Abstract
Despite the growth of fruit production, the challenge of postharvest fruit loss particularly in tropical and subtropical fruits due to spoilage, decay, and natural deterioration remains a critical issue, impacting the global food supply chain by reducing both the quantity and quality of fruits postharvest. Edible coatings have emerged as a sustainable solution to extending the shelf life of fruits and decreasing postharvest losses. The precise composition and application of these coatings are crucial in determining their effectiveness in preventing microbial growth and preserving the sensory attributes of fruits. Furthermore, the integration of nanotechnology into edible coatings has the potential to enhance their functionalities, including improved barrier properties, the controlled release of active substances, and increased antimicrobial capabilities. Recent advancements highlighting the impact of edible coatings are underscored in this review, showcasing how they help in prolonging shelf life, preserving quality, and minimizing postharvest losses of subtropical fresh fruits worldwide. The utilization of edible coatings presents challenges in terms of production, storage, and large-scale application, all while ensuring consumer acceptance, food safety, nutritional value, and extended shelf life. Edible coatings based on polysaccharides and proteins encounter difficulties due to inadequate water and gas barrier properties, necessitating the incorporation of plasticizers, emulsifiers, and other additives to enhance their mechanical and thermal durability. Moreover, high levels of biopolymers and active components like essential oils and plant extracts could potentially impact the taste of the produce, directly influencing consumer satisfaction. Therefore, ongoing research and innovation in this field show great potential for reducing postharvest losses and strengthening food security. This paper presents a comprehensive overview of the latest advancements in the application of edible coatings and their influence on extending the postharvest longevity of main subtropical fruits, emphasizing the importance of maintaining the quality of fresh and fresh-cut subtropical fruits, prolonging their shelf life, and protecting them from deterioration through innovative techniques.
1. Introduction
Most tropical and subtropical fruits are produced in developing countries, which often involves grappling with insufficient infrastructure, unreliable power sources, and a heavier reliance on basic storage methods. Moreover, the supply chain for these fruits is intricate, requiring the involvement of various handlers before they reach consumers. These obstacles create additional pressure on fruits that are inherently more perishable than those grown in temperate climates [1]. Tropical and subtropical fruits serve as vital commodities that significantly contribute to human nutrition and health, with citrus fruits, bananas, mangoes, and pineapples standing out as the main traded tropical fruits. In contrast, less popular subtropical fruits are not extensively traded but are predominantly grown and consumed locally or regionally [2] mainly due to their high nutritional quality, medicinal, and economical aspects.
Globally, fruit losses display alarming statistical trends, with around one-third of food produced being lost or wasted throughout the supply chain, underscoring the substantial impact on food security and sustainability [3]. In developing nations, postharvest losses for fruits can range from 40% to 50%, emphasizing the difficulties in reducing these losses [4]. Freshly harvested horticultural products quickly lose their freshness, affecting their market value. Additionally, tropical and subtropical fruits are highly perishable, vulnerable to various physiological and microbial deterioration processes, and prone to mechanical damage during harvesting, transportation, and storage [5]. Consequently, the horticultural industry has been exploring new methods to control ripening and prolong shelf life. Traditional techniques like cold storage and modified atmosphere storage are employed to extend the shelf life of horticultural products. However, many subtropical fruits have a limited storage capacity, while certain fruits such as table grapes, nuts, and dried fruits can be stored for longer periods [6]. The primary postharvest issues affecting subtropical fruits are chilling injury, decay, and insect infestation.
Edible coatings have emerged as a viable solution for extending the shelf life of fruits, thereby reducing postharvest food waste and losses [7]. These coatings, made of biodegradable natural polymers, are applied as thin layers on food surfaces to enhance quality, prolong shelf life, and minimize postharvest losses [8]. The main categories used in producing edible coatings include polysaccharides like pectin, starch, gums, alginate, chitosan, cellulose, proteins such as gelatin, egg albumin, wheat gluten, zein, whey protein, casein, soy protein, and lipid compounds like fatty acids and waxes [9]. By incorporating essential oils or nanoparticles, edible coatings can further improve their physicochemical properties and provide antioxidant or antimicrobial effects [10,11,12].
This paper presents a review of recent advancements in using edible coatings and their impact on prolonging the postharvest life of major subtropical fruits, underlining the significance of preserving fresh/fresh-cut subtropical fruits, extending shelf life, and safeguarding them from spoilage using innovative methods. Important original research and review papers published during 2018–2024 were selected for this review.
2. Important Quality Factors of Fresh/Fresh-Cut Fruits
Fresh-cut fruits are susceptible to rapid quality degradation due to enzymatic activities and physiological changes. When choosing the type of edible coating, attention should be paid to the quality characteristics of the fruits [13]. Maintaining the quality of fresh-cut fruits post-harvest involves monitoring and controlling various indicators, including texture, color, nutritional content, microbial safety, sensory attributes, and storage conditions. Implementing appropriate handling and storage practices based on these indicators can help ensure product quality and safety. Scientific sources have provided several key quality indicators for fresh-cut fruits during post-harvest storage [14]. These indicators include physical, chemical, microbiological and sensory aspects that affect shelf life, safety and consumer acceptance.
Texture degradation, primarily due to enzymatic activity, is a significant concern in fresh-cut fruits. The texture is related to the cell wall components (pectin, hemicellulose, and cellulose), generating the softening of fresh-cut fruits during storage by enzymatic or non-enzymatic mechanisms. Enzymes such as β-galactosidase and polygalacturonase contribute to cell-wall modification and pectin degradation, leading to textural defects [15].
Color changes, often resulting from enzymatic browning, affect consumer perception and are influenced by factors like temperature and handling. Color is significantly affected by factors such as the particular cultivar, temperature and relative humidity conditions, and postharvest handling procedures. Enzymatic activity, particularly that of polyphenol oxidase (PPO), leads to browning in fruits like apples and avocados [16]. Additionally, one of the most important nutritional factors of fresh fruits is the vitamin C content. The vitamin C content declines during storage, with the rate of degradation varying among different fruits. Results have demonstrated that the ascorbic acid content of fresh-cut fruits of all species declines differently during storage [17].
Fresh-cut fruits are also susceptible to microbial contamination due to the exposure of internal tissues during processing. Fresh-cut fruits are prone to the invasion and colonization of microorganisms such as mesophiles and psychrophiles, molds, and yeasts. Pathogens like Listeria monocytogenes and Bacillus cereus have been detected in fresh-cut fruits, emphasizing the need for stringent hygiene practices [18].
Sensory qualities such as flavor and aroma are influenced by physicochemical variables, which can serve as indicators of sensory changes. Physicochemical variables such as weight, volume, pH, titratable acidity, the soluble solids content, and volatiles can be used as indicators of the sensory changes [19]. In addition, proper storage conditions are crucial for maintaining the quality of fresh/fresh-cut fruits. Cold storage at appropriate temperatures can extend shelf life and preserve quality [20,21].
Edible films and coatings are widely employed to enhance the shelf life and quality of fresh/fresh-cut fruits by acting as protective barriers against moisture loss, oxidation, and microbial contamination. The effectiveness of these films depends on their composition and the mechanisms by which they interact with the fruit’s surface and internal physiology.
Fruit loss, whether during development, harvest, or postharvest, can be caused by a combination of physiological, environmental, and biochemical factors. Key contributors include moisture loss, carbon dioxide (CO2) imbalance, ethylene accumulation and several others that lead to quality degradation, weight loss, and spoilage [22]. Moisture loss leads to shrinkage, weight loss, softening, and a loss of visual appeal, which can occur due to high ambient temperatures, low humidity, or improper packaging. Excessive moisture reduction can lead to dissatisfaction and ultimately consumer rejection [22]. CO2 imbalances can cause off-flavors or spoilage in fruits by altering respiration and metabolic processes [23]. Low CO2 encourages excessive respiration, which causes the rapid ripening and aging of produce. On the other hand, high CO2 (e.g., in modified atmospheres) may reduce spoilage but, if too high, can cause physiological disorders, anaerobic respiration, and off-flavors in fresh produce [24]. Therefore, the balance of CO2 and O2 is crucial for maintaining fruit quality during storage. Ethylene is a key plant hormone that plays a central role in fruit ripening, senescence, and eventual spoilage. While ethylene is essential for ripening in climacteric fruits, its excessive or uncontrolled presence can lead to fruit loss both before and after harvest [25].
Maintaining fruit quality postharvest requires managing ethylene exposure, moisture retention, temperature, and mechanical safety. Using edible coatings, cold storage, and sanitary practices significantly extends shelf life and preserves commercial and nutritional value. Figure 1 presents the factors affecting the quality of fruits during the postharvest process.
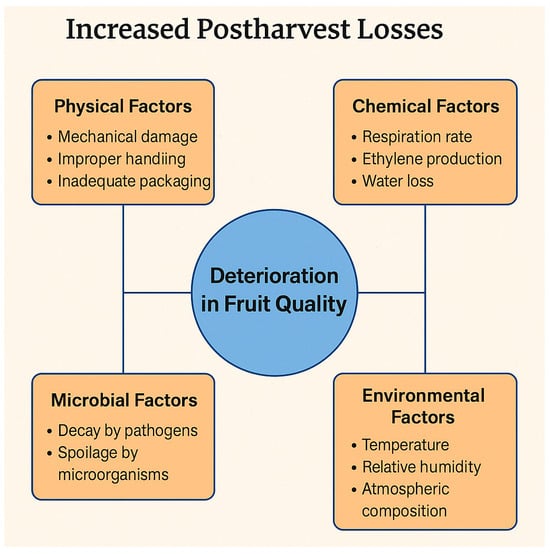
Figure 1.
Factors affecting the decline in the post-harvest quality of fruits.
Recent studies have highlighted the significant benefits of edible coatings in extending the shelf life and preserving the quality of various fruits. For instance, chitosan-based coatings have been shown to reduce respiration rates and inhibit enzymatic browning, thereby maintaining firmness and reducing weight loss in fruits such as strawberries [26]. Similarly, composite coatings combining biopolymers like gum Arabic and beeswax have effectively delayed senescence in tomatoes, extending their shelf life by up to 35 days under refrigeration [27]. Additionally, the application of carboxymethylcellulose coatings has demonstrated antimicrobial properties, preserving the firmness and reducing weight loss in avocados [28]. In the case of pineapples, composite edible coatings combined with modified atmosphere packaging have been effective in maintaining storage quality and microbiological properties [29]. These findings underscore the potential use of edible coatings as a sustainable and effective solution for prolonging the shelf life and enhancing the quality of fruits, thereby reducing postharvest losses and contributing to food security.
3. Edible Coatings, Mechanism of Action and Applications in the Food Industry
Edible coatings play a vital role in preventing moisture loss from fruits during storage by creating a semipermeable barrier that controls the rate of water vapor diffusion from the fruit to the surrounding atmosphere [30]. The ability of a coating to retain moisture is dependent on its composition, thickness, and the humidity of the storage environment [7]. Coatings containing polysaccharides such as pectin and alginate are effective at retaining moisture due to their hydrophilic properties, forming a gel-like structure that prevents dehydration and maintains fruit turgor, texture, and appearance [31].
The permeability of gases like oxygen and carbon dioxide is influenced by the composition and structure of edible coatings, which are crucial for the respiration and ripening processes of fruits [32]. For example, coatings made from hydrophobic polymers like zein act as barriers to oxygen diffusion, slowing down respiration and delaying the ripening processes [33]. This controlled exchange of gases helps to maintain oxygen levels for respiration and reduces the buildup of ethylene, a ripening hormone that speeds up fruit senescence [34]. Additionally, edible coatings provide an effective defense against microbial contamination, which is a major cause of fruit spoilage [35]. Certain coating materials, such as chitosan, have antimicrobial properties that help in combating fungi. Furthermore, by altering pH levels, oxygen availability, and the nutrient content around the fruit surface, coatings can establish an environment that is not conducive to microbial growth [36]. This antimicrobial effect impedes the growth of bacteria, yeasts, and molds, thereby prolonging the shelf life of fruit and reducing spoilage [37].
Utilizing standardized components, edible coatings are formulated to enhance food safety, quality, and nutrient levels, while prolonging their shelf life and minimizing gas transfer [38]. Diverse methodologies, such as immersion, spraying, brushing, and vacuum infusion, are engaged in the technology of edible coatings [39]. However, with regard to the application of edible coatings on fruits, the techniques of immersion and spraying are commonly employed. Specific parameters, like hue, texture, mass reduction, and nutritional worth, are customized based on the category of product and storage circumstances, allowing for meticulous supervision.
An extensive range of edible coating substances are accessible, each possessing distinct characteristics that impact their efficacy in preserving fruits. This section provides a summary of commonly used edible coating materials, including their characteristics and applications in food preservation, as discussed in several review articles [39,40,41]. Chitosan, derived from chitin, is an edible coating with film-forming and antimicrobial characteristics. Its positively charged nature enables interaction with microorganisms, impeding their proliferation [42]. Chitosan coatings obstruct gases and dampness, conserving fruit quality. Due to their distinctive attributes, these substances hold promise for diverse applications [43]. The molecular weight and deacetylation level influence its traits. It can be obtained from crustaceans, fungi, and insects for commercial manufacturing [44]. Carboxymethylcellulose (CMC) is a water-soluble polysaccharide employed in food as a thickening agent. It generates robust coatings that mitigate fruit dampness loss [45]. Coatings based on CMC safeguard fruit quality by decelerating gas transfer and microbial expansion. CMC is extensively utilized in the food sector and sanctioned by regulatory entities [45]. It is utilized in the production of edible films and in composite films with nanoparticles to confer antimicrobial characteristics [46]. Alginate, derived from seaweed, is an edible coating material that curtails moisture loss in fruits. It restrains spoilage microorganisms and extends fruit’s shelf life [37]. Alginate coatings boost the postharvest treatment of food [47]. They enhance product quality by conserving color and reducing mass loss. Zein, from maize, is an edible coating material that averts moisture loss from fruits. It exhibits barrier properties against gases and encapsulates antioxidants [48]. Zein is environmentally friendly and adaptable for applications in the food industry. Pectin, a natural polysaccharide from fruits, is extensively employed in edible coatings. Its biodegradability and low toxicity render it ideal for food packaging, enhancing the shelf life and providing a shielding barrier [49]. Gums are intricate carbohydrates that bind water and develop various types of gels, like seed gums and seaweed gums. Edible coatings employing gums such as tragacanth and guar gum exhibit potential in maintaining fruit and vegetable attributes during storage [50]. Aloe vera gel, a natural moisturizer with antimicrobial features, amplifies coating effects, refining fruit preservation [51,52]. Essential oils possess diverse medicinal properties, including antimicrobial effects, and are deemed eco-friendly alternatives to synthetic chemicals for food preservation [53]. Calcium chloride heightens the film-forming characteristics of edible coatings, efficiently reducing moisture loss and gas transfer. It plays a crucial role in several applications of edible coatings [54].
The mechanism of action of various edible coatings on fruits involves a combination of physical, biochemical, and functional effects that collectively enhance fruit preservation. These coatings—made from natural biopolymers such as polysaccharides, proteins, and lipids—form thin, consumable films that serve specific roles depending on their composition.
3.1. Polysaccharide-Based Coatings
The main polysaccharide-based edible coatings include alginate, chitosan, cellulose, pectin, and starch.
This type of edible coating is an excellent barrier against oxygen and carbon dioxide, helping to reduce respiration and delay fruit ripening. Moisture permeability in this type of edible coating is at an average level and is not as effective as lipids. The antimicrobial effects of polysaccharide-based edible coatings (especially chitosan) are that these coatings disrupt microbial membranes and bind to negatively charged microbial cell walls, inhibiting growth. In this regard, chitosan-coated strawberries showed reduced microbial spoilage and delayed fungal growth due to the coating’s antimicrobial properties [55].
3.2. Protein-Based Coatings
Some of the main ingredients that fall into this category of edible coatings include casein, whey protein, soy protein, zein, and gelatin.
This edible coating is a good barrier against O2 and CO2 due to its dense protein matrix. Also, this category of edible coating provides structural support and reduces physical damage. Protein-based edible coatings are useful for incorporating antioxidants and antimicrobial agents due to their diverse amino acid functional groups [56].
3.3. Lipid-Based Coatings
Examples of lipid-based edible coatings ingredients include beeswax, carnauba wax, fatty acids, and acetylated monoglycerides.
These types of edible coatings are excellent barriers to moisture loss and shriveling in fruits. They also increase the visual appeal of the fruit by creating a gloss on the surface, which increases the visual appeal for consumers. However, lipid-based edible coatings have poor gas barrier properties, so they may cause anaerobic respiration in the product if not used properly [57].
3.4. Composite Coatings
When different edible coatings are combined, the advantages of several edible coatings can be achieved simultaneously. The use of this type of edible coating provides moisture protection and balanced gas exchange. It also improves the performance of the edible coating because it allows for multi-purpose applications (antimicrobial + mechanical strength + antioxidant). Furthermore, specific ratios can be tailored to different fruit types and storage conditions [58].
3.5. Nanoemulsion and Encapsulated Coatings
Nanoemulsified essential oils, lipid nanoparticles, and polymer-encapsulated antioxidants are among the compounds that fall into this category.
The use of nanoscale particles provides a uniform coating and greater surface contact with the products. Another advantage of these edible coatings is controlled release, so that active agents (e.g., antimicrobials, antioxidants) are released gradually and over time. This feature makes the edible coatings stable and protects sensitive bioactive ingredients from degradation [59]. Figure 2 summarizes the characteristics of each edible coating.
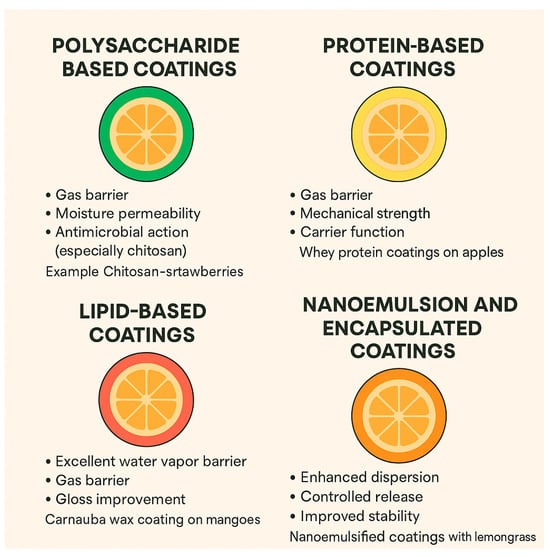
Figure 2.
Mechanism of action of various edible coatings.
4. Edible Coating Application and Its Effects on Subtropical Fresh/Fresh-Cut Fruits
Studying regional strategic fruits such as avocadoes, pomegranates, jujubes, dates, pistachios, and persimmons is important for several nutritional, economic, environmental, and cultural reasons, especially in regions with arid and semi-arid climates. These fruits are considered cash crops, contributing to rural livelihoods, export earnings, and value-added industries. Studying them improves supply chains, processing techniques, and market competitiveness. On the other hand, these fruits face unique postharvest challenges (e.g., perishability, microbial spoilage), which necessitates research into preservation, storage, and packaging technologies. This would enable reduced waste and longer market access, particularly for international trade. These fruits have a long history of use in traditional diets and medicine, particularly in the Middle East, Central Asia, and the Mediterranean. Their conservation and understanding support biodiversity, indigenous knowledge, and heritage products.
The global demand for exotic and nutrient-rich fruits is rising due to health trends and culinary diversity. Studying these fruits supports better postharvest management and reduces economic losses. Scientific studies can inform consumers about the nutritional value, safe consumption, and preparation of unfamiliar fruits. This fosters wider acceptance and consumption in new markets.
4.1. Avocado (Persea americana)
Postharvest losses of avocado are linked to harvesting injuries, mechanical damage, postharvest diseases, and physiological disorders during storage, transportation, and marketing, emphasizing the need for improved handling practices and the use of new technologies to minimize losses [60]. Edible avocado coatings, such as those based on alginate and mucilage, avocado seed starch, Aloe vera gel, corn starch, cassava peel starch with bay leaf extract, and other biodegradable materials, have been studied extensively for their ability to extend the shelf life of avocados by reducing mass loss, maintaining firmness, enhancing color retention, and decreasing decay [61,62,63]. These coatings act as barriers against environmental factors, controlling gas and moisture transfer, and some even possess additional properties, such as antioxidant and antifungal activity, which further contribute to preserving the fruit [64]. The application of edible coatings has shown promising results in minimizing waste, increasing consumer acceptance, and reducing the economic losses associated with avocado spoilage, making them a viable technology for enhancing the quality and marketability of avocados, especially in high-latitude markets where the fruit is scarce [61]. Garcia et al. [65] investigated how coatings comprising zein nanoparticles, zein and ε-polylysine nanoparticles, a zein solution and an ε-polylysine solution impact the quality and shelf life of avocados. The results of their study revealed that biopolymeric coatings significantly reduced decay on day 15 of ambient storage compared with the control. Additionally, the coated fruit presented lower weight loss and respiration rates. These authors concluded that their coatings have the potential to extend the fruit’s shelf life and reduce postharvest losses. Postharvest avocado losses primarily result from anthracnose disease, which is induced by Colletotrichum gloeosporioides. Funes et al. [64] indicated the effectiveness of coatings based on chitin nanocrystals, silk fibroins and melatonin on avocado, as the coatings were able to decrease the severity of anthracnose by 45%, indicating a similar efficacy to that of chemical fungicides. The research conducted highlights the significant potential of utilizing chitin nanocrystals and silk fibroins in conjunction with active compounds for managing anthracnose in ‘Hass’ avocados.
4.2. Date Palm (Phoenix dactylifera)
Issues such as a small fruit size, cuts, and browsing contribute to date palm losses during harvest time in different regions, emphasizing the need for effective mitigation strategies. The primary causes of postharvest losses in date palm crops include quality deterioration and a reduced shelf life [66]. Date palm fruits can benefit significantly from edible coatings to increase their quality and prolong their shelf life. Various studies have explored the use of different edible coatings, such as jasmine oil, black cumin oil, and Jojoba oil (JO) [67], chitosan, chitosan nanoparticles, and CaCl2 [54], free cinnamaldehyde (CA) and CA-loaded nanostructured lipid carriers [68], Jojoba oil, gum Arabic, and paraffin oil (PA) [69], and calcium and sodium alginate [70]. These coatings have shown promising results in reducing weight loss, delaying decay, improving total soluble solids, increasing total sugars, and inhibiting microbial growth. Rahemi et al. [71] investigated the effectiveness of different edible coatings, including pectin, methyl cellulose, and olive oil, on date palm. Dry and semidry date fruits were subjected to heat treatment at a temperature of 50 °C for 3 h, followed by the application of various coating formulations. The date fruits subsequently underwent a process of drainage, drying, and storage for periods of 3 and 6 months at an ambient temperature within zip-lock plastic bags and brown paper bags. Quality parameters such as the fruit moisture percentage, TSS, firmness, total tannin content, and total phenolic content were evaluated. The outcomes revealed that the date fruits coated with the formulations generally presented elevated levels of moisture in comparison with the untreated fruits. Notably, compared with other coating alternatives, the pectin-based coating demonstrated superior efficacy in prolonging the shelf life of date fruits.
The application of these edible coatings has proven effective in maintaining fruit quality, increasing storability, and reducing the need for refrigeration, thus offering practical solutions for extending the shelf life of date palm fruits. A recent study by Khafar et al. [72] explored the impact of different nano edible coatings, including AgNO3/ZnONPs, chitosan, and gelatin, combined with Thyme leaf extract/Green coffee extract/Luria leaf extract on date palm when stored at 2–4 °C. Compared with the control samples the coated date palm samples presented significantly lower microbial bar, mold and yeast contents and greater sensorial properties. These authors noted that the use of nano edible coatings has great potential for improving the quality of date palms for export.
4.3. Fig (Ficus carica)
Postharvest losses in figs primarily occur due to factors such as decay, weight loss, softening, and nutrient degradation [73]. Various studies have explored the use of edible coatings to increase the quality and shelf life of figs. Research has shown that coatings incorporating aloe gel and Opuntia ficus-indica mucilage can reduce the microbial load, preserve firmness, and minimize weight loss in fig cultivars during cold storage [74]. This was in addition to extracts from Diplazium esculentum (Retz). The addition of Stenochlaena palustris to sodium alginate coatings combined with a modified packaging atmosphere extended the shelf life of figs by up to 25 days, maintaining ideal gas composition, firmness, and acidity levels and preventing color changes and microbial growth [75]. Furthermore, the application of an edible coating containing pomegranate peel extract, alginic acid sodium salt (1% in H2O), and agar was found to be effective in preserving the quality of figs by increasing their antioxidant activity and extending their shelf life [76]. Additionally, in line with earlier reports, Saavedra et al. [77] demonstrated the potential use of edible coatings, including those with nanochitosan biodegradable coatings functionalized with natural extracts (cinnamon essential oil and Roselle calyces), for improving the postharvest quality and safety of figs [77]. They reported that nanochitosan biodegradable coatings maintained the quality and microbiological quality of unripe figs stored at 5 °C for 3 weeks.
4.4. Guava (Psidium guajava)
Edible coatings, including CMC and sodium alginate, extend the shelf life of guava fruits by creating a protective barrier against oxygen, carbon dioxide, and water vapor, thus delaying the ripening process [78]. Various studies have highlighted the effectiveness of different edible coatings in preserving the quality attributes of guava [79], especially chitosan-based coatings, which were optimized at 1% and showed significant microbial growth inhibition by maintaining the physicochemical characteristics of fresh-cut guava, leading to an extended shelf life under refrigeration [80]. Recently, the most effective coating for extending the shelf life of guava fruit was found to consist of 2.50% chitosan and 2% Tween 80 [81]. Additionally, composite coatings containing gum Arabic, beeswax, and coconut oil have been optimized to reduce weight loss and increase the total soluble solids content, ensuring the improved marketability and appearance of guava fruits during storage [82]. The optimized coating emulsion was made up of gum Arabic (6.6% w/v), beeswax (5.5% w/v) and coconut oil (3.6% w/v), with Tween 80 (3% w/v) as a surfactant. Furthermore, Zaidi et al. [83] studied the influence of different natural coatings, including garlic and ginger extracts, gum Arabic, and Aloe vera gel, on “Surahi” guava fruit stored at 25 °C for 15 days. They reported that garlic extract had the greatest preservative effect on guava, extending its shelf life. Additionally, nanosilicon dioxide, nanoschitosan, and nanosodium alginate coatings extended the shelf life of guava fruit [84]. These findings collectively emphasize the importance of edible coatings in enhancing the postharvest quality and market value of guava fruits.
Some examples of recent advances in the formulation and effects of edible coatings for avocado, date palm, fig, and guava fresh/fresh-cut fruits are described in Table 1.

Table 1.
Recent advances in the application and effects of edible coating in avocado, date palm, fig, and guava fresh/fresh-cut fruits.
4.5. Jujube (Ziziphus jujuba)
Jujube fruits, which are known for their high nutritional value, have a short storage life because of their perishable nature [91]. To address this issue, researchers have explored the use of edible coatings made from substances such as polysaccharides, proteins, and lipids, which are safe for both the environment and human consumption [92]. Studies have shown that incorporating tea polyphenols into alginate-based coatings can significantly reduce the respiration rate, electrolyte leakage, and malonaldehyde content in fresh jujube while maintaining important nutritional components and antioxidant enzyme activities, thus increasing fruit quality and shelf life [93]. Moradinezhad et al. [94] investigated the impact of different edible coatings (Aloe vera gel, CMC, and pectin) on the quality and shelf life of jujube fruit. These authors reported that Aloe vera and CMC coatings significantly improved the quality and prolonged the shelf life of jujube fruit. Additionally, Islam et al. [95] examined the effects of Aloe vera gel (AV) and modified atmosphere packing (MAP) on jujube fruit. The use of MAP, AV and the combination of MAP and AV has been shown to be an efficient technique for slowing the ripening process of jujube fruit while preserving its quality during cold storage. The application of MAP and AV coatings significantly decreased jujube weight loss and respiration rates, prolonged flesh firmness, delayed skin color changes, and maintained TSS and TA levels.
4.6. Litchi (Litchi chinensis)
The postharvest preservation of litchi fruit has been a significant challenge because of its short storage life and susceptibility to rapid deterioration. The application of edible coatings has been investigated in multiple studies to prolong the shelf life and preserve the quality of litchi fruit. For example, research has shown that an alginate-based coating incorporating cellulose nanocrystals can effectively reduce browning, the decay rate, and moisture loss in litchi while also enhancing the mechanical properties and increasing the soluble solid content [96]. Additionally, coatings made from guar gum, xanthan gum, and methyl cellulose at different concentrations (0.5, 1, 1.5, and 2%) were used on litchi, and the fruits were then stored at 4 °C. The results revealed that guar gum significantly improved the storage life of litchi by maintaining important biochemical attributes, such as the total soluble solids, ascorbic acid, and sugar content, thereby extending the fruit’s marketability and reducing postharvest losses [97]. Furthermore, an edible chitosan:pullulan blended coating enriched with pomegranate peel extract extended the storage life, maintained the quality, and enhanced the sensory attributes of litchi fruit [98]. These edible coatings offer promising solutions for enhancing the preservation and quality of litchi fruit, benefiting both producers and consumers.
4.7. Persimmon (Diospyros kaki)
Postharvest losses in persimmon are due mainly to rapid ripening, premature senescence, weight loss, decay, and oxidative damage. These losses can be mitigated through the application of various postharvest treatments. Studies have shown that the use of hydrocolloid-based edible coatings such as tragacanth gum (TCG) can significantly reduce respiration rates, ethylene production, weight loss, decay incidence, and oxidative stress while maintaining increased levels of bioactive compounds and enzymatic antioxidant activities [99]. Research has shown that applying Aloe vera gel combined with hydrocolloids such as gum Arabic significantly reduces weight loss, decay, the respiration rate, and ethylene production in stored persimmons, preserving their biochemical attributes and sensory qualities [100,101]. Additionally, Aloe vera-based coatings enriched with ascorbic acid, citric acid, and calcium chloride in modified atmosphere packaging have been found to be effective at maintaining total carotenoids and ascorbic acid and controlling microbial growth, enhancing the shelf life of fresh-cut persimmon fruits [102]. Furthermore, the use of chitosan nanoparticles, rosmarinic-acid-biomediated selenium nanoparticles, and their composites as edible coatings has shown promising results in reducing postharvest fungal infestations, particularly the black rot caused by Alternaria alternata, preserving fruit firmness, and maintaining fruit shelf life, highlighting their potential for enhancing persimmon quality and market value [103].
4.8. Pistachio (Pistacia vera)
Fresh pistachios are prone to postharvest losses due to physiological and biochemical changes after harvest. Aspergillus flavus is a significant concern in pistachio orchards because of its ability to produce aflatoxins, particularly aflatoxin B1 (AFB1), during postharvest storage. Various techniques have been explored to combat this issue. Edible pistachio coatings have been extensively studied for their ability to increase shelf life and reduce contamination. Research has shown that coatings such as chitosan combined with cold plasma treatment can effectively preserve pistachios by maintaining hardness and color and reducing peroxide, aflatoxin, and mold and yeast counts [104]. Similarly, methylcellulose coatings have demonstrated significant inhibitory effects on microbial and aflatoxin contamination in pistachios, improving sensory attributes as well [105]. Furthermore, alginate coatings enriched with thyme essential oil have been found to maintain phenolic content and antioxidant activity and reduce mold and yeast growth while lowering the peroxide value and free fatty acid content of pistachios during storage [106]. Additionally, the application of edible coatings such as chitosan and salicylic acid has been found to increase postharvest quality by reducing weight loss, maintaining enzyme activity, improving color, and inhibiting microbial growth during storage [107]. Nazoori et al. [108] explored how the storage life and quality of fresh pistachio fruit are affected by CMC-based coatings incorporating calcium oxide and GABA. They reported that coated pistachios had a significantly better fruit quality, lower weight loss, and overall acceptance during 50 days of storage. Similarly, Khajeh-Ali et al. [109] investigated the impact of a CMC-edible coating with Astragalus honey on the storage duration of pistachio kernels. These findings indicated that pistachio kernels coated with honey (2%) and CMC (1%) presented the lowest microbial counts, weight loss, peroxide levels, and textural hardness throughout the storage period. These studies collectively highlight the potential of edible coatings in enhancing the quality and safety of pistachios, offering a promising approach for commercial application and export.
4.9. Pitaya (Selenicereus undatus)
Pitaya, a non-climacteric fruit with a high moisture content, faces challenges such as water loss, which affects its quality during handling and storage [110]. Various postharvest treatments have been explored to maintain the quality of pitaya, including the application of edible coatings. Studies have shown that coatings such as carnauba-based and vegetable oil-based coatings can delay exocarp shriveling and maintain the quality of fruit for up to 15 days at 7 °C and 85% RH [110]. Additionally, the combination of a chitosan coating with hydrophobic components such as oleic acid has been found to preserve the quality of desalinated pitayas, extending their shelf life to 15 days by minimizing weight loss and delaying fungal growth [111]. These findings highlight the potential of edible coatings, such as those based on chitosan and hydrophobic components, to maintain the quality and extend the shelf life of pitaya fruit. Utama et al. [112] investigated the impact of alginate-based edible coatings infused with vanilla essential oil on the shelf life of fresh-cut red pitaya. This study revealed that the incorporation of 0.6% vanilla essential oil into alginate-based edible coatings facilitated the preservation of fresh-cut red pitaya for up to 9 days.
4.10. Pomegranate (Punica granatum)
Pomegranates are nonclimacteric fruits with a low respiration rate. However, postharvest losses in pomegranate are a significant concern due to sunburn, cracking, chilling injury [113], and fungal pathogens causing decay [114], leading to economic and nutritional losses [115,116]. Studies have shown that alternative compounds such as chitosan, plant protein hydrolysate, and red seaweed extract can effectively reduce postharvest decay in pomegranate [117]. Research has shown that the use of additives such as salicylic acid, canola oil, and Tween 80 in methylcellulose coatings can optimize quality management by affecting mass loss, TSS, the total phenolic content, and antioxidant activity [118]. The incorporation of zinc oxide nanoparticles (ZnONPs) with active phenol compounds from pomegranate peel into chitosan films can reduce microbial growth, extend shelf life, and improve physiochemical stability [119]. Coatings with flaxseed oil, black seed oil, and chitosan have been found to decrease weight loss, maintain visual quality, reduce chilling injury, and increase the total soluble solids and anthocyanin content in pomegranate juice, ultimately improving the overall fruit quality during storage [120]. Moradinezhad et al. [121] examined the effects of ascorbic acid coating and MAP on the quality and shelf life of pomegranate arils. They reported that coating arils with ascorbic acid prolonged the lag time of microorganisms and extended the shelf life of arils to more than 20 days during cold storage at 3 °C. Most recently, Seifi and Bekran [122] evaluated the effects of different edible coatings consisting of Aloe vera gel, chitosan, honey, and ascorbic acid and their combination on the quality of pomegranate arils. These authors reported that the combination of Aloe vera gel and ascorbic acid had the greatest effect on the quality, antioxidant content and sensorial properties of arils compared with those of the control samples. These findings highlight the significant potential of edible coatings in preserving fresh/fresh-cut pomegranate fruit and enhancing their nutritional value and marketability.
Some examples of recent advances in the formulation and effects of edible coatings on jujube, litchi, persimmon, pistachio, pitaya, and pomegranate fresh/fresh-cut fruits are described in Table 2.

Table 2.
Recent advances in the application and effects of edible coatings on jujube, litchi, persimmon, pistachio, pitaya, and pomegranate fresh/fresh-cut fruits.
5. Challenges of Using Edible Coatings
The use of edible coatings is a promising post-harvest technology, but it is accompanied by several challenges and disadvantages related to technical performance, safety, cost, and consumer acceptance [131]. Table 3 provides a categorized overview of these issues across different types of edible coatings:

Table 3.
Disadvantages of different types of edible coatings that limit their use.
Coatings may be applied unevenly due to the unevenness of the fruit surface (e.g., avocado, pitaya), which reduces their performance. Also, natural biopolymers often require specific conditions (temperature, pH) and may not be easily adapted to industrial-scale production. In addition, some coatings degrade quickly and have limited usability without preservatives. The feature that most concerns consumers is the sensory changes that occur in the products after the application of edible coatings. Coatings may change the texture, gloss or taste of the fruit, leading to consumer rejection. In addition, some consumers prefer unprocessed fruits and may associate coatings with unnatural or artificial additives.
6. Future Directions and Challenges
Incorporating antimicrobial elements, antioxidants, or enzymes into active coatings could provide extra protection against microbial spoilage, oxidative decay, and physiological decline. This requires the investigation of natural and synthetic active ingredients that can be seamlessly integrated into the coating structure. It is crucial to ensure consumers’ acceptance of edible coatings for successful implementation. However, utilizing edible coatings poses specific challenges related to production, storage, and large-scale usage while aiming to guarantee consumer approval, food safety, nutrition, and an extended shelf life [132]. Edible films based on polysaccharides and proteins encounter difficulties with low water and gas barrier properties, requiring the addition of plasticizers, emulsifiers, and other components to enhance their mechanical and thermal resistance [133]. Furthermore, heightened levels of biopolymers and active components like essential oils and plant extracts could potentially alter the taste of the food products, directly impacting consumer satisfaction. This is also associated with the potential harmfulness of the substances. Lastly, there are minimal safety and regulatory guidelines pertaining to the concentrations of active ingredients in edible coatings. Therefore, it is crucial to promote consumer awareness and regulations regarding the benefits of edible packaging for the environment and consumers in order to address challenges regarding consumer acceptance on a large scale [134].
7. Conclusions
The precise formulation and application of these coatings play a crucial role in determining their effectiveness in inhibiting the growth of microorganisms, reducing enzymatic browning, and maintaining the sensory qualities of fruits. Further research is needed to improve the use of edible coatings for different types of fruits and develop new approaches that tackle issues related to prolonging the shelf life and preserving the freshness of fruit. Although edible coatings have shown significant promise in fruit preservation, there are various future paths and challenges that must be addressed to promote their widespread adoption and advancement. Research efforts should focus on identifying and creating new, environmentally friendly, and biodegradable coating materials obtained from sustainable sources. This involves investigating neglected agricultural by-products and exploring the potential of biopolymers like proteins, polysaccharides, and lipids. Furthermore, the integration of nanotechnology into edible coatings has the potential to improve their properties, for example through improved barrier properties, controlled drug release, improved antibacterial efficacy, etc. This involves investigating nanoencapsulation and the application of nanoparticles to increase the effectiveness of coatings.
Author Contributions
F.M.: Supervision, Methodology, Writing, and Editing. A.A.: Reviewing and Editing. A.R.: Writing and Reviewing. M.D.: Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The authors will follow the Ethical Responsibilities of Authors and COPE rules. This study does not involve any human or animal testing.
Informed Consent Statement
On behalf of all co-authors I believe the participants are giving informed consent to participate in this study. I, Farid Moradinezhad give my consent for submitted manuscript to be published in Horticulturae.
Data Availability Statement
All data presented in the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Yahia, E.M. (Ed.) Fundamental issues. In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Fundamental Issues; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Philadelphia, PA, USA, 2011; Volume I. [Google Scholar] [CrossRef]
- Belmonte-Herrera, B.H.; Domínguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Preciado-Saldaña, A.M.; Salazar-López, N.J.; López-Martínez, L.X.; Yahia, E.M.; Robles-Sánchez, R.M.; González-Aguilar, G.A. Lesser-consumed tropical fruits and their by-products: Phytochemical content and their antioxidant and anti-inflammatory potential. Nutrients 2022, 14, 3663. [Google Scholar] [CrossRef] [PubMed]
- Blanckenberg, A.; Fawole, O.A.; Opara, U.L. Postharvest losses in quantity and quality of pear (cv. Packham’s Triumph) along the supply chain and associated economic, environmental and resource impacts. Sustainability 2022, 14, 603. [Google Scholar] [CrossRef]
- Silveira, A.C.; Rodríguez, S.; Kluge, R.; Palaretti, L.F.; Inestroza, C.; Escalona, V.H. Non-destructive techniques for mitigating losses of fruits and vegetables. Agrociencia Urug. 2021, 25, e850. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest technology of horticultural crops-An overview from farm to fork. Ethiop. J. Appl. Sci. Technol. 2013, 1–8. [Google Scholar]
- Kader, A.A.; Yahia, E.M. Postharvest biology of tropical and subtropical fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Sawston, UK, 2011; pp. 79–111. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of edible coating in extension of fruit shelf life. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Barciela, P.; Carpena, M.; Prieto, M.A. Edible coatings as a natural packaging system to improve fruit and vegetable shelf life and quality. Foods 2023, 12, 3570. [Google Scholar] [CrossRef] [PubMed]
- Aaqil, M.; Peng, C.; Kamal, A.; Nawaz, T.; Gong, J. Recent Approaches to the formulation, uses, and impact of edible coatings on fresh peach fruit. Foods 2024, 13, 267. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chem. Biol. Technol. Agric. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Hedayati, S.; Ansarifar, E. Assessment of zataria multiflora essential oil—Incorporated electrospun polyvinyl alcohol Fiber mat as active packaging. Polymers 2023, 15, 1048. [Google Scholar] [CrossRef]
- Kanwar, P.; Rana, P.; Vatsalya Swaroop, M.; Kumar, N.S. Nano-technology enhanced edible coating application on climacteric and non-climacteric fruits: A review. Int. J. Adv. Biochem. Res. 2024, 8, 58–68. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chanasut, U.; Kumpoun, W. Enzymatic browning and its amelioration in fresh-cut tropical fruits. In Fresh-Cut Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 51–76. [Google Scholar] [CrossRef]
- Suriati, L.; Utama, I.; Harjosuwono, B.A.; Gunam, I.B.W. Physicochemical characteristics of fresh-cut tropical fruit during storage. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 1731–1736. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.J.; Su, G.; Zhang, M.; Grierson, D.; Chen, K.S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Betancur, D.P.; Zapata-Vahos, I.C.; Henao-Rojas, J.C.; Martinez-Saldarriaga, J.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M. Inhibitory effect of fermented avocado seed extract (Persea americana Mill. cv. Hass) on Polyphenol Oxidase and its application as anti-browning agent in avocado, apple, and banana pulps. Heliyon 2025, 11, e42588. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Effect of alternative preservation steps and storage on vitamin C stability in fruit and vegetable products: Critical review and kinetic modelling approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef]
- Singh, A.; Walia, D.; Batra, N. Fresh-cut fruits: Microbial degradation and preservation. In Microbial Contamination and Food Degradation; Academic Press: Cambridge, MA, USA, 2018; pp. 149–176. [Google Scholar] [CrossRef]
- Song, Q.; Li, R.; Song, X.; Clausen, M.P.; Orlien, V.; Giacalone, D. The effect of high-pressure processing on sensory quality and consumer acceptability of fruit juices and smoothies: A review. Food Res. Int. 2022, 157, 111250. [Google Scholar] [CrossRef]
- Quandoh, E.; Albornoz, K. Fresh-cut watermelon: Postharvest physiology, technology, and opportunities for quality improvement. Front. Genet. 2025, 16, 1523240. [Google Scholar] [CrossRef]
- Çandır, E. Fresh-cut fruits. In Minimally Processed Refrigerated Fruits and Vegetables; Food Engineering Series; Springer: Boston, MA, USA, 2017; pp. 327–384. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Siddiqui, M.W. Factors affecting postharvest quality of fresh fruits. In Postharvest Quality Assurance of Fruits; Springer: Cham, Switzerland, 2015; pp. 7–32. [Google Scholar] [CrossRef]
- Ali, M.; Batool, S.; Khalid, N.; Manzoor, M.F.; Zhao, X.; Li, X.; Li, F.; Xinhua, Z.; Aadil, R.M. Alcoholic Off-Flavor Disorders in Fresh Fruits. J. Food Biochem. 2023, 2023, 3959653. [Google Scholar] [CrossRef]
- Hajizadeh, H.S. Importance of Atmospheric Composition for Postharvest Handling of Fruits and Vegetables. In Postharvest Physiology and Handling of Horticultural Crops; CRC Press: Boca Raton, FL, USA, 2023; pp. 133–166. [Google Scholar] [CrossRef]
- Tipu, M.M.; Sherif, S.M. Ethylene and its crosstalk with hormonal pathways in fruit ripening: Mechanisms, modulation, and commercial exploitation. Front. Plant Sci. 2024, 15, 1475496. [Google Scholar] [CrossRef] [PubMed]
- Aparna, M.; Geetha Lekshmi, P.R. Chitosan Based Edible Coatings: Enhancing Shelf Life and Quality in Fruits and Vegetables. J. Adv. Biol. Biotechnol. 2024, 27, 178–191. [Google Scholar] [CrossRef]
- Kathirvelu, T.; Xavier, J.R.; Innasimuthu, N.; Chauhan, O.P. Exploring composite edible coatings for shelf life extension and quality preservation of tomato (Solanum lycopersicum L.). Future Postharvest Food 2024, 1, 401–413. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A review on edible coatings and films: Advances, composition, production methods, and safety concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xing, Y.; Fan, X.; Qiu, Y.; Xu, Q.; Liu, X. Effect of composite edible coatings combined with modified atmosphere packaging on the storage quality and microbiological properties of fresh-cut pineapple. Foods 2023, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Firdous, N.; Moradinezhad, F.; Farooq, F.; Dorostkar, M. Advances in formulation, functionality, and application of edible coatings on fresh produce and fresh-cut products: A review. Food Chem. 2023, 407, 135186. [Google Scholar] [CrossRef]
- Ayoubi, A.; Balvardi, M.; Mahmoudi-Kordi, F. Maintaining the nutritional quality and increasing the shelf life of dried apricot using sodium alginate and pectin as edible coating. J. Food Meas. Charact. 2022, 16, 4025–4035. [Google Scholar] [CrossRef]
- MileriMileriene, J.; Serniene, L.; Henriques, M.; Gomes, D.; Pereira, C.; Kondrotiene, K.; Kasetiene, N.; Lauciene, L.; Sekmokiene, D.; Malakauskas, M. Effect of liquid whey protein concentrate–based edible coating enriched with cinnamon carbon dioxide extract on the quality and shelf life of Eastern European curd cheese. J. Dairy Sci. 2021, 104, 1504–1517. [Google Scholar] [CrossRef]
- Almeida, C.L.; Figueiredo, L.R.F.; Ribeiro, D.V.M.; Santos, A.M.C.; Souza, E.L.; Oliveira, K.A.; Oliveira, J.E.; Medeiros, E.S. Antifungal edible coatings for fruits based on zein and chitosan nanowhiskers. J. Food Sci. 2024, 89, 404–418. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent developments in edible films and coatings for fruits and vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Talesh, A.A.; Amiri, S.; Radi, M.; Hosseinifarahi, M. Effect of nanocomposite alginate-based edible coatings containing thymol-nanoemulsion and/or thymol-loaded nanostructured lipid carriers on the microbial and physicochemical properties of carrot. Int. J. Biol. Macromol. 2024, 308, 129196. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.C.; Cerqueira, M.A.; Michelin, M.; Fuciños, P.; Pastrana, L. Antiviral edible coatings and films: A strategy to ensure food safety. Trends Food Sci. Technol. 2023, 138, 551–563. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Weerasooriya, A.D. A systematic review on plant-based edible coatings for quality improvement and extended postharvest life of fresh fruits and vegetables. J. Hortic. Postharvest Res. 2025, 8, 177–198. [Google Scholar] [CrossRef]
- Majeed, H.; Iftikhar, T.; Zohaib, M. Extension of guava shelf life through the application of edible coating formulated with mango and lemon leaves extracts. Ind. Crops Prod. 2024, 216, 118671. [Google Scholar] [CrossRef]
- Andriani, V.; Handayani, N.A. Recent technology of edible coating production: A review. Mater. Today Proc. 2023, 87, 200–206. [Google Scholar] [CrossRef]
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Shirani, N.; Musavi, S.H. Edible coating and films as promising packaging: A mini review. J. Food Meas. Charact. 2021, 15, 4205–4214. [Google Scholar] [CrossRef]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent advances in edible coating of food products and its legislations: A review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Application of cellulose-and chitosan-based edible coatings for quality and safety of deep-fried foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1418–1437. [Google Scholar] [CrossRef]
- Nitu, N.J.; Ullah, M.S.; Howlader, P.; Mehedi, M.N.; Meem, H.Z.; Bose, S.K. Chitosan oligosaccharides maintained postharvest quality and increased shelf life of mango. J. Hortic. Postharvest Res. 2025, 8, 43–66. [Google Scholar] [CrossRef]
- Adiletta, G.; Di Matteo, M.; Petriccione, M. Multifunctional role of chitosan edible coatings on antioxidant systems in fruit crops: A review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, Q.; Ruan, C.; Xu, D.; Zeng, K. Application of carboxymethyl cellulose and its edible composite coating in fruit preservation. Packag. Technol. Sci. 2024, 37, 781–792. [Google Scholar] [CrossRef]
- Kong, P.; Rosnan, S.M.; Enomae, T. Carboxymethyl cellulose–chitosan edible films for food packaging: A review of recent advances. Carbohydr. Polym. 2024, 346, 122612. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, X.; Wang, L.; Wang, H.; Hu, Z.; Ju, X.; Yuan, Y. A review of food preservation based on zein: The perspective from application types of coating and film. Food Chem. 2023, 424, 136403. [Google Scholar] [CrossRef] [PubMed]
- Rohasmizah, H.; Azizah, M. Pectin-based edible coatings and nanoemulsion for the preservation of fruits and vegetables: A review. Appl. Food Res. 2022, 2, 100221. [Google Scholar] [CrossRef]
- Tiamiyu, Q.O.; Adebayo, S.E.; Yusuf, A.A. Gum Arabic edible coating and its application in preservation of fresh fruits and vegetables: A review. Food Chem. Adv. 2023, 2, 100251. [Google Scholar] [CrossRef]
- Sarker, A.; Grift, T.E. Bioactive properties and potential applications of Aloe vera gel edible coating on fresh and minimally processed fruits and vegetables: A review. J. Food Meas. Charact. 2021, 15, 2119–2134. [Google Scholar] [CrossRef]
- Alhassan, N.; Ndomakaah, A. Aloe vera gel coating maintains physicochemical parameters, extends the storage life, and preserves the qualities of Lantundan and Cavendish bananas. J. Hortic. Postharvest Res. 2024, 7, 287–300. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; El-Masry, A.M.; Fikry, M.; El-Kholy, M.F.; Shaban, A.E.; Sami, R.; Algarni, E.; Alshehry, G.; Aljumayi, H.; Benajiba, N.; et al. Utilization of active edible films (chitosan, chitosan nanoparticle, and CaCl2) for enhancing the quality properties and the shelf life of date palm fruits (Barhi cultivar) during cold storage. Coatings 2022, 12, 255. [Google Scholar] [CrossRef]
- Pillai, A.R.; Eapen, A.S.; Zhang, W.; Roy, S. Polysaccharide-based edible biopolymer-based coatings for fruit preservation: A review. Foods 2024, 13, 1529. [Google Scholar] [CrossRef]
- Purewal, S.S.; Kaur, A.; Bangar, S.P.; Singh, P.; Singh, H. Protein-based films and coatings: An innovative approach. Coatings 2023, 14, 32. [Google Scholar] [CrossRef]
- Devi, L.S.; Jaiswal, A.K.; Jaiswal, S. Lipid incorporated biopolymer based edible films and coatings in food packaging: A review. Curr. Res. Food Sci. 2024, 8, 100720. [Google Scholar] [CrossRef]
- Jia, Q.; Lin, X.; Yang, Y.; Duan, B. Multifunctional edible chitin nanofibers/ferulic acid composite coating for fruit preservation. J. Polym. Sci. 2024, 62, 338–352. [Google Scholar] [CrossRef]
- Putra, P.S.U.; Adhika, D.R.; Genecya, G.; Al Madanie, M.S.; Asri, L.A.T.W. Evaluation of Chitosan-Encapsulated Lemongrass (Cymbopogon citratus) Essential Oil Nanoemulsion for Fruit Edible Coating. OpenNano 2025, 24, 100246. [Google Scholar] [CrossRef]
- Abebe, A.; Kuma, B.; Zemedu, L. Assessment of postharvest loss of avocado at producers level (case of Wolaita and KembataTembaro zones). J. Agric. Crops 2022, 8, 364–374. [Google Scholar] [CrossRef]
- Luna-Zapién, E.A.; Zegbe, J.A.; Meza-Velázquez, J.A. Applying an alginate and mucilage-based edible coating to avocado halves favors some physical attributes and consumer acceptance. J. Prof. Assoc. Cactus Dev. 2023, 25, 244–256. [Google Scholar] [CrossRef]
- Berihu, H.; Zegeye, A. Enhancement of quality and storability of avocado (Persea americana) fruit using a blend of Aloe vera gel and corn starch as surface coating. Int. J. Food Eng. Technol. 2022, 6, 21. [Google Scholar] [CrossRef]
- Garcia, F.; Davidov-Pardo, G. Recent advances in the use of edible coatings for preservation of avocados: A review. J. Food Sci. 2021, 86, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Funes, C.F.; Larach, A.; Besoain, X.; Serrano, D.D.; Hadad, C.; Pedreschi, R.; Van Nhien, A.N.; Fuentealba, C. Active coatings based on oxidized chitin nanocrystals and silk fibroins for the control of anthracnose in ‘Hass’ avocados. Int. J. Biol. Macromol. 2023, 253, 126673. [Google Scholar] [CrossRef]
- Garcia, F.; Lin, W.J.; Mellano, V.; Davidov-Pardo, G. Effect of biopolymer coatings made of zein nanoparticles and ε-polylysine as postharvest treatments on the shelf-life of avocados (Persea americana Mill. Cv. Hass). J. Agric. Food Res. 2022, 7, 100260. [Google Scholar] [CrossRef]
- Al Shaibani, F.Y.Y.; Kaur, N.; Ahmed, Z.F.R. Reducing postharvest loss by improving fruit quality, shelf life, bioactive compounds of Rutab date (Phoenix dactylifera L. Barhi) using natural elicitors. In V International Conference on Postharvest and Quality Management of Horticultural Products of Interest for Tropical Regions 1340; International Society for Horticultural Science: Leuven, Belgium, 2021; pp. 119–124. [Google Scholar] [CrossRef]
- Alotaibi, M.M.; Alsubeie, M.S.; Almuziny, M.; Alghamdi, S.A.; Alzuaibr, F.M.; Alasmari, A.; Albalawi, B.F.; Ismail, K.A.; Khalifa, S.M.; Dawood, A.S.; et al. Preserving postharvest quality of Medjool date palm fruits by edible oil emulsions application. Sustainability 2024, 16, 5528. [Google Scholar] [CrossRef]
- Akhavan, H.R.; Hosseini, F.S.; Amiri, S.; Radi, M. Cinnamaldehyde-loaded nanostructured lipid carriers extend the shelf life of date palm fruit. Food Bioprocess Technol. 2021, 14, 1478–1489. [Google Scholar] [CrossRef]
- Aboryia, M.S.; Omar, A.S. Effectiveness of some edible coatings on storage ability of Zaghloul date palm fruits. J. Plant Prod. 2020, 11, 1477–1485. [Google Scholar] [CrossRef]
- Samra, N.R.; Shalan, A.M.; Basma, T.E. Maintaining storability of Brahee date palm fruits with postharvest edible coating by using alginate salts. J. Plant Prod. 2019, 10, 983–993. [Google Scholar] [CrossRef]
- Rahemi, M.; Roustai, F.; Sedaghat, S. Use of edible coatings to preserve date fruits (Phoenix dactylofera L.). J. Packag. Technol. Res. 2020, 4, 79–84. [Google Scholar] [CrossRef]
- Khafar, E.A.A.; Al-Jahani, G.M.; Alhajji, H.A.; Anean, H.E.A. Novel in nano-edible films applications in the production of high-quality dates Al Hulwah and Soukari for export. EC Nutr. 2023, 18, 4–24. [Google Scholar] [CrossRef]
- Kucuker, E.; Aglar, E.; Sakaldaş, M.; Şen, F.; Gundogdu, M. Impact of postharvest putrescine treatments on phenolic compounds, antioxidant capacity, organic acid contents and some quality characteristics of fresh fig fruits during cold storage. Plants 2023, 12, 1291. [Google Scholar] [CrossRef]
- Sortino, G.; Guccione, E.; Casales, F.G.; de Chiara, M.L.V.; Passafiume, R.; Gallotta, A.; Allegra, A. Application of Opuntia ficus-indica mucilage and aloe gel-based edible coating to enhance postharvest quality and microbiological aspects of fresh figs (Ficus carica L.). Horticulturae 2024, 10, 482. [Google Scholar] [CrossRef]
- Samad, A.; Makhbar, S.; Sharifulazar, H.; Basri, A.M.; Lim, S.A. The effects of Diplazium esculentum Retz. and Stenochlaena palustris incorporated with sodium alginate as edible coating on packaged figs (Ficus carica L.): A preliminary study. J. Food Process. Preserv. 2022, 46, e16611. [Google Scholar] [CrossRef]
- Paolucci, M.; Di Stasio, M.; Sorrentino, A.; La Cara, F.; Volpe, M.G. Active edible polysaccharide-based coating for preservation of fresh figs (Ficus carica L.). Foods 2020, 9, 1793. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, S.C.; Ventura-Aguilar, R.I.; Bautista-Baños, S.; Barrera-Necha, L.L. Biodegradable chitosan coating for improving quality and controlling Alternaria alternata growth in figs. World J. Adv. Res. Rev. 2020, 7, 115–125. [Google Scholar] [CrossRef]
- Singh, A.; Singh, B.K.; Barman, K.; Singh, A.K. Effect of pre-storage edible coating on postharvest quality of guava fruits cv. Lalit under ambient condition. Int. J. Environ. Clim. Change 2023, 13, 1136–1148. [Google Scholar] [CrossRef]
- Supa, S.A.; Howlader, P.; Ali, M.; Rupa, R.A.; Bose, S.K. Kumar Edible coatings maintained postharvest quality and increased shelf life of guava fruits. J. Hortic. Postharvest Res. 2024, 7, 15–34. [Google Scholar] [CrossRef]
- Kumari, J.; Pooja. Optimisation of edible coating conditions using statistical Response Surface Methodology for shelf life extension of fresh-cut guava. Acta Aliment. 2023, 52, 163–176. [Google Scholar] [CrossRef]
- Singh, M.; Saroj, R.; Kaur, D. Optimized chitosan edible coating for guava and its characterization. Meas. Food 2024, 14, 100145. [Google Scholar] [CrossRef]
- Ting, U.H.; Abidin, M.Z.; Abdullah, M.S.; Ab Razak, A.F.; Salleh, M.H.; Basri, S.M. Optimisation of Edible Coating to Improve the Postharvest Shelf-life of Guava Using Response Surface Methodology. ASM Sci. J. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Zaidi, M.; Akbar, A.; Ali, S.; Akram, H.; Ercisli, S.; Ilhan, G.; Sakar, E.; Marc, R.A.; Sonmez, D.A.; Ullah, R.; et al. Application of plant-based edible coatings and extracts influences the postharvest quality and shelf life potential of “Surahi” guava fruits. ACS Omega 2023, 8, 19523–19531. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, V.; Singh, J. Enhancing shelf life of winter guava “Allahabad Safeda” fruits using nanoemulsion coatings. J. Food Chem. Nanotechnol. 2023, 9 (Suppl. S1), S594–S601. [Google Scholar] [CrossRef]
- Coyotl-Pérez, W.A.; Morales-Rabanales, Q.N.; Lozoya-Gloria, E.; Becerra-Martínez, E.; Ramírez-García, S.A.; Mosso-González, C.; Villa-Ruano, N. Fungistatic films containing cinnamon essential oil: New coatings to preserve the nutraceutical content of avocado fruit against fusariosis. Chem. Biodivers. 2022, 19, e202200441. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.d.J.; Ocampo-López, J.; Reyes-Munguía, A.; Carrillo-Inungaray, M.L.; Cawood, M.; Medina-Pérez, G.; Fernández-Luqueño, F.; Campos-Montiel, R.G. Influence of bioactive compounds incorporated in a nanoemulsion as coating on avocado fruits (Persea americana) during postharvest storage: Antioxidant activity, physicochemical changes and structural evaluation. Antioxidants 2019, 8, 500. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT 2021, 152, 112346. [Google Scholar] [CrossRef]
- Karthi, J.S.; Johar, V.; Singh, V.; Rani, S. Edible coatings: Innovation to improve the shelf life of guava. Int. J. Plant Soil Sci. 2023, 35, 125–135. [Google Scholar] [CrossRef]
- Shanta, S.S.; Ahmed, T.; Jubayer, F.; Sharma, M.; Sridhar, K.; Hoque, M.; Rana, R.; Inbaraj, B.S. Effect of Taro corm mucilage and black seed oil as edible coatings on the shelf-life and quality of fresh guava. Agronomy 2023, 13, 538. [Google Scholar] [CrossRef]
- Luna-Zapién, E.A.; Minjares-Fuentes, R.; Sierra-Campos, E.; Marszalek, J.E.; Barraza-Guerrero, S.I.; Meza-Velazquez, J.A. Conservation of commercial quality and bioactive compounds of guava pieces by application of an alginate-acemannan coating. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13080. [Google Scholar] [CrossRef]
- Zeraatgar, H.; Davarynejad, G.H.; Moradinezhad, F.; Abedi, B. Preharvest application effect of salicylic acid and calcium nitrate on physicochemical characteristics of fresh jujube fruit (Ziziphus jujuba. Mill) during storage. Erwerbs-Obstbau 2019, 61, 119–127. [Google Scholar] [CrossRef]
- Rout, C.K.; Singh, S. Efficacy of edible coatings on jujube (Ziziphus mauritiana lamk.) fruits: A review. Plant Arch. 2021, 21, 2665–2669. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Dong, Y.; Zhi, H.; Zong, W. Tea polyphenols incorporated into alginate-based edible coating for quality maintenance of Chinese winter jujube under ambient temperature. LWT 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Naeimi, A.; Farhangfar, H. Influence of edible coatings on postharvest quality of fresh Chinese jujube fruits during refrigerated storage. J. Hortic. Postharvest Res. 2018, 1, 1–14. [Google Scholar]
- Islam, A.; Acıkalın, R.; Ozturk, B.; Aglar, E.; Kaiser, C. Combined effects of Aloe vera gel and modified atmosphere packaging treatments on fruit quality traits and bioactive compounds of jujube (Ziziphus jujuba Mill.) fruit during cold storage and shelf life. Postharvest Biol. Technol. 2022, 187, 111855. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, Y.; Tang, Y.; Hu, Q.; Kong, D.; Xiao, W.; Gan, L.; Huang, J.; Zhang, Y. An alginate coating with networks of crosslink and percolation for preservation of litchi. Available SSRN 4578113 2023. [CrossRef]
- Shubham, N.K.; Rai, R.; Dongariyal, A.; Kumar, R.; Pratap, T. Effect of edible coating and packaging on physiological and sensory attributes of litchi (Litchi chinensis Sonn.) fruits. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 517–526. [Google Scholar] [CrossRef]
- Kumar, N.; Neeraj, P.; Singla, M. Enhancement of storage life and quality maintenance of litchi (Litchi chinensis Sonn.) fruit using chitosan: Pullulan blend antimicrobial edible coating. Int. J. Fruit Sci. 2020, 20 (Suppl. S3), S1662–S1680. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Ercisli, S.; Ilhan, G.; Marc, R.A.; Skrovankova, S.; Mlcek, J. Improvement of postharvest quality and bioactive compounds content of persimmon fruits after hydrocolloid-based edible coating application. Horticulturae 2022, 8, 1045. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ali, S.; Canan, I. Postharvest application of gum Arabic edible coating delays ripening and maintains quality of persimmon fruits during storage. J. Food Process. Preserv. 2020, 44, e14583. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Mosa, W.F.A.; Ali, S.; Sardar, H.; Ali, M.M.; Ullah, S.; Ali, H.M.; Lisek, A.; Anjum, M.A. Biocomposite coatings delay senescence in stored Diospyros kaki fruits by regulating antioxidant defence mechanism and delaying cell wall degradation. Horticulturae 2023, 9, 351. [Google Scholar] [CrossRef]
- Sortino, G.; Allegra, A.; Gallotta, A.; Saletta, F.; Passafiume, R.; Gaglio, R.; Inglese, P.; Farina, V. Effects of combinational use of controlled atmosphere, cold storage and edible coating applications on shelf life and quality attributes of fresh-cut persimmon fruit. Chem. Biol. Technol. Agric. 2022, 9, 60. [Google Scholar] [CrossRef]
- Salem, M.F.; Tayel, A.A.; Alzuaibr, F.M.; Bakr, R.A. Innovative approach for controlling black rot of persimmon fruits by means of nanobiotechnology from nanochitosan and rosmarinic acid-mediated selenium nanoparticles. Polymers 2022, 14, 2116. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Mahdavi, S.; Mirzazadeh, M.; Alam, Z.; Solaimanimehr, S. The effect of chitosan coating combined with cold plasma on the quality and safety of pistachio during storage. Food Sci. Nutr. 2023, 11, 4296–4307. [Google Scholar] [CrossRef]
- Moslehi, Z.; Mohammadi Nafchi, A.; Moslehi, M.; Jafarzadeh, S. Aflatoxin, microbial contamination, sensory attributes, and morphological analysis of pistachio nut coated with methylcellulose. Food Sci. Nutr. 2021, 9, 2576–2584. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Shakerardekani, A.; Mirdehghan, S.H. Effect of alginate coating enriched with Shirazi thyme essential oil on quality of the fresh pistachio (Pistacia vera L.). J. Food Sci. Technol. 2021, 58, 34–43. [Google Scholar] [CrossRef]
- Molamohammadi, H.; Pakkish, Z.; Akhavan, H.R.; Saffari, V.R. Effect of salicylic acid incorporated chitosan coating on shelf life extension of fresh in-hull pistachio fruit. Food Bioprocess Technol. 2020, 13, 121–131. [Google Scholar] [CrossRef]
- Nazoori, F.; Afrashteh, S.; Mirdehghan, S.H. Impacts of carboxymethyl cellulose-based coatings with calcium oxide and GABA on storage life and quality maintenance of fresh pistachio fruit. J. Hortic. Sci. Biotechnol. 2023, 98, 365–373. [Google Scholar] [CrossRef]
- Khajeh-Ali, S.; Shahidi, F.; Sedaghat, N. Evaluation of the effect of carboxy methyl cellulose edible coating containing Astragalus honey (Astragalus gossypinus) on the shelf-life of pistachio kernel. Food Control 2022, 139, 109094. [Google Scholar] [CrossRef]
- Razali, N.A.; Sargent, S.A.; Sims, C.A.; Brecht, J.K.; Berry, A.D.; Cheng, G. Potential of postharvest coatings to maintain freshness of red-fleshed pitaya (Hylocereus costaricensis). Agriculture 2021, 11, 892. [Google Scholar] [CrossRef]
- Espinal-Hernández, P.; Colinas-León, M.T.; Ybarra-Moncada, M.C.; Méndez-Zúñiga, S.M.; Corrales-García, J. Postharvest effects of 1-mcp and chitosan/oleic acid coating in pitaya (Stenocereus griseus H.). J. Prof. Assoc. Cactus Dev. 2021, 23, 43–57. [Google Scholar] [CrossRef]
- Utama, N.A.; Setiawan, C.K.; Fajri, I. Effect of alginate based edible coating enriched with vanilla essential oil on shelf-life of fresh-cut red pitaya (Hylocereus polyrhizus). In IOP Conference Series: Earth and Environmental Science, Second International Conference on Sustainable Agriculture 30–31 July 2019, Yogyakarta, Indonesia; IOP Publishing: Bristol, UK, 2020; Volume 458, p. 012046. [Google Scholar] [CrossRef]
- Ranjbari, F.; Moradinezhad, F.; Khayyat, M. Efficacy of nitric oxide and film wrapping on quality maintenance and alleviation of chilling injury on pomegranate fruit. J. Agric. Sci. Technol. 2018, 20, 1025–1036. [Google Scholar]
- Dorostkar, M.; Moradinezhad, F. Postharvest quality responses of pomegranate fruit (cv. Shishe-kab) to ethanol, sodium bicarbonate dips and modified atmosphere packaging. Adv. Hortic. Sci. 2022, 36, 107–117. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ranjbar, A. Advances in postharvest diseases management of fruits and vegetables: A review. Horticulturae 2023, 9, 1099. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ranjbar, A. Role of plant growth regulators and eco-friendly postharvest treatments on alleviating chilling injury and preserving quality of pomegranate fruit and arils: A review. J. Plant Growth Regul. 2024, 43, 1368–1383. [Google Scholar] [CrossRef]
- Mincuzzi, A.; Picciotti, U.; Sanzani, S.M.; Garganese, F.; Palou, L.; Addante, R.; Ragni, M.; Ippolito, A. Postharvest diseases of pomegranate: Alternative control means and a Spiderweb effect. J. Fungi 2023, 9, 808. [Google Scholar] [CrossRef]
- Mwelase, S.; Kaseke, T.; Fawole, O.A. Development and optimization of methylcellulose-based edible coating using response surface methodology for improved quality management of ready-to-eat pomegranate arils. CyTA-J. Food 2023, 21, 656–665. [Google Scholar] [CrossRef]
- Anean, H.A.; Mallasiy, L.O.; Bader, D.M.; Shaat, H.A. Nano edible coatings and films combined with zinc oxide and pomegranate peel active phenol compounds has been to extend the shelf life of minimally processed pomegranates. Materials 2023, 16, 1569. [Google Scholar] [CrossRef]
- Ali, H.W.R.; Aljabary, A.M.A.O. Maintenance of pomegranate fruit quality by coating with flaxseed, black seed oils, and chitosan during different storage periods. Iraqi J. Agric. Sci. 2023, 54, 1689–1702. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ansarifar, E.; Mohammadian Moghaddam, M. Extending the shelf life and maintaining quality of minimally-processed pomegranate arils using ascorbic acid coating and modified atmosphere packaging. J. Food Meas. Charact. 2020, 14, 3445–3454. [Google Scholar] [CrossRef]
- Seifi, E.; Bekran, A. The effect of some edible coating treatments on shelf life of pomegranate arils cultivar “Malas-e Saveh”. J. Hortic. Postharvest Res. 2024, 7, 35–46. [Google Scholar] [CrossRef]
- Su, X.; Lin, H.; Fu, B.; Mei, S.; Lin, M.; Chen, H.; Zheng, Z.; Bo, H.; Yang, D.-P.; Lin, Y. Egg-yolk-derived carbon dots@ albumin bio-nanocomposite as multifunctional coating and its application in quality maintenance of fresh litchi fruit during storage. Food Chem. 2023, 405, 134813. [Google Scholar] [CrossRef]
- Fernández-Catalán, A.; Palou, L.; Taberner, V.; Grimal, A.; Argente-Sanchis, M.; Pérez-Gago, M.B. Hydroxypropyl methylcellulose-based edible coatings formulated with antifungal food additives to reduce Alternaria black spot and maintain postharvest quality of cold-stored ‘Rojo Brillante’ persimmons. Agronomy 2021, 11, 757. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Mirdehghan, S.H.; Shakerardekani, A.; Golding, J.B. Incorporation of Zataria multiflora Boiss essential oil into gum Arabic edible coating to maintain the quality properties of fresh in-hull pistachio (Pistacia vera L.). Food Packag. Shelf Life 2021, 30, 100724. [Google Scholar] [CrossRef]
- Tavakolipour, H.; Kalbasi-Ashtari, A.; Mokhtarian, M. Effects of coating pistachio kernels with mixtures of whey protein and selected herbal plant extracts on growth inhibition of Aspergillus flavus and prevention of aflatoxin during storage. J. Food Saf. 2020, 40, e12711. [Google Scholar] [CrossRef]
- Hernández-Valencia, C.G.; Román-Guerrero, A.; Aguilar-Santamaría, Á.; Cira, L.; Shirai, K. Cross-linking chitosan into hydroxypropylmethylcellulose for the preparation of neem oil coating for postharvest storage of pitaya (Stenocereus pruinosus). Molecules 2019, 24, 219. [Google Scholar] [CrossRef]
- Mwelase, S.; Fawole, O.A. Application of chitosan-based edible coating enriched with 24-epibrassinolide to maintain quality of late-harvested pomegranate fruit (Â WonderfulÂ). In V International Symposium on Pomegranate and Minor Mediterranean Fruits 1349; International Society for Horticultural Science: Leuven, Belgium, 2022; pp. 669–678. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Khayyat, M.; Saeb, H. Combination effects of postharvest treatments and modified atmosphere packaging on shelf life and quality of Iranian pomegranate fruit cv. Sheshi-kab. Int. J. Postharvest Technol. Innov. 2013, 3, 244–256. [Google Scholar] [CrossRef]
- Mokhtarizadeh Naeini, M.; Jafari, A.; Gholamnejad, J.; Vazifeshenas, M. Optimizing shelf life of pomegranate fruits during traditional storage by Tragacanth gum coating. J. Hortic. Postharvest Res. 2020, 3, 49–60. [Google Scholar]
- Radev, R. Edible films and coatings for food products-advantages and disadvantages. Известия на Съюза на учените-Варна. Серия Икoнoмически науки 2021, 10, 43–51. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha Prasad, J.; Yadav, A.; Upadhyay, A.; Neeraj Shukla, S.; Trajkovska Petkoska, A.; Heena Suri, S.; Gniewosz, M.; Kieliszek, M. Recent trends in edible packaging for food applications—Perspective for the future. Food Eng. Rev. 2023, 15, 718–747. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-based functional films for packaging applications: A review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef] [PubMed]
- Duguma, H.T. Potential applications and limitations of edible coatings for maintaining tomato quality and shelf life. Int. J. Food Sci. Technol. 2022, 57, 1353–1366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).