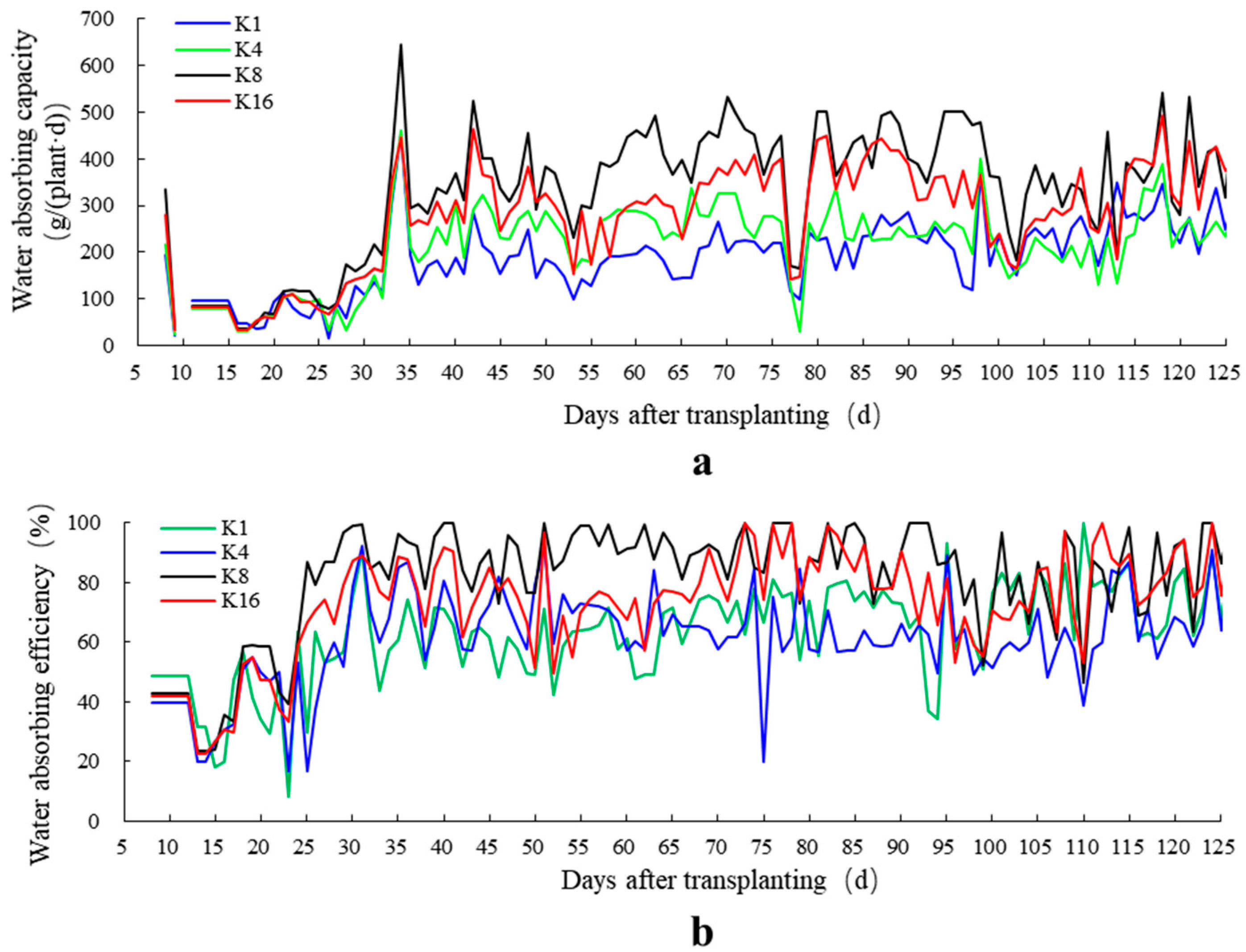
3.3. Effects of K+ Supply in Nutrient Solution on Water Absorption Efficiency
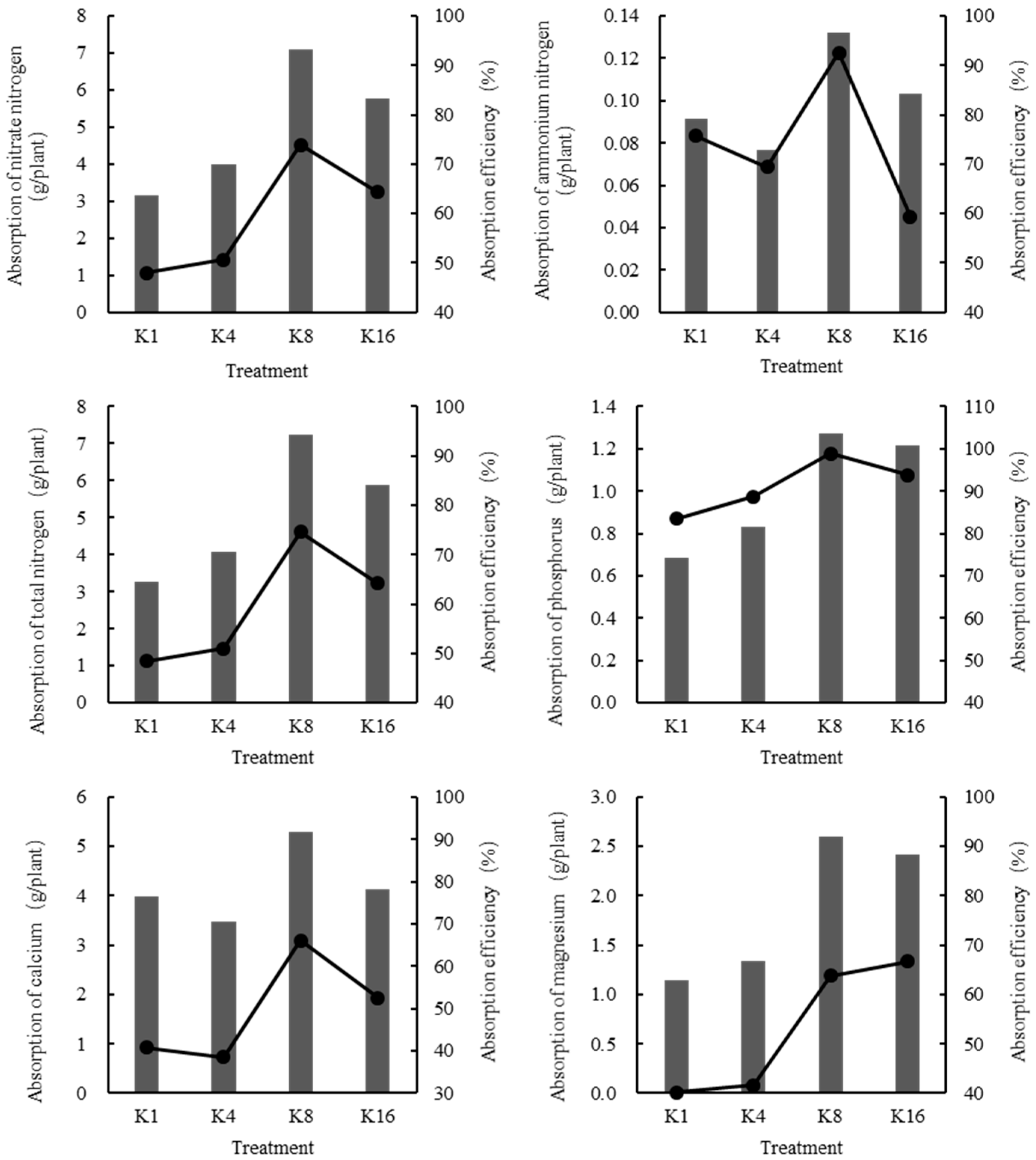
3.4. Effects of K+ Supply in Nutrient Solution on Absorption Capacity and Absorption Efficiency of Other Elements
4. Discussion
4.1. Potassium Utilization Efficiency
4.2. Potassium Absorption Efficiency
4.3. Water Absorption Efficiency
4.4. Absorption Capacity and Absorption Efficiency of Other Elements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rengel, Z.; Damon, P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, D.; Wang, J.; Mao, H. How to diagnose potassium abundance and deficiency in tomato leaves at the early cultivation stage. Horticulturae 2023, 9, 1225. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Li, Z.; Huang, X.; Shen, T.; Zou, X. Simultaneous and nondestructive diagnostics of nitrogen/magnesium/potassium-deficient cucumber leaf based on chlorophyll density distribution features. Biosyst. Eng. 2021, 212, 458–467. [Google Scholar] [CrossRef]
- Qu, Z.; Qi, X.; Liu, Y.; Liu, K.; Li, C. Interactive effect of irrigation and polymer-coated potassium chloride on tomato production in a greenhouse. Agric. Water Manag. 2020, 235, 106149. [Google Scholar] [CrossRef]
- Ramírez, S.L.F.; Díaz, S.F.R.; Muro, E.J. Relation between soilless tomato quality and potassium concentration in nutritive solution. Acta Hortic. 2012, 947, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Liu, X.; Wang, J.; Tang, Z.; Yu, J. Effect of potassium application rate on growth physiology, yield and quality of tomato cultivated in facility substrate. Acta Bot. Boreali–Occident. Sin. 2021, 41, 1725–1735. [Google Scholar]
- Li, J.; Li, M.; Mao, H.; Zhu, W. Diagnosis of potassium nutrition level in Solanum lycopersicum based on electrical impedance. Biosyst. Eng. 2016, 147, 130–138. [Google Scholar]
- Johnson, R.; Vishwakarma, L.; Shahadat, H.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, L.; Ju, F.; Wang, Z.; Xiong, C.; Yu, H.; Yu, K.; Huo, Y.; Khattak, W.A.; Hu, W.; et al. Potassium application increases cotton (Gossypium hirsutum L.) fiber length by improving K+/Na+ homeostasis and potassium transport capacity in the boll-leaf system under moderate salinity. Agronomy 2022, 12, 2962. [Google Scholar] [CrossRef]
- Gierth, M.; Mäser, P. Potassium transporters in plants—Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007, 581, 2348–2356. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Volker, R.; Ernest, A.K. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar]
- Javed, Q.; Wu, Y.; Xing, D.; Azeem, A.; Ullah, I.; Zaman, M. Re-watering: An effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chil. J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef]
- Mao, H.; Liu, Y.; Wang, Y.; Ma, G.; Wang, B.; Du, X.; Shi, Q.; Ni, J. Response of growth, photosynthesis, dry matter partition and roots to combined nitrogen-potassium stress in cucumber. Qual. Assur. Saf. Crops Foods 2022, 14, 45–53. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Shabbir, A.; Ullah, M.S.; Jabran, K.; Javed, Q.; Buttar, N.A.; Azeem, A. Physiological response of tomato plants under different irrigation levels and nutrient concentrations in greenhouse. Pak. J. Agric. Sci. 2020, 57, 599–608. [Google Scholar]
- Wang, W.; Liu, M.; Chen, J.; Wu, Z.; Ding, H.; Wang, B.; Wang, L.; Ji, Y. Effects of cultivation substrate and liquid supply method on yield, quality and ion accumulation in root zone of tomato. J. Plant Nutr. Fertil. 2025, 31, 176–187. [Google Scholar]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The effects of potassium fertilization on water–use efficiency in crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Xue, Z.; Li, Q. Water and potassium utilization efficiency and yield and quality of cucumber (Cucumis sativus L.). Sci. Hortic. 2024, 330, 113025. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guo, C.; Zhao, M.; Wang, H.; Hu, W.; Cao, N.; Zhou, Z.; Wang, X.; Zhao, W. Potassium application alleviates the drought–induced reduction in photoassimilates synthesis and distribution within the middle and upper fruiting branches, enhancing subtending cotton boll weight. Plant Physiol. Biochem. 2025, 223, 109849. [Google Scholar] [CrossRef]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Sulpice, R.; Miller, A.J.; Stitt, M.; Amtmann, A.; Gibon, Y. Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiol. 2009, 150, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ma, G.; Wang, B.; Du, X.; Shi, Q.; Ni, J.; Mao, H. Mechanical properties of stem and physiological-biochemical responses of cucumber under different N and K conditions. Qual. Assur. Saf. Crops Foods 2022, 14, 64–74. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilizers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Gao, H.; Gong, L.; Ni, J.; Li, Q. Metabolomics analysis of lettuce (Lactuca sativa L.) affected by low potassium supply. Agriculture 2022, 12, 1153. [Google Scholar] [CrossRef]
- Shabbir, A.; Mao, H.; Ullah, I.; Buttar, N.; Ajmal, M.; Solangi, K. Improving water use efficiency by optimizing the root distribution patterns under varying drip emitter density and drought stress for cherry tomato. Agronomy 2021, 11, 3. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; He, D.; Tuskagoshi, S.; Kozai, T.; Shinohara, Y. Estimating EC and ionic EC contribution percentage of nutrient solution based on ionic activity. Int. J. Agric. Biol. Eng. 2019, 12, 42–48. [Google Scholar] [CrossRef]
- Jin, J.; Bai, Y.; Yang, L. High Efficiency Soil Nutrient Testing Technology and Equipment; China Agricultural Press: Beijing, China, 2006; pp. 75–84. [Google Scholar]
- Yi, Z.; Wang, P.; Chen, P.; Tu, N. Effect of different types of nitrogen fertilizer on nitrogen absorption and utilization of summer maize (Zea mays L.). J. Plant Nutr. Fertil. 2008, 14, 472–478. [Google Scholar]
- Zhao, X.; Yu, H.; Wen, J.; Wang, X.; Du, Q.; Wang, J.; Wang, Q. Response of root morphology, physiology and endogenous hormones in maize (Zea mays L.) to potassium deficiency. J. Integr. Agric. 2016, 15, 785–794. [Google Scholar] [CrossRef]
- Erel, R.; Yermiyahu, U.; Ben-gal, A.; Dag, A.; Shapira, O.; Schwartz, A. Modification of non-stomatal limitation and photoprotection due to K and Na nutrition of olive trees. J. Plant Physiol. 2015, 177, 1–10. [Google Scholar] [CrossRef]
- Hou, M.; Fan, F.; Song, G.; Su, Y.; Ji, F. Effect of K application rate on yield and K use efficiency of buckwheat. J. Plant Nutr. Fertil. 2013, 19, 340–346. [Google Scholar]
- Tavallali, V.; Esmaili, S.; Karimi, S. Nitrogen and potassium requirements of tomato plants for the optimization of fruit quality and antioxidative capacity during storage. Food Meas. 2018, 12, 755–762. [Google Scholar] [CrossRef]
- Çolpan, E.; Zengin, M.; Özbahçe, A. The effects of potassium on the yield and fruit quality components of stick tomato. Hortic. Environ. Biotechnol. 2013, 54, 20–28. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Alemán, F.; Martínez, V.; Rubio, F. K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 2014, 171, 688–695. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaie, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, T.N.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef]
- Savita, B.; Bharat, K.; Dhriti, K.; Usha, T.; Yanchen, D.; Ali, R. Manifold roles of potassium in mediating drought tolerance in plants and its underlying mechanisms. Plant Sci. 2025, 351, 112337. [Google Scholar]
- Chen, Z.; Yamaji, N.; Horie, T.; Che, J.; Li, J.; An, G.; Ma, J. A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiol. 2017, 174, 1837–1849. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Zhang, C.; Javed, Q.; Azeem, A. Optimization of irrigation and nutrient concentration based on economic returns, substrate salt accumulation and water use efficiency for tomato in greenhouse. Arch. Agron. Soil Sci. 2017, 63, 1748–1762. [Google Scholar] [CrossRef]
- Cui, Y.; Xia, Z.; Ma, Q.; Wang, W.; Chai, W.; Wang, S. The synergistic effects of sodium and potassium on the xerophyte Apocynum venetum in response to drought stress. Plant Physiol. Biochem. 2019, 135, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Darwish, T.; Fadel, A.; Chahine, S.; Baydoun, S.; Jomaa, I.; Atallah, T. Effect of potassium supply and water stress on potato drought tolerance and water productivity. Commun. Soil Sci. Plant Anal. 2022, 53, 1100–1112. [Google Scholar] [CrossRef]
- Li, M.; Wang, F.; Zhao, J.; Li, B.; Fei, N.; Fang, X. Effects of irrigation quota and potassium application rate on the growth, yield and water and fertilizer use efficiency of potato under drip irrigation and plastic film mulching. J. China Agric. Univ. 2022, 27, 100–110. [Google Scholar]
- Qin, X.; Xing, D.; Wu, Y.; Wang, W.; Li, M.; Solangi, K. Diurnal variation in transport and use of intracellular leaf water and related photosynthesis in three karst plants. Agronomy 2022, 12, 2758. [Google Scholar] [CrossRef]
- Xing, D.; Wu, Y.; Fu, W.; Li, Q.; Hu, L.; Wu, Y. Regulated deficit irrigation scheduling of Orychophragmus violaceus based on photosynthetic physiological response traits. Trans. ASABE 2016, 59, 1853–1860. [Google Scholar]
- Wang, Y.; Ma, G.; Du, X.; Liu, Y.; Wang, B.; Xu, G.; Mao, H. Effects of nutrient solution irrigation quantity and downy mildew infection on growth and physiological traits of greenhouse cucumber. Agronomy 2020, 10, 1921. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of deficit irrigation and reduced N fertilization on plant growth, root morphology and water use efficiency of tomato grown in soilless culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.; Wang, Z.; Chen, S.; Ullah, I.; Ali, M.; Saifullah, M. Effect of fertigation levels on water consumption, soil total nitrogen, and growth parameters of Brassica chinensis under straw burial. Commun. Soil Sci. Plant Anal. 2020, 52, 32–44. [Google Scholar] [CrossRef]
- Darko, R.O.; Yuan, S.; Yan, H.; Liu, J.; Abbey, A. Calibration and validation of AquaCrop for deficit and full irrigation of tomato. Int. J. Agric. Biol. Eng. 2016, 9, 104–110. [Google Scholar]
- Darko, R.O.; Yuan, S.; Hong, L.; Liu, J.; Yan, H. Irrigation, a productive tool for food security—A review. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2016, 66, 191–206. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.; Wang, Z.; Ali, M.U.; Chen, S.; Zhang, S.; Wu, Q.; Ullah, M.S. Coupling fertigation and buried straw layer improves fertilizer use efficiency, fruit yield, and quality of greenhouse tomato. Agric. Water Manag. 2020, 239, 106239. [Google Scholar] [CrossRef]
- Lan, W.; Wang, W.; Wang, S.; Li, L.; Buchanan, B.B.; Lin, H.; Gao, J.; Luan, S. A rice high–affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc. Natl. Acad. Sci. USA 2010, 107, 7089–7094. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, B.P.; Wiesman, Z. Effect of potassium magnesium chloride in the fertigation solution as partial source of potassium on growth, yield and quality of greenhouse tomato. Sci. Hortic. 2004, 99, 279–288. [Google Scholar] [CrossRef]
- Lv, Z.; Lu, G. A new curve of critical leaf potassium concentration based on the maximum root dry matter for diagnosing potassium nutritional status of sweet potato. Front. Plant Sci. 2021, 12, 714279. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y.; Zhang, H.; Rengel, Z.; Ai, Y.; Zhang, Y.; Zhu, G. Potassium starvation affects biomass partitioning and sink-source responses in three sweet potato genotypes with contrasting potassium-use efficiency. Crop Pasture Sci. 2018, 69, 506–514. [Google Scholar] [CrossRef]
- Coimbra, K.G.; Peixoto, J.R.; Santim, M.R.; Nunes, M.S. Effect of fertilizers and compounds resistance inducers in the agronomic performance of cultivars of processing tomato. Acta Hortic. 2019, 1249, 91–96. [Google Scholar] [CrossRef]



| Treatment | TKA | KPE | KUE |
|---|---|---|---|
| g/Plant | g/g | g/g | |
| K1 | 0.71 ± 0.07 d | 95.1 ± 4.7 a | 18.2 ± 4.4 a |
| K4 | 2.19 ± 0.26 c | 47.0 ± 3.3 b | 9.8 ± 0.4 b |
| K8 | 3.24 ± 0.15 b | 35.0 ± 1.3 c | 9.9 ± 0.8 b |
| K16 | 5.09 ± 0.62 a | 27.9 ± 1.4 d | 8.7 ± 1.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhang, R.; Fu, B.; Chen, H.; Song, X.; Lv, G.; Zhang, R. Effects of Potassium Supply in Nutrient Solution on Water and Nutrient Absorption of Substrate-Grown Tomato Plants. Horticulturae 2025, 11, 629. https://doi.org/10.3390/horticulturae11060629
Song J, Zhang R, Fu B, Chen H, Song X, Lv G, Zhang R. Effects of Potassium Supply in Nutrient Solution on Water and Nutrient Absorption of Substrate-Grown Tomato Plants. Horticulturae. 2025; 11(6):629. https://doi.org/10.3390/horticulturae11060629
Chicago/Turabian StyleSong, Jinxiu, Rong Zhang, Bingyan Fu, He Chen, Xiaoming Song, Gaoqiang Lv, and Rongqiang Zhang. 2025. "Effects of Potassium Supply in Nutrient Solution on Water and Nutrient Absorption of Substrate-Grown Tomato Plants" Horticulturae 11, no. 6: 629. https://doi.org/10.3390/horticulturae11060629
APA StyleSong, J., Zhang, R., Fu, B., Chen, H., Song, X., Lv, G., & Zhang, R. (2025). Effects of Potassium Supply in Nutrient Solution on Water and Nutrient Absorption of Substrate-Grown Tomato Plants. Horticulturae, 11(6), 629. https://doi.org/10.3390/horticulturae11060629






